Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Colon Cancer from Primary Tumor and Liver Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Samples
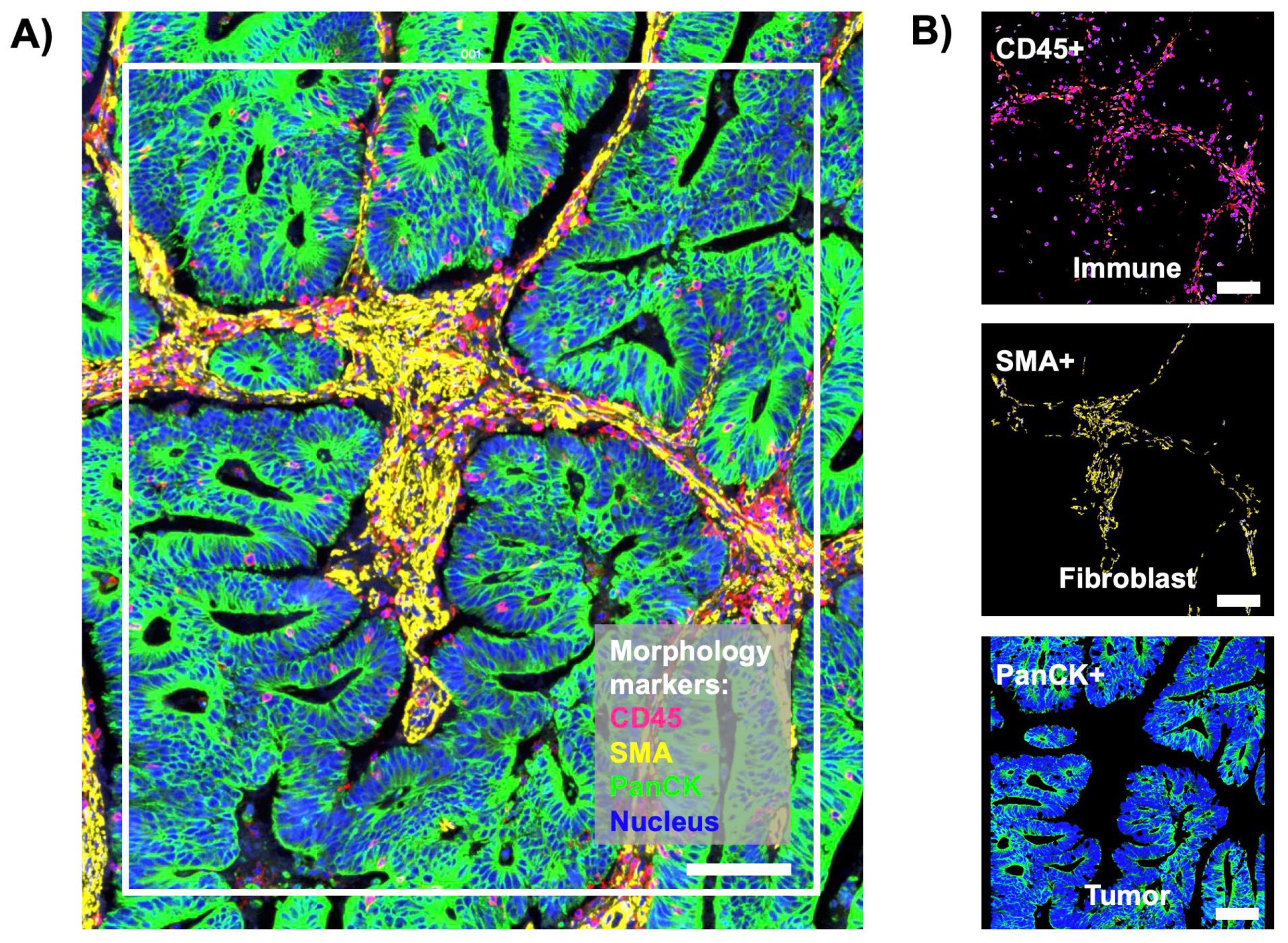
2.2. Spatial Transcriptomic Analysis
2.3. IHC Staining of TSPAN8 Protein in Primary and Metastatic Liver Tissues of Stage 4 CC
3. Results
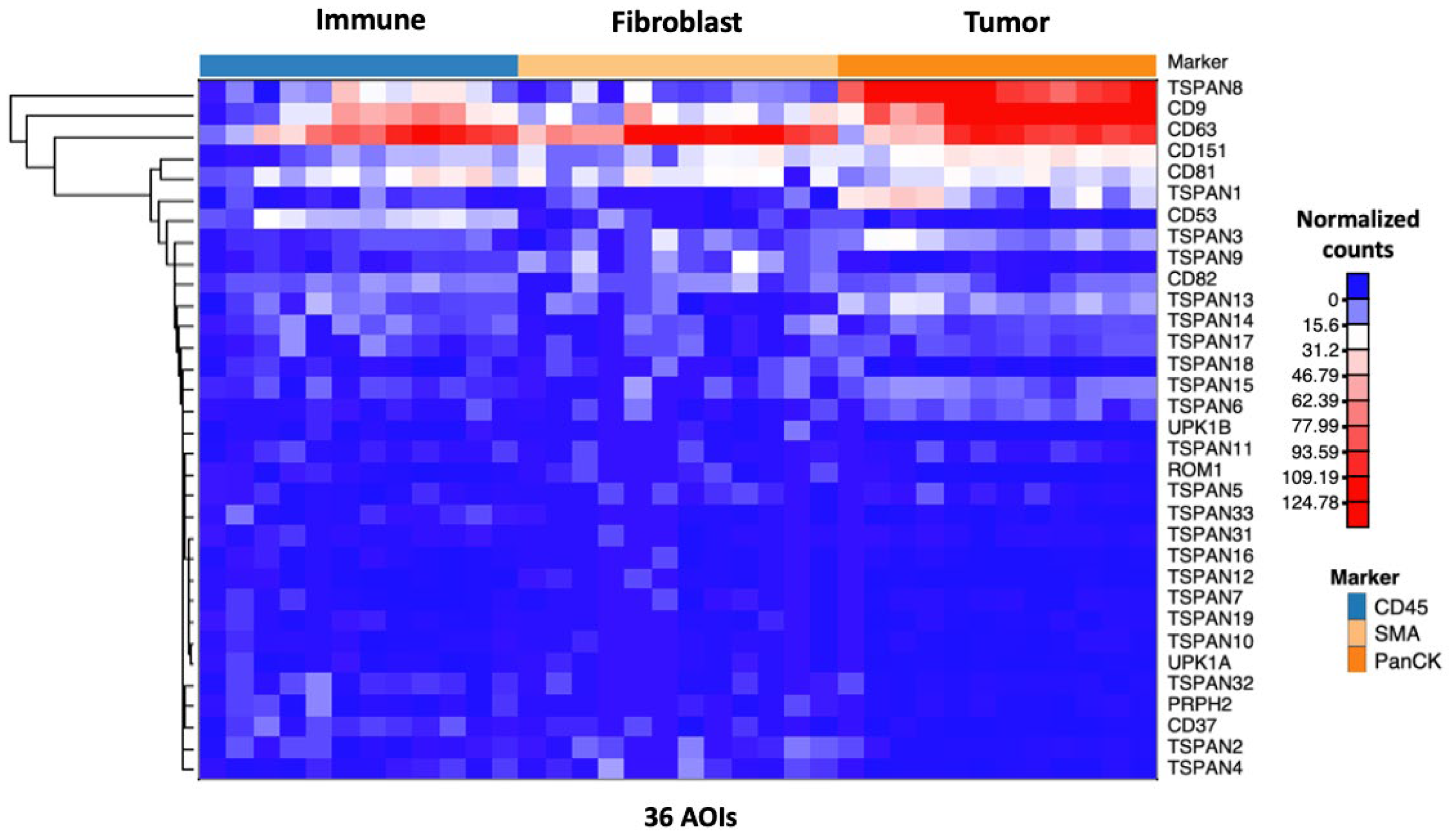
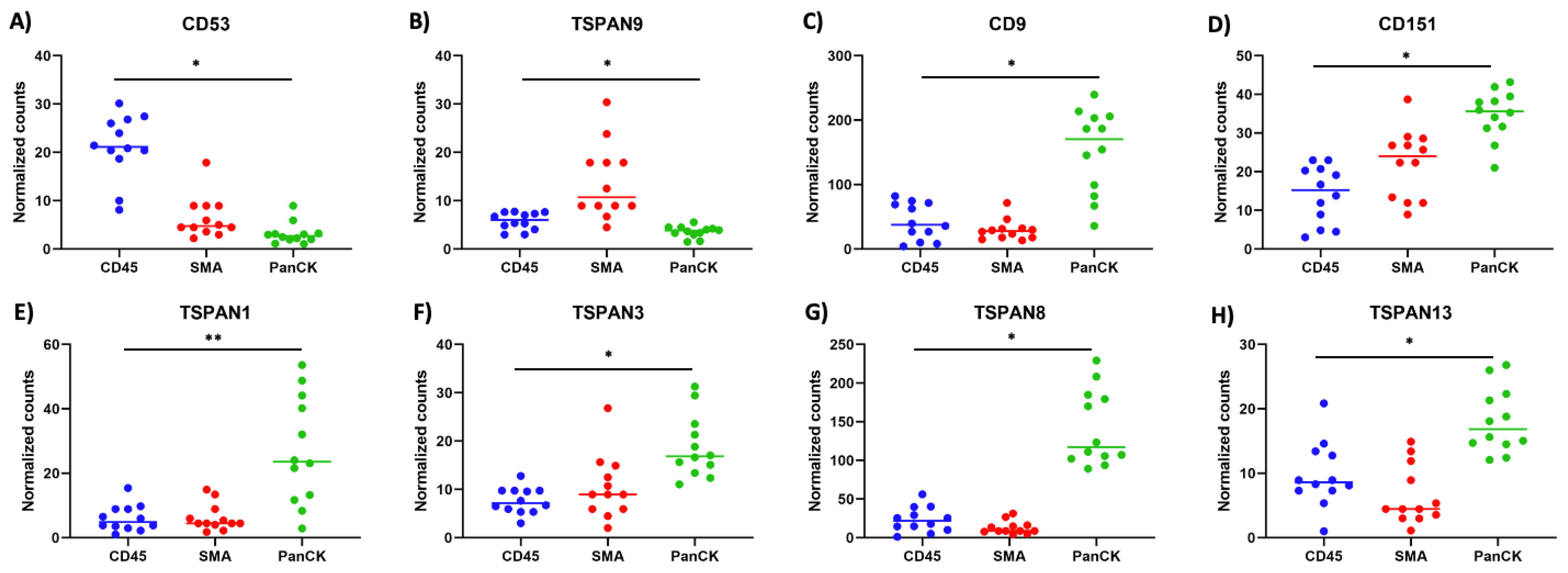
3.1. Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Primary CC Tissues
3.2. Spatially Resolved Differential Gene Expression Levels of Tetraspanins in Primary and Metastatic Liver Tissues of Stage 4 CC
3.3. IHC Staining of TSPAN8 Protein in Primary and Metastatic Liver Tissues of Stage 4 CC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Dominguez, O.; Yilmaz, S.; Steele, S.R. Stage IV Colorectal Cancer Management and Treatment. J. Clin. Med. 2023, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Duong, H.Q. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127 Pt 17, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, Z.; Zhou, L.; Yang, M.; He, S. The versatile roles of testrapanins in cancer from intracellular signaling to cell-cell communication: Cell membrane proteins without ligands. Cell Biosci. 2023, 13, 59. [Google Scholar] [CrossRef]
- Suwatthanarak, T.; Usuba, K.; Kuroha, K.; Tanaka, M.; Okochi, M. Screening of EWI-2-Derived Peptides for Targeting Tetraspanin CD81 and Their Effect on Cancer Cell Migration. Biomolecules 2023, 13, 510. [Google Scholar] [CrossRef]
- Titu, S.; Grapa, C.M.; Mocan, T.; Balacescu, O.; Irimie, A. Tetraspanins: Physiology, Colorectal Cancer Development, and Nanomediated Applications. Cancers 2021, 13, 5662. [Google Scholar] [CrossRef]
- Suwatthanarak, T.; Ito, K.; Tanaka, M.; Sugiura, K.; Hoshino, A.; Miyamoto, Y.; Miyado, K.; Okochi, M. A peptide binding to the tetraspanin CD9 reduces cancer metastasis. Biomater. Adv. 2023, 146, 213283. [Google Scholar] [CrossRef]
