Abstract
The use of medicinal plants in the management of diabetes mellitus (DM) is extensively reported. However, there is still very limited information on the role of these plants as markers of oxidative stress in DM. This current review evaluated the effect of Amaranthus spinosus, Amaranthus hybridus, and Abelmoschus esculentus on markers of oxidative stress in rodent models of DM. Current findings indicate that these plants have the potential to reduce prominent markers of oxidative stress, such as serum malondialdehyde and thiobarbituric acid-reactive substances, while increasing enzymes that act as antioxidants, such as superoxide dismutase, catalase, glutathione, and glutathione peroxidase. This may reduce reactive oxygen species and further ameliorate oxidative stress in DM. Although the potential benefits of these plants are acknowledged in rodent models, there is still a lack of evidence showing their efficacy against oxidative stress in diabetic patients. Therefore, we recommend future clinical studies in DM populations, particularly in Africa, to evaluate the potential effects of these plants. Such studies would contribute to enhancing our understanding of the significance of incorporating these plants into dietary practices for the prevention and management of DM.
1. Introduction
Diabetes mellitus (DM) is a chronic, life-threatening disease that has caused more than 6.7 million deaths worldwide [1]. This condition affects approximately 537 million adults (20–79 years old) [1]. According to the International Diabetes Federation, the number of people living with diabetes is predicted to reach 643 million by 2030 [1]. There are three commonly known types of DM: type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (GDM). T2DM is considered the most common, and it affects [2] at least 95% of the diabetic population [3]. In 2021, the prevalence of T2DM was reported to be 10.5% [4], and most of the population is from low- and middle-income countries (LMICs) [1].
Diabetic patients in LMICs face many challenges, which include a lack of awareness and knowledge about the disease, difficulty accessing health care systems, including medications, and inadequate diabetes management strategies, which likely result from a poor socio-economic background [5]. Biological risk factors associated with DM include older age, increased body mass index (BMI), obesity, stress, physical inactivity, and chronic inflammation due to other infectious diseases [6,7,8]. DM is associated with health complications such as cardiovascular diseases, kidney diseases, vision impairment, and neurological conditions [9]. Oxidative stress has been reported to play a major role in the pathophysiology of DM-related complications [10].
In 2021, it was documented that DM caused at least 966 billion USD in health expenditure, with 9% of total spending on adults [1]. Low- and middle-income countries already have overwhelming health burdens resulting from other common diseases such as tuberculosis and the human immunodeficiency virus [11]. Therefore, healthcare systems in place must implement efficacious medicines that are less toxic and cost-effective in the management of DM.
While medical and pharmacological drugs are currently available for managing DM, these are still associated with severe side effects in different individuals, increased complications, and a rising mortality rate in DM. For instance, using sodium-glucose cotransporter 2 inhibitors increases the risk of hypotension, diabetic ketoacidosis, kidney injury, and bone fractures [12]. Regrettably, prolonged use of glucophage is linked to cobalamin deficiency, increasing the risk of additional complications, including anemia, in T2DM patients [13]. Given the mentioned drawbacks of pharmaceutical medications, there has been a burgeoning interest in the utilization of functional foods and herbal remedies for the treatment and management of DM. This interest is partly attributable to their inherent properties. Numerous studies have highlighted the antioxidant characteristics of medicinal plants in the treatment and management of DM.
For instance, our team recently found the potential beneficial effects of Corchorus olitorius and curcumin in a rodent model of obesity and DM and T2DM, respectively [14,15].
Interestingly, functional fruits have also demonstrated potential benefits, especially on oxidative stress in DM [16]. Although there are rising calls for more research support for medicinal plant use in the treatment and management of DM [11], there is still limited clinical evidence to support their efficacy, especially in DM. Moreover, evidence from previous preclinical studies has not focused on common markers of oxidative stress. In this study, we aim to gather evidence from preclinical studies evaluating the effect of Amaranthus hybridus, Amaranthus spinosus, and Abelmoschus esculentus in DM primarily due to their beneficial properties and safety profile [17], with the main focus on various biomarkers of oxidative stress. Therefore, this review will highlight and document the potential benefits of these selected medicinal plants in DM.
2. Oxidative Stress and Diabetes Mellitus
Oxidative stress occurs as a result of an imbalance between the production and clearance of reactive oxygen species (ROS) [18] and contributes to the pathogenesis and pathophysiology of DM [19,20]. DM is a metabolic disorder characterized by increased blood glucose levels resulting from insulin resistance and impaired insulin secretion [21].
In DM, several factors contribute to oxidative stress, including hyperglycemia, dyslipidemia, insulin resistance, and inflammation. Hyperglycemia and hyperlipidemia can lead to increased cellular oxidative stress through mitochondrial electron leak or incomplete fatty acid oxidation, the formation of advanced glycation end products (AGEs), lipid hydroperoxides, and induced free fatty acids (FFA), diacylglycerol (DAG), and ceramides. Lipid peroxidation has been identified as one factor that leads to DM development [22,23]. This occurs when there is uncontrolled high blood glucose and free fatty acids, which in turn activate DAG and protein kinase C (PKC) [24,25,26]. Activation of PKC induces the inflammatory response by promoting the secretion of endothelin 1 (ET-1), vascular cell adhesion molecule (VCAM-1), intercellular adhesion molecule (ICAM-1), nuclear factor kappa-light chain enhancer of activated β cells (NF-κβ), and NADPH oxidase [27,28,29]. Notably, NADPH oxidase activation mediates ROS generation through superoxide [30,31]. Excessive ROS production damages cells, resulting in a pronounced inflammatory response [32]. Hence, it is comprehensible why diabetes is frequently linked to inflammation [32,33,34,35]. Conversely, catalase (CAT), an active enzyme, functions as an antioxidant by catalyzing the conversion of hydrogen peroxide into water and oxygen [36]. Nevertheless, diminished CAT activity results in oxidative stress in the pancreatic beta cells, which contain numerous mitochondria. This excess production of reactive oxygen species (ROS) ultimately leads to dysfunction in β-cells and the onset of diabetes [18]. Therefore, this would subject the cells or organs to oxidative stress by allowing the accumulation of harmful oxidants and free radicals. Reactive oxygen species can also increase insulin resistance, leading to further hyperglycemia [37,38,39,40,41]. This occurs when caloric intake exceeds energy expenditure, thereby causing an increase in citric acid cycle activity. This subsequently leads to excess mitochondrial NADH (mNADH) and ROS [42]. Inflammation, which is common in DM, can also contribute to oxidative stress by activating immune cells that produce ROS [43]. Several studies have indicated that oxidative stress can contribute to the development of diabetic complications such as neuropathy, retinopathy, and nephropathy [43,44,45,46]. Therefore, controlling oxidative stress may be an important DM management strategy [44]. This can be achieved through lifestyle modifications such as regular exercise, a healthy diet, and smoking cessation [47]. Antioxidant supplements such as vitamin C, vitamin E, and alpha-lipoic acid may also be beneficial in reducing oxidative stress [48]. Some medicinal plants, such as Amaranthus spinosus, Amaranthus hybridus, and Abelmoschus esculentus, have been shown to have antioxidant effects.
3. Amaranthus Species
Amaranthus, a herbaceous plant native to Central America, has been cultivated for centuries due to its valuable properties [49]. It has spread to various nations, successfully established itself, and naturalized in numerous regions across the globe [50]. In Africa, it is esteemed as a traditional food plant, thus providing a valuable source of nutrition. All parts of the plant, including seeds, roots, leaves, and stems, are recognized for their edible and medicinal attributes [51]. In addition, Amaranthus is affordable and cost-effective [52], making it significant in improving nutrition, ensuring food security, and alleviating poverty, especially in low- and middle-income countries [53]. Amaranthus has been recognized as a superfood, making it an interesting plant for further exploration in research [54]. Among the various Amaranthus species, the common ones include Amaranthus thunbergii, A. greazican, A. deflexus, A. hypochondriacus, A. viridis, A. spinosus, and A. hybridus. This pivotal plant possesses an abundance of vital nutrients, making it a rich source of essential nutrients, including vitamins and minerals [54]. These micronutrients have been extensively studied for their crucial role in promoting optimal well-being. More interestingly, Amaranthus has been reported to possess various compounds, including amino acids such as lysine, arginine, histidine, leucine, cysteine, phenylalanine, isoleucine, valine, threonine, and methionine [55].
The Amaranthus plant also contains important active compounds that promote its activities [56,57,58] (Figure 1). The use of Amaranthus in the public interest extends beyond its nutritional benefits to its therapeutic properties, especially in the management of cholesterol and blood glucose levels in DM [59,60]. While many studies reporting on the therapeutic properties of Amaranthus in DM have focused on inflammatory markers [61,62,63], only a limited number of studies have explored its benefits in alleviating oxidative stress in DM. The present study will review studies reporting the potential benefits of Amaranthus spinosus and hybridus on markers of oxidative stress in DM. This will help to improve current knowledge on the importance of consuming these plants, especially in diabetic individuals, in order to manage the condition and in non-diabetic individuals to prevent the risk of developing DM.
