The Effects of Tocotrienol on Gut Microbiota: A Scoping Review
Abstract
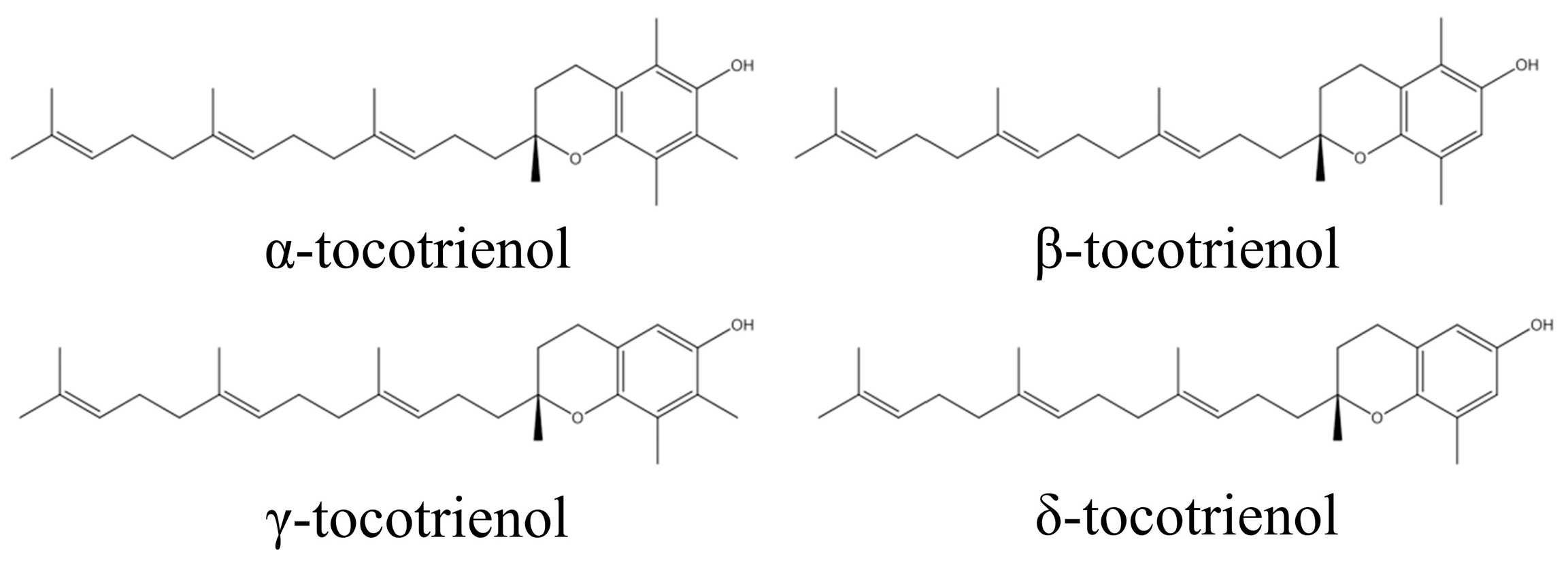
:1. Introduction
2. Methodology
2.1. Defining the Research Question
2.2. Identifying Relevant Studies
2.3. Study Selection
2.4. Extracting the Data
2.5. Collating, Summarising, and Reporting the Results
3. Results
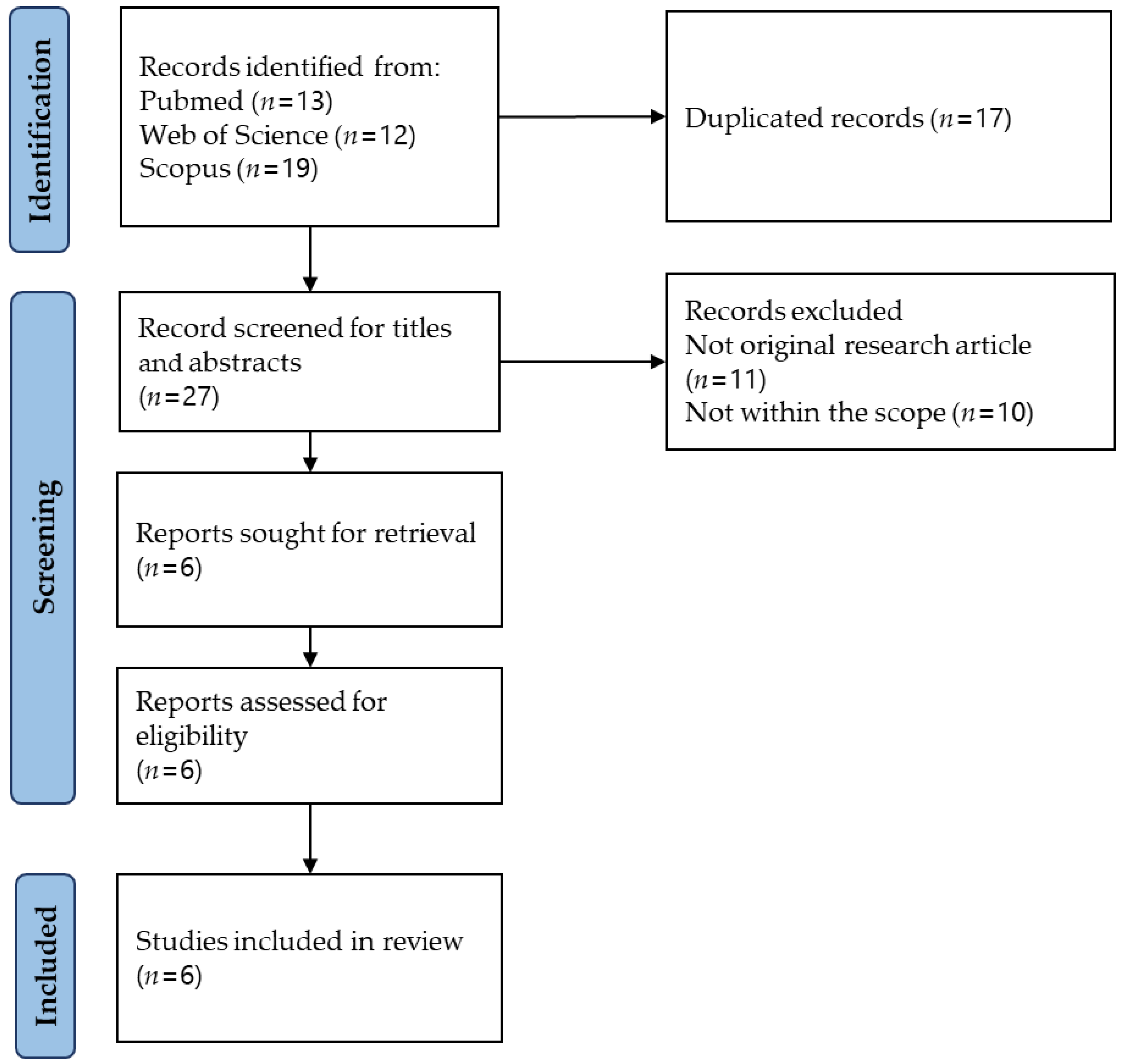
3.1. Study Selection
3.2. Study Characteristics
3.3. Study Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Cai, X.; Fei, W.; Ren, F.; Wang, F.; Luan, X.; Chen, F.; Zheng, C. Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation. Front. Pharmacol. 2020, 11, 594042. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.M.; Lampe, J.W.; Gibson, G. Targeted Approaches for In Situ Gut Microbiome Manipulation. J. Parenter. Enter. Nutr. 2020, 44, 581–588. [Google Scholar] [CrossRef]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Baumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Siranosian, B.A.; Brooks, E.F.; Andermann, T.; Rezvani, A.R.; Banaei, N.; Tang, H.; Bhatt, A.S. Rare transmission of commensal and pathogenic bacteria in the gut microbiome of hospitalized adults. Nat. Commun. 2022, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv. Nutr. 2023, 14, 840–857. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Caudet, J.; Trelis, M.; Cifre, S.; Soriano, J.M.; Rico, H.; Merino-Torres, J.F. Interplay between Intestinal Bacterial Communities and Unicellular Parasites in a Morbidly Obese Population: A Neglected Trinomial. Nutrients 2022, 14, 3211. [Google Scholar] [CrossRef]
- Huang, R.; Liu, P.; Bai, Y.; Huang, J.; Pan, R.; Li, H.; Su, Y.; Zhou, Q.; Ma, R.; Zong, S.; et al. Changes in the gut microbiota of osteoporosis patients based on 16S rRNA gene sequencing: A systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 2022, 23, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef] [PubMed]
- Saleri, R.; Borghetti, P.; Ravanetti, F.; Cavalli, V.; Ferrari, L.; De Angelis, E.; Andrani, M.; Martelli, P. Effects of different short-chain fatty acids (SCFA) on gene expression of proteins involved in barrier function in IPEC-J2. Porc. Health Manag. 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Perez-Reytor, D.; Puebla, C.; Karahanian, E.; Garcia, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Belancic, A. Gut microbiome dysbiosis and endotoxemia—Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Malavolta, M.; Pierpaoli, E.; Giacconi, R.; Basso, A.; Cardelli, M.; Piacenza, F.; Provinciali, M. Anti-inflammatory Activity of Tocotrienols in Age-related Pathologies: A SASPected Involvement of Cellular Senescence. Biol. Proced. Online 2018, 20, 22. [Google Scholar] [CrossRef]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Springer: Berlin/Heidelberg, Germany, 2016; Volume 928, pp. 97–130. [Google Scholar]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef]
