Abstract
Lawsonia inermis, known as henna, has traditionally been utilized in cosmetics and folk medicine because of their valuable health effects. A lack of information about the processes that increase or decrease release, as well as the biological activities of constituents of natural origin, is an important pharmacological problem. This investigation evaluates the influence of moist heat on the flavonoid and phenolic contents of henna powder and their biological activities. HPLC analysis reflected the existence of 20 and 19 compounds of flavonoids and phenolics in the extract of unpre-treated henna by moist heat (UPMH) and pre-treated henna by moist heat (PMH). Several compounds such as chlorogenic acid, ellagic acid, rutin, rosmarinic acid, kaempferol, and pyrocatechol occurred with high concentrations of 57,017.33, 25,821.09, 15,059.88, 6345.08, 1248.42, and 819.19 µg/mL UPMH while occurred with low concentrations of 44,286.51, 17,914.26, 3809.85, 5760.05, 49.01, and 0.0 µg/mL, respectively in PMH. C. albicans, C. tropicalis, and G. candidum were more affected by UPMH with inhibition zones of 30.17 ± 0.29, 27 ± 0.5, and 29 ± 1.5 mm than PMH with inhibition zones of 29 ± 0.5, 25.33 ± 0.58, and 24.17 ± 0.29 mm, respectively. UPMH henna exhibited less MIC and MFC against the tested yeasts than PMH. Moreover, UPMH henna showed good wound healing, where the rat of migration, wound closure %, and area difference % were 14.806 um, 74.938 um2, and 710.667% compared with PMH henna 11.360 um, 59.083 um2, 545.333%, respectively. Antioxidant activity of UPMH and PMH henna. Promising antioxidant activity was recorded for both UPMH or PMH henna with IC50 5.46 µg/mL and 7.46 µg/mL, respectively. The docking interaction of chlorogenic acid and ellagic acid with the crystal structures of G. candidum (4ZZT) and C. albicans (4YDE) was examined. The biological screening demonstrated that the compounds had favorable docking results with particular proteins. Chlorogenic acid had robust behavior in the G. candidum (4ZZT) active pocket and displayed a docking score of −7.84379 Kcal/mol, higher than ellagic acid’s −6.18615 Kcal/mol.
1. Introduction
Lawsonia inermis (Lythraceae family) is commonly recognized as henna and is native to subtropical areas of North Africa and Asia. Traditionally, it has been utilized as a dandruff-fighting and a controller for fungi when functional to the hair, feet, and hands, besides coloring of skin, hair, and nails was attributed to henna [1,2]. Several pharmacological properties were associated with henna extract, such as alleviating and ameliorating wound healing, antifungal, antibacterial, antioxidant, nootropic, hepatoprotective, anti-ulcer, anti-cancer, anti-inflammatory, and anti-cancer activity. However, the leaves of this plant represent the most valuable part, but roots, stem, and bark have been utilized in ethno medicine conventional medicine for over nine centuries [3,4].
Species of Candida are pathogenic yeast forming mucocutaneous and systemic complaints in humans, particularly in diabetes patients, transplant recipients, xerostomia, malignancy, malnutrition, and lowly oral hygiene (immunocompromised patients) [5,6]. Several species of Candida become resistant to antifungal compounds [7]. Therefore, there is a requisite for novel compounds to fight pathogenic yeasts with greater efficiency and low toxicity. Yiğit [3] tested the paste of L. inermis extract against several clinical Candida isolates including Candida albicans, C. parapsilosis, C. glabrata, C. tropicalis, C. krusei, C. kefyr with different levels of inhibition, where the inhibition zone was more than 20 mm against 35.4% of the isolates, and was up to 15 mm against 38.0%, while the rest of isolates were resistance to the extract of L. inermis. As reported by Samadi et al. [8], henna extract can be applied to treat oral cavity infections resulting from C. albicans because of the promising anti-candidal activity of the extract, with 2.8 mg/ mL as the minimum inhibitory concentration. Besides Candida spp., the henna leaves extract reflected a fungicidal effect against filamentous fungi, including Penicillium ochrochloron, P. funiculosum, Aspergillus flavus, A. ochraceus due to the presence of apigenin 5-glucoside in the extract [9]. Trichophyton spp. Curvularia spp. and Geotrichum spp. were affected by henna leaf extract because of the existence of terpenes, aliphatic compounds, and flavonoids [10].
Different extraction solvents (chloroform, ethanol, and methanol) and methods (hot sequential extraction) were applied to evaluate the biological activities of L. inermis [11]. Despite the use of natural materials since the beginning of humanity, and even the demand for them is increasing daily, there is a big gap between how to apply, the correct extraction methods, and pre-treatments to evaluate its effects on the type and quantity of active ingredients. In the present investigation, moist heat was applied to the powder of henna before the extraction process and its biological activities.
Some plants, such as Laurus nobilis, were subjected to microwave, and high temperature of oven up to 120 °C [12]. Also, Al-Rajhi et al. [13] studied the effect of moist heat on the phenolic and flavonoid contents of L. nobilis, where moist heat induced the release of constituents and increased its biological activities. To date, numerous studies have reported the biological activities of henna using several extraction solvents, but few, if any, investigations have assessed the phytochemical characterization and biological effect of pre-treated henna by moist heat. Therefore, the current investigation aimed to study the effect of moist heat on henna before extraction, anti-yeast and antioxidant activity, and healing properties. Also, the molecular docking interaction of the most detected constituents of henna extract with some tested yeasts.
