Evaluation of the Effects of Radiation Therapy on Muscle Contractibility and Skin Healing: An Experimental Study of the Cancer Treatment Implications
Abstract
1. Introduction
2. Materials and Methods
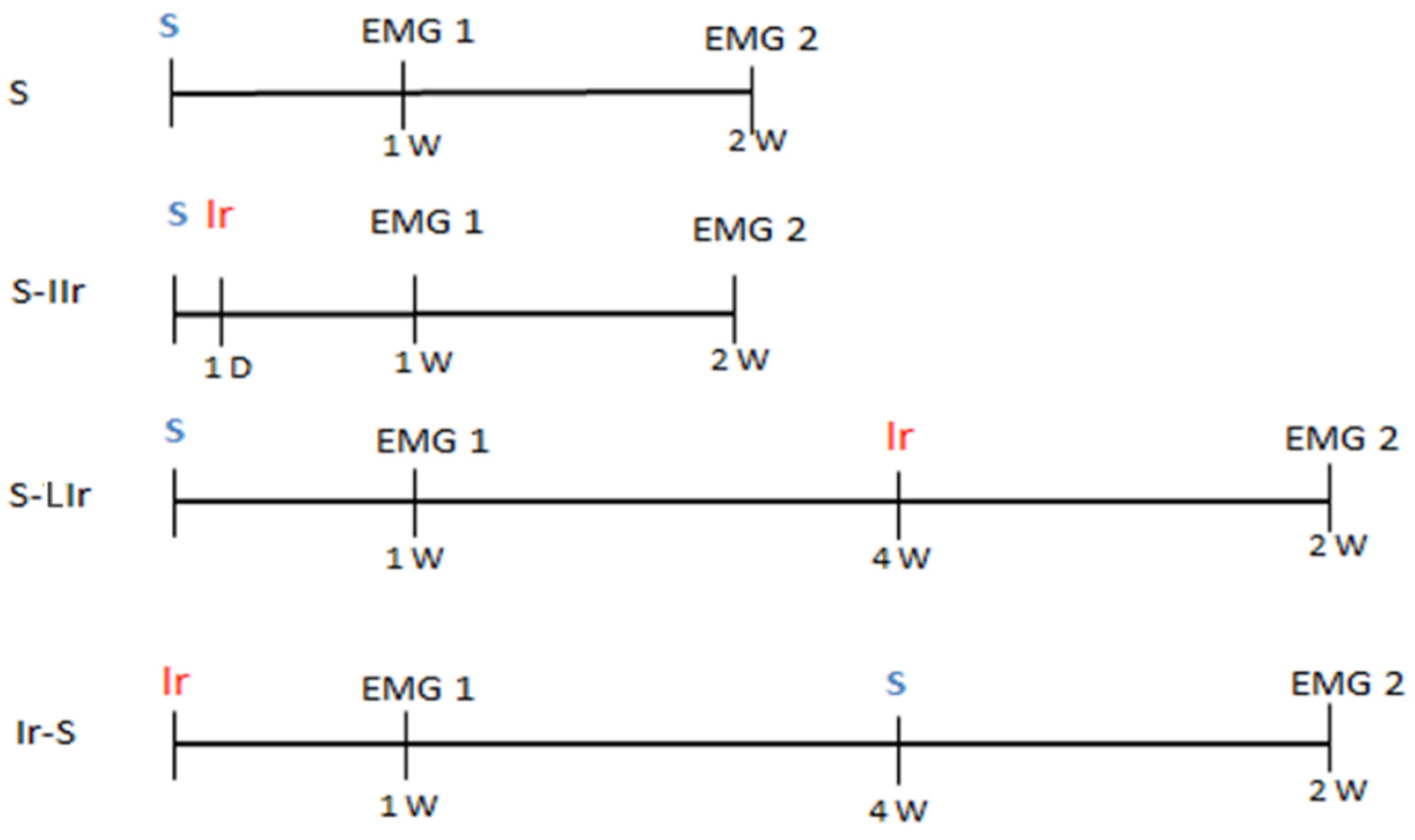
2.1. Experimental Setup
2.2. Surgical Procedure to Surgical Wound
2.3. Irradiation Procedure
2.4. Analysis of Muscle Contractibility
2.5. Analysis of Soft Tissues
