Pigeon during the Breeding Cycle: Behaviors, Composition and Formation of Crop Milk, and Physiological Adaptation
Abstract
:1. Introduction
2. Artificial Breeding of Pigeons
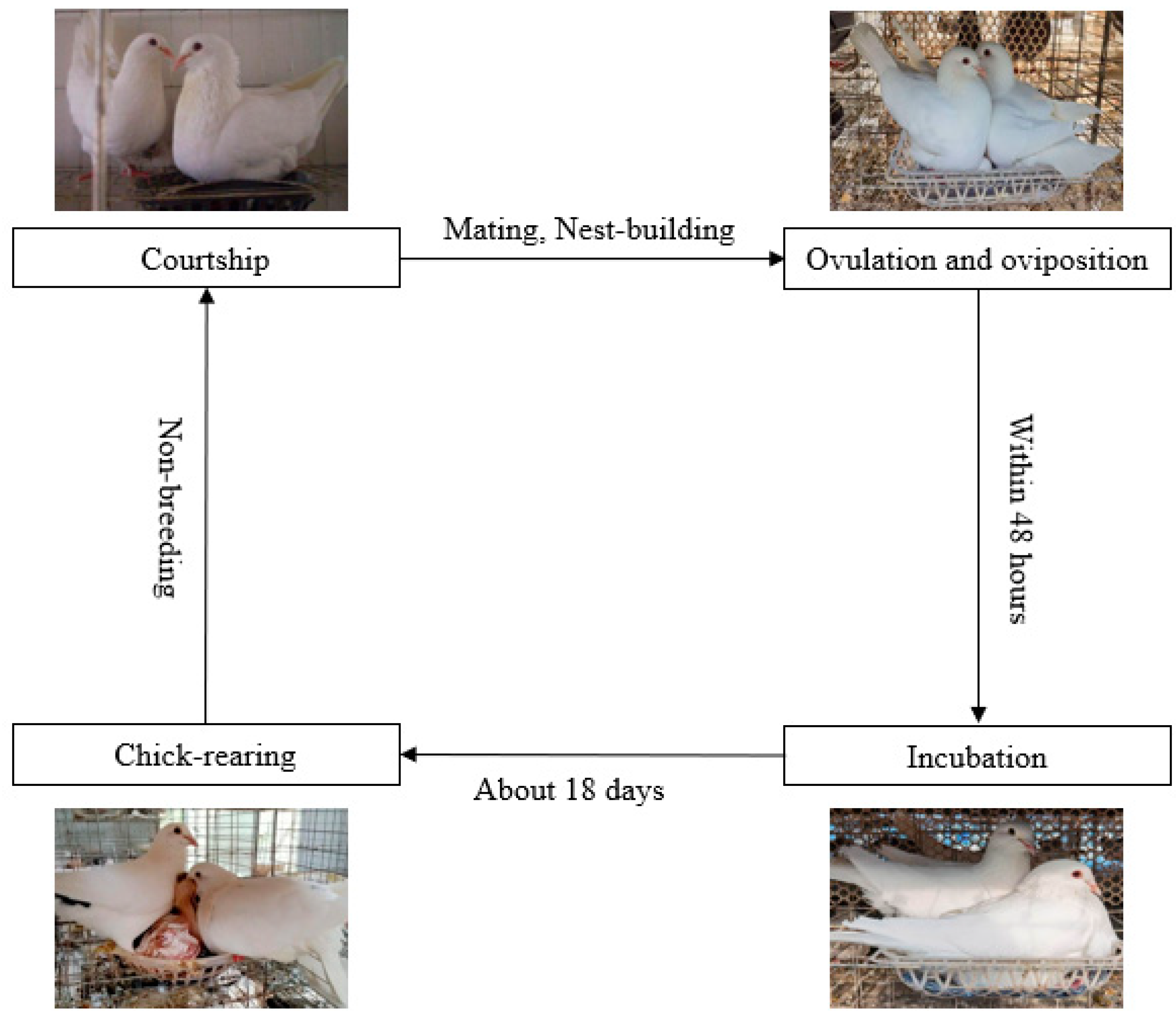
3. Behaviors during the Breeding Cycle
4. The Composition of Pigeon Crop Milk
4.1. Protein
4.2. Lipids
4.3. Carbohydrate
4.4. Mineral and Vitamin
4.5. Active Ingredients
4.5.1. Growth Factor and Immune Active Substance
4.5.2. Active Enzyme
4.6. Microorganism
5. The Formation Mechanism of Pigeon Milk
5.1. The Proliferation of Pigeon Crop Epidermal Cells
5.1.1. Morphological Changes in the Crop
5.1.2. The Regulation of Hormones on Crop Proliferation
5.1.3. The Regulation of Crop Proliferation by Non-Coding RNA
5.2. Accumulation of Nutrients in Pigeon Crop Epithelial Cells
5.2.1. Synthesis of Protein in Crop Epidermal Cells
5.2.2. Synthesis of Lipid in Crop Epidermal Cells
5.2.3. Synthesis of Carbohydrates in Crop Epidermal Cells
5.3. Shedding of Crop Epidermal Cells
6. Physiological Adaptation in Pigeons during the Breeding Period
6.1. Changes in Intestine Morphology and Function
6.2. Changes in Liver Metabolism
7. Can Artificial Crop Milk Be Successful?
8. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, J.; Liao, N.; Zheng, Y.; Yang, L.; Zhou, H.; Xu, K.; Han, C.; Luo, H.; Qin, C.; Tang, C.; et al. The composition and function of pigeon milk microbiota transmitted from parent pigeons to squabs. Front. Microbiol. 2020, 11, 1789. [Google Scholar] [CrossRef]
- Fattah, A.F.A. Parental Care during Incubation, Brooding and Growth Rates of Egyptian Baladi Pigeon Nestlings. Brit. J. Poult. Sci. 2015, 4, 29–33. [Google Scholar]
- Silver, R.; Andrews, H.; Ball, G.F. Parental care in an ecological perspective: A quantitative analysis of avian subfamilies. Integ. Comp. Biol. 1985, 25, 823–840. [Google Scholar] [CrossRef]
- Gayathri, K.L.; Hegde, S.N. Influence of breeding activity on the haematology of domestic pigeons, Columba livia, Pavo. Indian J. Ornithol. 1994, 32, 39–45. [Google Scholar]
- Nepote, K.H. Pigeons as Laboratory Animals. Poult. Avian. Biol. Rev. 1999, 10, 109–115. [Google Scholar]
- Shetty, S.; Jacob, R.T.; Shenoy, K.B.; Hegde, S.N. Patterns of Breeding Behaviour in the Domestic Pigeon. Bird Behav. 1990, 9, 14–19. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Domyan, E.T. Domestic pigeons. Curr. Biol. 2013, 23, 302–303. [Google Scholar] [CrossRef]
- Gillespie, M.J.; Haring, V.R.; McColl, K.A.; Monaghan, P.; Donald, J.A.; Nicholas, K.R.; Moore, R.J.; Crowley, T.M. Histological and global gene expression analysis of the ‘lactating’ pigeon crop. BMC Genom. 2011, 12, 452. [Google Scholar] [CrossRef]
- Gillespie, M.J.; Crowley, T.M.; Haring, V.R.; Wilson, S.L.; Harper, J.A.; Payne, J.S.; Green, D.; Monaghan, P.; Donald, J.A.; Nicholas, K.R.; et al. Transcriptome analysis of pigeon milk production-role of cornification and triglyceride synthesis genes. BMC Genom. 2013, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Rendón, M.A.; Garrido, A.; Rendón-Martos, M.; Ramirez, J.M.; Amat, J.A. Assessing sex-related chick provisioning in greater flamingo Phoenicopterus roseus parents using capture-recapture models. J. Anim. Ecol. 2014, 83, 479–490. [Google Scholar] [CrossRef]
- Fretwell, P.T.; Trathan, P.N.; Wienecke, B.; Kooyman, G.L. Emperor penguins breeding on iceshelves. PLoS ONE 2014, 9, 85285. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, S.; An, Y.; Wang, Z.; Shao, Y.X.; Du, S.H.; Li, X.; Sun, X.S. Influence of dietary phosphorus concentrations on the performance of rearing pigeons (Columba livia), and bone properties of squabs. Poult. Sci. 2022, 101, 101744. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Q.; Wang, X.H.; Hu, X.C.; Yan, H.C.; Wang, X.Q. Effects of dietary crude protein levels on growth performance, carcass characteristics, meat quality of squabs and laying performance of breeding pigeons. J. South China Agric. Univ. 2016, 37, 1–6. (In Chinese) [Google Scholar]
- Li, F.H.; Chang, L.L.; Qiu, H.J.; Bu, Z.; Tang, Q.P.; Chen, W.B.; Zhang, R.; Fu, S.Y.; Mu, C.Y. Comparative evaluation of meat quality and main nutritional components of breast muscle from different squab breeds. Meat Res. 2019, 33, 7–11. (In Chinese) [Google Scholar]