2. Materials and Methods
2.1. Chemical Used
Methanol, acetonitrile, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (Steinheim, Germany). Potato dextrose agar (PDA) medium was obtained from Oxoid Ltd., Basingstoke, Hampshire, UK.
2.2. Henna Source and Its Pre-Treated with Moist Heat
Dried henna leaves were obtained from the company of Abnaa Sayed Elobied Agro-Export, P.O. Box 10725, Khartoum, Sudan. The plant was validated by Prof. Marei A. Hamed, Prof. of the plant. The sample of leaves was kept under herbarium number SH 4325 in the Faculty of Science, Al-Azhar University, Egypt. The leaves were ground by a mill, and then passed via a 40-mesh sieve. The powder of Sudanian Henna was used in the current investigation. Henna powder (250 g) was autoclaved for 10 min at 100 °C, then cooled at room temperature (25 °C) and became pretreated by moist heat (PMH). At the same time, 250 g of henna was kept without pre-treatment by moist heat (UPMH) at 30 °C for 10 min with a humidity of 32%. The PMH and UPMH henna powders were extracted by mixing with 600 mL of methanol on the magnetic stirrer for 12 h. For removing any remains of the powders, the mixture at 5000 rpm for 10 min was centrifuged. Via rotary evaporator, the supernatant was concentrated to get a known weight, followed by re-dissolved in 0.5 mL of dimethyl sulfoxide (DMSO) [13].
2.3. Assessment of Phenolic and Flavonoid Constituents by HPLC
UPMH and PMH henna extract were subjected to HPLC (Agilent 1260 series) for phenolic and flavonoid constituents’ detection. The separation process was performed via Zorbax Eclipse Plus C8 column (4.6 mm × 250 mm i.d., 5 μm). The flow rate of the mobile phase (MP) of water (W) and acetonitrile containing 0.05% trifluoroacetic acid (A) was 0.9 mL/min. The MP was automated sequentially in a linear gradient in the flowing order: 0 min (82% W); 82% W from 0–1 min; 75% W from 1–11 min; 60% W from 11–18 min; 82% W from 18–24 min. The ultraviolet (UV) detector was adopted at 280 nm and 330 nm for phenolic and flavonoid constituents’ detection, respectively. The solution of tested samples was injected in volume 5 μL with the column maintained at 40 °C. The input data of standard molecules of phenolic and flavonoids was used for the quantitative determination of the extract’s compounds [14].
2.4. Anti-Yeast Activity of UPMH and PMH Henna Extracts
The anti-yeast activity of the henna extracts was assessed according to Al-Rajhi et al. [15] with some modification via cup-plate agar diffusion technique against Candida albicans (ATCC 10231), Geotrichum candidum (RCMB 027016), and Candida tropicalis. The tested yeasts were standardized (Corresponding to 0.5 McFarland scale), sowed in molten sterile Sabouraud dextrose agar medium, and poured into petri dishes. After solidification, via sterile cork borer (6 mm radius), four cups were cut and removed. Via automatic microlitre pipette, 100 µL of 20 µg/mL of each extract was injected in each cup, and then kept in the refrigerator at 4 °C for 30 min to allow the extract to diffuse through the agar layer. Followed by the incubation at 35 °C for 48 h. The visualized inhibition zones were recorded using a calibrated ruler in millimeters.
2.5. Evaluation of Minimum Inhibitory Concentration of UPMH and PMH Henna Extracts
The extract of henna, including the unpre-treated and pre-treated henna powders, was tested to detect minimum inhibitory concentration against tested yeasts. According to the CLSI M27-A3 standard manner. For each species of yeasts, a dilution was prepared in an equivalent to 0.5 McFarland. A dilution of each extract was prepared (1 mg/mL). Using 96-well plates, 100 µL of RPMI 1640 broth adjusted at pH 7 using a buffer of MOPS were transferred into each well. 100 μL of each extract was mixed with RPMI in wells of 1st column, and then serial dilution was performed. From the suspensions of tested yeast, 100 μL (0.5 McFarland) were added to each well, and wells without yeast cell suspensions were used as a negative control. Followed by incubation for one day at 34 ± 2 °C. The lowest concentration of extract that gave 50% declined growth was defined as MIC compared to the controls. The antifungal nystatin was used as a positive control, while the solvent of the extraction (methanol) was used as a negative control, respectively.
2.6. Estimation of Minimum Fetal Concentration (MFC) of UPMH and PMH Henna Extracts
To assess the MFC, 50 μL of the clear homogenized well suspension (devoid of visual growth) was cultivated using Sabouraud Dextrose Agar plates, followed by incubation for 48 h at 35 °C. The lowest dose of the extract affected growth inhibition (99.9%) compared to growth at control (without treatment) was MFC. The count of each yeast colony (CFU/mL) at different doses was compared with the count of each yeast colony at control (without treatment).