2.6. Statistical Analysis
3. Results
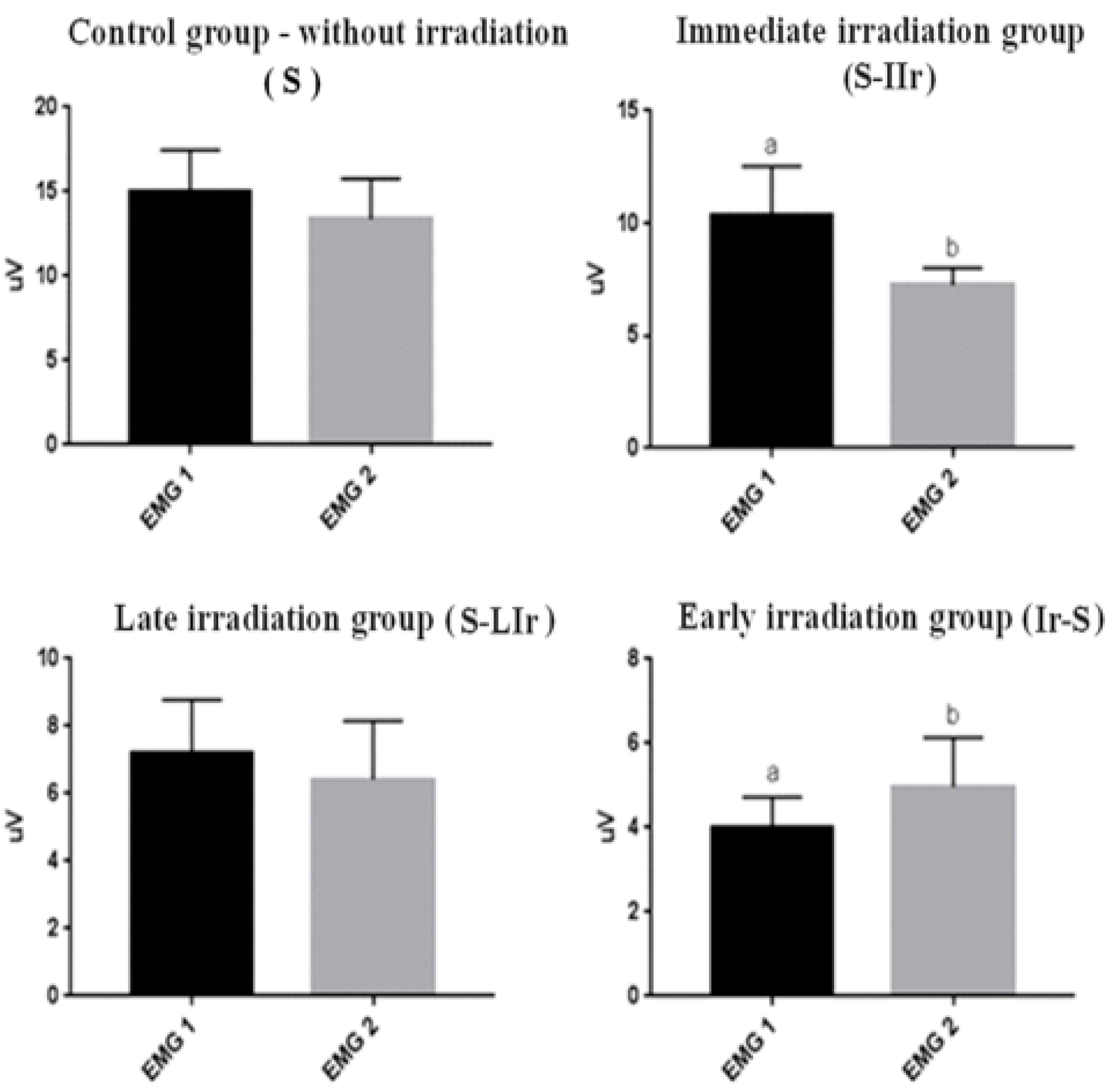
3.1. Analysis of Muscular Contractibility
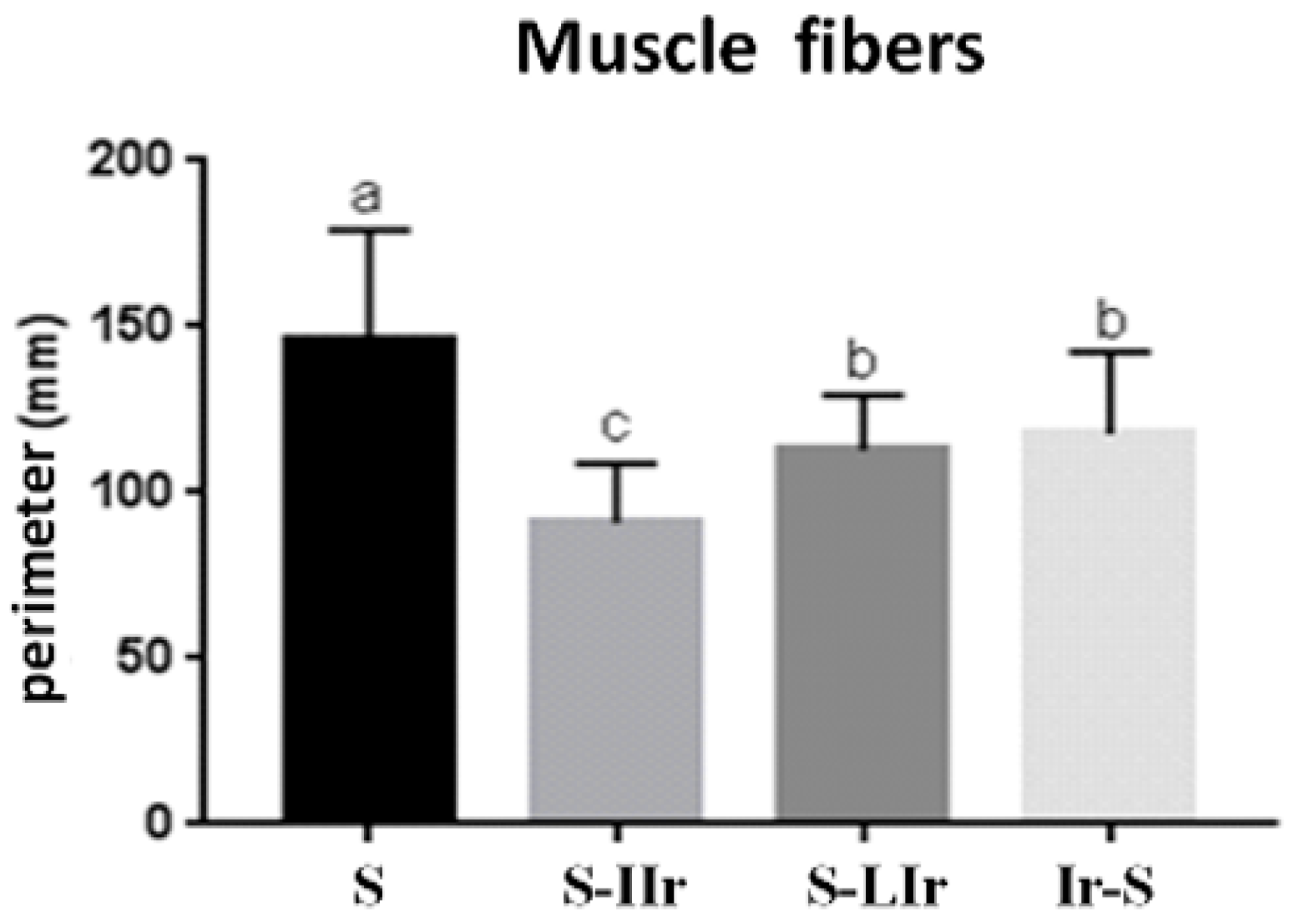
3.2. Histological Analysis of Muscle Tissue