- Khor, B.-H.; Tiong, H.-C.; Tan, S.C.; Wong, S.K.; Chin, K.-Y.; Karupaiah, T.; Ima-Nirwana, S.; Abdul Gafor, A.H. Effects of tocotrienols supplementation on markers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2021, 16, e0255205. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory gamma- and delta-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Self-emulsified annatto tocotrienol improves bone histomorphometric parameters in a rat model of oestrogen deficiency through suppression of skeletal sclerostin level and RANKL/OPG ratio. Int. J. Med. Sci. 2021, 18, 3665–3673. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Tejpal Singh, H.S.; Aminuddin, A.A.; Pang, K.-L.; Ekeuku, S.O.; Chin, K.-Y. The Role of Tocotrienol in Arthritis Management—A Scoping Review of Literature. Pharmaceuticals 2023, 16, 385. [Google Scholar]
- Pierpaoli, E.; Orlando, F.; Cirioni, O.; Simonetti, O.; Giacometti, A.; Provinciali, M. Supplementation with tocotrienols from Bixa orellana improves the in vivo efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in a mouse model of infected wound. Phytomedicine 2017, 36, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol Inhibits Nuclear Factor-κB Signaling Pathway through Inhibition of Receptor-interacting Protein and TAK1 Leading to Suppression of Antiapoptotic Gene Products and Potentiation of Apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, T.; Xu, Y.; Luo, Y.; Zhong, X.; Shi, L.; Hu, T.; Guo, T.; Nie, Y.; Luo, F.; et al. δ-Tocotrienol, Isolated from Rice Bran, Exerts an Anti-Inflammatory Effect via MAPKs and PPARs Signaling Pathways in Lipopolysaccharide-Stimulated Macrophages. Int. J. Mol. Sci. 2018, 19, 3022. [Google Scholar] [CrossRef]
- Qureshi, A.A. Tocotrienols: Exciting Biological and Pharmacological Properties of Tocotrienols and other Naturally Occurring Compounds, Part I. Ann. Clin. Case Rep. 2022, 7, 2194. [Google Scholar] [PubMed]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFkB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Feng, L.; Xia, Y.; Garcia, G.E.; Hwang, D.; Wilson, C.B. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J. Clin. Investig. 1995, 95, 1669–1675. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Liu, P.L.; Ng, L.T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol. Nutr. Food Res. 2008, 52, 921–929. [Google Scholar] [CrossRef]
- Elmassry, M.; Chung, E.; Hamood, A.; Shen, C.L. Supplementation of Geranylgeraniol and Tocotrienols to High-Fat Diet Shifts the Gut Microbiome Composition and Function in Type 2 Diabetic Mice. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 393. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Chung, E.; Cao, J.J.; Hamood, A.N.; Shen, C.L. Osteoprotective effect of green tea polyphenols and annatto-extracted tocotrienol in obese mice is associated with enhanced microbiome vitamin K(2) biosynthetic pathways. J. Nutr. Biochem. 2020, 86, 108492. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef]
