Special Staining and Protein Expression of VEGF/EGFR and P53/NF-κB in Cryptorchid Tissue of Erhualian Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Materials
2.3. Preparation and Staining of Paraffin Sections of Testicular Tissue
2.4. Counting of the Number of Convoluted Tubules
2.5. Measurement of the Diameter of Convoluted Tubules
2.6. Protein Extraction and Detection
2.7. Immunohistochemical Detection of VEGF/EGFR and P53/NF-κB
2.8. Immunofluorescent Detection of VEGF/EGFR and P53/NF-κB
2.9. Data Statistics and Analysis
3. Results
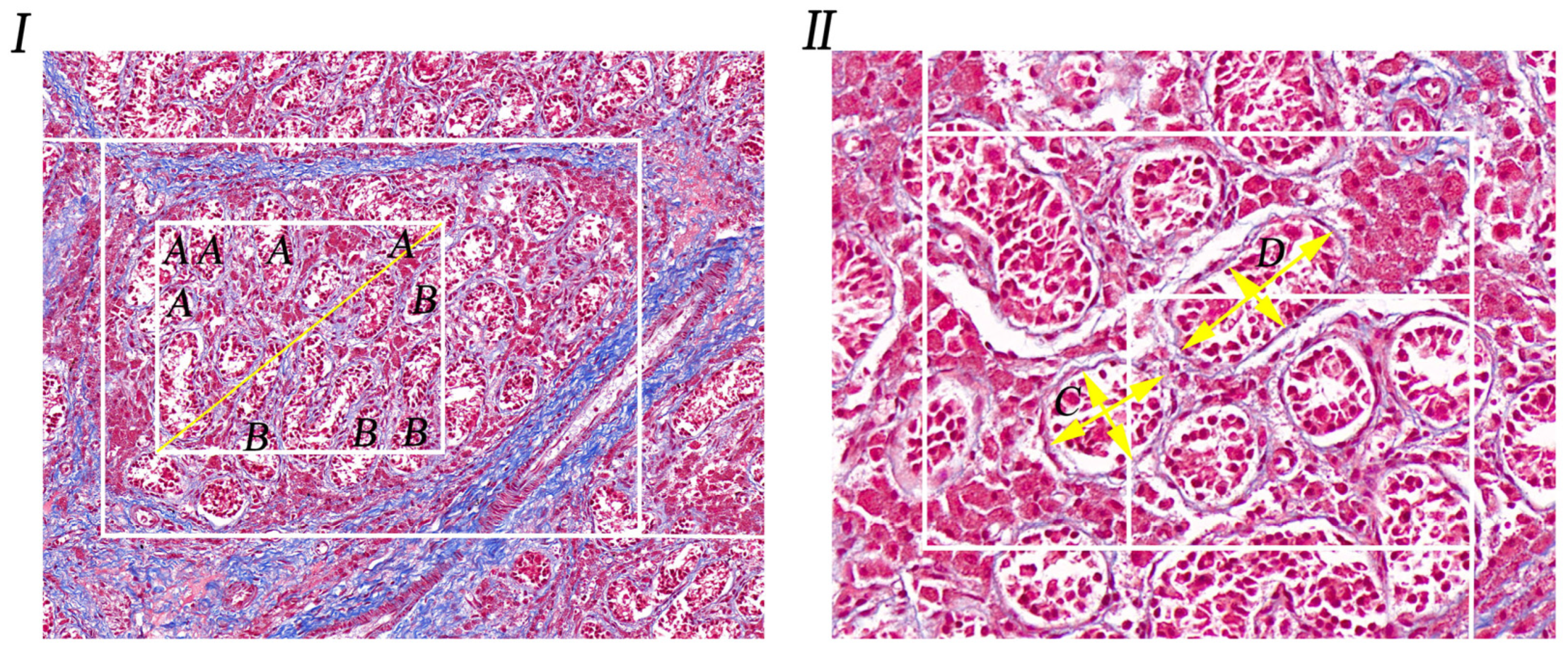
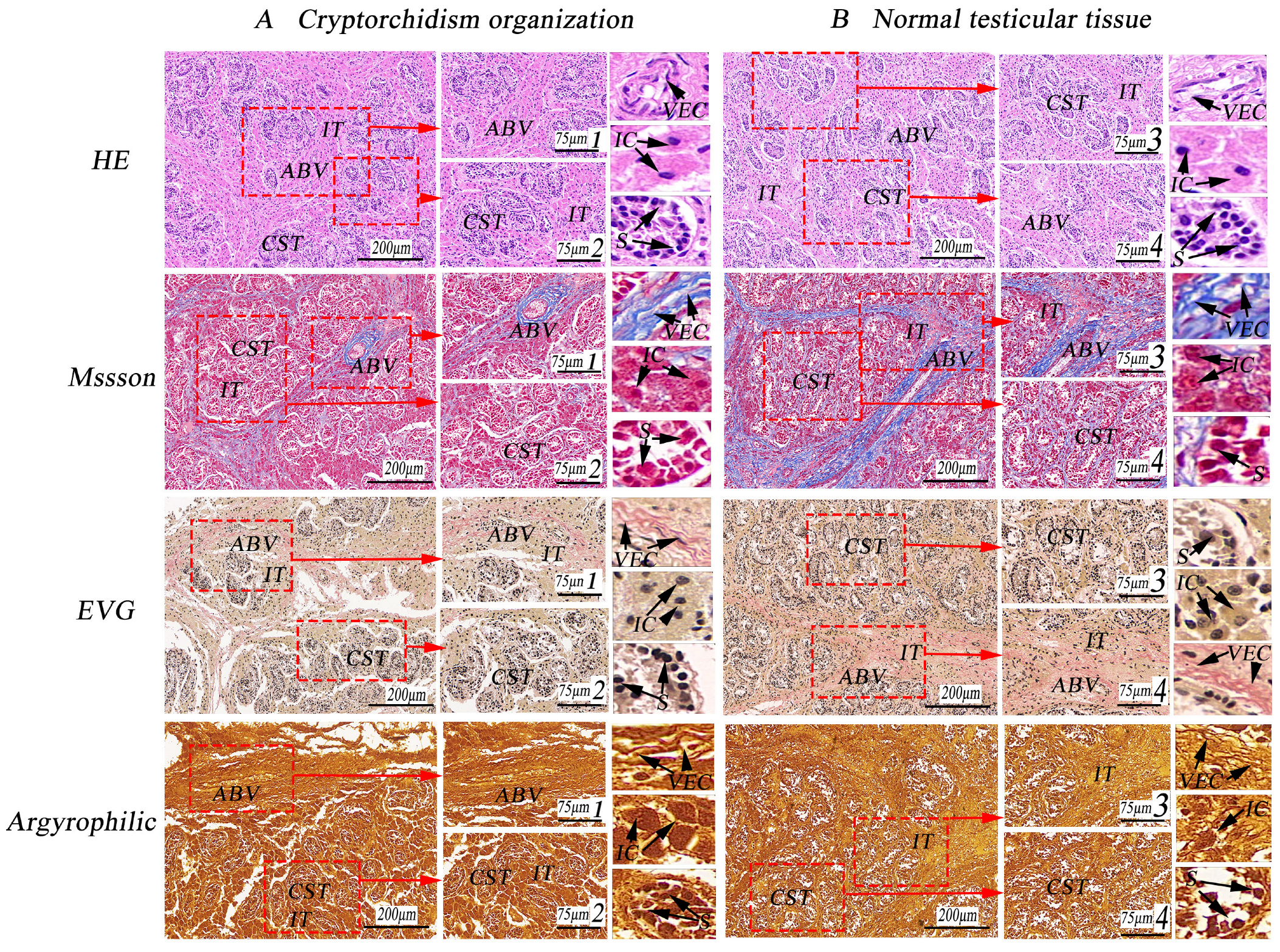
3.1. Observation of Special Staining of Cryptorchid Tissue
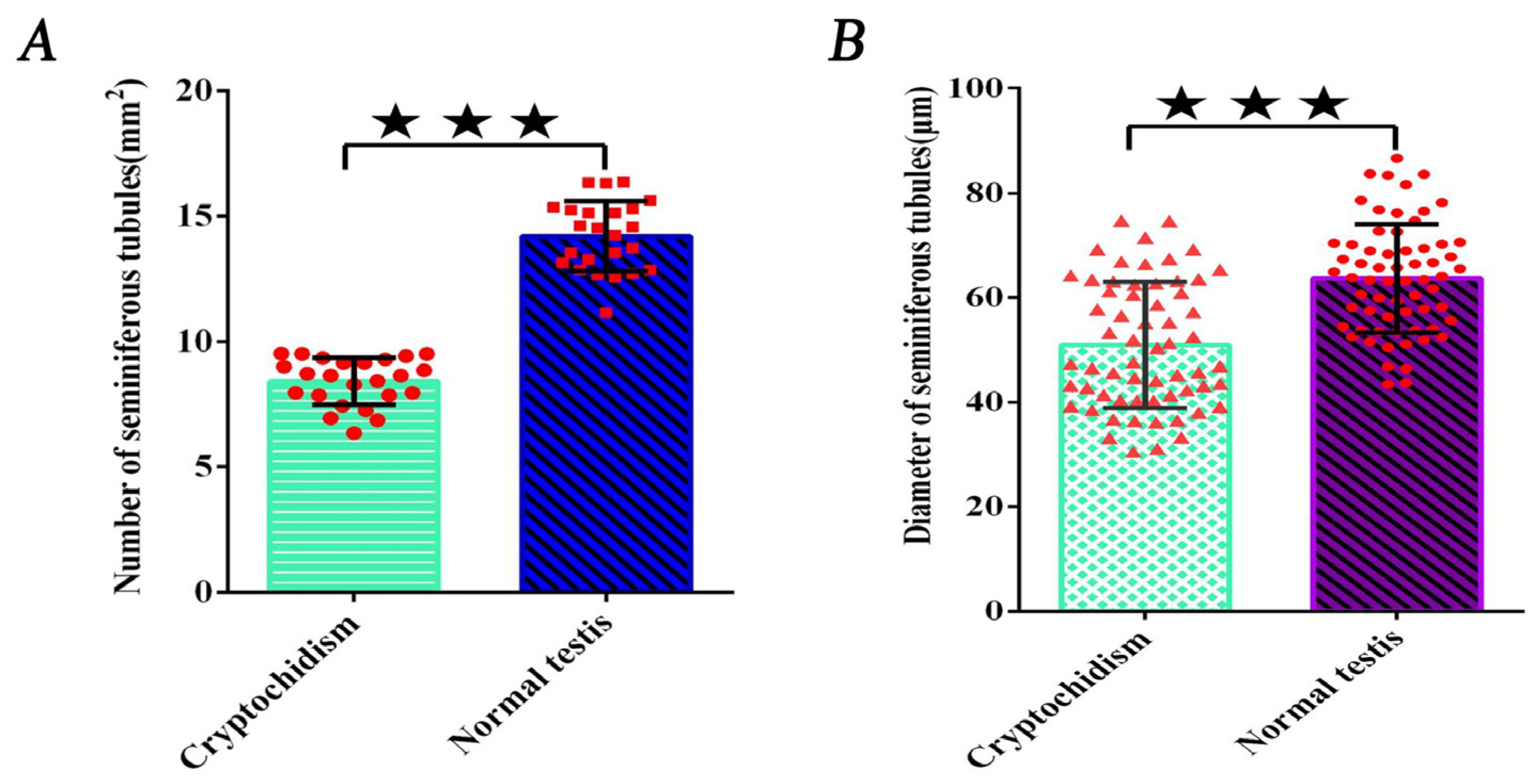
3.2. Counting the Number of Convoluted Tubules
3.3. Measurement of the Diameter of Convoluted Tubules
3.4. Analysis of VEGF/EGFR and P53/NF-κB Protein Expression
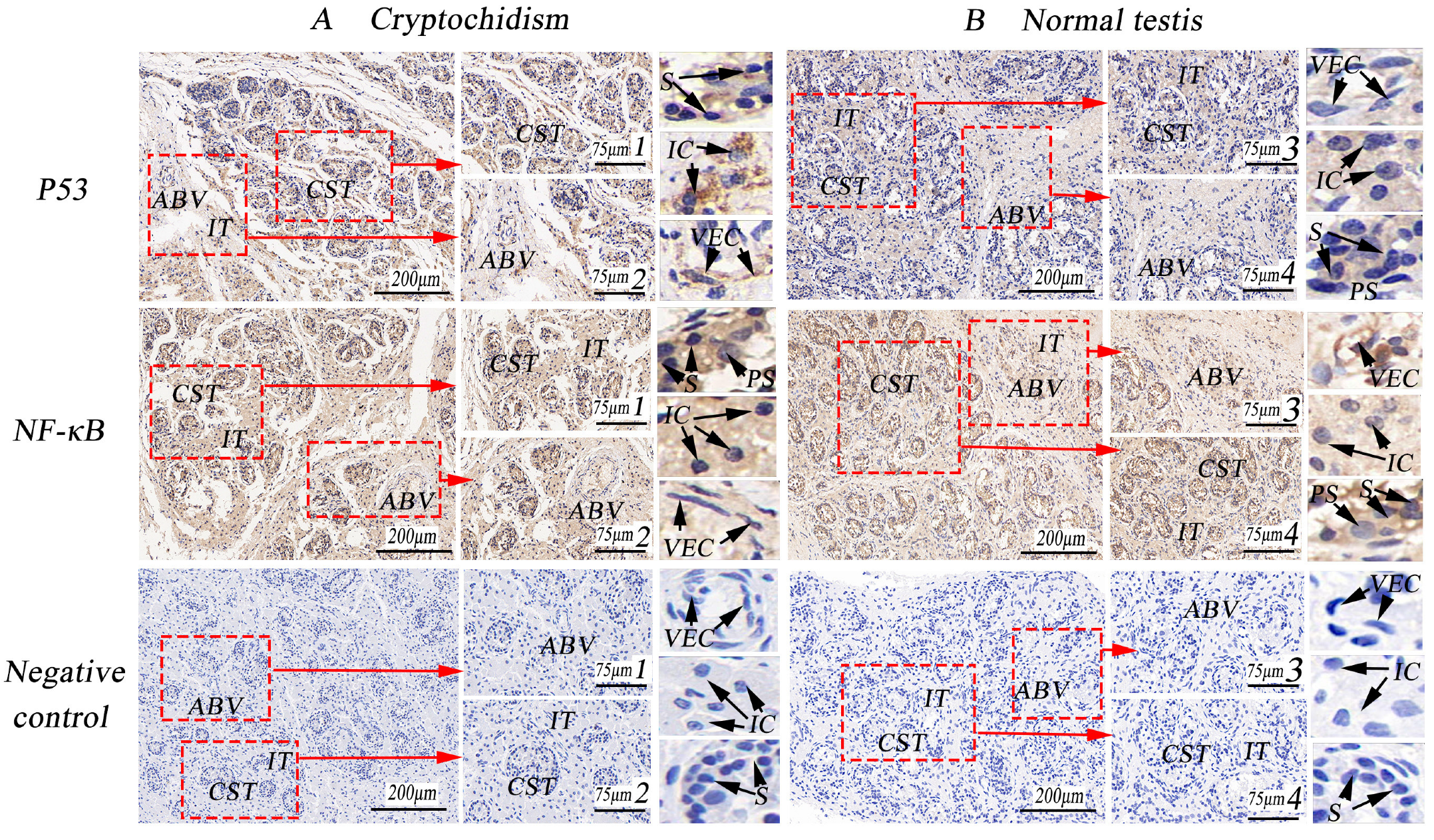
3.5. Immunohistochemical Expression Analysis of VEGF/EGFR and P53/NF-κB
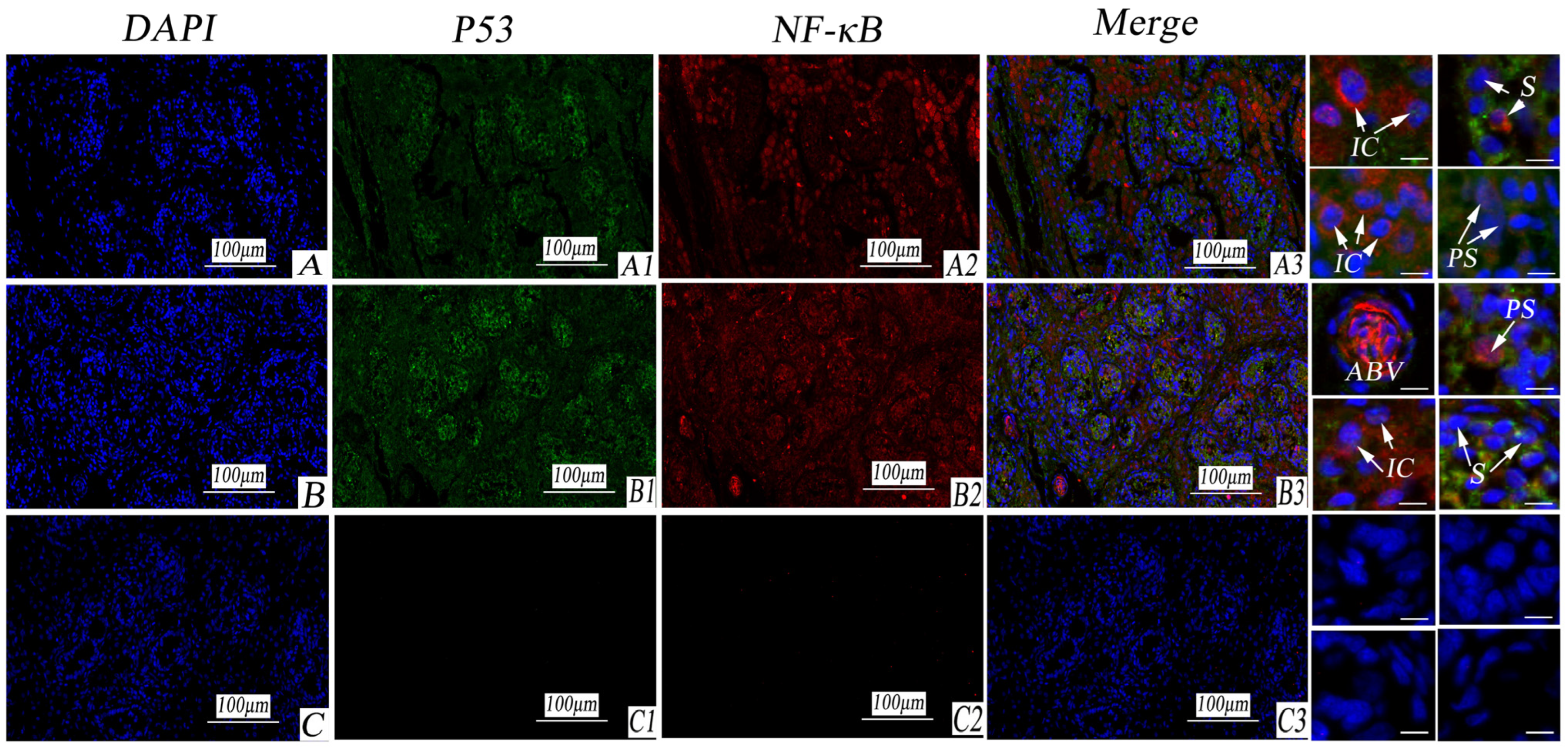
3.6. Immunofluorescence Expression Analysis of VEGF/EGFR and P53/NF-κB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.B.; López-Cortegano, E.; Oyelami, F.O.; Zhang, Z.; Ma, P.P.; Wang, Q.S.; Pan, Y.C. Conservation priorities analysis of Chinese indigenous pig breeds in the Taihu Lake Region. Front. Genet. 2021, 12, 242–251. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015, 108, 98–104. [Google Scholar] [CrossRef]
- Paul, C.; Teng, S.; Saunders, P. A Single, Mild, Transient Scrotal Heat Stress Causes Hypoxia and Oxidative Stress in Mouse Testes, Which Induces Germ Cell Death. Biol. Reprod. 2009, 80, 913–919. [Google Scholar] [CrossRef]