2.7. Antioxidant Activity of UPMH and PMH Henna Extracts
The 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH), developed by Elansary et al. [9] with minor modifications, was used to study the antioxidant scavenging activity. Various dilutions (1 mL) containing different concentrations of the plant extracts were combined with 1 mL of 0.2 mM DPPH dissolved in methanolic. Using a Helios spectrophotometer (Unicam, Cambridge, UK), the absorbance at 520 nm was measured following a 30-min incubation time at 25 °C. The same process was used in a blank experiment to create a solution devoid of the tested plant extract, and the absorbance was recorded. The percent inhibition of each solution’s free radical-scavenging activity was then determined using the following equation:
Antioxidant activity was itemized as IC50, which is the amount of the tested extracts needed to result in a 50% drop in the initial DPPH concentration.
2.8. Healing Properties of UPMH and PMH Henna Extracts
A multiwell plate was used for scratch wound examination. The plate was coated with an extracellular matrix substrate of 10 μg/mL fibronectin. Followed by incubation at 37 °C for 2 h. Then, the unbound extracellular matrix was removed and washed with phosphate-buffered saline. The growing cells from a dish containing tissue culture were detached with trypsin. The cells were developed on the scratch wound assay plate, followed by incubation to permit cells to spread and to obtain a confluent monolayer. The monolayer cell, including the confluent monolayer, was scraped using a pipette tip. Once scratched, slightly wash the monolayer of cells to remove separated cells. Then, replace with fresh medium containing tested extracts. The plate was incubated at 37 °C in the incubator of cell culture for 24–48 h. After the end of the incubation period, the cell monolayer was washed using phosphate-buffered saline. Then, the cells were fixed for 15 minutes using 3.7% paraformaldehyde. The cells were stained for 10 min using crystal violet (1% in ethanol). Then, the cell culture was examined using a phase-contrast microscope [16]. The following analysis was calculated according to the following equations:
where, Wi = average of initial wound width (um), Wf = average of final wound width (um), t = time span of the assay in hours
where, At0 = intial wound area, At∆t = wound area after n hours
2.9. Molecular Docking Investigation
Docking studies were carried out using the MOE (Molecular Operating Environment) software 2019.0102 program.
Ligand preparation: Chemical structures of substrate molecules (chlorogenic acid and ellagic acid) were drawn using ChemDraw Ultra 15.0, and this structure was saved as MDL files (“.sdf”) for MOE to show. These structures were optimized by adding hydrogens, and energies were minimized with parameters (gradient: 0.05, Force Field: MMFF94X).
Preparation of receptor structure: The G. candidum and C. albicans models were predicted through homology modeling. The best model was selected for docking analysis. This model is subjected to 3D protonation and energy minimization using parameters (gradient: 0.05, Force Field: MMFF94X + Solvation). The minimized structure was used as the receptor protein for Docking. The protein molecules utilised throughout our investigation were obtained from Protein Data Bank (http://www.rcsb.org/pdb accessed on 23 September 2015) using PDB codes (4ZZT) and (4YDE), respectively for G. candidum and C. albicans.
Docking Run: The MOE docking program with default parameters was used to bind the selected ligands with receptor proteins and to find the correct conformation of the substrate. Free energy of binding of the ligand from a given pose was estimated by MOE London dG scoring function. The top five poses were determined using hydrogen bonds with lengths under 3.5 Å and binding free energies (S, kcal/mol) between substances and amino acids that are part of proteins. Additionally, the RMSD and RMSD-refine fields were used to compare the results pose-with-pose in the co-crystal ligand position and before and after amendment, respectively.
2.10. Statistical Analysis
Standard deviation was calculated from the average three replicates of the obtained results via Microsoft programs of Excel version 365 and SPSS v.25. For variance analysis, one-way ANOVA, besides the test of post hoc Tukey, were applied to values analysis with a parametric distribution. The confidence interval was set to 95%, and the border of the accepted error was set up to 5%.
3. Results and Discussion
3.1. Flavonoid and Phenolic Contents of Unpre-Treated and Pre-Treated Henna by Moist Heat
The effect of moist heat was evaluated on the contents of flavonoid and phenolic in Henna extract, as well as its anti-yeast properties and other biological activities were performed (Figure 1). From HPLC analysis, the extract of unpre-treated henna by moist heat (UPMH) reflected the existence of 20 compounds, while the extract of pre-treated henna by moist heat (PMH) reflected the existence of 19 compounds of flavonoids and phenolics with different retention times, area, area %, and concentrations (Figure 2 and Figure 3 and Table 1). Chlorogenic acid, ellagic acid, gallic acid, rosmarinic acid, and rutin represent the highest concentrations in both UPMH and PMH henna extracts. The results indicated that the concentrations of the most detected compounds decreased in PMH henna extract compared to UPMH. For example, the concentrations of chlorogenic acid, ellagic acid, rutin, rosmarinic acid, kaempferol, and pyrocatechol were 57,017.33, 25,821.09, 15,059.88, 6345.08, 1248.42, and 819.19 µg/mL in UPMH, while become 44,286.51, 17,914.26, 3809.85, 5760.05, 49.01, and 0.0 µg/mL in PMH henna extract On the other hand, quercetin, naringenin, gallic acid, and coumaric acid concentrations were 96.76, 133.45, 9349.90, and 270.56 µg/mL in UPMH while becoming 1269.47, 2146.89, 32,349.91, and 402.02 µg/mL in PMH henna extract, respectively. Some studies reported that the effect of moist heat induced the discharge of extract constituents, unlike the current study. For example, Juániz et al. [17] found that phenolic constituents of vegetables liberated more if they were pretreated with heat due to cell wall destruction by heat. The decrease of most compounds in pre-treated henna extract or missing compound (pyrocatechol) indicated that these compounds are unstable at high temperatures or heat-sensitive and, at the same time, may easily oxidize and transform into other compounds. This explanation may match with Khoddami et al. [14]. While the concentration of some compounds increased, it may be due to high temperature causing the destruction of plant cell walls, causing the release of internal compounds. Another clarification of this phenomenon is that some phenolic and flavonoid constituents exist in an insoluble form. Heat may break these constituents, leading to the discharge of these bound constituents.