- Cossiga, V.; Lembo, V.; Nigro, C.; Mirra, P.; Miele, C.; D’Argenio, V.; Leone, A.; Mazzone, G.; Veneruso, I.; Guido, M.; et al. The Combination of Berberine, Tocotrienols and Coffee Extracts Improves Metabolic Profile and Liver Steatosis by the Modulation of Gut Microbiota and Hepatic miR-122 and miR-34a Expression in Mice. Nutrients 2021, 13, 1281. [Google Scholar] [CrossRef]
- Ran, L.; Liu, A.B.; Lee, M.J.; Xie, P.; Lin, Y.; Yang, C.S. Effects of antibiotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors 2019, 45, 450–462. [Google Scholar] [CrossRef]
- Farhana, L.; Sarkar, S.; Nangia-Makker, P.; Yu, Y.; Khosla, P.; Levi, E.; Azmi, A.; Majumdar, A.P.N. Natural agents inhibit colon cancer cell proliferation and alter microbial diversity in mice. PLoS ONE 2020, 15, e0229823. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Acute Ischemic Stroke and the Subsequent Risk for Poor Functional Outcomes. J. Parenter. Enter. Nutr. 2021, 45, 518–529. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef]
- Ikeda, T.; Kamohara, H.; Suda, S.; Nagura, T.; Tomino, M.; Sugi, M.; Wajima, Z. Comparative Evaluation of Endotoxin Activity Level and Various Biomarkers for Infection and Outcome of ICU-Admitted Patients. Biomedicines 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All About Diet? Nutr. Clin. Pract. 2015, 30, 760–779. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Gornicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Tey, B.T.; Chan, E.S.; Lai, O.M.; Chang, H.W.; Tan, T.B.; Liu, Y.; Wang, Y.; Tan, C.P. Stabilization and Release of Palm Tocotrienol Emulsion Fabricated Using pH-Sensitive Calcium Carbonate. Foods 2021, 10, 358. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ahmad, F.; Ima-Nirwana, S. Regulation of inflammatory response and oxidative stress by tocotrienol in a rat model of non-alcoholic fatty liver disease. J. Funct. Foods 2020, 74, 104209. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, X.; Lei, Y.; Xia, W.; Cai, F.; Zhu, D.; An, Y.; Xi, Y.; Niu, X.; Wang, Z.; et al. gamma-Tocotrienol inhibits T helper 17 cell differentiation via the IL-6/JAK/STAT3 signaling pathway. Mol. Immunol. 2022, 151, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Anandha Rao, J.S.; Ramdas, P.; Ng, M.H.; Kannan Kutty, M.; Selvaduray, K.R.; Radhakrishnan, A.K. Reduced infiltration of regulatory T cells in tumours from mice fed daily with gamma-tocotrienol supplementation. Clin. Exp. Immunol. 2021, 206, 161–172. [Google Scholar] [CrossRef]