- Gao, H.J. Production situation of China pigeon industry in 2021 and trend outlook. Anim. Agric. 2022, 4, 44–46. (In Chinese) [Google Scholar]
- Kokoszyński, D.; Stęczny, K.; Żochowska-Kujawska, J.; Sobczak, M.; Kotowicz, M.; Saleh, M.; Fik, M.; Arpášová, H.; Hrnčár, C.; Włodarczyk, K. Carcass characteristics, physicochemical properties, and texture and microstructure of the meat and internal organs of carrier and king pigeons. Animals 2020, 10, 1315. [Google Scholar] [CrossRef]
- Shen, F. Current Situation and Development Countermeasures of Meat Pigeon Breeding in Chongming District. Shanghai J. Anim. Hus. Vet. Med. 2019, 6, 56–57. (In Chinese) [Google Scholar]
- Zhu, X.P.; Mao, X.G.; Chen, X.Q. Effect of egg weight on hatchability and growth and development of pigeon squab. China Poult. 2009, 31, 43–44. (In Chinese) [Google Scholar]
- Aggrey, S.E.; Cheng, K.M. Genetic and posthatch parental influences on growth in pigeon squabs. J. Hered. 1993, 84, 184–187. [Google Scholar] [CrossRef]
- Chang, L.L.; Xie, P.; Bu, Z.; Wang, Q.; Fu, S.Y.; Mu, C.Y. Effect of dietary lysine level on performance, egg quality and serum biochemical indices of laying pigeons. J. Appl. Poult. Res. 2018, 27, 152–158. [Google Scholar] [CrossRef]
- Chang, L.L.; Zhang, R.; Fu, S.Y.; Mu, C.Y.; Tang, Q.P.; Bu, Z. Effects of different dietary calcium levels on the performance, egg quality, and albumen transparency of laying pigeons. Animals 2019, 9, 110. [Google Scholar] [CrossRef]
- Shao, Y.X.; Li, X.; Du, S.H.; Sun, X.S.; Wang, Y.Y.; Zhao, D.D.; Wang, Z. Effect of dietary supplemental zinc on laying performance, egg quality, and plasma hormone levels of breeding pigeons. Biol. Trace Elem. Res. 2023, 201, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Jie, P.; Huang, W.Y.; Liang, Y.Y.; Zhang, W.; Zhang, Y.L.; Yang, M.L.; Zheng, S.Q.; Lv, Y.T.; Gou, Z.Y.; Cheng, C.S.; et al. Optimal dietary energy and protein levels for breeding pigeons in the winter “2+3” lactation pattern. Poult. Sci. 2023, 102, 102964. [Google Scholar]
- Xie, P.; Jiang, X.Y.; Bu, Z.; Fu, S.Y.; Zhang, S.Y.; Tang, Q.P. Free choice feeding of whole grains in meat-type pigeons: 1. effect on performance, carcass traits and organ development. Brit. Poult. Sci. 2016, 57, 699–706. [Google Scholar] [CrossRef]
- Darwati, S.; Martojo, H.; Sihombing, D.T.H.; Sumantri, C. Productivity of local pigeon fed with cafetaria method in intensive rearing. Anim. Prod. 2012, 1, 315–319. [Google Scholar]
- Wang, Y.; Li, Y.B.; Yang, H.M.; Wang, Z.Y. Effect of monochromatic lights on egg production, sex hormone levels, and expression of their receptors in pigeons. Livest. Sci. 2018, 216, 233–236. [Google Scholar] [CrossRef]
- Cooper, J.B. Light intensity and housing for pigeons. Poult. Sci. 1976, 55, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Cazelles, B.; Prevot-Julliard, A.C.; Leboucher, G.; Gasparini, J. Reproduction management affects breeding ecology and reproduction costs in feral urban pigeons (Columba livia). Can. J. Zool. 2010, 88, 781–787. [Google Scholar] [CrossRef]
- Fabricius, E.; Jansson, A.M. Laboratory observations on the reproductive behaviour of the pigeon (Columba livia) during the pre-incubation phase of the breeding cycle. Anim. Behav. 1963, 11, 534–547. [Google Scholar] [CrossRef]
- Lehrman, D.S. The reproductive behavior of ring doves. Sci. Am. 1964, 211, 48–54. [Google Scholar] [CrossRef]
- Murton, R.K.; Thearle, R.J.; Lofts, B. The endocrine basis of breeding behaviour in the feral pigeon (Columba livia). I. Effects of exogenous hormones on the pre-incubation behaviour of intact males. Anim. Behav. 1969, 17, 286–306. [Google Scholar] [CrossRef]
- Whitman, C.O. Posthumous Works: The Behaviour of Pigeons; Harvey, A., Ed.; Carnegie Institution of Washington: Washington, DC, USA, 1919. [Google Scholar]
- Mohamed, R.A.; Shukry, M.; Mousa-Balabel, T.M.; Elbassiouny, A.A. Assessment of plasma prolactin and nest defense behaviour during breeding cycle of pigeon (Columba livia domestica). J. Environ. Agric. Sci. 2016, 7, 19–22. [Google Scholar]