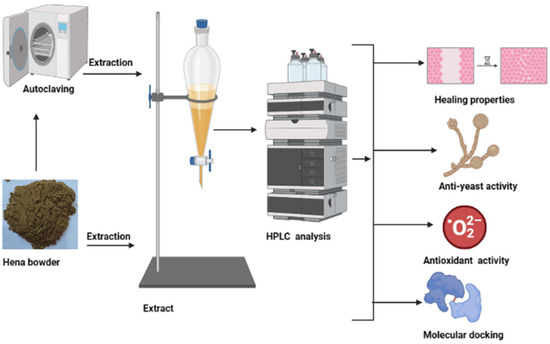
Figure 1.
Designed tests for unpre-treated (UPMH) and pre-treated henna powder by moist heat (PMH) in an autoclave with different assessments, including HPLC analysis, healing properties, anti-yeast activity, antioxidant activity, and molecular docking of the main constituents of henna extract.
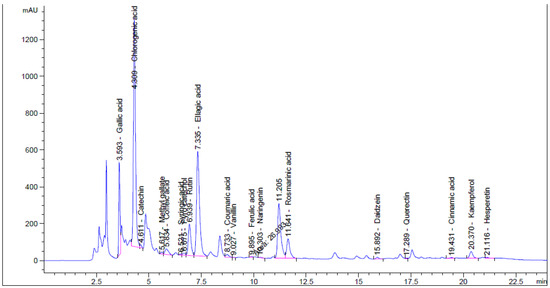
Figure 2.
Phenolic and flavonoid compounds detection in UPMH henna extract by moist heat indicated by HPLC chromatogram.
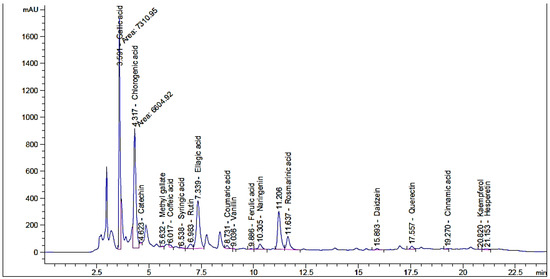
Figure 3.
Phenolic and flavonoid compounds detection in PMH henna extract by moist heat indicated by HPLC chromatogram.

Table 1.
Identified phenolic and flavonoid compounds in unpre-treated (UPMH) and pre-treated (PMH) henna extracts by moist heat.
Previously, Routray and Orsat [18] revealed that some phenolic ingredients were degraded when the natural plant extracts were exposed to the high power of the microwave. Dezashibi et al. [19] found that ultrasound of henna extract increments the phenolic constituents. Moreover, the content of total phenolic was affected by storage temperature and period. Other reports indicated that rises in temperature from 40 to 80 °C caused rises in total phenolic content [20]. Effects of boiling, microwaving, and boiling were reported on the content of ascorbic acid, β-carotene, and vitamin E in some plants, where visualized changes were observed [21].
3.2. Anti-Yeast Activity of Unpre-Treated and Pre-Treated Henna by Moist Heat
From the anti-yeast activity experiment, the extract of UPMH henna reflected significantly more inhibitory action with inhibition zones, 30.17 ± 0.29, 27 ± 0.5, and 29 ± 1.5 mm against C. albicans, C. tropicalis, and G. candidum compared with extract of PMH henna with inhibition zones, 29 ± 0.5, 25.33 ± 0.58, and 24.17 ± 0.29 mm, respectively (Table 2 and Figure 4). Moreover, the MIC and MFC of the extract of UPMH henna were less than the extract of PMH henna against all tested yeasts but with different degrees of sensitivity. Both types of extracts exhibited the highest anti-yeast activity than standard antifungal agents. According to the MIC and MFC, G. candidum was more sensitive, followed by C. albicans and C. tropicalis. The calculated index of MFC/MIC (≤2) indicated the cidal properties of both extracts. L. inermis showed anti-yeast activity but with different levels of inhibition depending on several factors, such as solvent extract, as mentioned previously by Suleiman and Mohamed [22], where the extraction by ethanol exhibited similar activity to nystatin, but the petroleum ether extract reflected more activity. Also, differences between the activities may be due to the region, the plant’s development, climatic changes, soil fertilizers, and the tested fungal population. Petroleum ether and ethanol extract of henna at 10 mg/mL demonstrated 26.3- and 25.3-mm inhibition zones against Saccharomyces cerevisiae 22.7-and-17 mm inhibition zones, respectively, against C. albicans. The inhibitory potential of the extract by the two types of solvents was more than that observed by the nystatin but did not give antifungal action against Pichia fabianii [22]. Kouadri [23] recorded strong anti-C. albicans activity of Saudi henna with an inhibition zone of 26 mm with a low MIC of 3.12 mg/mL due to several biologically active constituents. In vivo study, the henna (4%) was formulated as a vaginal cream, which showed promising management for infection caused by C. albicans in female rats. Moreover, it gave a similar effect to the antifungal agent (clotrimazole) [24].