- Bose, S.; Steussy, C.N.; López-Pérez, D.; Schmidt, T.; Kulathunga, S.C.; Seleem, M.N.; Lipton, M.; Mesecar, A.D.; Rodwell, V.W.; Stauffacher, C.V. Targeting Enterococcus faecalis HMG-CoA reductase with a non-statin inhibitor. Commun. Biol. 2023, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa Mdo, S.; Braga, M.F.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fan, J.; Fan, Z.; Zhang, K. γ-Tocotrienol reverses multidrug resistance of breast cancer cells through the regulation of the γ-Tocotrienol-NF-κB-P-gp axis. J. Steroid Biochem. Mol. Biol. 2021, 209, 105835. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]
| Researchers | Study Design | Findings |
|---|---|---|
| Yang et al., 2021 [53] | Animals: male BALB/c mice (6–7 weeks old) Disease model: colitis-associated colon cancer induced via i.p. injection with AOM at 9.5 mg/kg body weight and 1.5% DSS in drinking water for 1 week. Treatment: δ/γ-tocotrienol (T3) (8/1) 0.035% (~2.2 μM daily) and δ-T3-13-COOH 0.04% (~2.3 μM daily) diet; 2 months Control diet: AIN-93G diet | Gut microbiota composition: Firmicutes-to-Bacteroidetes ratio ↓ δ-T3 and δ-T3-13-COOH vs. AIN-93G diet Family ↑ Streptococccaceae in δ-T3 and δ-T3-13-COOH vs. AIN-93G diet ↑ Eubacterium coprostanoligenes in δ-T3 vs. δ-T3-13-COOH and AIN-93G ↓ Clostridiales vadinBB60 group in δT3 vs. δ-T3-13-COOH and AIN-93G Genus ANCOM analysis δ-T3-13-COOH partially ↑ in genus Roseburia in AOM/DSS ↑ Lactococcus species in δ-T3 and δ-T3-13-COOH vs. AIN-93G LEfSe analysis ↑ Parabacteroides goldsteinii CL02T12C30 and Bacteroides in δ-T3 and δ-T3-13-COOH vs. AIN-93G diet Canonical correspondence analysis (CCA) δ-T3 and δ-T3-13-COOH changed the gut microbiota composition Pro-inflammatory cytokines: ↑ granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α) and Interleukin-1 beta (IL-1β) in AOM/DSS vs. AIN-93G ↓ GM-CSF and MCP-1 in δT3-13-COOH vs. AIN-93G ↓ IL-1β in δ-T3 vs. AIN-93G Health effects: δ-T3-13-COOH reduced total and large-size tumors, δ-T3 only reduced tumor size |
| Elmassry et al., 2020 [54] | Animals: male C57BL/6J mice (5 weeks old) Disease model: high-fat diet (HFD) in obese mice Treatment: annatto tocotrienol (AT) treatment (400 mg/kg diet) and Green tea polyphenols treatment (0.5% (w/v) GTP in drinking water) for 14 weeks | Gut microbiome composition: Phylum level GTP and GTP+AT: ↓ Firmicutes A. muciniphila and Clostridium species (in the Clostridiaceae family) ↑ in GTP vs. negative control group Clostridium symbiosum, Dorea longicatena, Ruminococcus lactaris, and Sporobacter termitidis ↓ in GTP vs. negative control group R. lactaris ↑ in GTP vs. negative control group Clostridium (in the Clostridiaceae family), Clostridum saccharogumia, and Subdoligranulum variabile ↑ in GTP+AT vs. negative