- Weng, B.; Ran, M.L.; Chen, B.; He, C.Q.; Dong, L.H.; Peng, F.Z. Genome-wide analysis of long non-coding RNAs and their role in postnatal porcine testis development. Genomics 2017, 109, 446–456. [Google Scholar] [CrossRef]
- Sargent, K.M.; Lu, N.X.; Clopton, D.T.; Pohlmeier, W.E.; Brauer, V.M.; Ferrara, N.; Silversides, D.W.; Cupp, A.S. Loss of vascular endothelial growth factor A (VEGFA) isoforms in granulosa cells using pDmrt-1-Cre or Amhr2-Cre reduces fertility by arresting follicular development and by reducing litter size in female mice. PLoS ONE 2015, 10, e0116332. [Google Scholar] [CrossRef]
- Del, V.F.; Poels, J.; Vermeulen, M.; Ucakar, B.; Giudice, M.G.; Kanbar, M.; des Rieux, A.; Wyns, C. Accelerated and improved vascular maturity after transplantation of testicular tissue in hydrogels supplemented with VEGF-and PDGF-loaded nanoparticles. Int. J. Mol. Sci. 2021, 22, 5779–5796. [Google Scholar]
- Nazari, A.; Valizadeh, M.R.; Hassanshahi, G.; Khorramdelazad, H. Expression of vascular endothelial growth factor and its receptors in infertile men with varicocele. J. Reprod. Immunol. 2020, 140, 103131–103135. [Google Scholar] [CrossRef]
- Gualdoni, G.S.; Jacobo, P.V.; Sobarzo, C.M.; Pérez, C.V.; Durand LA, H.; Theas, M.S.; Lustig, L.; Guazzone, V.A. Relevance of angiogenesis in autoimmune testis inflammation. Mol. Hum. Reprod. 2021, 27, gaaa073. [Google Scholar] [CrossRef]
- Barthold, J.S.; Kryger, J.V.; Derusha, A.M.; Duel, B.P.; Jednak, R.; Skafar, D.F. Effects of an environmental endocrine disruptor on fetal development, estrogen receptor alpha and epidermal growth factor receptor expression in the porcine male genital tract. J. Urol. 1999, 162, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.S.; Lu, R.K.; Chen, Z.D. Clinical significance of EGF and EGFR expression changes in cryptorchid boys. Asian J. Androl. 2002, 4, 255–258. [Google Scholar] [PubMed]