Table 2.
Anti-yeast activity, MIC, MFC, and MFC/MIC index of UPMH, PMH henna extracts, positive control (Nystatin) and negative control (solvent used).
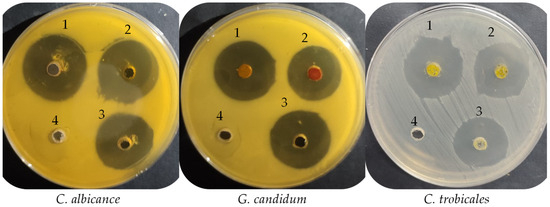
Figure 4.
Anti-yeast activity of UPMH (1), PMH (2) Henna extracts, positive control (3) and negative control (4) against C. albicance, G. candidum and C. trobicales.
3.3. Healing Properties of Unpre-Treated and Pre-Treated Henna by Moist Heat
There is a difference between the healing properties of the extract of UPMH and PMH henna, where the extract of UPMH henna provides reliable healing compared to the extract of PMH henna and control (cells without treatment) (Table 3 and Figure 5). The greatest common data resulting from the assessment of wound healing is the gap closure rate, which determines the rapidity of the cells’ collective motion. Rat of migration (RM), wound closure % and area difference % were 14.806 um, 74.938 um2, and 710.667% using extract of UPMH henna; 11.360 um, 59.083 um2, 545.333% using extract of PMH henna, compared to control cells, 11.554 um, 58.903 um2, and 554.667%, respectively with significant differs (Table 3). Daemi et al. [25] demonstrated that the healing process was accelerated by henna via minimizing tissue inflammation and increasing the uptake of glucose. An ointment containing henna extract shows a promising effect in managing episiotomy wounds [26]. El Massoudi et al. [27] explained the healing properties of henna. They mentioned that henna is richneed with different active molecules such as flavonoids, saponins, polyphenols, and others, which are vital in lowering oxidative stress and accelerating wound healing.

Table 3.
Healing properties of the extract of UPMH and PMH henna extract.
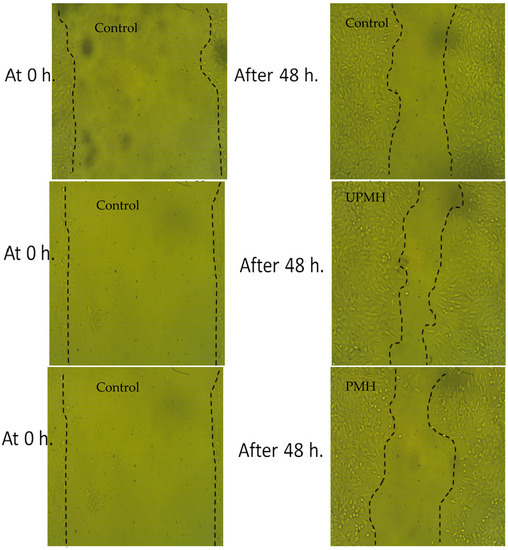
Figure 5.
Images of scratch test illustrated the effect of UPMH and PMH henna extract by moist heat on the wounding area at 0 and 48 h.
3.4. Antioxidant Properties of Unpre-Treated and Pre-Treated Henna by Moist Heat
The antioxidant activity of UPMH and PMH henna by moist heat was recorded in Table 4, compared with standard (Ascorbic acid). Generally, henna extract of either UPMH or PMH exhibited promising DPPH scavenging %. However, the extract of UPMH henna reflected more activity than the extract of PMH henna with IC50 5.46 µg/mL and 7.46 µg/mL, respectively, compared with the IC50 value of ascorbic acid 2.52 µg/mL. The low activity of antioxidants for the PMH henna may be due to a low concentration of active compounds, as mentioned in HPLC analysis, due to exposure to moist heat. From Table 4, the antioxidant potential increments with the increasing dosage of the tested extracts with concentration-dependent liner. Réblov [28] studied the antioxidant activity of several phenolic acids under stress of temperature. For instance, vanillic acid was effective at 90 °C, while gallic and caffeic acids presented antioxidant potential at 150 °C. In in vivo investigation, henna protected them from oxidative stress and possessed hepatoprotective properties in Wistar rats [29]. Çubukçu et al. [30] reported that treatment by heat had harmful effects on the antioxidant activities of some plants, such as garlic and onion, while freezing enhanced the antioxidant properties of garlic and had negative effects on onion.

Table 4.
Antioxidant activity of unpre-treated (UPMH), pre-treated (PMH) henna extract by moist heat and ascorbic acid.
3.5. Molecular Docking of Chlorogenic Acid and Ellagic Acid with 4ZZT Protein of G. candidum and 4YDE Protein of C. albicans
In the current decade, molecular docking has attracted the attention of several investigators in drug design, development, and discovery. Structure-based computer modeling of ligand-receptor interactions is widely used in modern drug development. To identify conformational changes that vary with the environment and to characterize the interaction of the molecule with the protein with which it interacts inside the body, molecular docking calculations are commonly used in structure-based drug design investigations.