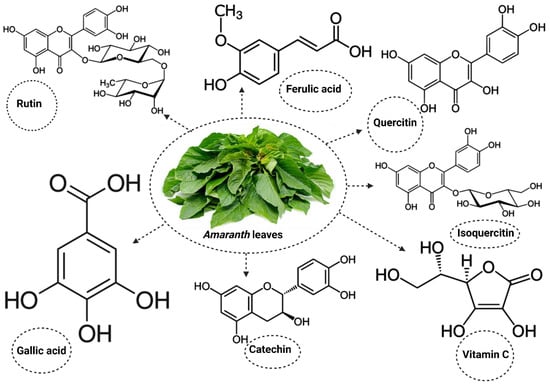
Figure 1.
Some of the active compounds present in Amaranthus.
3.1. Amaranthus spinosus
As presented in Table 1, Amaranthus spinosus has been shown to alleviate oxidative stress. Amaranthus spinosus has been shown to reduce hyperglycemia-associated oxidative stress significantly in rodent models of obesity [64]. A study by Kumar et al. (2011) reported that methanol extract of Amaranthus spinosus at a dose of 200 and 400 mg/kg for 15 days had antioxidant effects against DM [65]. This was demonstrated by a reduction in the serum level of malondialdehyde (MDA) concomitant with the increased activity of enzymes that act as antioxidants, such as glutathione (GSH) and catalase (CAT), in alloxan-induced diabetic rats. Furthermore, the same study showed an increase in total thiols. Similar findings were observed by Mishra et al. (2012), whereby Amaranthus spinosus leaf extract (ASEt) at doses of 250 and 500 mg/kg for 21 days reduced oxidative stress and improved pancreatic cell function in diabetic rats [66]. These positive impacts were demonstrated by a significant increase in superoxide dismutase (SOD), CAT, GSH, and glutathione peroxidase (GPx). An increase in antioxidant enzymes observed after Amaranthus treatment shows its potential as an antioxidant remedy (Figure 2). Therefore, this suggests that this plant could play an important role in alleviating oxidative stress.

Table 1.
Overview of studies evaluating the antioxidant effect of Amaranthus rodents in a model of diabetes mellitus.
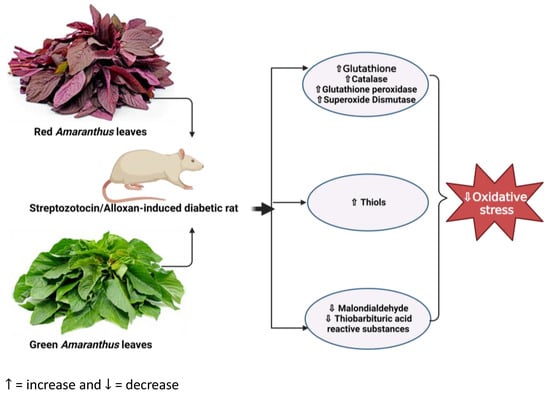
Figure 2.
Overview showing the impact of Amaranthus on oxidative stress in diabetic rats. Administration of Amaranthus in diabetic rats ameliorates oxidative stress by reducing malondialdehydes and thiobarbituric acid while increasing the activity of antioxidant enzymes such as glutathione, glutathione peroxidase, catalase, and superoxide dismutase. (https://www.seeds-gallery.eu/9135-large_default/amaranth-red-garnet-seeds-Amaranthus-tricolor.jpg (accessed on 25 July 2023), https://specialtyproduce.com/produce/Green_Amaranth_12831.php (accessed on 25 July 2023).
3.2. Amaranthus hybridus
Similarly, Amaranthus hybridus has been reported to have anti-oxidative stress effects in DM [67]. Amaranthus hybridus ethanol leaf extract (AHELE) has been identified to have a nephron protective effect against oxidative damage in streptozotocin (STZ)-induced diabetic rats [68]. In a study by Balasubramanian et al. (2016), this was indicated by a significant reduction in the marker of lipid peroxidation, thiobarbituric acid reactive substances (TBARS) (p < 0.001), and a significant increase in antioxidant markers such as superoxide dismutase (SOD) (p < 0.001) and CAT (p < 0.01). In support of their findings, they also discovered that AHELE possessed both nephroprotective and hepatoprotective effects by reducing the levels of MDA in the liver and kidneys of STZ-induced diabetic rats [68]. Therefore, the evidence from rat models of diabetes induced by STZ or alloxan indicates the potential of the Amaranthus plant as an anti-oxidative stress agent (Figure 2, Table 1). MDA is an end-product of fatty acid peroxidation [69], and its high level is an indication of lipid peroxidation [70]. During lipid peroxidation, lipids are degraded in the cell membrane, thus leading to cell damage [69,71]. Lipid peroxidation has been identified as one of the factors leading to the development of DM [22,23].
4. Abelmoschus esculentus
Abelmoschus esculentus L. is also known as okra and belongs to the Malvaceae plant family. Although this plant is found in Africa, it is widely distributed in Asia, America, and Southern Europe [72]. The fruit, seeds, roots, leaves, flowers, and pods of Abelmoschus esculentus contain vital bioactive chemicals that contribute to this plant’s beneficial effects [73,74] (Figure 3). For example, the seed contains oligomeric catechins and flavonol derivatives [74,75,76]. The root contains carbohydrates and flavonol glycosides [77], while the leaves contain minerals, tannins, and flavonol glycosides [78]. The pod contains carotene, folic acid, thiamine, riboflavin, protein, fiber, calcium, iron, zinc, niacin, vitamin C, oxalic acid, and amino acids [79,80,81]. These compounds mediate the various functions that Abelmoschus esculentus possesses. Such potential benefits include but are not limited to anti-hyperglycemic, anti-inflammatory, and antioxidant effects [82,83,84].
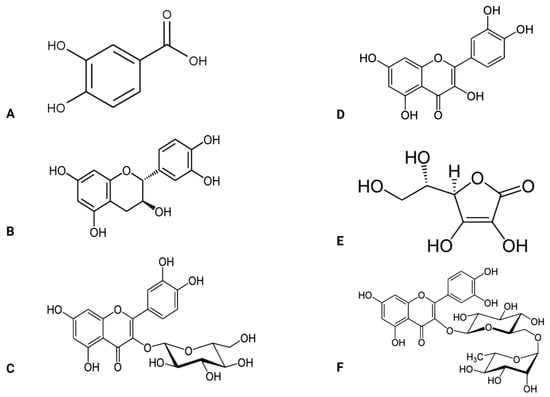
Figure 3.
Active compounds found in the Abelmoschus esculentus plant. (A) protocatechuic acid, (B,C) catechin, (D) quercetin, (E) vitamin C, (F) rutin.
4.1. Antioxidant Effect of Abelmoschus esculentus
The antioxidant properties of Abelmoschus esculentus have been revealed by previous research [83,84,85,86], and this is attributable mainly to its active compounds, such as polyphenols and flavonoids [87] (Figure 3). Polyphenols mediate antioxidant activity by reducing MDA and increasing SOD, GPx, and catalase activity [77,78,82,84,85,86,87,88,89,90,91,92,93]. Some of the active polyphenol compounds include isoquercetin, quercetin, quercetin-3-O-gentiobioside, quercetin-3-O-glucoside, protocatechuic acid, and rutin. All these phenolic compounds exhibit free radical scavenging and ferric-reducing properties [92] and further inhibit the activities of α-glucosidase and α-amylase [94]. Specifically, the Abelmoschus esculentus seeds are excellent sources of phenols, including procyanidin B1 and B2, which facilitate the free radical scavenging activities of 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-casino-bis (3-ethylbenzothi azoline-6-sulfonic acid (ABTS) [90,95,96].
4.1.1. Effect of Abelmoschus esculentus on Oxidative Stress in Animal Models of Diabetes Mellitus
Mice and rats have been used for decades to mimic DM observed in humans, primarily to explore the beneficial effects, toxicity, and desirable doses of different compounds against various metabolic disorders [97]. Oxidative stress is implicated in the progression of insulin resistance into DM due to the increased production of free radical molecules. These ROS molecules (hydrogen peroxide, superoxide anion, and hydroxyl radicals) are generated by the partial reduction of oxygen molecules [98]. However, when these molecules are excessively produced in the body, they cause damage to cellular proteins, membrane lipids, and nucleic acids and reduce lifespan [99,100].