- Thirouard, L.; Holota, H.; Monrose, M.; Garcia, M.; Haze, A.; Damon-Soubeyrand, C.; Renaud, Y.; Saru, J.P.; Perino, A.; Schoonjans, K.; et al. Identification of a Crosstalk among TGR5, GLIS2, and TP53 Signaling Pathways in the Control of Undifferentiated Germ Cell Homeostasis and Chemoresistance. Adv. Sci. 2022, 9, 2200626–2200649. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hu, J.; Li, Y.; Zhao, J.; Li, Z.; Liu, X.; Yao, L.; Zhang, Y. Altered expression of NDRG2 in the testes of experimental rat model of cryptorchidism. Urology 2010, 75, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Huang, H.; Lu, S.; Ou, B.; Feng, J.; Shan, W.; Li, H.; Wang, Z.; Hong, A.; Ma, Y. PACAP ameliorates fertility in obese male mice via PKA/CREB pathway-dependent Sirt1 activation and p53 deacetylation. J. Cell. Physiol. 2020, 235, 7465–7483. [Google Scholar] [CrossRef]
- Shen, H.; Liao, K.; Wu, H.F.; Lu, H.C.; Li, Y.; Li, Z.; Zhang, W. In utero exposure of high-dose di-n-butyl phthalate resulted in opposite effects on testicular cell apoptosis in late embryonic and pubertal male rat offspring. Hum. Exp. Toxicol. 2017, 36, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.M.; Andreassen, C.H.; Jørgensen, A.; Nielsen, J.E.; Juel, M.L.; Boisen, I.M.; Schwarz, P.; Toppari, J.; Baron, R.; Lanske, B. RANKL regulates male reproductive function. Nat. Commun. 2021, 12, 2450. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H.; et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J. Cell. Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef]