- Roh, S.; Kim, S.; Hong, I.; Lee, M.; Kim, H.J.; Ahn, T.S.; Kang, D.H.; Baek, M.J.; Kwak, H.J.; Kim, C.J.; et al. High Expression of Tetraspanin 5 as a Prognostic Marker of Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 6476. [Google Scholar] [CrossRef]
- Maisonial-Besset, A.; Witkowski, T.; Navarro-Teulon, I.; Berthier-Vergnes, O.; Fois, G.; Zhu, Y.; Besse, S.; Bawa, O.; Briat, A.; Quintana, M.; et al. Tetraspanin 8 (TSPAN 8) as a potential target for radio-immunotherapy of colorectal cancer. Oncotarget 2017, 8, 22034–22047. [Google Scholar] [CrossRef]
- Zhang, H.S.; Liu, H.Y.; Zhou, Z.; Sun, H.L.; Liu, M.Y. TSPAN8 promotes colorectal cancer cell growth and migration in LSD1-dependent manner. Life Sci. 2020, 241, 117114. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Li, M.; Yang, L.; Han, Y.; Liu, C.; Zhang, B.; Wu, M.; Wang, G.; Zhang, Z.; et al. CD151 promotes Colorectal Cancer progression by a crosstalk involving CEACAM6, LGR5 and Wnt signaling via TGFβ1. Int. J. Biol. Sci. 2021, 17, 848–860, Erratum in Int. J. Biol. Sci. 2023, 19, 3290–3291. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; You, L.; Hardillo, J.A.U.; Chien, M.P. Spatial Transcriptomic Technologies. Cells 2023, 12, 2042. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Ke, R. Spatial Transcriptomics for Tumor Heterogeneity Analysis. Front. Genet. 2022, 13, 906158. [Google Scholar] [CrossRef] [PubMed]
- Tanjak, P.; Chaiboonchoe, A.; Suwatthanarak, T.; Acharayothin, O.; Thanormjit, K.; Chanthercrob, J.; Suwatthanarak, T.; Wannasuphaphol, B.; Chumchuen, K.; Suktitipat, B.; et al. The KRAS-Mutant Consensus Molecular Subtype 3 Reveals an Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Cancers 2023, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, S.N.; Goh, D.; Cheung, C.C.L.; Nga, P.Q.Y.; Lim, J.C.T.; Yeong, J.P.S. Transcriptional Spatial Profiling of Cancer Tissues in the Era of