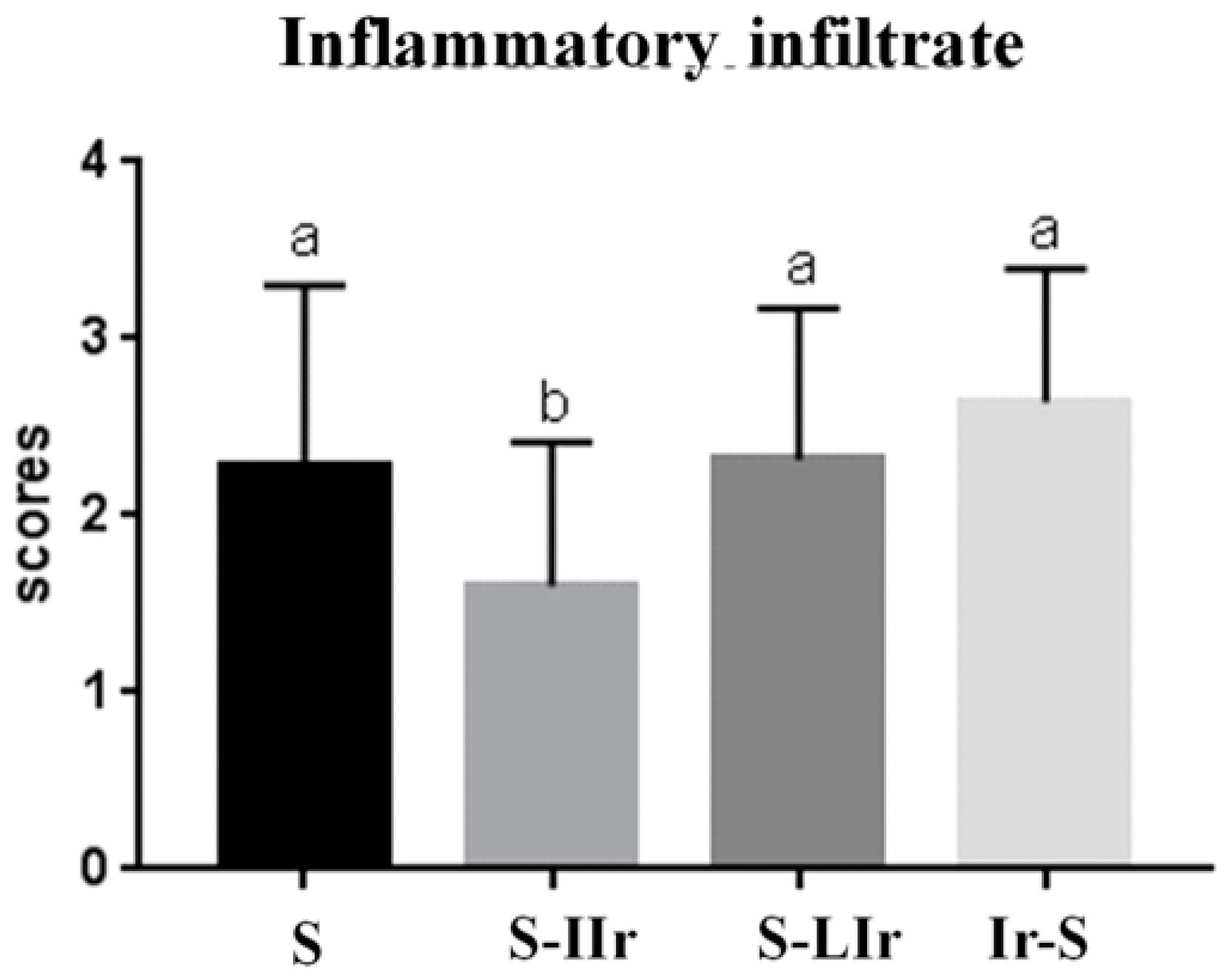
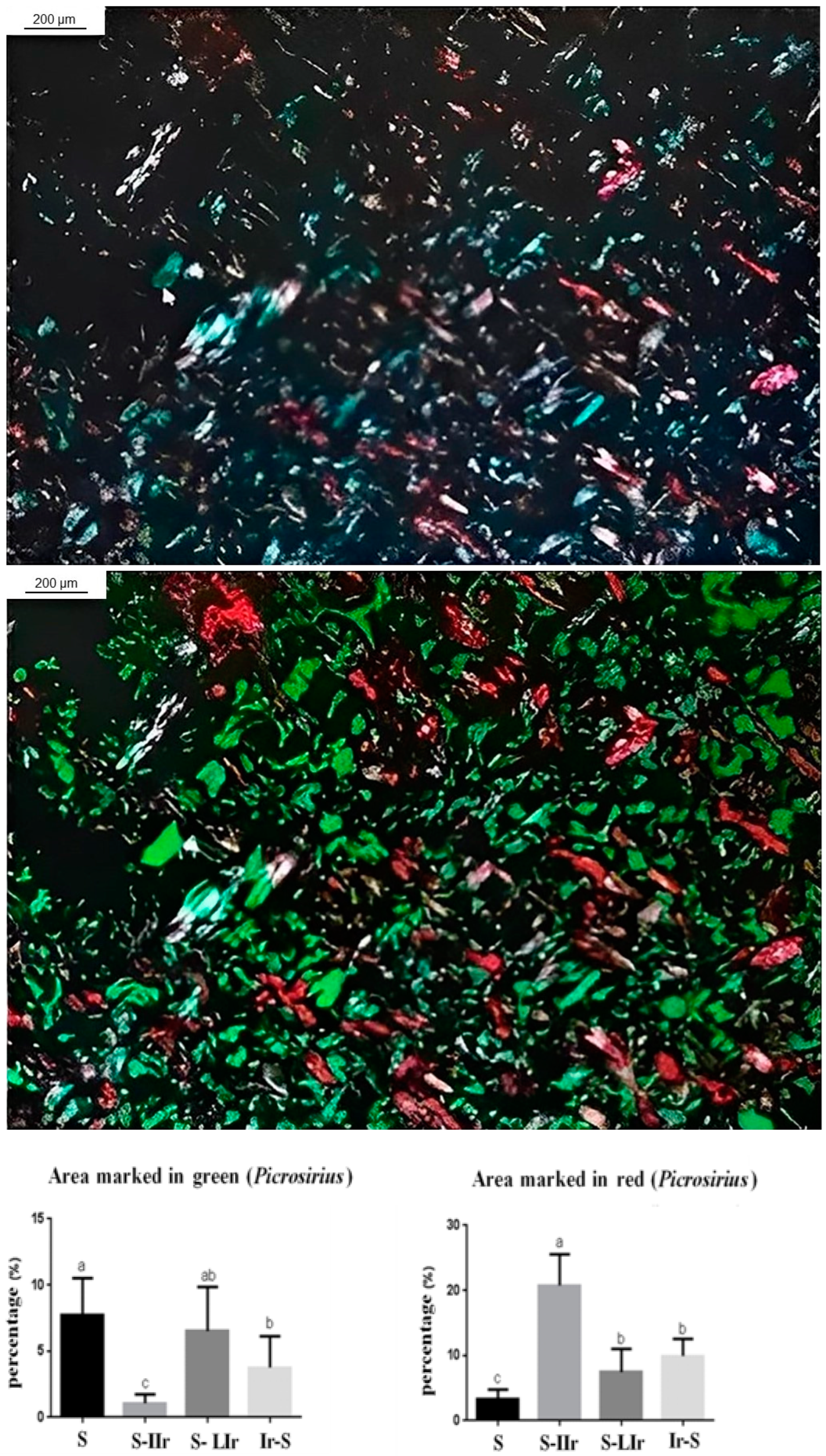
3.3. Histological Analysis of the Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Ries, L.A.G.; Eisner, M.P.; Kosary, C.L. SEER Cancer Statistics Review, 1973–1999; National Cancer Institute: Bethesda, MD, USA, 2002. Available online: https://seer.cancer.gov/archive/csr/1973_1999/ (accessed on 14 May 2021).