- Wallman, J.; Grabon, M.; Silver, R. What determines the pattern of sharing of incubation and brooding in ring doves? J. Comp. Physiol. Psy. 1979, 93, 481. [Google Scholar] [CrossRef]
- Saxena, V.L.; Pandey, E.; Agarwal, S.; Saxena, A.K. Execution of breeding and nidification behaviour in Pigeon (Columba livia) and Dove (Streptopelia chinensis). Asian J. Exp. Sci. 2008, 22, 405–410. [Google Scholar]
- Andersson, M.; Wiklund, G.; Rundgren, H. Parental defence of offspring: A model and an example. Anim. Behav. 1980, 28, 536–542. [Google Scholar] [CrossRef]
- Oniki, Y.; Willis, E.O. Nesting behavior of the Picazuro pigeon, Columba Picazuro (Columbidae, Aves). Braz. J. Biol. 2000, 60, 663–666. [Google Scholar] [CrossRef]
- Smith, S. The instinctive nature of nest sanitation. Part II Brit. Birds 1993, 36, 186–188. [Google Scholar]
- Hinton, A., Jr.; Buhr, R.J.; Ingram, K.D. Physical, chemical, and microbiological changes in the crop of broiler chickens subjected to incremental feed withdrawal. Poult. Sci. 2000, 79, 212–218. [Google Scholar] [CrossRef]
- Vaughn, L.E.; Holt, P.S.; Gast, R.K. Cellular assessment of crop lymphoid tissue from specific-pathogen-free white leghorn chickens after Salmonella enteritidis challenge. Avian Dis. 2008, 52, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Rawski, M.; Długosz, J.; Swiatkiewicz, S.; Jozefiak, D. Avian crop function-a review. Ann. Anim. Sci. 2016, 16, 653–678. [Google Scholar] [CrossRef]
- Vandeputte-Poma, J. Feeding, growth, and metabolism of the pigeon, Columba livia domestica: Duration and role of crop milk feeding. J. Comp. Physiol. 1980, 135, 97–99. [Google Scholar] [CrossRef]
- Shetty, S.; Bharathi, L.; Shenoy, K.B.; Hegde, S.N. Biochemical properties of pigeon milk and its effect on growth. J. Comp. Physiol. 1992, 162, 632–636. [Google Scholar] [CrossRef]
- Yang, M.C.; Vohra, P. Protein and metabolizable energy requirements of hand-fed squabs from hatching to 28 days of age. Poult. Sci. 1987, 66, 2017–2023. [Google Scholar] [CrossRef]
- Sales, J.; Janssens, G.P.J. Nutrition of the domestic pigeon (Columba livia domestica). World’s Poult. Sci. J. 2003, 59, 221–232. [Google Scholar] [CrossRef]
- Davies, W.L. The composition of the crop-milk of pigeons. Biochem. J. 1939, 33, 898–901. [Google Scholar] [CrossRef]
- Leash, A.; Liebman, J.; Taylor, A.; Limbert, R. An analysis of the crop contents of White Carneaux Pigeons (Columba livia), days one through twenty-seven. Lab. Anim. Sci. 1971, 21, 86–90. [Google Scholar] [PubMed]
- Sim, J.S.; Hickman, A.M.R.; Nwokolo, E. Nutrient composition of squabs crop contents during the first 8 days post hatch. Poult. Sci. 1986, 65, 17. [Google Scholar]
- Hu, X.C.; Gao, C.Q.; Wang, X.H.; Yan, H.C.; Chen, Z.S.; Wang, X.Q. Crop milk protein is synthesised following activation of the IRS1/Akt/TOR signalling pathway in the domestic pigeon (Columba livia). Br. Poult. Sci. 2016, 57, 855–862. [Google Scholar] [CrossRef]
- Xie, W.Y.; Fu, Z.; Pan, N.X.; Yan, H.C.; Wang, X.Q.; Gao, C.Q. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia). Poult. Sci. 2019, 98, 5514–5524. [Google Scholar] [CrossRef]
- Chen, M.J.; Pan, N.X.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. Methionine promotes crop milk protein synthesis through the JAK2-STAT5 signaling during lactation of domestic pigeons (Columba livia). Food Funct. 2020, 11, 10786–10798. [Google Scholar] [CrossRef]
- Fox, P.F.; Mulvihill, D.M. Casein. Food. Gels. 1990, 4, 121–173. [Google Scholar]
- West, D.W. Structure and function of the phosphorylated residues of casein. J. Dairy. Res. 1986, 53, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D.; Buntin, J.D. Regulation of pigeon cropmilk secretion and parental behaviors by prolactin. Annu. Rev. Nutr. 1995, 15, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ma, W.; Ji, F.; Sun, X.; Du, S.; Li, X.; Li, Q.; Wang, Z. Exploration of proteomics analysis of crop milk in pigeons (Columba livia) during the lactation period. ACS Omega 2021, 6, 27726–27736. [Google Scholar] [CrossRef]
- Zhu, J.G.; Xie, P.; Zheng, M.D.; Meng, Y.; Wei, M.L.; Liu, Y.; Liu, T.W.; Gong, D.Q. Dynamic changes in protein concentrations of keratins in crop milk and related gene expression in pigeon crops during different incubation and chick- rearing stages. Br. Poult. Sci. 2023, 64, 100–109. [Google Scholar] [CrossRef]