Chlorogenic and ellagic acids were docked using MOE (Molecular Operating Environment) in a vital trial to get insight into the potential pathways through which these compounds exert their antibacterial action. Both target proteins interact effectively with inhibitor compounds. The docking results of compounds’ interaction with the crystal structures of G. candidum (4ZZT) and C. albicans (4YDE) (4YDE code of the farnesyltransferase enzyme, and 4ZZT code of the enzyme cellobiohydrolase enzyme, which represent one of the virulence factors in yeast cells) revealed that chlorogenic acid was found the most promising than ellagic acid. Chlorogenic acid revealed substantial binding interactions within the (4ZZT) active site with a binding score of −7.84379 Kcal mol−1 according to five conventional hydrogen bonds between GLU 217 and O 19 with 2.80 Å, GLN 175 and O 17 with 2.97 Å, HIS 228 and O 19 with 3.24 Å, TRP 380 and C 1 with 3.19 Å, and TRP 371 and 6-ring with 3.61 Å. Also, ellagic acid outlined four significant interactions with active site residues of G. candidum (4ZZT) between GLU 217 and O 20 with 2.67 Å, THR 226 and O 22 with 3.20 Å, ARG 399 and O 24 with 3.08 Å, and ARG 251 and 6-ring with 4.09 Å.
Similarly, ranked the inhibitor compounds with C. albicans (4YDE) showed similar behavior and adopted direct H-donor and H-acceptor bonds with active site residues. Chlorogenic acid highlighted a binding score of −5.69876 Kcal mol−1, and exhibited several key interactions with (4YDE) with ASP 527, ASP 569, and LYS 512 via C 26, O 40, and O 23, respectively. While ellagic acid had a binding score of −4.5145 Kcal mol−1, and only one donor interaction between the O 20 atom and the ASP 527 amino acid residue, it demonstrated reduced effectiveness to (4YDE). The 2D and 3D docking interactions are given in Figure 6, Figure 7, Figure 8 and Figure 9, and the obtained results are shown in Table 5, Table 6, Table 7 and Table 8. Several reports confirmed the biological activities of drugs theoretically via molecular docking interaction [31,32,33,34,35,36]. The most potent constituent in henna (3a, 4a-Dihydroxy-a-tetralone) was docked with sterol 14-demethylase protein of Staphylococcus aureus, which reflected a docking score of −7.196 kcal mol−1 [37]. Alteration of yeast cells from the yeast shape to filamentous shape is linked with pathogenicity [38]. Therefore, the inhibitors of these enzymes (4YDE and 4ZZT) are potentially therapeutic against infection caused by yeasts.
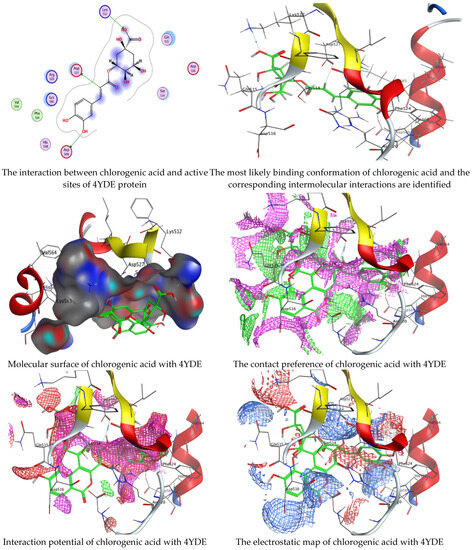
Figure 6.
Molecular docking process of chlorogenic acid with the crystal structure of C. albicans (4YDE).
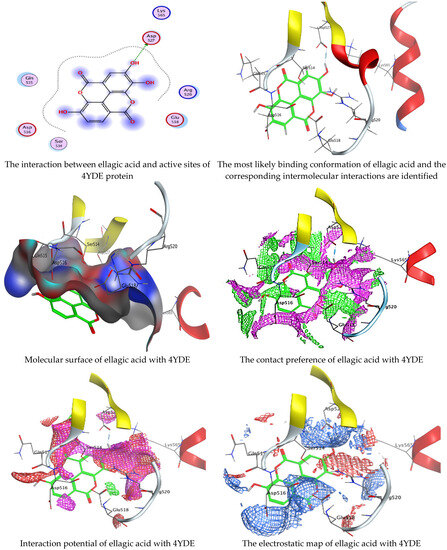
Figure 7.
Molecular docking process of ellagic acid with the crystal structure of C. albicans (4YDE).
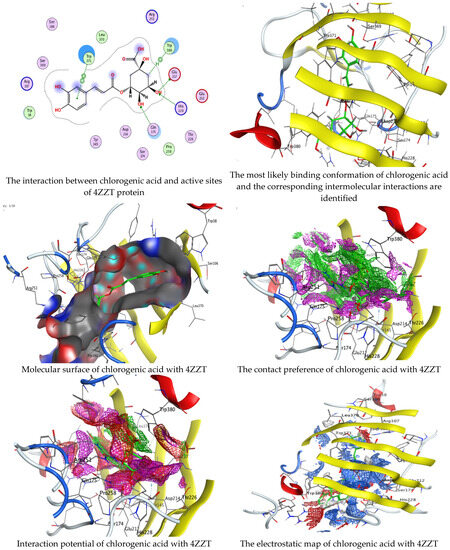
Figure 8.