control group Clostridium (in the Lachnospiraceae family), C. symbiosum, Defluviitalea saccharophila, Ruminococcus lactaris, and Sporobacter termitidis ↓ in GTP+AT vs. negative control group Functional profile of the gut microbiome ⟷ in AT vs. negative control group Glycerol degradation, toluene, D-glucarate, allantoin, D-galactarate, and catechol ↓ in GTP vs. negative control Vitamin K2 and urate biosynthesis/inosine-5′-phosphate degradation ↑ in GTP+AT vs. negative control group Amino acid, glycogen, starch, and mannan degradation, and inosine-5′-phosphate biosynthesis ↓ in GTP+AT vs. negative control group Health effects: AT and GTP individually and in combination improved bone health and reduced white adipose tissue. Skeletal effects of GTP > those of AT. |
| Cossiga et al., 2021 [56] | Animals: male C57BL/6J mice (4 weeks old) Disease model: HFD in a mouse model of non-alcoholic fatty liver disease (NAFLD) Treatment: HFD enriched with plant extracts (HFD+E) (140 mg/kg diet) with 87.84 mg of berberine, 5.27 mg of tocotrienols, and 5.28 mg of chlorogenic acid for 24 weeks. Normal control: standard diet Negative control: HFD | Gut microbiome composition: ⟷ in HFD vs. standard diet ⟷ in HFD+E vs. HFD and standard diet Phylum level Actinobacteria and Firmicutes ↑ in HFD vs. HFD+E and standard diet Bacteroidetes ↑ in HFD+E and standard diet vs. HFD Deferribacteres ↑ in HFD+E vs. standard diet and HFD Verrucomicrobia ↓ in HFD+E vs. standard diet and HFD Firmicutes-to-Bacteroidetes ratio ↓ in standard diet vs. HFD Restored partially in HFD+E Genus level Bacteroides ↑ in HFD+E vs. standard diet and HFD Health effects: The plant extract reduced fasting blood glucose and prevented hyperinsulinemia, though it did not prevent liver steatosis |
| Ran et al., 2019 [57] | Animals: male C57BL/6J mice (22 g; 7 weeks old) Disease model: alteration of gut microbiota through administration of antibiotics in drinking water; ampicillin (1 mg), sulfamethoxazole (1.6 mg), and trimethoprim (0.32 mg) for 12 days Treatment: mice were intragastrically (i.g.) administered a mixture of vitamin E (mVE, containing α-T, γ-T, δ-T, γ-T3, and δ-T3, each at a dose of 75 mg/kg) in 0.1 mL corn oil daily for 17 days Groups: A groups A1: + antibiotics + vitamin E A2: − antibiotics − vitamin E C groups C1: − antibiotics + vitamin E C2: − antibiotics − vitamin E | Faecal genomic DNA: ↓ in A groups (1–6 ng) vs. C groups (80–125 ng) Not sufficient for 16S rRNA sequencing analysis The antibiotics depleted the gut commensal bacteria Effect of antibiotics on blood levels of tocopherols, tocotrienols, and their metabolites: α-T ⟷ in C1 vs. C2 ↑ in A1 vs. C1 (by 40%) ⟷ in A2 vs. C2 δ-T and γ-T ↑in A1 vs. C1 (by 125%) ⟷ in A2 vs. C2 δ-T3 and γ-T3 ↑ in A1 vs. C1 (by 150–157%) ⟷ in A2 vs. C2 