Immunotherapy: The Potential and Promise. Cancers 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Nadel, B.B.; Lopez, D.; Montoya, D.J.; Ma, F.; Waddel, H.; Khan, M.M.; Mangul, S.; Pellegrini, M. The Gene Expression Deconvolution Interactive Tool (GEDIT): Accurate cell type quantification from gene expression data. Gigascience 2021, 10, giab002. [Google Scholar] [CrossRef]
- Nakano, A.; Harada, T.; Morikawa, S.; Kato, Y. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta Pathol. Jpn. 1990, 40, 107–115. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.; Sun, K.H.; Zhou, L. α-smooth muscle actin is not a marker of fibrogenic cell activity in skeletal muscle fibrosis. PLoS ONE 2018, 13, e0191031. [Google Scholar] [CrossRef]
- Menz, A.; Gorbokon, N.; Viehweger, F.; Lennartz, M.; Hube-Magg, C.; Hornsteiner, L.; Kluth, M.; Völkel, C.; Luebke, A.M.; Fraune, C.; et al. Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors. Int. J. Surg. Pathol. 2023, 31, 927–938. [Google Scholar] [CrossRef]
- Huan, J.; Gao, Y.; Xu, J.; Sheng, W.; Zhu, W.; Zhang, S.; Cao, J.; Ji, J.; Zhang, L.; Tian, Y. Overexpression of CD9 correlates with tumor stage and lymph node metastasis in esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3054–3061. [Google Scholar] [PubMed]
- Chen, L.; Zhu, Y.Y.; Zhang, X.J.; Wang, G.L.; Li, X.Y.; He, S.; Zhang, J.B.; Zhu, J.W. TSPAN1 protein expression: A significant prognostic indicator for patients with colorectal adenocarcinoma. World J. Gastroenterol. 2009, 15, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.C.; Doran, P.P.; Macmathuna, P. In Silico Promoter Analysis can Predict Genes of Functional Relevance in Cell Proliferation: Validation in a Colon Cancer Model. Transl. Oncogenomics 2007, 2, 1–16. [Google Scholar] [PubMed]