Molecular docking process of chlorogenic acid with the crystal structure of G. candidum (4ZZT).
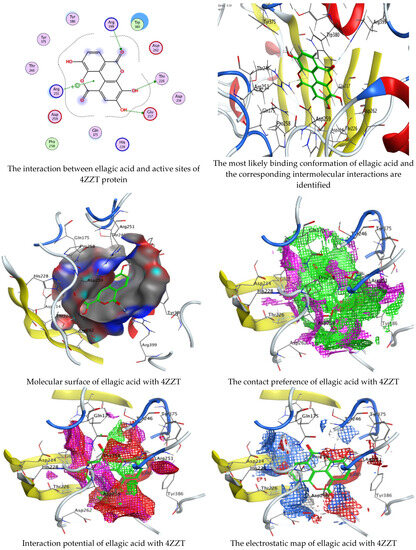
Figure 9.
Molecular docking process of ellagic acid with the crystal structure of G. candidum (4ZZT).

Table 5.
Docking scores and energies of chlorogenic acid and ellagic acid with the crystal structure of C. albicans (4YDE).

Table 6.
Docking scores and energies of chlorogenic acid and ellagic acid with the crystal structure of G. candidum (4ZZT).

Table 7.
Interaction of chlorogenic acid and ellagic acid with the crystal structure of C. albicans (4YDE).

Table 8.
Interaction of chlorogenic acid and ellagic acid with the crystal structure of G. candidum (4ZZT).
4. Conclusions
The obtained results indicated that pre-treated henna powder with moist heat provided undesirable findings, where several phenolic and flavonoid compounds were decreased as exposure to moist heat. Moreover, the anti-yeast, antioxidant, and healing properties of un-pretreated henna were acceptable compared to pretreated henna by moist heat. In the search for new bioactive analogues, chlorogenic acid and ellagic acid exhibited good potential for G. candidum and C. albicans inhibition. According to docking data, chlorogenic acid performed the best interaction with the crystal structure of G. candidum (4ZZT), and the results can be utilized to guide future experimental studies. This investigation recommended the application of henna without any cocked process.
Author Contributions
Investigation, methodology, supervision, S.A.A.; formal analysis, Writing—review and editing, M.I.A.; investigation, resources, M.J.; investigation, writing—original draft preparation, conceptualization, T.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RP23038), Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and funding the current study (grant number IMSIU-RP23038).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jothiprakasam, V.I.; Ramesh, S.A.; Rajasekharan, S.K. Preliminary phytochemical screening and antibacterial activity of Lawsonia inermis Linn (Henna) leaf extracts against reference bacterial strains and clinically important AMPC beta-lactamases producing Proteus mirabilis. Int. J. Pharm. Pharm. Sci. 2013, 5, 219–222. [Google Scholar]
- Rahmoun, N.; Boucherit-Otmani, Z.; Boucherit, K.; Benabdallah, M.; Choukchou-Braham, N. Antifungal activity of the Algerian Lawsonia inermis (henna). Pharm. Biol. 2013, 51, 131–135. [Google Scholar] [CrossRef]
- Yiğit, D. Antifungal activity of Lawsonia inermis L. (Henna) against clinical Candida isolates. Erzincan Univ. J. Sci. Technol. 2017, 10, 196–202. [Google Scholar]
- Supian, F.N.A.; Osman, N.I. Phytochemical and Pharmacological Activities of Natural Dye Plant, Lawsonia inermis L. (Henna). J. Young Pharm. 2023, 15, 201–211. [Google Scholar] [CrossRef]
- Coronado-Castellote, L.; Jimenez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286. [Google Scholar] [CrossRef] [PubMed]
- Hemaid, A.S.S.; Abdelghany, M.M.E.; Abdelghany, T.M. Isolation and identification of Candida spp. from immunocompromised patients. Bull. Natl. Res. Cent. 2021, 45, 163. [Google Scholar] [CrossRef]
- Badiee, P.; Alborzi, A.; Shakiba, E.; Farshad, S.; Japoni, A. Susceptibility of Candida species isolated from immunocompromised patients to antifungal agents. East. Mediterr. Health J. 2011, 17, 425–430. [Google Scholar] [CrossRef]
- Samadi, F.M.; Suhail, S.; Sonam, M.; Sharma, N.; Singh, S.; Gupta, S.; Dobhal, A.; Pradhan, H. Antifungal efficacy of herbs. J. Oral. Biol. Craniofacial Res. 2019, 9, 28–32. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.A.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef]
- Abirami, S.; Raj, B.E.; Soundarya, T.; Kannan, M.; Sugapriya, D.; Al-Dayan, N.; Mohammed, A.A. Exploring antifungal activities of acetone extract of selected Indian medicinal plants against human dermal fungal pathogens. Saudi J. Biol. Sci. 2021, 28, 2180–2187. [Google Scholar] [CrossRef]
- Chowdhury, M.M.H.; Kubra, K.; Ahmed, S.R. Antimicrobial, Phytochemical and toxicological evaluation of Lawsonia inermis extracts against clinical isolates of pathogenic bacteria. Res. J. Med. Plant 2014, 8, 187–195. [Google Scholar] [CrossRef]