Several biomarkers have been widely considered predictors of oxidative stress; these include SOD, MDA, CAT, GPx, and GSH. The existing research suggests that Abelmoschus esculentus has antioxidant potential, partly due to its high phenolic and flavonoid content. For instance, two groups recently demonstrated a very high content of phenols and flavonoids in Abelmoschus esculentus mucilage and seed peel, respectively [101,102]. In contrast, other studies showed an increased half-maximal inhibitory concentration (IC50) in Abelmoschus esculentus extracts and mucilage compared to vitamin C [101,103]. This suggests the reduced antioxidant capability of Abelmoschus esculentus. IC50 refers to the number of antioxidant compounds necessary to scavenge 50% of the initial DPPH radicals. The compound’s increased effectiveness in scavenging DPPH radicals results in a reduced IC50 value, indicating an ideal level of antioxidant activity for the compound [104]. The summarized effect of Abelmoschus esculentus in rodent models of diabetes is presented in Table 2. Although the potential benefits are acknowledged, different studies have contradictory findings, with others showing negative results on oxidative stress markers. Below, we outline the effects of Abelmoschus esculentus on various markers of oxidative stress.
4.1.2. Abelmoschus esculentus on Oxidative Stress with a Focus on ROS
In general, the evidence supports using Abelmoschus esculentus treatment as a possible antioxidant in at least three preclinical studies (Table 2). These studies revealed a significant (p < 0.05) reduction in the levels of ROS in a rodent model of DM and, therefore, an attenuation of oxidative stress [105,106,107]. Elevated ROS levels are associated with oxidative stress and organ damage [18]. Therefore, the potential of Abelmoschus esculentus to reduce these excess ROS may be of importance in reducing organ and tissue damage in diabetes mellitus. Abelmoschus esculentus potential to reduce ROS is associated with its high polyphenols, flavonoids, and vitamin C content as they scavenge free radical molecules, thus alleviating oxidative stress [79,80,81]. These compounds accomplish this activity by transferring hydrogen atoms to unstable ROS molecules, stabilizing them, and subsequently preventing any cell, tissue, or organ damage [108,109]. Similarly, Abelmoschus esculentus is rich in quercetin and catechin, which have antioxidant properties [74,75,76]. These compounds reduce NADPH oxidase activity, reducing ROS production and further oxidative stress [110,111].
4.1.3. Abelmoschus esculentus on Oxidative Stress with a Focus on SOD
SOD is an antioxidant enzyme that protects cells against ROS if it is upregulated in the body [112]. In a model of DM, the central feature is oxidative stress, which exacerbates the condition. The administration of antioxidants, however, seems important as they reduce oxidative stress by increasing SOD levels. For example, as presented in Table 2, Abelmoschus esculentus is significantly (p < 0.05) associated with an increased SOD [96,106,113,114]. SOD is a crucial antioxidant that defends the body’s organs and cells from oxidative damage. Therefore, an increase in SOD in the body is important to help break down potentially harmful oxygen molecules in cells. An improvement of antioxidant status by Abelmoschus esculentus extract, as demonstrated by a significant increase in SOD, is commendable and thus may be relevant to attenuating oxidative stress (Figure 4). Such an effect of okra may further lead to ameliorating secondary complications associated with oxidative stress. However, other studies showed a significant (p < 0.05) decrease in SOD following the administration of Abelmoschus esculentus [91,102,115,116]. This suggests the limitation of Abelmoschus esculentus as an antioxidant; reduction of SOD in hyperglycemia or overproduction of ROS may subject the cells to damage and subsequently to apoptosis.
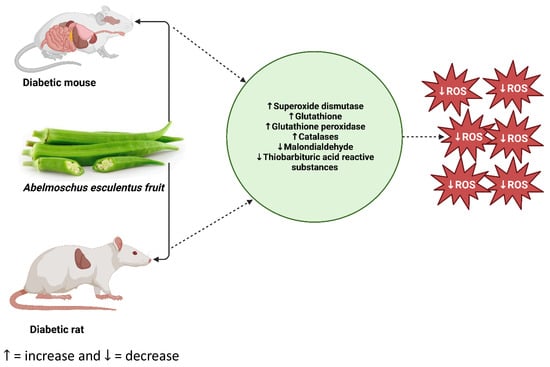
Figure 4.
Potential mode of action of Abelmoschus esculentus in rodent models of diabetes. In a nutshell, oral administration of Abelmoschus esculentus ameliorates oxidative stress in rodent models of diabetes induced by either alloxan monohydrate or a high-fat diet coupled with streptozotocin. ROS: reactive oxygen species (https://prove.es/en/unknown-but-really-healthy-vegetable-called-okra/ (accessed on 25 July 2023)).
4.1.4. Abelmoschus esculentus on Oxidative Stress with a Focus on CAT Activity
CAT is an active enzyme involved in the catalysis of hydrogen peroxide into water and oxygen [36]. However, due to reduced CAT activity, beta cells of the pancreas that contain many mitochondria undergo oxidative stress by producing excess ROS that leads to β-cells dysfunction and, ultimately, diabetes [18]. Interestingly, evidence presented in Table 2 showed that Abelmoschus esculentus treatment in rodent models of diabetes significantly increases CAT activity [91,95,105,106,113,115,117]. This suggests that Abelmoschus esculentus may ameliorate oxidative stress, further reduce complications of DM, or prevent DM in non-diabetics (Figure 4). Although the potential benefits of Abelmoschus esculentus on CAT activity have been noted in DM rodent models, another group of researchers has reported contradictory findings, as shown by significantly reduced CAT activity [116]. This, disappointingly, suggests a limited beneficial impact of Abelmoschus esculentus in improving the activity of CAT enzymes in diabetic models and, thus, its limited efficacy in reducing oxidative stress.
4.1.5. Effects of Abelmoschus esculentus on Oxidative Stress: Focusing on MDA and TBARS
Additional oxidative stress markers include MDA, TBARS, lipid hydroperoxides (LH), and 4-hydroxy-2-Nonenal (4-HNE), the end products of lipid peroxidation. However, among these markers, MDA is widely studied and is regarded as an ideal marker of oxidative stress [118]. MDA seems to be elevated in DM, thus increasing the likelihood of developing complications associated with oxidative stress. It is assumed that the reduction of these markers can substantially alleviate oxidative stress and associated complications. Interestingly, preclinical evidence gathered in Table 2 showed Abelmoschus esculentus promising potential in reducing MDA amongst rodent models of DM [91,95,105,106,113,115,117]. Disappointingly, evidence from a preclinical model of GDM reported by Tian [113] showed different findings, which suggest the limitation of Abelmoschus esculentus in alleviating oxidative stress in GDM. Although the results were unfavorable, we believe this is due to insulin resistance occurring in the late stage of pregnancy and different pathophysiological mechanisms between GDM and DM, thus limiting the antioxidant potential of Abelmoschus esculentus [119]. Consistently, TBARS, a derivative of thiobarbituric acid and MDA, increases in response to oxidative stress [120]. Notably, an increased level of TBARS is observed in T1DM and T2DM, signifying oxalate toxicity induced by lipid peroxidation [121,122]. However, existing evidence showed a significant decrease in TBARS levels following Abelmoschus esculentus treatment in rodent models of DM [95,107]. Therefore, this reduction would suggest Abelmoschus esculentus potential for attenuating oxalate toxicity and a further reduction in oxidative stress.

Table 2.
Overview of studies evaluating the antioxidant effect of Abelmoschus esculentus in a rodent model of diabetes mellitus.
Table 2.
Overview of studies evaluating the antioxidant effect of Abelmoschus esculentus in a rodent model of diabetes mellitus.