Antibiotics treatment ↑ bioavailability of newly administered VE. Serum delta-carboxyethyl hydroxychroman (δ-CEHC), gamma-carboxyethyl hydroxychroman (γ-CEHC), and alpha-carboxyethyl hydroxychroman (α-CEHC) ↑ in C groups vs. A groups Serum delta-carboxylmethylbutyl hydroxychroman (δ-CMBHC) and gamma-carboxylmethylbutyl hydroxychroman (γ-CMBHC) ↑ in C groups vs. A groups Serum α-CMBHC ⟷ in A groups and C groups Serum levels of longer side-chain degradation metabolites of δ- and γ-forms of VE ↑ in A groups vs. C groups ↓ in long-chain vs. short-chain Liver levels of tocopherols, tocotrienols, and their metabolites Same as serum levels but ↑ in α-T and γ-T3 (3–5 times) vs. serum levels ↑ in A for mVE (by 80–100%), except for α-T CEHC and CMBHC ↓ in A groups vs. C groups, except α-CEHC ↑ γ-CEHC, γ-CMBHC, and α-CMBHC (6 times) vs. serum levels. ↓ in α-CEHC ↑ α-CMBHC in liver vs. α-CMBHC in serum levels in A groups Kidney levels of tocopherols, tocotrienols, and their metabolites Not affected by treatment ↓ in δ-T and γ-T, ½ of γ-T3 but 2(δ-T3) C1 kidney vs. C1 liver CEHC ↓ in A groups vs. C groups, except low levels of α-CEHC α-CEHC ↓ in kidney and liver samples in C1 and C2 vs. α-CMBHC Urine levels of tocopherols, tocotrienols, and their metabolites Tocopherols and tocotrienols were not detected ↑ VE metabolites in C groups vs. blood and tissue levels δ- and γ-forms of metabolites ↑ in the first 12 days but ↓ after antibiotic treatment α-CEHC and CMBHC ↑ after day 10 Faecal levels of tocopherols, tocotrienols, and their metabolites: ↓ 5 VE forms following antibiotic treatment VE metabolites ↑ in first 12 days but ↓ after antibiotic treatment α-CMBHC completely blocked by antibiotic treatment α-CEHC not detected Long-chain metabolites ↓ from 13 to 9 carbons following antibiotic treatment |
| Chung et al., 2020 [55] | Animals: male C57BL/6J mice (5 weeks old) Disease model: HFD-fed mice Treatment: Annatto-extracted tocotrienol (800 mg/kg diet) (AT) and metformin (200 mg/kg diet) (MET) for 14 weeks Normal control: LFD Negative control: HFD | Gut microbiota profile: RDA Significant association between dietary treatment and variation in gut microbiome Alpha diversity ⟷ LFD, HFD, AT, and MET Most abundant phyla: Firmicutes, Verrucomicrobia, Bacteroides, and Actinobacteria GM in HFD mice Firmicutes-to-Bacteroidetes ratio ↑ in HFD vs. LFD ↓ in AT vs. HFD Ruminococcus lactaris and Alistipes massiliensis ↑ in HFD vs. LFD Bifidobacterium bifidum, Clostridium disporicum, Barnesiella, Allobaculum, and rc4 -4 ↓ in HFD vs. LFD GM in AT and MET mice Lachnospiraceae family ↓ in MET vs. HFD Firmicutes and D. longicatena ↓ in AT vs. MET Firmicutes ↓ in AT vs. LFD, HFD, and MET Verrucomicrobia ↑ in AT vs. LFD Ruminococcus lactaris, Dorea longicatena, and Lachnospiraceae families ↓ in AT vs. HFD Health effects: AT decreased resistin, leptin, IL-6, and glucose, but did not affect fat pad weight |