- Tang, X.; Li, D.; Zhao, T.; Zhu, S.; Gao, X.; Zhou, R.; Deng, F.; Fu, W.; Jia, W.; Liu, G. The inhibition of CFTR in the descended testis of SD rats with unilateral cryptorchidism induced by di-(2-ethylhexyl) phthalate (DEHP). Environ. Sci. Pollut. Res. 2022, 29, 77047–77056. [Google Scholar] [CrossRef]
- Chen, J.; Fok, K.L.; Chen, H.; Zhang, X.H.; Xu, W.M.; Chan, H.C. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2. Hum. Reprod. 2012, 27, 2585–2597. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Nyengaard, J.R.; Mayhew, T.M. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc. Pathol. 2009, 19, 133–135. [Google Scholar] [CrossRef]
- Charleston, J.S.; Hansen, K.R.; Thyer, A.C.; Charleston, L.B.; Gougeon, A.; Siebert, J.R.; Klein, N.A. Estimating human ovarian non-growing follicle number: The application of modern stereology techniques to an old problem. Hum. Reprod. 2007, 22, 2103–2110. [Google Scholar] [CrossRef][Green Version]
- Song, X. Preliminary Study on Expression and Distribution of Immune-Related Regulatory Factors in Testis and Cryptorchidism of Tibetan Sheep in Plateau Pastoral Area. Master Thesis, Gansu Agricultural University, Lanzhou, China, 2018. (In Chinese). [Google Scholar]
- Chen, S.M.; Yang, W.; Zhang, X.Y.; Jin, J.Y.; Liang, C.; Wang, J.D.; Zhang, J.H. Melamine induces reproductive dysfunction via down-regulated the phosphorylation of p38 and downstream transcription factors Max and Sap1a in mice testes. Sci. Total Environ. 2021, 770, 144727–144738. [Google Scholar] [CrossRef]
- Yuan, L.G.; Lu, Y.R.; Tao, J.Z.; Zhang, Y. Histochemical characteristics and ultrastructural comparison of extracellular matrix associated proteins in testis and cryptorchidism of Bactrian came. Theriol. Sin. 2017, 37, 189–199. [Google Scholar]