| Experimental Model | Treatment and Duration | Experimental Outcomes | Country | Reference |
|---|---|---|---|---|
| Alloxan monohydrate induced diabetes in Swiss albino female and male mice. | The suspension was prepared by dissolving the powdered peel seed (PPS) and Abelmoschus esculentus mucilage (PM) of Abelmoschus esculentus into distilled water. PPS and PM were administered orally at 150 and 200 mg/kg body weight for three weeks. | Total flavonoid content was higher in PPS than in PM. At the same time, the total phenol content was higher in PM than PPS. Abelmoschus esculentus had reduced antioxidant capabilities, as shown by a higher IC50 value in Abelmoschus esculentus PM and PPS compared to vitamin C. | Bangladesh | [101] |
| High-fat diet (HFD)-streptozotocin (STZ)-induced diabetes in SPF-grade C57BL/6 male mice. | Abelmoschus esculentus powder was isolated using distilled water and 80% ethanol precipitation from Abelmoschus esculentus and orally administered at 200 and 400 mg/kg of Abelmoschus esculentus powder for eight weeks. | Abelmoschus esculentus in diabetic mice reduced the level of reactive oxygen species (ROS) in diabetic mice compared to mice in the control group. A dose of 400 Abelmoschus esculentus powder in diabetic mice significantly increased superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) and decreased malondialdehyde (MDA) in the kidney when compared to the control group. The same pattern was observed at 200; however, this was not significantly different from diabetic controls. | China | [106] |
| HFD-fed-specific pathogen-free (SPF)-grade C57BL/6 male mice administered with STZ to induce diabetes. | Abelmoschus esculentus powder was dissolved in distilled water, and 200 or 400 mg/kg of body weight was orally administered for eight weeks. | Abelmoschus esculentus powder in diabetic mice decreased ROS and MDA and increased SOD, glutathione peroxidase (GPx), and CAT in the liver compared to the diabetic control. Nuclear factor erythroid 2–related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and superoxide dismutase 2 (SOD2) expression were significantly upregulated by Abelmoschus esculentus powder. | China | [105] |
| Streptozotocin-induced diabetes in male Wistar albino rats. | Abelmoschus esculentus peel powder (AEPP) and Abelmoschus esculentus seed powder (AESP) were mixed with distilled water and administered orally at a dose of (100 or 200 mg/kg bw) for 28 days. | Administration of both doses of AEPP and AESP in diabetic rats significantly increased liver, kidney, and pancreatic SOD, CAT, GPx, and GSH levels compared to the diabetic controls. | India | [95] |
| STZ-induced diabetes in male Wistar rats. | Abelmoschus esculentus mucilage extract and Abelmoschus esculentus seed aqueous solution were made by dissolving the powder in ethanol. The extract was orally administered at 150 and 200 mg/kg of body weight for 30 days. | Abelmoschus esculentus in diabetic rats significantly decreased MDA and increased catalase, SOD, and GSH activity compared to diabetic controls. | Saudi Arabia | [115] |
| STZ-induced diabetes in male Wistar rats. | About 50 g of Abelmoschus esculentus powder was used to make the following extracts: (ethanolic extract 75%, ethanolic extract 90%, aqueous extract, and ethyl acetate extract). Abelmoschus esculentus extracts were orally administered at 200 and 400 mg/kg doses for eight weeks. | Abelmoschus esculentus extracts had reduced antioxidant properties, as revealed by an increased half-maximal inhibitory concentration (IC50) level compared to vitamin C and quercetin. | Iran | [103] |
| STZ-induced diabetes mellitus in adult Wistar rats. | Abelmoschus esculentus powder was mixed with food and given as a food pellet to the rats. | Abelmoschus esculentus-mixed diets in diabetic rats significantly reduced SOD, CAT, GSH, MDA, and α-amylase. However, GPx in Abelmoschus esculentus was not significantly different from diabetic controls. | Nigeria | [116] |
| Alloxan-induced diabetes in female and male Wistar rats. | Whole Abelmoschus esculentus (WAE), Abelmoschus esculentus peel (AEP), and Abelmoschus esculentus seed (AES). Abelmoschus esculentus samples (WAE, AEP, and AES) were administered at 100, 200, and 300 mg/kg by single forced oral feeding once daily for 21 days. | Abelmoschus esculentus in diabetic rats significantly increased CAT activity and GSH and decreased MDA compared to diabetic controls. | Nigeria | [117] |
| HFD fed Spraque–Dawley male rats were injected with STZ to induce diabetes. | Abelmoschus esculentus subfractions (F1, F2, and FR), the ethanol-extracted subfraction, and distilled water residue of Abelmoschus esculentus oral feed for 12 weeks. | Abelmoschus esculentus in diabetic rats has benefits in regulating dipeptidyl peptidase-4 (DPP-4) and the glucagon-like peptide 1 receptor (GLP-1R), thus reducing oxidative stress. Abelmoschus esculentus in diabetic rats significantly decreased serum and kidney TBARS compared to diabetic controls. All extracts lowered peroxidation except for fraction 1. | Taiwan | [107] |
| Alloxan-induced diabetes in male Wistar strain rats. | Abelmoschus esculentus powder (2 g/kg rat body weight) was mixed well in 0.2% carboxy methyl cellulose (CMC) and fed to animals by gavage technique at 2 g/kg body weight for 35 days. | Abelmoschus esculentus in diabetic rats significantly increased lipid peroxidation in erythrocytes, GSH, and decreased MDA in the kidney compared to diabetic controls. | India | [123] |
| Alloxan monohydrate induced diabetes in male and female Wistar rats. | The animal diet was prepared by mixing 33 g of the Abelmoschus esculentus powder with 67 g of normal rat feed, and 66 g of Abelmoschus esculentus was mixed with 34 g of normal rat feed to obtain the 33% and 66% supplement ratios and fed to rats for 16 days. | Abelmoschus esculentus powder in diabetic rats significantly decreased SOD and MDA levels and increased GSH and CAT activity compared to diabetic controls. | Nigeria | [91] |
| STZ-induced diabetes in male Sprague–Dawley rats. | The Abelmoschus esculentus powder samples (250 g) were subjected to extraction procedures using ten volumes of either 75% ethanol or distilled water. 100 mL/kg of solution (aqueous extract, ethanol extract, and aqueous extract of Indole Acetic Acid (IAA) from Abelmoschus esculentus) was administered orally daily for six weeks. | Abelmoschus esculentus powder significantly decreased SOD in diabetic rats that received ethanol extract compared to diabetic controls. Diabetic rats that received an aqueous extract of Abelmoschus esculentus at 100 mg/kg had significantly increased liver total phenolic content. | Canada | [102] |
| STZ-induced hyperglycemia in male Wistar rats. | Three fresh Abelmoschus esculentus pods were sliced and infused in 250 mL of 3.6 mL Abelmoschus esculentus infusion water for 28 days. | Abelmoschus esculentus in diabetic rats significantly increased SOD when compared to diabetic controls. | Bangladesh | [114] |
| STZ-induced gestational diabetes (GDM) in Spraque–Dawley rats. | The intervention group was administered orally a solution containing 200 mg/kg of Abelmoschus esculentus extract. | Abelmoschus esculentus in GDM rats significantly increased SOD, MDA, CAT, GSH, and GPx in the liver and pancreas compared to GDM controls. | China | [113] |
SOD: superoxide dismutase, MDA: malondialdehyde, CAT: catalase, GSH: glutathione, GPx: glutathione peroxidase, CMC: carboxy methyl cellulose, IAA-AE: Indole Acetic Acid-Abelmoschus esculentus, AEPP: Abelmoschus esculentus peel powder, AESP: Abelmoschus esculentus seed powder, GDM: gestational diabetes, F1: fraction 1, FR: fractional residue, DPP-4: dipeptidyl peptidase-4, GLP-1R: glucagon-like peptide 1 receptor, WAE: whole Abelmoschus esculentus, AEP: Abelmoschus esculentus peel, AES: Abelmoschus esculentus seed, SPF: specific pathogen-free, IC50: half maximal inhibitory concentration, TT: total thiols, TBARS: thiobarbituric acid reactive substances, Nrf2: Nuclear factor erythroid 2–related factor 2.
4.1.6. Abelmoschus esculentus on Glutathione and Glutathione Peroxidase
GSH is a group of enzymes that protect the body from oxidative stress by reducing lipid hydroperoxides and free hydrogen peroxide [124,125]. The general overview of the effect of Abelmoschus esculentus on markers of GPx and GSH is outlined in Table 2.
However, a decrease in its activity subjects the cells or organs to oxidative stress by allowing the accumulation of harmful oxidants and free radicals. Therefore, antioxidant compounds that increase the activity of GSH and GPx can be used as an alternative therapy to ameliorate oxidative stress and protect the cells from oxidative damage (Figure 4). Our current review found contradictory reports from various rodent models of DM induced by STZ or alloxan monohydrate. Of interest was that several studies reported a significant increase in the activity of GSH [91,95,105,106,115,123]. Additionally, Tian et al. [113] and Aleisa et al. [115] have reported a noteworthy augmentation in the activity of GPx, further substantiating the potential of Abelmoschus esculentus and its constituents as antioxidant agents.
In contrast to the aforementioned encouraging discoveries, other researchers [91,95,105,106,115,116,123] have recently identified a notable decline in GSH levels after administering Abelmoschus esculentus treatment to rodent models with diabetes. This once again highlights a potential limitation of Abelmoschus esculentus as an antioxidant. Abelmoschus esculentus ability to regulate oxidative stress seems to be attributable to its high content of phenols, flavonoids, and associated minerals, as presented in Figure 3.