- Hegde, S.N. The amino-acid composition of pigeon milk. Curr. Sci. India. 1972, 41, 23–24. [Google Scholar]
- Zhang, X.Y.; Zhang, N.N.; Wan, X.P.; Li, L.L.; Zou, X.T. Gene expression of amino acid transporter in pigeon (Columbia livia) intestine during post-hatch development and its correlation with amino acid in pigeon milk. Poult. Sci. 2017, 96, 1120–1131. [Google Scholar] [CrossRef]
- Xie, P.; Han, M.X.; Chen, W.X.; Wan, X.P.; Xu, Y.G.; Gong, D.Q. The profiling of amino acids in crop milk and plasma and mRNA abundance of amino acid transporters and enzymes related to amino acid synthesis in the crop tissue of male and female pigeons during incubation and chick-rearing periods. Poult. Sci. 2020, 99, 1628–1642. [Google Scholar] [CrossRef]
- Jin, C.L.; He, Y.A.; Jiang, S.G.; Wang, X.Q.; Yan, H.C.; Tan, H.Z.; Gao, C.Q. Chemical composition of pigeon crop milk and factors affecting its production: A review. Poult. Sci. 2023, 102, 102681. [Google Scholar] [CrossRef]
- Xie, P.; Wang, X.P.; Bu, Z.; Zou, X.T. Differential expression of fatty acid transporters and fatty acid synthesis-related genes in crop tissues of male and female pigeons (Columba livia domestica) during incubation and chick rearing. Br. Poult. Sci. 2017, 58, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Desmeth, M.; Vandeputte-Poma, J. Lipid composition of pigeon cropmilk-I. Total lipids and lipid classes. Comp. Biochem. Physiol. 1980, 66, 129–133. [Google Scholar] [CrossRef]
- Desmeth, M. Lipid composition of pigeon cropmilk-Ⅱ. Fatty acids. Comp. Biochem. Physiol. 1980, 66, 135–138. [Google Scholar] [CrossRef]
- Shetty, S.; Hegde, S.N. Changes in lipids of pigeon “milk” in the first week of its secretion. Lipids 1991, 26, 930–933. [Google Scholar] [CrossRef]
- Shetty, S.; Salimath, P.V.; Hegde, S.N. Carbohydrates of pigeon milk and their changes in the first week of secretion. Arch. Physiol. Biochem. 1994, 102, 277–280. [Google Scholar] [CrossRef]
- Zhu, J.G.; Xie, P.; Song, C.; Liu, T.W.; Gong, D.Q. Differential expression of glucose metabolism-related genes and AMP-activated protein kinases in crop tissue of male and female pigeons (Columba livia domestica) during the incubation and chick-rearing periods. J. Anim. Physiol. Anim. Nutr. 2023, 107, 680–690. [Google Scholar] [CrossRef]
- Carr, R.H.; James, C.M. Synthesis of adequate protein in the glands of the pigeon crop. Am. J. Physiol. 1931, 97, 227–231. [Google Scholar] [CrossRef]
- Shetty, S.; Jacob, R.T.; Hegde, S.N. Mineral composition of pigeon milk. Experientia 1990, 46, 449–451. [Google Scholar] [CrossRef]
- Hegde, S.N. Composition of pigeon milk and its effect on growth in chicks. Indian J. Exp. Biol. 1973, 11, 238–239. [Google Scholar]
- Bharathi, L.; Shenoy, K.B.; Hegde, S.N. In vivo and in vitro growth-stimulatory effects of pigeon milk. Comp. Biochem. Physiol. Comp. Physiol. 1994, 108, 451–459. [Google Scholar] [CrossRef]
- Bharathi, L.; Shenoy, K.B.; Mojamdar, M.; Hegde, S.N. In vitro growth-stimulatory property of pigeon milk. Biochem. Cell. Biol. 1993, 71, 303–307. [Google Scholar] [CrossRef]
- Shetty, S.; Hegde, S.N. Pigeon milk: A new source of growth factor. Experientia 1993, 49, 925–928. [Google Scholar] [CrossRef]
- Shetty, S.; Hegde, S.N.; Bharathi, L. Purification of a growth factor from pigeon milk. Biochim. Biophys. Acta 1992, 1117, 193–198. [Google Scholar] [CrossRef]
- Xie, P.; Wan, X.P.; Bu, Z.; Diao, E.J.; Gong, D.Q.; Zou, X.T. Changes in hormone profiles, growth factors, and mRNA expression of the related receptors in crop tissue, relative organ weight, and serum biochemical parameters in the domestic pigeon (Columba livia) during incubation and chick-rearing periods under artificial farming conditions. Poult. Sci. 2018, 97, 2189–2202. [Google Scholar]
- Gillespie, M.J.; Stanley, D.; Chen, H.; Donald, J.A.; Nicholas, K.R.; Moore, R.J.; Crowley, T.M. Functional similarities between pigeon ‘milk’ and mammalian milk: Induction of immune gene expression and modification of the microbiota. PLoS ONE 2012, 7, 48363. [Google Scholar] [CrossRef]