- Pompa, G.; Saccucci, M.; Di Carlo, G.; Brauner, E.; Valentini, V.; Di Carlo, S.; Gentile, T.; Guarino, G.; Polimeni, A. Survival of dental implants in patients with oral cancer treated by surgery and radiotherapy: A retrospective study. BMC Oral Health 2015, 15, 5. [Google Scholar] [CrossRef]
- Gallet, P.; Phulpin, B.; Merlin, J.L.; Leroux, A.; Bravetti, P.; Mecellem, H.; Tran, N.; Dolivet, G. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS ONE 2011, 6, e29399. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, W.; Yang, S.; Huang, L.; Chen, J.; Gong, C.; Fu, Z.; Zhang, L.; Tan, J. Bone marrow mesenchymal stem cell implantation for the treatment of radioactivity-induced acute skin damage in rats. Mol. Med. Rep. 2015, 12, 7065–7071. [Google Scholar] [CrossRef]
- Mazeron, R.; Tao, Y.; Lusinchi, A.; Bourhis, J. Current concepts of management in radiotherapy for head and neck squamous-cell cancer. Oral Oncol. 2009, 45, 402–408. [Google Scholar] [CrossRef]
- Freitas, D.A.; Caballero, A.D.; Pereira, M.M.; Oliveira, S.K.M.; Silva, G.P.; Hernández, C.I.V. Sequelas bucais da radioterapia de cabeça e pescoço. Rev. CEFAC Minas Gerais 2011, 13, 1103–1108. [Google Scholar] [CrossRef][Green Version]
- Kent, M.L.; Brennan, M.T.; Noll, J.L.; Fox, P.C.; Burri, S.H.; Hunter, J.C.; Lockhart, P.B. Radiation-induced trismus in head and neck cancer patients. Support. Care Cancer 2008, 16, 305–309. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; Kalk, W.W.; Roodenburg, J.L. Trismus in head and neck oncology: A systematic review. Oral Oncol. 2004, 40, 879–889. [Google Scholar] [CrossRef]
- Astradsson, T.; Laurell, G.; Ahlberg, A.; Nikolaidis, P.; Johansson, H.; Ehrsson, Y.T. Trismus in patients with head and neck cancer and 5-year overall survival. Acta Otolaryngol. 2018, 138, 1123–1127. [Google Scholar] [CrossRef]
- Rolim, A.E.H.; Costa, L.J.; Ramalho, L.M.P. Repercussões da radioterapia na região orofacial e seu tratamento. Radiol. Bras. 2011, 44, 388–395. [Google Scholar] [CrossRef]
- Epstein, J.B.; Robertson, M.; Emerton, S.; Phillips, N.; Stevenson-Moore, P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 2001, 23, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Katsura, K.; Sasai, K.; Sato, K.; Saito, M.; Hoshina, H.; Hayashi, T. Relationship between oral health status and development of osteoradionecrosis of the mandible: A retrospective longitudinal study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 731–738. [Google Scholar] [CrossRef]
- Bonomo, P.; Loi, M.; Desideri, I.; Olmetto, E.; Delli Paoli, C.; Terziani, F.; Greto, D.; Mangoni, M.; Scoccianti, S.; Simontacchi, G.; et al. Incidence of skin toxicity in squamous cell carcinoma of the head and neck treated with radiotherapy and cetuximab: A systematic review. Crit. Rev. Oncol. Hematol. 2017, 120, 98–110. [Google Scholar] [CrossRef]
- Pazdrowski, J.; Dańczak-Pazdrowska, A.; Polańska, A.; Kaźmierska, J.; Barczak, W.; Szewczyk, M.; Golusiński, P.; Adamski, Z.; Żaba, R.; Golusiński, W. An ultrasonographic monitoring of skin condition in patients receiving radiotherapy for head and neck cancers. Skin Res. Technol. 2019, 25, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- Vegian, M.R.C.; Costa, B.C.A.; Federico, C.; Tango, E.K.; Herrera, F.; Neves, R.M.; Prado, R.F.; Tango, R.N.; Avelino, S.O.M.; Vasconcellos, L.M.R. Systemic and local effects of radiotherapy: An experimental study on implants placed in rats. Clin. Oral Investig. 2020, 6, 785–797. [Google Scholar] [CrossRef]
- Goldstein, M.; Maxymiw, W.G.; Cummings, B.J.; Wood, R.E. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 365–373. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006, 35, 337–342. [Google Scholar] [CrossRef]
- Tolentino, E.D.S.; Centurion, B.S.; Ferreira, L.H.C.; Souza, A.P.; De Damante, J.H.; Rubira-Bullen, I.R.F. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [CrossRef]