5. Limitations
The current review has several limitations; for instance, the evidence gathered here is primarily from preclinical studies, with mainly mice and rats used for such experimentation. Additionally, the majority of models of diabetes presented here were primarily developed by using the administration of either STZ or alloxan monohydrate, with a few using HFD to induce diabetes. It is commonly known that these two drugs cause pancreatic damage, and the model developed through their administration mimics that of T1DM, while HFD resembles that of T2DM seen in humans. Since the pathogenesis of these conditions differs, the interpretation may be skewed toward T1DM, as most studies have induced diabetes through drug intervention. While evidence from the preclinical studies supports the use of both Amaranthus and Abelmoschus esculentus as herbal treatments for DM against oxidative stress, it is still not clear as to which part of these plants is more beneficial, as some studies used leaves, seeds, and pods.
The exact dose or form by which these plants are administered is also not specified, as a powder has been used to prepare the extract or given to rodents as a food-powder mixture. Although other clinical studies have been conducted on Abelmoschus esculentus in diabetic populations, ranging from randomized controlled trials to quasi-experiments, such studies did not focus on oxidative stress or related markers [126]. Moreover, regarding Amaranthus, only one trial has been conducted in diabetic patients, and the results are promising [127]. However, since then, clinical evidence has been scarce. Lastly, Amaranthus, and Abelmoschus esculentus have been proven effective in reducing diabetes. However, to the best of our knowledge, no studies have been conducted using a combined treatment of both of these plants in diabetic models or clinical trials. As the evidence reviewed here is derived from preclinical studies, it is important to note that in some cases, the evidence from experimental studies is not fully translatable to humans due to different physiological systems and functions.
6. Conclusions and Recommendations
The study discussed the effects of different parts of Amaranthus and Abelmoschus esculentus, highlighting their potential as alternative remedies to attenuate oxidative stress in diabetes models. Current evidence on the recommended dosages for these plants has proven these plants to be considered safe in the management of DM. Both plants are rich in carbohydrates, proteins, fatty acids, vitamins, fiber, minerals, and other bioactive phytochemicals that promote good health and are less expensive, making them economically affordable natural antioxidants. Evidence exploring the efficacy and safety of Abelmoschus esculentus in diabetes on other markers exists [17,101], but studies in this population still lack exploration of oxidative stress. While the potential benefits of Amaranthus have been extensively explored in diabetic models, there is limited evidence in clinical trials, especially on oxidative stress. The preclinical evidence gathered in this review revealed that Amaranthus treatment in diabetes could ameliorate hyperglycemic-associated oxidative stress (Figure 2). Remarkably, our summarized evidence also demonstrated that the administration of Abelmoschus esculentus to diabetic rodents also attenuates oxidative stress (Figure 4). Although there have been limited clinical trials involving Abelmoschus esculentus in diabetic populations [126], its potential benefits are widely recognized within the broader research community, and its safety has been confirmed. However, since there are currently no trials investigating the effects of these plants on oxidative stress, it is recommended that future trials be conducted, especially in African countries where the prevalence of DM is high. Therefore, we plan to explore oxidative stress with well-designed and adequately powered clinical studies. Therefore, future studies may be necessary to fully understand its efficacy, optimal dosage, and impact on oxidative stress. These studies should be well-designed and adequately powered, focusing on diabetic patients.
Author Contributions
Conceptualization, W.N.P. and K.M.; methodology, W.N.P. and K.M.; validation, W.N.P. and K.M.; investigation, W.N.P. and K.M.; data curation, W.N.P. and K.M.; writing—original draft preparation, W.N.P. and K.M.; writing—review and editing, W.N.P. and K.M.; visualization, W.N.P. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of a bigger study entitled “The use and functional properties of African indigenous fruits and vegetables in alleviating household food and nutrition insecurity for local communities” which is funded by the University of South Africa Women in Research (WiR-2023). Opinions expressed, and conclusions arrived at, are those of the author and not necessarily to be attributed to the (WiR-2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
K.M. is partially funded by Research Development Grants for nGAP Scholars (NGAP23022780506) and the Research Excellence Award for Next Generation Researchers (NONF230515106418). However, the content presented in this manuscript is the authors’ sole responsibility and does not necessarily represent the official views of the NRF nGAP or the funders.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
DM: Diabetes mellitus; T2DM: Type 2 diabetes mellitus; T1DM: Type 1 diabetes mellitus; AHELE: Amaranthus hybridus ethanol leaf extract; CAT: Catalase; GSH: Glutathione; GPx: Glutathione peroxidase; SOD: Superoxide dismutase; MDA: Malondialdehyde; TBARS: Thiobarbituric acid reactive substances; and STZ: Streptozotocin; IAA: Indole Acetic Acid; PM: Powdered nucillage; PPS: powdered peel seed; ROS: Reactive oxygen species; AEP: Abelmoschus esculentus powder; HFD: High-fat diet; AEPP: Abelmoschus escuelentus Peel powder; AESP: Abelmoschus esculentus seed powder; DPPH: 1,1-diphenyl-2-picrylhydrazyl; ABTS: 2,2′-casino-bis-3-ethylbenzothiazoline-6-sulfonic acid; LMICs: low- and middle-income countries; FFA: Free fatty acids; AGEs: advanced glycation end products; mNADH: mitochondrial NADH; DAG: diacylglycerol; PKC: Protein kinase C; ET-1: endothelin 1; VCAM-1: vascular cell adhesion molecule; ICAM-1: intercellular adhesion molecule; NF-κβ: nuclear factor kappa-light-chain-enhancer of activated β cell; CMC: Carboxy methyl cellulose; GDM: gestational diabetes; F1: Fraction 1; FR: Fractional residue; GLP-1R: Glucagon like peptide 1 receptor; WAE: Whole Abelmoschus esculentus; SPF: Specific pathogen-free.
References
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF Diabetes Atlas: Global Estimates of Undiagnosed Diabetes in Adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Gojka Roglic WHO Global Report on Diabetes: A Summary. Int. J. Non.-Commun. Dis. 2016, 1, 3–8. [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, F.; Simatos, G.; Simatou, A. The Challenges in the Development of Diabetes Prevention and Care Models in Low-Income Settings. Front. Endocrinol. 2020, 11, 518. [Google Scholar] [CrossRef]
- Harris, M.L.; Oldmeadow, C.; Hure, A.; Luu, J.; Loxton, D.; Attia, J. Stress Increases the Risk of Type 2 Diabetes Onset in Women: A 12-Year Longitudinal Study Using Causal Modelling. PLoS ONE 2017, 12, e0172126. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Pham, N.M.; Lee, A.H.; Binns, C.W. Prevalence of and Risk Factors for Type 2 Diabetes Mellitus in Vietnam: A Systematic Review. Asia Pac. J. Public Health 2015, 27, 588–600. [Google Scholar] [CrossRef]
- Sanada, H.; Yokokawa, H.; Yoneda, M.; Yatabe, J.; Yatabe, M.S.; Williams, S.M.; Felder, R.A.; Jose, P.A. High Body Mass Index Is an Important Risk Factor for the Development of Type 2 Diabetes. Intern. Med. 2012, 51, 1821–1826. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications Diabetes Special Issue. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Shao, Y.; Williamson, C. The HIV-1 Epidemic: Low- to Middle-Income Countries. Cold Spring Harb. Perspect. Med. 2012, 2, a007187. [Google Scholar] [CrossRef] [PubMed]