- Engberg, R.M.; Kaspers, B.; Schranner, I.; Kosters, J.; Losch, U. Quantification of the immunoglobulin classes IgG and IgA in the young and adult pigeon (Columba livia). Avian Pathol. 1992, 21, 409–420. [Google Scholar] [CrossRef]
- Goudswaard, J.; van der Donk, J.A.; van der Gaag, I.; Noordzij, A. Peculiar IgA transfer in the pigeon from mother to squab. Dev. Comp. Immunol. 1979, 3, 307–319. [Google Scholar] [CrossRef]
- Frelinger, J.A. Maternally derived transferrin in pigeon squabs. Science 1971, 171, 1260–1261. [Google Scholar] [CrossRef]
- Bharathi, L.; Shenoy, K.B.; Hegde, S.N. Biochemical differences between crop tissue and crop milk of pigeons (Columba livia). Comp. Biochem. Physiol. 1997, 116, 51–55. [Google Scholar] [CrossRef]
- Hegde, S.N.; Neelakantan, B. The digestive enzymes of the pigeon crop-milk. Ind. Zool. 1970, 1, 75–80. [Google Scholar]
- Shahani, K.M.; Kwan, A.J.; Friend, B.A. Role and significance of enzymes in human milk. Am. J. Clin. Nutr. 1980, 33, 1861–1868. [Google Scholar] [CrossRef]
- Shetty, S.; Sridhar, K.R.; Shenoy, K.B.; Hegde, S.N. Observations on bacteria associated with pigeon crop. Folia Microbiol. 1990, 35, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatr. Obes. 2017, 12, 3–17. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser, L.J.; Bordenstein, S.R. Mom knows best: The universality of maternal microbial transmission. PLoS Biol. 2013, 11, e1001631. [Google Scholar] [CrossRef]
- Beams, H.W.; Meyer, R.K. The formation of pigeon “milk”. Physiol. Zool. 1931, 4, 486–500. [Google Scholar] [CrossRef]
- Riddle, O.; Bates, R.W.; Dykshorn, S. The preparation, identification and assay of prolactin-a hormone of the anterior pituitary. Am. J. Physiol. 1933, 105, 191–216. [Google Scholar] [CrossRef]
- Dumont, J.N. Prolactin-induced cytologic changes in the mucosa of the pigeon crop during crop-milk formation. Z. Zellforsch. Mikrosk. Anat. 1965, 68, 755–782. [Google Scholar] [CrossRef]
- Riddle, O.; Bates, W.R.; Dykshorn, W.S. A new hormone of the anterior pituitary. Exp. Biol. Med. 1932, 29, 1211–1212. [Google Scholar] [CrossRef]
- Bates, R.W.; Riddle, O. Effect of Route of Administration on the Bioassay of Prolactin. Proc. Soc. Exp. Biol. Med. 1936, 34, 847–849. [Google Scholar] [CrossRef]
- Horseman, N.D.; Nollin, L.J. The mitogenic, but not differentiative, response of crop tissue to prolactin is circadian phase dependent. Endocrinology 1985, 116, 2085–2089. [Google Scholar] [CrossRef]
- Nicoll, C.S.; Bern, H.A. Further analysis of the occurrence of pigeon crop sac-stimulating activity (Prolactin) in the vertebrate adenohypophysis. Gen. Comp. Endocrinol. 1968, 11, 5–20. [Google Scholar] [CrossRef]
- Pukac, L.A.; Horseman, N.D. Regulation of pigeon crop gene expression by prolactin. Endocrinology 1984, 114, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Pitts, D.S.; Nicoll, C.S. Prolactin’s mitogenic action on the pigeon crop-sac mucosal epithelium involves direct and indirect mechanisms. Gen. Comp. Endocrinol. 1984, 54, 236–246. [Google Scholar] [CrossRef]
- Hirvonen, A. Ornithine decarboxylase activity and the accumulation of its mRNA during early stages of liver regeneration. Biochim. Biophys. Acta. 1989, 1007, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.S.; Zieve, L. Effects of partial and sham hepatectomy on ornithine decarboxylase and thymidine kinase activities and mRNA contents. Biochem. Int. 1990, 20, 761–765. [Google Scholar] [PubMed]
- Nishiguchi, Y.; Hibasami, H.; Komada, Y.; Sakurai, M.; Nakashima, K. Human promyelocytic cell line hl60 has the specific binding sites for prolactin and its ornithine decarboxylase, dna synthesis and cellular proliferation are induced by prolactin. Leuk. Res. 1993, 17, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Bani, D. Relaxin: A pleiotropic hormone. Gen. Pharmacol. 1997, 28, 13–22. [Google Scholar] [CrossRef]
- Bani, G.; Bigazzi, M. Morphological changes induced in mouse mammary gland by porcine and human relaxin. Actu. Anat. 1984, 119, 149–154. [Google Scholar] [CrossRef]
- Bani, G.; Bigazzi, M.; Bani, D. Effects of relaxin on the mouse mammary gland. I. The myoepithelial cells. J. Endocrinol. Investig. 1985, 8, 207–215. [Google Scholar] [CrossRef]