- Hu, J.; Qiu, B.; Wang, S.; Qu, B.J.; Wang, F.Q.; Zhang, X.; Ying, Y. A Case of Histopathological Observation of Inborn Cryptorchid in Beagle Dog. Prog. Vet. Med. 2016, 37, 133–135. [Google Scholar]
- Nakada, T.; Nagai, R.; Mizumachi, S.; Ookura, N.; Uechi, S.; Tatemoto, H.; Kawamoto, H. Effects of the induced crytorchidism on rat testicular histology. In The Science Bulletin of the College of Agriculture; University of Ryukyus: Nakagami-gun, Japan, 2009; pp. 43–47. [Google Scholar]
- Cho, Y.M.; Chou, J.C.; Fang, C.M.; Hu, S.; Wang, K.L.; Wang, S.W.; Wang, P.S. Chronic intermittent hypoxia stimulates testosterone production in rat Leydig cells. Life Sci. 2019, 233, 116694. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; Mokhtar, D.M.; Fernández-Ortiz, M.; Escames, G.; Acuña-Castroviejo, D. Retinoid-related orphan nuclear receptor alpha (RORα)-deficient mice display morphological testicular defects. Lab. Investig. 2019, 99, 1835–1849. [Google Scholar] [CrossRef]
- Gorowska-Wojtowicz, E.; Duliban, M.; Kotula-Balak, M.; Bilinska, B. Modulatory Effects of Estradiol and Its Mixtures with Ligands of GPER and PPAR on MAPK and PI3K/Akt Signaling Pathways and Tumorigenic Factors in Mouse Testis Explants and Mouse Tumor Leydig Cells. Biomedicines 2022, 10, 1390–1412. [Google Scholar] [CrossRef]
- Nalbandian, A.; Dettin, L.; Dym, M.; Ravindranath, N. Expression of vascular endothelial growth factor receptors during male germ cell differentiation in the mouse. Biol. Reprod. 2003, 69, 985–994. [Google Scholar] [CrossRef][Green Version]