- Pittampalli, S.; Upadyayula, S.; Hema, H.M.; Mekala, M.; Lippmann, S. Risks vs Benefits for SGLT2 Inhibitor Medications. Fed. Pract. 2018, 35, 45–48. [Google Scholar] [PubMed]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A Review of Its Therapeutic Efficacy and Adverse Effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Phoswa, W.N. Corchorus Olitorius Extract Exhibit Anti-Hyperglycemic and Anti-Inflammatory Properties in Rodent Models of Obesity and Diabetes Mellitus. Front. Nutr. 2023, 10, 1099880. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin Supplementation Improves Biomarkers of Oxidative Stress and Inflammation in Conditions of Obesity, Type 2 Diabetes and NAFLD: Updating the Status of Clinical Evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Dlamini, S.; Phoswa, W.N.; Modjadji, P.; Lebelo, S.L. The Impact of Punica Granatum Linn and Its Derivatives on Oxidative Stress, Inflammation, and Endothelial Function in Diabetes Mellitus: Evidence from Preclinical and Clinical Studies. Antioxidants 2023, 12, 1566. [Google Scholar] [CrossRef]
- Tavakolizadeh, M.; Peyrovi, S.; Ghasemi-Moghaddam, H.; Bahadori, A.; Mohkami, Z.; Sotoudeh, M.; Ziaee, M. Clinical Efficacy and Safety of Okra (Abelmoschus esculentus (L.) Moench) in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Acta Diabetol. 2023. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Kuyvenhoven, J.P.; Meinders, A.E. Oxidative Stress and Diabetes Mellitus Pathogenesis of Long-Term Complications. Eur. J. Intern. Med. 1999, 10, 9–19. [Google Scholar] [CrossRef]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [CrossRef] [PubMed]
- de Souza Bastos, A.; Graves, D.T.; de Melo Loureiro, A.P.; Júnior, C.R.; Corbi, S.C.T.; Frizzera, F.; Scarel-Caminaga, R.M.; Câmara, N.O.; Andriankaja, O.M.; Hiyane, M.I.; et al. Diabetes and Increased Lipid Peroxidation Are Associated with Systemic Inflammation Even in Well-Controlled Patients. J. Diabetes Complicat. 2016, 30, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Troendle, E.P.; Barabas, P.; McAleese, C.A.; Friedel, T.; Stitt, A.W.; Curtis, T.M. The Role of Lipoxidation in the Pathogenesis of Diabetic Retinopathy. Front. Endocrinol. 2021, 11, 621938. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, P.; Chacinska, M.; Charkiewicz, K.; Baranowski, M.; Gorski, J.; Blachnio-Zabielska, A.U. Effect of Metformin on Bioactive Lipid Metabolism in Insulin-Resistant Muscle. J. Endocrinol. 2017, 233, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-Evoked Activation of PKC and PKD Isoforms in Regulation of Glucose and Lipid Metabolism: A Review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Mechanisms for Vascular Cell Adhesion Molecule-1 Activation of ERK1/2 during Leukocyte Transendothelial Migration. PLoS ONE 2011, 6, e26706. [Google Scholar] [CrossRef]
- Kawanami, D.; Maemura, K.; Takeda, N.; Harada, T.; Nojiri, T.; Saito, T.; Manabe, I.; Imai, Y.; Nagai, R. C-Reactive Protein Induces VCAM-1 Gene Expression through NF-ΚB Activation in Vascular Endothelial Cells. Atherosclerosis 2006, 185, 39–46. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.M.; Song, H.S.; Park, K.Y.; Kim, Y.M.; Kim, M.S.; Pak, Y.K.; Lee, I.K.; Lee, J.D.; Park, S.J.; et al. Oleic Acid Induces Endothelin-1 Expression through Activation of Protein Kinase C and NF-ΚB. Biochem. Biophys. Res. Commun. 2003, 303, 891–895. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH Oxidases, Reactive Oxygen Species, and the Kidney: Friend and Foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications Review-Article. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Zhang, E.; Miramini, S.; Patel, M.; Richardson, M.; Ebeling, P.; Zhang, L. Role of TNF-α in Early-Stage Fracture Healing under Normal and Diabetic Conditions. Comput. Methods Programs Biomed. 2022, 213, 106536. [Google Scholar] [CrossRef]
- Adams, A.P. Is Diabetic Retinopathy an inflammatory Disease? Br. J. Ophthalmol. 2000, 86, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 Diabetes Mellitus: From a Metabolic Disorder to an Inflammatory Condition. World J. Diabetes 2015, 6, 598. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Désiré, A.-L.D.; Rosette, M.A.-L.E. Chapter2.2 Catalase. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 81–90. ISBN 978-0-12-819096-8. [Google Scholar]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 Emission and Cellular Redox State Link Excess Fat Intake to Insulin Resistance in Both Rodents and Humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.H.; Neufer, P.D. Linking Mitochondrial Bioenergetics to Insulin Resistance via Redox Biology. Trends Endocrinol. Metab. 2012, 23, 142–153. [Google Scholar] [CrossRef]
- Iwakami, S.; Misu, H.; Takeda, T.; Sugimori, M.; Matsugo, S.; Kaneko, S.; Takamura, T. Concentration-Dependent Dual Effects of Hydrogen Peroxide on Insulin Signal Transduction in H4IIEC Hepatocytes. PLoS ONE 2011, 6, e27401. [Google Scholar] [CrossRef]
- Berdichevsky, A.; Guarente, L.; Bose, A. Acute Oxidative Stress Can Reverse Insulin Resistance by Inactivation of Cytoplasmic JNK. J. Biol. Chem. 2010, 285, 21581–21589. [Google Scholar] [CrossRef]
- Tucker, P.S.; Fisher-Wellman, K.; Bloomer, R.J. Can Exercise Minimize Postprandial Oxidative Stress in Patients with Type 2 Diabetes? Curr. Diabetes Rev. 2008, 4, 309–319. [Google Scholar] [CrossRef]
- Maddux, B.A.; See, W.; Lawrence, J.C.; Goldfine, A.L.; Goldfine, I.D.; Evans, J.L. Protection against oxidative stress-induced insulin resistance in rat l6 muscle cells by micromolar concentrations of-lipoic acid. Diabetes 2001, 50, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. ISBN 9780128032701. [Google Scholar]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Zhang, Z.; Mima, A.; King, G.L. Molecular Mechanisms of Diabetic Vascular Complications. J. Diabetes Investig. 2010, 1, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Tripathi, D.N.; Kumar, A.; Singh, S. Oxidative Stress and Inflammation in Diabetic Complications. Int. J. Endocrinol. 2014, 2014, 679754. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Ilias, I.; Alevizaki, M. The Role of Diet and Lifestyle in Primary, Secondary, and Tertiary Diabetes Prevention: A Review of Meta-Analyses. Rev. Diabet. Stud. 2010, 7, 26–35. [Google Scholar] [CrossRef]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of Dietary Vitamin C, Vitamin E, and Alpha-Lipoic Acid Supplementation on the Antioxidant Defense System and Immune-Related Gene Expression in Broilers Exposed to Oxidative Stress by Dexamethasone. Poult. Sci. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Thapa, R.; Edwards, M.; Blair, M.W. Relationship of Cultivated Grain Amaranth Species and Wild Relative Accessions. Genes 2021, 12, 1849. [Google Scholar] [CrossRef]
- Das, S. Distribution and Maintenance of Amaranth Germplasm Worldwide. In Amaranthus: A Promising Crop of Future; Das, S., Ed.; Springer: Singapore, 2016; pp. 99–106. ISBN 978-981-10-1469-7. [Google Scholar]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth—Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; van Rensburg, E.J.; Mabelebele, M. The Probable Use of Genus Amaranthus as Feed Material for Monogastric Animals. Animals 2020, 10, 1504. [Google Scholar] [CrossRef]
- Yadav, K.; Bhatia, A.L.; Sisodia, R. Modulation of Radiation Induced Biochemical Changes in Testis of Swiss Albino Mice by Amaranthus Paniculatus Linn. Asian J. Exp. Sci. 2004, 18, 63–74. [Google Scholar]
- Ruth, O.N.; Unathi, K.; Nomali, N.; Chinsamy, M. Underutilization versus Nutritional-Nutraceutical Potential of the Amaranthus Food Plant: A Mini-Review. Appl. Sci. 2021, 11, 6879. [Google Scholar] [CrossRef]