- Bani, G.; Bigazzi, M.; Bani, D. Effects of relaxin on the mouse mammary gland. II. The epithelial cells. J. Endocrinol. Investig. 1986, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bigazzi, M.; Bani, G.; Sacchi, T.B.; Petrucci, F.; Bianchi, S. Relaxin: A mammotropic hormone promoting growth and differentiation of the pigeon crop sac mucosa. Acta Endocrinol. 1988, 117, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Bani, G.; Sacchi, T.B.; Cecchi, R.; Bigazzi, M. The effects of relaxin on the pigeon crop sac mucosa: Light and electron microscopic study. Z. Mikrosk. Annt. Forsch. 1987, 101, 577–596. [Google Scholar]
- Bani, G.; Sacchi, T.B.; Bigazzi, M. Response of the pigeon crop sac to mammotrophic hormones: Comparison between relaxin and prolactin. Gen. Comp. Endocr. 1990, 80, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Mayer, G.L.; Hebert, N.; Nicoll, C.S. Interactions among prolactin, epidermal growth factor, and proinsulin on the growth and morphology of the pigeon crop-sac mucosal epithelium in vivo. Endocrinology 1987, 120, 1258–1264. [Google Scholar] [CrossRef]
- Tanaka, T.; Haneda, S.; Imakawa, K.; Sakai, S.; Nagaoka, K. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 2009, 77, 181–187. [Google Scholar] [CrossRef]
- Dysin, A.P.; Barkova, O.Y.; Pozovnikova, M.V. The role of microRNAs in the mammary gland development, health, and function of cattle, goats, and sheep. Non-Coding RNA 2021, 7, 78. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhou, H.T.; Hickford, J.G.H.; Hao, Z.Y.; Gong, H.; Hu, J.; Liu, X.; Li, S.B.; Shen, J.Y.; Ke, N.; et al. Identification and characterization of circular RNAs in mammary gland tissue from sheep at peak lactation and during the nonlactating period. J. Dairy Sci. 2021, 104, 2396–2409. [Google Scholar] [CrossRef]
- Ge, P.; Ma, H.; Li, Y.; Ni, A.; Isa, A.M.; Wang, P.; Bian, S.; Shi, L.; Zong, Y.; Wang, Y.; et al. Identification of microRNA-Associated-ceRNA Networks Regulating Crop Milk Production in Pigeon (Columba livia). Genes 2020, 12, 39. [Google Scholar] [CrossRef]
- Ma, H.; Ge, P.; Bian, S.; Li, Y.; Ni, A.; Zhang, R.; Wang, Y.; Zhao, J.; Zong, Y.; Yuan, J.; et al. miR-193-5p negatively regulates PIK3CD to promote crop fibrocyte proliferation in pigeon (Columba livia). Poult. Sci. 2023, 102, 102378. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Wu, Z.; Hou, Y.; Bazer, F.W.; Wu, G. Amino acids and mammary gland development: Nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 2016, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, Q.L.; Pacheco, D.; Mccoard, S.A. 2015. Administration of exogenous growth hormone is associated with changes in plasma and intracellular mammary amino acid profiles and abundance of the mammary gland amino acid transporter SLC3A2 in mid-lactation dairy cows. PLoS ONE 2015, 10, e0134323. [Google Scholar] [CrossRef] [PubMed]
- Apelo, S.I.; Singer, L.M.; Ray, W.K.; Helm, R.F.; Lin, X.Y.; Mcgilliard, M.L.; St-pierre, N.R.; Hanigan, M.D. Casein synthesis is independently and additively related to individual essential amino acid supply. J. Dairy Sci. 2014, 97, 2998–3005. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429. [Google Scholar] [CrossRef]
- Appuhamy, J.A.D.R.N.; Bell, A.L.; Nayananjalie, W.A.D.; Escobar, J.; Hanigan, M.D. Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2011, 141, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Tardito, S.; Barilli, A.; Bianchi, M.G.; Dall, A.V.; Bussolati, O. Glutamine stimulates mTORC1 independent of the cell content of essential amino acids. Amino Acids 2012, 43, 2561–2567. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Tee, A.R. Mammalian target of rapamycin complex 1: Signalling inputs, substrates and feedback mechanisms. Cell. Sig. 2009, 21, 827–835. [Google Scholar] [CrossRef]
- Chen, M.J.; Fu, Z.; Jiang, S.G.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. Targeted disruption of TORC1 retards young squab growth by inhibiting the synthesis of crop milk protein in breeding pigeon (Columba livia). Poult. Sci. 2020, 99, 416–422. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Singer, L.M.; Lin, X.Y.; McGilliard, M.L.; St-Pierre, N.R.; Haniga, M.D. Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J. Dairy Sci. 2014, 97, 1047–1056. [Google Scholar] [CrossRef]