- Tian, W.J.; Jeon, S.H.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Piao, J.J.; Xin, Z.C.; Chen, Y.G.; Feng, H.Y.; et al. Effect of Li-ESWT on Testicular Tissue and Function in Androgen-Deficient Rat Model. Oxidative Med. Cell. Longev. 2022, 2022, 5213573. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, L.J.; de Cuevas, M.; Le, K.H.; Viveiros, J.M.; Matunis, E.L. Activation of the EGFR/MAPK pathway drives transdifferentiation of quiescent niche cells to stem cells in the Drosophila testis niche. Elife 2022, 11, e70810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhu, Q.; Yuan, K.; Su, Z.; Ge, F.; Ge, R.S.; Huang, Y. Epidermal growth factor regulates the development of stem and progenitor Leydig cells in rats. J. Cell. Mol. Med. 2020, 24, 7313–7330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, X.; Qiao, C.; Wang, M.; Chen, W.; Luan, X.; Yan, Y.; Shen, C.; Fang, J.; Hu, X.; et al. Somatic CG6015 mediates cyst stem cell maintenance and germline stem cell differentiation via EGFR signaling in Drosophila testes. Cell Death Discov. 2021, 7, 68. [Google Scholar] [CrossRef]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Testis-specific isoform of Na/K-ATPase (ATP1A4) Interactome in raft and non-raft membrane fractions from capacitated bovine sperm. Int. J. Mol. Sci. 2019, 20, 3159–3173. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 2021, 263, 128161–128171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Wu, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Multiple transcriptomic profiling: p53 signaling pathway is involved in DEHP-induced prepubertal testicular injury via promoting cell apoptosis and inhibiting cell proliferation of Leydig cells. J. Hazard. Mater. 2021, 406, 124316–124325. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, B.; Xu, S.; Wang, J.; Gao, L.; Song, Y.P.; Lv, J.W.; Xu, F.X.; Li, J.; Chen, J.; et al. Reactive oxygen species-evoked genotoxic stress mediates arsenic-induced suppression of male germ cell proliferation and decline in sperm quality. J. Hazard. Mater. 2021, 406, 124768–124779. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tang, D.; Cao, S.; Li, T.; Gao, H.; Ma, T.; Yao, T.; Li, J.; Chang, B. Effect of different exercise loads on testicular oxidative stress and reproductive function in obese male mice. Oxidative Med. Cell. Longev. 2020, 2020, 3071658. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, X.; Liu, Y.; Li, Y.; Long, S.; Gu, C.; Ye, J.; Xie, S.; Cao, S. Japanese Encephalitis Virus infection induces inflammation of swine testis through RIG-I—NF-ĸB signaling pathway. Vet. Microbiol. 2019, 238, 108430–108440. [Google Scholar] [CrossRef]
- Mai, H.N.; Chung, Y.H.; Shin, E.J.; Jeong, J.H.; Jung, T.W.; Sharma, N.; Lei, X.G.; Nah, S.Y.; Jang, C.G.; Kim, D.J.; et al. Overexpression of glutathione peroxidase-1 attenuates cocaine-induced reproductive dysfunction in male mice by inhibiting nuclear factor κB. Chem. Biol. Interact. 2019, 307, 136–146. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Yadegari, P.; Imani, A.; Taghdir, M. Vitamin D3 protects against lead-induced testicular toxicity by modulating Nrf2 and NF-κB genes expression in rat. Reprod. Toxicol. 2021, 103, 36–45. [Google Scholar] [CrossRef]




| Number | Cryptochidism (Number/mm2) | Normal Testis (Number/mm2) |
|---|---|---|
| Sample 1 | 8.62 ± 0.71 | 13.74 ± 1.04 |
| Sample 2 | 7.87 ± 1.14 | 14.58 ± 0.31 |
| Sample 3 | 8.89 ± 0.81 | 13.36 ± 0.83 |
| Sample 4 | 7.66 ± 0.57 | 15.11 ± 0.61 |