- Li, P.; Dong, M.; Wang, Z. Downregulation of TSPAN13 by miR-369-3p inhibits cell proliferation in papillary thyroid cancer (PTC). Bosn. J. Basic Med. Sci. 2019, 19, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Piao, L.; Wang, J.; Guo, L.; Wang, J.; Wang, L.; Tong, L.; Yuan, X.; Han, X.; Fang, S.; et al. Tspan9 Induces EMT and Promotes Osteosarcoma Metastasis via Activating FAK-Ras-ERK1/2 Pathway. Front. Oncol. 2022, 12, 774988. [Google Scholar] [CrossRef]
- Schaper, F.; van Spriel, A.B. Antitumor Immunity Is Controlled by Tetraspanin Proteins. Front. Immunol. 2018, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef]
- Bonnet, M.; Maisonial-Besset, A.; Zhu, Y.; Witkowski, T.; Roche, G.; Boucheix, C.; Greco, C.; Degoul, F. Targeting the Tetraspanins with Monoclonal Antibodies in Oncology: Focus on Tspan8/Co-029. Cancers 2019, 11, 179. [Google Scholar] [CrossRef]
- Dunlock, V.E. Tetraspanin CD53: An overlooked regulator of immune cell function. Med. Microbiol. Immunol. 2020, 209, 545–552. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Z.; Zhang, N.; Zhu, C.; Lin, X.; Yu, J.; Chen, Z.; Lan, P.; Wan, Y. Increase in CD4+FOXP3+ regulatory T cell number and upregulation of the HGF/c-Met signaling pathway during the liver metastasis of colorectal cancer. Oncol. Lett. 2020, 20, 2113–2118. [Google Scholar] [CrossRef]
- Ahrends, T.; Borst, J. The opposing roles of CD4+ T cells in anti-tumour immunity. Immunology 2018, 154, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Gong, J.; Zhou, Z.; Tian, D.; Wang, Z. TCF4 enhances hepatic metastasis of colorectal cancer by regulating tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death Dis. 2021, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef] [PubMed]
- Pein, M.; Oskarsson, T. Microenvironment in metastasis: Roadblocks and supportive niches. Am. J. Physiol. Cell Physiol. 2015, 309, C627–C638. [Google Scholar] [CrossRef]