- Reyad-ul-Ferdous, M.D. Present Biological Status of Potential Medicinal Plant of Amaranthus Viridis: A Comprehensive Review. Am. J. Clin. Exp. Med. 2015, 3, 12. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Adetutu, A.; Olaniyi, T.D. Antioxidant Activity of Amaranthus Species from the Amaranthaceae Family A Review. South. Afr. J. Bot. 2020, 133, 111–117. [Google Scholar] [CrossRef]
- Mishra, A.; Jha, S.K.; Ojha, P. Study on Zero Energy Cool Chamber (ZECC) for Storage of Vegetables. Int. J. Sci. Res. Publ. (IJSRP) 2020, 10, p9767. [Google Scholar] [CrossRef]
- Gins, V.K.; Motyleva, S.M.; Kulikov, I.M.; Tumanyan, A.F.; Romanova, E.V.; Baikov, A.A.; Gins, E.M.; Terekhin, A.A.; Gins, M.S. Antioxidant Profile of Amaranthus Paniculatus L. Of the Pamyat of Kovas Variety. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012152. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Sarojini, G.; Devi, N.L. Hypocholesterolemic Effect of Amaranth Seeds (Amaranthus Esculantus). Plant Foods Hum. Nutr. 1993, 44, 63–70. [Google Scholar] [CrossRef]
- Jia, W.; Gaoz, W.; Tang, L. Antidiabetic Herbal Drugs Officially Approved in China. Phytother. Res. 2003, 17, 1127–1134. [Google Scholar] [CrossRef]
- Kar, A.; Bhattacharjee, S. Bioactive Polyphenolic Compounds, Water-Soluble Vitamins, in Vitro Anti-Inflammatory, Anti-Diabetic and Free Radical Scavenging Properties of Underutilized Alternate Crop Amaranthus Spinosus L. from Gangetic Plain of West Bengal. Food Biosci. 2022, 50, 102072. [Google Scholar] [CrossRef]
- Schröter, D.; Neugart, S.; Schreiner, M.; Grune, T.; Rohn, S.; Ott, C. Amaranth’s 2-Caffeoylisocitric Acid—An Anti-Inflammatory Caffeic Acid Derivative That Impairs NF-ΚB Signaling in LPS-Challenged RAW 264.7 Macrophages. Nutrients 2019, 11, 571. [Google Scholar] [CrossRef]
- Nawale, R.B.; Mate, G.S.; Wakure, B.S. Ethanolic Extract of Amaranthus Paniculatus Linn. Ameliorates Diabetes-Associated Complications in Alloxan-Induced Diabetic Rats. Integr. Med. Res. 2017, 6, 41–46. [Google Scholar] [CrossRef]
- Prince, M.R.U.; Zihad, S.M.N.K.; Ghosh, P.; Sifat, N.; Rouf, R.; Al Shajib, G.M.; Alam, M.A.; Shilpi, J.A.; Uddin, S.J. Amaranthus Spinosus Attenuated Obesity-Induced Metabolic Disorders in High-Carbohydrate-High-Fat Diet-Fed Obese Rats. Front. Nutr. 2021, 8, 653918. [Google Scholar] [CrossRef] [PubMed]
- Ashok Kumar, B.S.; Lakshman, K.; Nandeesh, R.; Arun Kumar, P.A.; Manoj, B.; Kumar, V.; Sheshadri Shekar, D. In Vitro Alpha-Amylase Inhibition and in Vivo Antioxidant Potential of Amaranthus Spinosus in Alloxan-Induced Oxidative Stress in Diabetic Rats. Saudi J. Biol. Sci. 2011, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.B.; Verma, A.; Mukerjee, A.; Vijayakumar, M. Amaranthus Spinosus L. (Amaranthaceae) Leaf Extract Attenuates Streptozotocin-Nicotinamide Induced Diabetes and Oxidative Stress in Albino Rats: A Histopathological Analysis. Asian Pac. J. Trop. Biomed. 2012, 2, S1647–S1652. [Google Scholar] [CrossRef]
- Balasubramanian, T.; Karthikeyan, M.; Muhammed Anees, K.P.; Kadeeja, C.P.; Jaseela, K. Antidiabetic and Antioxidant Potentials of Amaranthus hybridus in Streptozotocin-Induced Diabetic Rats. J. Diet. Suppl. 2017, 14, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, T.; Karthikeyan, M. Therapeutic Effect of Amaranthus hybridus on Diabetic Nephropathy. J. Dev. Drugs 2015, 5, 1000147. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ghonimi, N.A.M.; Elsharkawi, K.A.; Khyal, D.S.M.; Abdelghani, A.A. Serum Malondialdehyde as a Lipid Peroxidation Marker in Multiple Sclerosis Patients and Its Relation to Disease Characteristics. Mult. Scler. Relat. Disord. 2021, 51, 102941. [Google Scholar] [CrossRef] [PubMed]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) Oxidation in Mitochondria: An Emerging Target in the Ageing Process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Jamaly, S.; Farjana, F.; Begum, N.; Zenat, E.A. A Review on Ethnomedicinal, Pharmacological, Phytochemical and Pharmaceutical Profile of Lady’s Finger (Abelmoschus esculentus L.) Plant. Pharmacol. Pharm. 2019, 10, 94–108. [Google Scholar] [CrossRef]
- Esmaeilzadeh, D.; Razavi, B.M.; Hosseinzadeh, H. Effect of Abelmoschus esculentus (Okra) on Metabolic Syndrome: A Review. Phytother. Res. 2020, 34, 2192–2202. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P. Identification and Quantification of Polyphenolic Compounds from Okra Seeds and Skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, C.I.; Chinomso, N.J.; Ndubuisi, N.S.; Obinna, A.; Ogochukwu, A.P.; Nnaemeka, U.E.; Gift, P.O.; Esther, O.; Virginus, U. Phytochemical And Proximate Composition of Igbo Okra (Abelmoschus esculentus) Seeds. World J. Pharm. Life Sci. 2021, 7, 20–24. [Google Scholar]
- Sunilson, J.; Anbu, J.; Jayaraj, P.; Mohan, M.S.; Anita, A.; Kumari, G.; Varatharajan, R. Antioxidant and Hepatoprotective Effect of the Roots of Hibiscus Esculentus Linn. Int. J. Green Pharm. 2008, 200–203. [Google Scholar]
- Maria, E.E.C.; Luciana, M.P.d.S.; Elba, d.S.F.; Amanda, P.d.F.; Carlos, A.d.A.G.; Jailane, d.S.A.; Tatiane, S.-G. Nutritional, Antinutritional and Phytochemical Status of Okra Leaves (Abelmoschus esculentus) Subjected to Different Processes. Afr. J. Biotechnol. 2015, 14, 683–687. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R. Chemical Composition, Nutritional Value and Antioxidant Properties of Mediterranean Okra Genotypes in Relation to Harvest Stage. Food Chem. 2018, 242, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Woldegiorgis, A.Z.; Rakshit, S.K. Proximate, Mineral, and Antinutrient Compositions of Indigenous Okra (Abelmoschus esculentus) Pod Accessions: Implications for Mineral Bioavailability. Food Sci. Nutr. 2016, 4, 223–233. [Google Scholar] [CrossRef]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C.F.R. Chemical Composition, Nutritional Value, and Biological Evaluation of Tunisian Okra Pods (Abelmoschus Esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Majd, N.E.; Tabandeh, M.R.; Shahriari, A.; Soleimani, Z. Okra (Abelmoscus Esculentus) Improved Islets Structure, and Down-Regulated PPARs Gene Expression in Pancreas of High-Fat Diet and Streptozotocin-Induced Diabetic Rats. Cell J. 2018, 20, 31–40. [Google Scholar] [CrossRef]
- Fabianová, J.; Šlosár, M.; Kopta, T.; Vargová, A.; Timoracká, M.; Mezeyová, I.; Andrejiová, A. Yield, Antioxidant Activity and Total Polyphenol Content of Okra Fruits Grown in Slovak Republic. Horticulturae 2022, 8, 966. [Google Scholar] [CrossRef]
- Puji, S.; Wahyuningsih, A.; Winarni, D.; Pramudya, M.; Setianingsih, N.; Ayubu Mwendolwa, A.; Nindyasari, F. Antioxidant Potential of Red Okra Pods (Abelmoschus esculentus Moench). EPIC Biol. Sci. 2021, 1, 158–163. [Google Scholar]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Rakshit, S.K.; Woldegiorgis, A.Z. Indigenous Ethiopian Okra (Abelmoschus esculentus) Mucilage: A Novel Ingredient with Functional and Antioxidant Properties. Food Sci. Nutr. 2018, 6, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, W.; Li, Y.; Prasad, N.; Tang, Z. Antioxidant Activity of Extract and Its Major Constituents from Okra Seed on Rat Hepatocytes Injured by Carbon Tetrachloride. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Savira, N.I.I.; Anggraini, D.W.; Winarni, D.; Suhargo, L.; Kusuma, B.W.A.; Nindyasari, F.; Setianingsih, N.; Mwendolwa, A.A. Antioxidant and Nephroprotective Effects of Okra Pods Extract (Abelmoschus esculentus L.) against Lead Acetate-Induced Toxicity in Mice. Scientifica 2020, 2020, 4237205-10. [Google Scholar] [CrossRef]
- Mousavi, A.; Pourakbar, L.; Siavash Moghaddam, S. Effects of Malic Acid and EDTA on Oxidative Stress and Antioxidant Enzymes of Okra (Abelmoschus esculentus L.) Exposed to Cadmium Stress. Ecotoxicol. Environ. Saf. 2022, 248, 114320. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Fachrisa, A.; Nisaâ€TM, N.; Kusuma, B.W.A.; Shoukat, N.; Ahmar, R.F.; Alifiyah, N.I. Potential of Red Okra Extract (Abelmoschus esculentus L. Moench) to Restore Kidney Damage Due to Sodium Nitrite. Biosaintifika J. Biol. Biol. Educ. 2021, 13, 84–91. [Google Scholar] [CrossRef]