| Sample 5 | 8.54 ± 1.05 | 14.07 ± 1.24 |
| Mean value | 8.31 ± 0.86 A | 14.17 ± 0.81 B |
| Diameter | Cryptochidism (μm) | Normal Testis (μm) |
|---|---|---|
| Sample 1 | 47.03 ± 6.02 | 71.13 ± 7.31 |
| Sample 2 | 42.83 ± 2.79 | 60.23 ± 3.35 |
| Sample 3 | 55.01 ± 3.97 | 61.33 ± 8.51 |
| Sample 4 | 57.87 ± 2.13 | 62.08 ± 4.11 |
| Sample 5 | 47.77 ± 3.65 | 62.49 ± 5.87 |
| Mean value | 50.11 ± 3.71 A | 63.45 ± 5.83 B |
| Integral Optical Density (IntDen/Area) | ||
|---|---|---|
| Cryptochidism | Normal Testis | |
| VEGF | 3.0754 ± 0.3725 A | 1.1502 ± 0.3254 B |
| EGFR | 1.1682 ± 0.2148 A | 3.4133 ± 0.4945 B |
| P53 | 3.6564 ± 0.3127 A | 1.4251 ± 0.1347 B |
| NF-κB | 4.7085 ± 0.5241 A | 5.1317 ± 0.3124 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Shao, Y.; Liu, C.; Lv, X.; Afedo, S.Y.; Bao, W. Special Staining and Protein Expression of VEGF/EGFR and P53/NF-κB in Cryptorchid Tissue of Erhualian Pigs. Life 2024, 14, 100. https://doi.org/10.3390/life14010100
Liu P, Shao Y, Liu C, Lv X, Afedo SY, Bao W. Special Staining and Protein Expression of VEGF/EGFR and P53/NF-κB in Cryptorchid Tissue of Erhualian Pigs. Life. 2024; 14(1):100. https://doi.org/10.3390/life14010100
Chicago/Turabian StyleLiu, Penggang, Yiming Shao, Caihong Liu, Xiaoyang Lv, Seth Yaw Afedo, and Wenbin Bao. 2024. "Special Staining and Protein Expression of VEGF/EGFR and P53/NF-κB in Cryptorchid Tissue of Erhualian Pigs" Life 14, no. 1: 100. https://doi.org/10.3390/life14010100
APA StyleLiu, P., Shao, Y., Liu, C., Lv, X., Afedo, S. Y., & Bao, W. (2024). Special Staining and Protein Expression of VEGF/EGFR and P53/NF-κB in Cryptorchid Tissue of Erhualian Pigs. Life, 14(1), 100. https://doi.org/10.3390/life14010100