- Yang, H.T.; Chien, M.Y.; Chiang, J.H.; Lin, P.C. Literature-based translation from synthetic lethality screening into therapeutics targets: CD82 is a novel target for KRAS mutation in colon cancer. Comput. Struct. Biotechnol. J. 2022, 20, 5287–5295. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Shen, X.; Cui, X.; Guo, Y. Identification of novel biomarkers and small molecule drugs in human colorectal cancer by microarray and bioinformatics analysis. Mol. Genet. Genom. Med. 2019, 7, e00713. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Li, G.; Chen, D.; Zhou, Q. Upregulation of TSPAN12 is associated with the colorectal cancer growth and metastasis. Am. J. Transl. Res. 2017, 9, 812–822. [Google Scholar]
- Liu, Y.; Ding, L.; Li, C.; Heng, L.; Chen, J.; Hou, Y. UPK1B promoted the invasion and stem cell characteristics of non-small cell lung cancer cells by modulating c-myc/Sox4 axis. Tissue Cell 2023, 85, 102250. [Google Scholar] [CrossRef]
- Andrijes, R.; Hejmadi, R.K.; Pugh, M.; Rajesh, S.; Novitskaya, V.; Ibrahim, M.; Overduin, M.; Tselepis, C.; Middleton, G.W.; Győrffy, B.; et al. Tetraspanin 6 is a regulator of carcinogenesis in colorectal cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2011411118. [Google Scholar] [CrossRef]
- Arencibia, J.M.; Martín, S.; Pérez-Rodríguez, F.J.; Bonnin, A. Gene expression profiling reveals overexpression of TSPAN13 in prostate cancer. Int. J. Oncol. 2009, 34, 457–463. [Google Scholar] [CrossRef][Green Version]
- Ma, X.; Qiu, S.; Tang, X.; Song, Q.; Wang, P.; Wang, J.; Xia, Q.; Wang, Z.; Zhao, Q.; Lu, M. TSPAN31 regulates the proliferation, migration, and apoptosis of gastric cancer cells through the METTL1/CCT2 pathway. Transl. Oncol. 2022, 20, 101423. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Ma, X.J.; Xu, D.; Luo, J. UPK1B promotes the invasion and metastasis of bladder cancer via regulating the Wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5471–5480. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882.e11, Erratum in Cancer Cell 2021, 39, 883. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.D.; Moseley, G.W.; van Spriel, A.B. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens 2004, 64, 533–542. [Google Scholar] [CrossRef]