- Futter, C.E.; Felder, S.; Schlessinger, J.; Ullrich, A.; Hopkins, C.R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell. Biol. 1993, 120, 77–83. [Google Scholar] [CrossRef]
- Emans, N.; Gorvel, J.P.; Walter, C.; Gerke, V.; Kellner, R.; Griffiths, G.; Gruenberg, J. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell. Biol. 1993, 120, 1357–1369. [Google Scholar] [CrossRef]
- Horseman, N.D. A prolactin-inducible gene product which is a member of the Calpactin/Lipocortin family. Mol. Endocrinol. 1989, 3, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, D.M.; Zhao, F.Q.; Liang, S.L.; Liu, J.X. AMPK-mTOR pathway is involved in glucose-modulated amino acid sensing and utilization in the mammary glands of lactating goats. J. Anim. Sci. Biotechnol. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Bian, S.; Li, Y.; Ni, A.; Zhang, R.; Ge, P.; Han, P.; Wang, Y.; Zhao, J.; Zong, Y.; et al. Analyses of circRNAs profiles of the lactating and nonlactating crops in pigeon (Columba livia). Poult. Sci. 2023, 102, 102464. [Google Scholar] [CrossRef]
- Ma, H.; Ni, A.; Ge, P.; Li, Y.; Shi, L.; Wang, P.; Fan, J.; Isa, A.M.; Sun, Y.; Chen, J. Analysis of Long Non-Coding RNAs and mRNAs Associated with Lactation in the Crop of Pigeons (Columba livia). Genes 2020, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.S.; Hu, S.L.; Yu, K.; Wang, H.; Wang, W.; Loor, J.; Luo, J. Lipoprotein lipase, tissue expression and effects on genes related to fatty acid synthesis in goat mammary epithelial cells. Int. J. Mol. Sci. 2014, 15, 22757–22771. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, D.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol. Genom. 2007, 28, 323–336. [Google Scholar] [CrossRef]
- Maningat, P.D.; Sen, P.; Rijnkels, M.; Sunehag, A.L.; Hadsell, D.L.; Bray, M.; Haymond, M.W. Gene expression in the human mammary epithelium during lactation: The milk fat globule transcriptome. Physiol. Genom. 2008, 37, 12–22. [Google Scholar] [CrossRef]
- Garrison, M.M.; Scow, R.O. Effect of prolactin on lipoprotein lipase in crop sac and adipose tissue of pigeons. Am. J. Physiol. 1975, 228, 1542–1544. [Google Scholar] [CrossRef]
- Clegg, R.A.; Barber, M.C.; Pooley, L.; Ernens, I.; Larondelle, Y.; Travers, M.T. Milk fat synthesis and secretion: Molecular and cellular aspects. Livest. Prod. Sci. 2001, 70, 3–14. [Google Scholar] [CrossRef]
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444. [Google Scholar] [CrossRef] [PubMed]
- Fielding, B.A.; Frayn, K.N. Lipoprotein lipase and the disposition of dietary fatty acids. Br. J. Nutr. 1998, 80, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Xie, P.; Zhu, J.G.; Wang, L.X.; Liu, Y.; Diao, E.J.; Gong, D.Q.; Liu, T.W. Lipid accumulation and oxidative stress in the crop tissues of male and female pigeons during incubation and chick-rearing periods. Poult. Sci. 2023, 102, 102289. [Google Scholar] [CrossRef]
- Feng, X.; Cai, Z.; Mu, T.; Yu, B.; Wang, Y.; Ma, R.; Liu, J.; Wang, C.; Zhang, J.; Gu, Y. CircRNA screening and ceRNA network construction for milk fat metabolism in dairy cows. Front. Vet. Sci. 2022, 9, 995629. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, Y.; Wang, C.; Wang, J.; Wang, L.; Liu, L.; Liu, L.; Gao, X.; Li, Q. Epigenetic regulation of miR-29s affects the lactation activity of dairy cow mammary epithelial cells. J. Cell. Physiol. 2015, 230, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, M.; Brown, M.; Downs, C.T. Does sugar content matter? Blood plasma glucose levels in an occasional and a specialist avian nectarivore. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 40–44. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell. Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Halse, R.; Fryer, L.G.; McCormack, J.G.; Carling, D.; Yeaman, S.J. Regulation of glycogen synthase by glucose and glycogen: A possible role for AMP-activated protein kinase. Diabetes 2003, 52, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Zhu, J.G.; Liu, Y.; Liu, T.W.; Xu, Y.G.; Gong, D.Q. Effect of Akt activation on apoptosis-related gene expression in the crop tissues of male and female pigeons (Columba livia). Poult. Sci. 2021, 100, 101392. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. Nf-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, B.Y.; De, S. Mitochondrial VDAC, the Na+/Ca2+ exchanger, and the Ca2+ uniporter in Ca2+ dynamics and signaling. Adv. Exp. Med. Biol. 2017, 9, 323–347. [Google Scholar]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef]