- Tanko, Y.; Idris, N.; Om, A.; Nm, G.; Muhammad, A.; Ka, M.; Yusuf, R. Evaluation of the Effect of Okra (Abelmoschus esculentus) Supplement on Blood Glucose Levels and Antioxidant Biomarkers on Alloxan Induced Diabetic Wistar Rats. J. Biomed. Sci. 2016, 1, 55–62. [Google Scholar]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of Fermentation Time on the Phenolic, Flavonoid and Vitamin C Contents and Antioxidant Activities of Okra (Abelmoschus esculentus) Seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of Ultrasound-Assisted Extraction of Okra (Abelmoschus esculentus (L.) Moench) Polysaccharides Based on Response Surface Methodology and Antioxidant Activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef]
- Wu, D.T.; Nie, X.R.; Shen, D.D.; Li, H.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; Qin, W. Phenolic Compounds, Antioxidant Activities, and Inhibitory Effects on Digestive Enzymes of Different Cultivars of Okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Investigation of in Vivo Antioxidant Property of Abelmoschus esculentus (L) Moench. Fruit Seed and Peel Powders in Streptozotocin-Induced Diabetic Rats. J. Ayurveda Integr. Med. 2012, 3, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Fekadu Gemede, H.; Desse Haki, G.; Beyene, F.; Woldegiorgis, A.Z.; Kumar Rakshit, S. Phenolic Profiles and Antioxidant of Ethiopian Indigenous Okra (Abelmoschus esculentus) Pod And Seed Accessions: A New Source of Natural Antioxidants. Ann. Food Sci. Technol. 2019, 20, 809–819. [Google Scholar]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental Animal Models for Diabetes and Its Related Complications—A Review. Lab. Anim. Res. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 23. [Google Scholar] [CrossRef]
- Uddin Zim, A.F.M.I.; Khatun, J.; Khan, M.F.; Hossain, M.A.; Haque, M.M. Evaluation of in Vitro Antioxidant Activity of Okra Mucilage and Its Antidiabetic and Antihyperlipidemic Effect in Alloxan-Induced Diabetic Mice. Food Sci. Nutr. 2021, 9, 6854–6865. [Google Scholar] [CrossRef]
- Adewale, M.E.; Masisi, K.; Le, K.; Olaiya, C.O.; Esan, A.M.; Aluko, R.E.; Moghadasian, M.H. The Effects of Okra (Abelmoschus esculentus (L) Moench) Fruit Extracts on Diabetes Markers in Streptozotocin-Induced Diabetic Rats. Arch. Diabetes Obes. 2021, 3, 296–304. [Google Scholar]
- Nasrollahi, Z.; ShahaniPour, K.; Monajemi, R.; Ahadi, A.M. Effect of Quercetin and Abelmoschus esculentus (L.) Moench on Lipids Metabolism and Blood Glucose through AMPK-α in Diabetic Rats (HFD/STZ). J. Food Biochem. 2022, 46, e14506. [Google Scholar] [CrossRef]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of Standardized Units for a Correct Interpretation of IC50 Values Obtained from the Inhibition of the DPPH Radical by Natural Antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Wang, J.; Yan, T.; Xu, F.; Wu, B.; Xiao, F.; Bi, K.; Niu, J.; Jia, Y. The Anti-Nephritic Activity of a Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) via Modulation of AMPK-Sirt1-PGC-1α Signaling Axis Mediated Anti-Oxidative in Type 2 Diabetes Model Mice. Int. J. Biol. Macromol. 2019, 140, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Lin, H.C.; Lin, C.L.; Wang, C.J.; Huang, C.N. Abelmoschus Esculentus Subfractions Improved Nephropathy with Regulating Dipeptidyl Peptidase-4 and Type 1 Glucagon-like Peptide Receptor in Type 2 Diabetic Rats. J. Food Drug Anal. 2019, 27, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Li, J. Comparison of Vitamin c and Its Derivative Antioxidant Activity: Evaluated by Using Density Functional Theory. ACS Omega 2020, 25467–25475. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin c—Antioxidative and pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Mhya, D.H.; Mohammed, A.; Dawus, T.T. Investigation of NADPH-Oxidase’s Binding Subunit(s) for Catechin Compounds Induce Inhibition. Eur. J. Adv. Chem. Res. 2023, 4, 10–18. [Google Scholar] [CrossRef]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant Effects of Vitamins C and E Are Associated with Altered Activation of Vascular NADPH Oxidase and Superoxide Dismutase in Stroke-Prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef]
- Karmakar, A.; Das, A.K.; Ghosh, N.; Sil, P.C. Chapter2.7 Superoxide Dismutase. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–166. ISBN 978-0-12-819096-8. [Google Scholar]
- Tian, Z.-H.; Miao, F.-T.; Zhang, X.; Wang, Q.-H.; Lei, N.; Guo, L.-C. Therapeutic Effect of Okra Extract on Gestational Diabetes Mellitus Rats Induced by Streptozotocin. Asian Pac. J. Trop. Med. 2015, 8, 1038–1042. [Google Scholar] [CrossRef]
- Tyagita, N.; Utami, K.P.; Zulkarnain, F.H.; Rossandini, S.M.; Pertiwi, N.P.; Rifki, M.A.; Safitri, A.H. Okra Infusion Water Improving Stress Oxidative and Inflammatory Markers on Hyperglycemic Rats. Bangladesh J. Med. Sci. 2019, 18, 748–752. [Google Scholar] [CrossRef]
- Aleissa, M.S.; AL-Zharani, M.; Alneghery, L.M.; Hasnain, M.S.; Almutairi, B.; Ali, D.; Alarifi, S.; Alkahtani, S. Comparative Study of the Anti-Diabetic Effect of Mucilage and Seed Extract of Abelmoschus Esculentus against Streptozotocin-Induced Diabetes in Rat Model. J. King Saud. Univ. Sci. 2022, 34. [Google Scholar] [CrossRef]
- Uadia, P.O.; Imagbovomwan, I.O.; Oriakhi, K.; Eze, I.G. Effect of Abelmoschus Esculentus (Okra)-Based Diet on Streptozotocin-Induced Diabetes Mellitus in Adult Wistar Rats. Trop. J. Pharm. Res. 2020, 19, 1737–1743. [Google Scholar] [CrossRef]
- Abbas, A.Y.; Muhammad, I.; Abdulrahman, M.B.; Bilbis, L.S. Antioxidant Effect of Ex-Maradi Okra Fruit Variety (Abelmuscus esculentus) on Alloxan-Induced Diabetic Rats. Trop. J. Nat. Prod. Res. 2020, 4, 105–112. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Ruszała, M.; Pilszyk, A.; Niebrzydowska, M.; Kimber-trojnar, Ż.; Trojnar, M.; Leszczyńska-gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus 2.0. Int. J. Mol. Sci. 2022, 23, 4364. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 2020, e61122. [Google Scholar] [CrossRef]
- Strom, A.; Kaul, K.; Brüggemann, J.; Ziegler, I.; Rokitta, I.; Püttgen, S.; Szendroedi, J.; Müssig, K.; Roden, M.; Ziegler, D. Lower Serum Extracellular Superoxide Dismutase Levels Are Associated with Polyneuropathy in Recent-Onset Diabetes. Exp. Mol. Med. 2017, 49, e394. [Google Scholar] [CrossRef]
- Puntel, R.L.; Roos, D.H.; Paixão, M.W.; Braga, A.L.; Zeni, G.; Nogueira, C.W.; Rocha, J.B.T. Oxalate Modulates Thiobarbituric Acid Reactive Species (TBARS) Production in Supernatants of Homogenates from Rat Brain, Liver and Kidney: Effect of Diphenyl Diselenide and Diphenyl Ditelluride. Chem. Biol. Interact. 2007, 165, 87–98. [Google Scholar] [CrossRef]
- Mishra, N.; Kumar, D.; Rizvi, S.I. Protective Effect of Abelmoschus Esculentus Against Alloxan-Induced Diabetes in Wistar Strain Rats. J. Diet. Suppl. 2016, 13, 634–646. [Google Scholar] [CrossRef]
- Hatai, B.; Ganguly, A.; Bandopadhyay, S.; Banerjee, S.; Hatai, J. Impact of Glutathione Peroxidase Activity (GPX) as Oxidative-Stress Marker and Its Role on Inflammation with Osteoarthritis Patients. Int. J. Adv. Res. 2017, 5, 1288–1294. [Google Scholar] [CrossRef]
- Hisalkar, P.J.; Patne, A.B.; Fawade, M.M.; Karnik, A.C. Evaluation of Plasma Superoxide Dismutase and Glutathione Peroxidase in Type 2 Diabetic Patients c Patients. Res. Artic. Biol. Med. 2012, 4, 65–72. [Google Scholar]
- Mokgalaboni, K.; Lebelo, L.S.; Modjadji, P.; Ghaffary, S. Okra Ameliorates Hyperglycaemia in Pre-Diabetic and Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of the Clinical Evidence. Front. Pharmacol 2023, 14, 1132650. [Google Scholar] [CrossRef] [PubMed]
- Yelisyeyeva, O.; Semen, K.; Zarkovic, N.; Kaminskyy, D.; Lutsyk, O.; Rybalchenko, V. Activation of Aerobic Metabolism by Amaranth Oil Improves Heart Rate Variability Both in Athletes and Patients with Type 2 Diabetes Mellitus. Arch. Physiol. Biochem. 2012, 118, 47–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).