| Farhana et al., 2020 [58] | Disease model: human colon cancer cells HT-29 and HCT-116; HCT-116 cells xenografted into SCID mice Treatment: essential turmeric oil + curcumin (ETO–Cur), tocotrienol-rich fraction (TRF), and ETO–Cur–TRF for 34 days Negative control: untreated tumor-grafted SCID mice | Microbial profiling: Diversity index ⟷ between negative control and ETO–Cur–TRF group Operational taxonomic unit (OTU) ↑ in ETO–Cur–TRF vs. negative control group Beta diversity in phylogenetic tree ↑ in ETO–Cur–TRF vs. negative control group Diversity of species number (n) ↑ in ETO–Cur–TRF vs. negative control group Phylum ↑ Proteobacteria and Actinobacteria in ETO–Cur–TRF group vs. negative control group. Tenericute was eliminated in ETO–Cur–TRF group Composition of microbial family ↑ Porphymonadaceae, Rickenellaceae, Lactobacillaeceae, Desulphovibrionaceae, Enterobacteriaceae, and Bifidobacteriaceae in ETO–Cur–TRF-treated group vs. negative control group ↓ Bacteroidaceae in ETO–Cur–TRF group vs. negative control group ↓ Bacteroides and Parabacteroides in ETO–Cur–TRF group vs. negative control group ↑ Clostridium XIVa, Lactobacillus, and Aliistipes in ETO–Cur–TRF group vs. negative control group ↓ Bacteroides uniformis in ETO–Cur–TRF group vs. negative control group Gut microbial changes in tumor ↑ Bifidobacteria, Lactobacillus, and Clostridium IV in ETO–Cur–TRF treated mice vs. negative control group Health effects: ETO–Cur–TRF was more effective than ETO–Cur and TRF in inhibiting cancer growth in vitro and in vivo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumareswaran, A.; Ekeuku, S.O.; Mohamed, N.; Muhammad, N.; Hanafiah, A.; Pang, K.-L.; Wong, S.K.; Chew, D.C.H.; Chin, K.-Y. The Effects of Tocotrienol on Gut Microbiota: A Scoping Review. Life 2023, 13, 1882. https://doi.org/10.3390/life13091882
Kumareswaran A, Ekeuku SO, Mohamed N, Muhammad N, Hanafiah A, Pang K-L, Wong SK, Chew DCH, Chin K-Y. The Effects of Tocotrienol on Gut Microbiota: A Scoping Review. Life. 2023; 13(9):1882. https://doi.org/10.3390/life13091882
Chicago/Turabian StyleKumareswaran, Aswini, Sophia Ogechi Ekeuku, Norazlina Mohamed, Norliza Muhammad, Alfizah Hanafiah, Kok-Lun Pang, Sok Kuan Wong, Deborah Chia Hsin Chew, and Kok-Yong Chin. 2023. "The Effects of Tocotrienol on Gut Microbiota: A Scoping Review" Life 13, no. 9: 1882. https://doi.org/10.3390/life13091882
APA StyleKumareswaran, A., Ekeuku, S. O., Mohamed, N., Muhammad, N., Hanafiah, A., Pang, K.-L., Wong, S. K., Chew, D. C. H., & Chin, K.-Y. (2023). The Effects of Tocotrienol on Gut Microbiota: A Scoping Review. Life, 13(9), 1882. https://doi.org/10.3390/life13091882