- Steffen, J.; Koehler, C.M. ER-mitochondria contacts: Actin dynamics at the ER control mitochondrial fission via calcium release. J. Cell. Biol. 2018, 217, 15–17. [Google Scholar] [CrossRef]
- Zhong, C.; Tong, D.Q.; Zhang, Y.R.; Wang, X.Q.; Yan, H.C.; Tan, H.Z.; Gao, C.Q. DL-methionine and DL-methionyl-DL-methionine increase intestinal development and activate Wnt/β-catenin signaling activity in domestic pigeons (Columba livia). Poult. Sci. 2022, 101, 101644. [Google Scholar] [CrossRef]
- Xie, P.; Wan, X.P.; Yang, C.X.; Zhu, J.G.; Xu, Y.G.; Gong, D.Q. Effects of incubation and chick rearing on intestinal morphology, digestive enzyme activities, and mRNA expression of nutrient transporter genes in the pigeon (Columba livia) under artificial farming conditions. Poult. Sci. 2020, 99, 2785–2797. [Google Scholar] [CrossRef]
- Palmer, M.F.; Rolls, B.A. Activities of some metabolic enzymes in the small intestinal mucosa during pregnancy and lactation in the rat. Reproduction 1980, 60, 231–236. [Google Scholar] [CrossRef]
- Bustamante, J.J.; Copple, B.L.; Soares, M.J.; Dai, G. Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int. 2010, 30, 406–415. [Google Scholar] [CrossRef]
- Haga, S.; Fujimoto, S.; Yonezawa, T.; Yoshioka, K.; Shingu, H.; Kobayashi, Y.; Takahashi, T.; Otani, Y.; Katoh, K.; Obara, Y. Changes in hepatic key enzymes of dairy calves in early weaning production systems. J. Dairy Sci. 2008, 91, 3156–3164. [Google Scholar] [CrossRef]
- Wan, X.P.; Xie, P.; Bu, Z.; Zou, X.T. Changes in hepatic glucose and lipid metabolism-related parameters in domestic pigeon (Columba livia) during incubation and chick rearing. J. Anim. Physiol. Anim. Nutr. 2018, 102, e558–e568. [Google Scholar] [CrossRef]
- Dong, X.Y.; Zhang, M.; Jia, Y.X.; Zou, X.T. Physiological and hormonal aspects in female domestic pigeons (Columba livia) associated with breeding stage and experience. J. Anim. Physiol. Anim. Nutr. 2013, 97, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Donsbough, A.L.; Powell, S.; Waguespack, A.; Bidner, T.D.; Southern, L.L. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult. Sci. 2010, 89, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Early weaning stress induces intestinal microbiota disturbance, mucosal barrier dysfunction and inflammation response activation in pigeon squabs. Front. Microbiol. 2022, 13, 877866. [Google Scholar] [CrossRef]
- Wen, J.S.; Xu, Q.Q.; Zhao, W.Y.; Hu, C.H.; Zou, X.T.; Dong, X.Y. Effects of early weaning on intestinal morphology, digestive enzyme activity, antioxidant status, and cytokine status in domestic pigeon squabs (Columba livia). Poult. Sci. 2022, 101, 101613. [Google Scholar] [CrossRef] [PubMed]

| Composition | Proportion (Based on Dry Weight) |
|---|---|
| Protein | about 64% |
| Lipid | about 30% |
| Mineral | about 5–6% |
| Carbohydrate | about 1–3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, J.; Xie, P.; Gong, D. Pigeon during the Breeding Cycle: Behaviors, Composition and Formation of Crop Milk, and Physiological Adaptation. Life 2023, 13, 1866. https://doi.org/10.3390/life13091866
Wang L, Zhu J, Xie P, Gong D. Pigeon during the Breeding Cycle: Behaviors, Composition and Formation of Crop Milk, and Physiological Adaptation. Life. 2023; 13(9):1866. https://doi.org/10.3390/life13091866
Chicago/Turabian StyleWang, Liuxiong, Jianguo Zhu, Peng Xie, and Daoqing Gong. 2023. "Pigeon during the Breeding Cycle: Behaviors, Composition and Formation of Crop Milk, and Physiological Adaptation" Life 13, no. 9: 1866. https://doi.org/10.3390/life13091866
APA StyleWang, L., Zhu, J., Xie, P., & Gong, D. (2023). Pigeon during the Breeding Cycle: Behaviors, Composition and Formation of Crop Milk, and Physiological Adaptation. Life, 13(9), 1866. https://doi.org/10.3390/life13091866