- Khodja, Y.K.; Dahmoune, F.; Bey, M.B.; Madani, K.; Khettal, B. Conventional method and microwave drying kinetics of Laurus nobilis leaves: Effects on phenolic compounds and antioxidant activity. Braz. J. Food Technol. 2020, 23, e2019214. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Anti-Helicobacter pylori, Antioxidant, Antidiabetic, and Anti-Alzheimer’s Activities of Laurel Leaf Extract Treated by Moist Heat and Molecular Docking of Its Flavonoid Constituent, Naringenin, against Acetylcholinesterase and Butyrylcholinesterase. Life 2023, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Abdelghany, T.M. Pharmacological Evaluation of Acacia nilotica Flower Extract against Helicobacter pylori and Human Hepatocellular Carcinoma In Vitro and In Silico. J. Funct. Biomater. 2023, 14, 237. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.-P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Dezashibi, Z.; Samarin, A.M.; Hematyar, N.; Khodaparast, M.H. Phenolics in Henna: Extraction and stability. Eur. J. Exp. Biol. 2013, 3, 38–41. [Google Scholar]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Garofulić, I.E. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2017, 27, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, E.A.; Mohamed, E.A. In vitro activity of Lawsonia inermis (Henna) on some pathogenic fungi. J. Mycol. 2014, 2014, 375932. [Google Scholar]
- Soliman, S.S.M.; Semreen, M.H.; El-Keblawy, A.A.; Abdullah, A.; Uppuluri, P.; Ibrahim, A.S. Assessment of herbal drugs for promising anti-Candida activity. BMC Complement. Altern. Med. 2017, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Kouadri, F. In vitro antibacterial and antifungal activities of the Saudi Lawsonia inermis extracts against some nosocomial infection pathogens. J. Pure Appl. Microbiol. 2018, 12, 281–286. [Google Scholar] [CrossRef]
- Yaralizadeh, M.; Abedi, P.; Namjoyan, F.; Fatahinia, M.; Chegini, S.N. A comparison of the effects of Lawsonia inermis (Iranian henna) and clotrimazole on Candida albicans in rats. J. Med. Mycol. 2018, 28, 419–423. [Google Scholar] [CrossRef]
- Daemi, A.; Farahpour, M.R.; Oryan, A.; Karimzadeh, S.; Tajer, E. Topical administration of hydroethanolic extract of Lawsonia inermis (henna) accelerates excisional wound healing process by reducing tissue inflammation and amplifying glucose uptake. Kaohsiung J. Med. Sci. 2019, 35, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.; Zibanejad, S.; Kopaei, M.R. Healing effect of Quercus persica and Lawsonia inermis ointment on episiotomy wounds in primiparous women. J. Res. Med. Sci. 2020, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- El Massoudi, S.; Zinedine, A.; Rocha, J.M.; Benidir, M.; Najjari, I.; El Ghadraoui, L.; Benjelloun, M.; Errachidi, F. Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia inermis) Aqueous Extracts. Cosmetics 2023, 10, 92. [Google Scholar] [CrossRef]
- Réblová, Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 2012, 30, 171–175. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, P.; Chandel, M.; Singh, A.P.; Jain, A.; Kaur, S. Antioxidant and hepatoprotective potential of Lawsonia inermis L. leaves against 2-acetylaminofluorene induced hepatic damage in male Wistar rats. BMC Complement. Altern. Med. 2017, 17, 56. [Google Scholar] [CrossRef]
- Çubukçu, H.C.; Kılıçaslan, N.S.D.; Durak, I. Different effects of heating and freezing treatments on the antioxidant properties of broccoli, cauliflower, garlic and onion. An experimental in vitro study. Sao Paulo Med. J. 2019, 137, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Alotaibi, K.; Aldarhami, A.; Bazaid, A.S.; Ganash, M.; Saeedi, N.H.; Ghany, T.A. Effectiveness of oil-based nanoemulsions with molecular docking of its antimicrobial potential. BioResources 2023, 18, 1554–1576. [Google Scholar] [CrossRef]
- Dehghan, M.; Fathinejad, F.; Farzaei, M.H.; Barzegari, E. In silico unraveling of molecular anti-neurodegenerative profile of Citrus medica flavonoids against novel pharmaceutical targets. Chem. Pap. 2023, 77, 595–610. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Ghany, T.M.A. Nanoemulsions of some edible oils and their antimicrobial, antioxidant, and anti-hemolytic activities. BioResources 2023, 18, 1465–1481. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Kavepour, N.; Bayati, M.; Rahimi, M.; Aliahmadi, A.; Ebrahimi, S.N. Optimization of aqueous extraction of henna leaves (Lawsonia inermis L.) and evaluation of biological activity by HPLC-based profiling and molecular docking techniques. Chem. Eng. Res. Des. 2023, 195, 332–343. [Google Scholar] [CrossRef]
- McGeady, P.; Logan, D.A.; Wansley, D.L. A protein-farnesyl transferase inhibitor interferes with the serum-induced conversion of Candida albicans from a cellular yeast form to a filamentous form. FEMS Microbiol. Lett. 2002, 213, 41–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).