- Levy, S.; Shoham, T. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 2005, 5, 136–148. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, M.; Wu, L. Spatial transcriptomics technology in cancer research. Front. Oncol. 2022, 12, 1019111. [Google Scholar] [CrossRef]
- Liu, H.T.; Chen, S.Y.; Peng, L.L.; Zhong, L.; Zhou, L.; Liao, S.Q.; Chen, Z.J.; Wang, Q.L.; He, S.; Zhou, Z.H. Spatially resolved transcriptomics revealed local invasion-related genes in colorectal cancer. Front. Oncol. 2023, 13, 1089090. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwatthanarak, T.; Thanormjit, K.; Suwatthanarak, T.; Acharayothin, O.; Methasate, A.; Chinswangwatanakul, V.; Tanjak, P. Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Colon Cancer from Primary Tumor and Liver Metastasis. Life 2024, 14, 126. https://doi.org/10.3390/life14010126
Suwatthanarak T, Thanormjit K, Suwatthanarak T, Acharayothin O, Methasate A, Chinswangwatanakul V, Tanjak P. Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Colon Cancer from Primary Tumor and Liver Metastasis. Life. 2024; 14(1):126. https://doi.org/10.3390/life14010126
Chicago/Turabian StyleSuwatthanarak, Thanawat, Kullanist Thanormjit, Tharathorn Suwatthanarak, Onchira Acharayothin, Asada Methasate, Vitoon Chinswangwatanakul, and Pariyada Tanjak. 2024. "Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Colon Cancer from Primary Tumor and Liver Metastasis" Life 14, no. 1: 126. https://doi.org/10.3390/life14010126
APA StyleSuwatthanarak, T., Thanormjit, K., Suwatthanarak, T., Acharayothin, O., Methasate, A., Chinswangwatanakul, V., & Tanjak, P. (2024). Spatial Transcriptomic Profiling of Tetraspanins in Stage 4 Colon Cancer from Primary Tumor and Liver Metastasis. Life, 14(1), 126. https://doi.org/10.3390/life14010126