- Olascoaga, A.; Vilar-Compte, D.; Poitevin-Chaco, A.; Contreras-Ruiz, J. Wound healing in radiated skin: Pathophysiology and treatment options. Int. Wound J. 2008, 5, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Meng, L.; Hou, X.; Qu, C.; Wang, B.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2018, 11, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Motevaseli, E.; Shirazi, A.; Geraily, G.; Rezaeyan, A.; Norouzi, F.; Rezapoor, S.; Abdollahi, H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. Int. J. Radiat. Biol. 2018, 94, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Rezaeyan, A.; Haddadi, G.H.; Hosseinzadeh, M.; Moradi, M.; Najafi, M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J. Med. Phys. 2016, 41, 182–191. [Google Scholar] [CrossRef]
- Garden, A.S.; Lewin, J.S.; Chambers, M.S. How to reduce radiation-related toxicity in patients with cancer of the head and neck. Curr. Oncol. Rep. 2006, 8, 140–145. [Google Scholar] [CrossRef]
- Villa, A.; Akintoye, S.O. Dental Management of Patients Who Have Undergone Oral Cancer Therapy. Dent. Clin. N. Am. 2018, 62, 131–142. [Google Scholar] [CrossRef]
- Kim, J.; Shin, E.S.; Kim, J.E.; Yoon, S.P.; Kim, Y.S. Neck muscle atrophy and soft-tissue fibrosis after neck dissection and postoperative radiotherapy for oral cancer. Radiat. Oncol. J. 2015, 33, 344–349. [Google Scholar] [CrossRef]
- McCaul, L.K. Oral and dental management for head and neck cancer patients treated by chemotherapy and radiotherapy. Dent. Update 2012, 39, 135–138, 140. [Google Scholar] [CrossRef]
- Pauli, N.; Mejersjö, C.; Fagerberg-Mohlin, B.; Finizia, C. Temporomandibular disorder in head and neck cancer patients undergoing radiotherapy: Clinical findings and patient-reported symptoms. Head Neck 2019, 41, 3570–3576. [Google Scholar] [CrossRef]
- Krasin, M.J.; Wiese, K.M.; Spunt, S.L.; Hua, C.H.; Daw, N.; Navid, F.; Davidoff, A.M.; McGregor, L.; Merchant, T.E.; Kun, L.E.; et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head-and-neck sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 355–360. [Google Scholar] [CrossRef]
- Lindblom, U.; Gärskog, O.; Kjellén, E.; Laurell, G.; Levring Jäghagen, E.; Wahlberg, P.; Zackrisson, B.; Nilsson, P. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014, 53, 620–627. [Google Scholar] [CrossRef]
- Bundgaard, T.; Tandrup, O.; Elbrønd, O. A functional evaluation of patients treated for oral cancer. A prospective study. Int. J. Oral Maxillofac. Surg. 1993, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Shiva Kumar, H.R.; Rai, K.K. Trismus in oral cancer patients undergoing surgery and radiotherapy. J. Oral Biol. Craniofacial Res. 2016, 6 (Suppl. S1), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Starmer, H.M. Dysphagia in head and neck cancer: Prevention and treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, K.A.; Bhayani, M.K.; Beadle, B.M.; Gold, K.A.; Shinn, E.H.; Lai, S.Y.; Lewin, J. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: Use it or lose it. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Beuren, A.G.; Paim, É.D.; Flores, N.D.S.; Martins, V.B.; Macagnan, F.E. Preventive measures for the progression of dysphagia in patients with cancer of head and neck subjected to radiotherapy: A systematic review with meta-analysis. Codas 2023, 35, e20210246. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avelino, S.O.M.; Neves, R.M.; Sobral-Silva, L.A.; Tango, R.N.; Federico, C.A.; Vegian, M.R.C.; de Almeida-Silva, L.A.; Kaminagakura, E.; Amorim, J.B.O.; Vasconcellos, L.M.R. Evaluation of the Effects of Radiation Therapy on Muscle Contractibility and Skin Healing: An Experimental Study of the Cancer Treatment Implications. Life 2023, 13, 1838. https://doi.org/10.3390/life13091838
Avelino SOM, Neves RM, Sobral-Silva LA, Tango RN, Federico CA, Vegian MRC, de Almeida-Silva LA, Kaminagakura E, Amorim JBO, Vasconcellos LMR. Evaluation of the Effects of Radiation Therapy on Muscle Contractibility and Skin Healing: An Experimental Study of the Cancer Treatment Implications. Life. 2023; 13(9):1838. https://doi.org/10.3390/life13091838
Chicago/Turabian StyleAvelino, Sarah O. M., Rafael M. Neves, Leonardo A. Sobral-Silva, Rubens N. Tango, Claudio A. Federico, Mariana R. C. Vegian, Luis Augusto de Almeida-Silva, Estela Kaminagakura, José Benedito O. Amorim, and Luana M. R. Vasconcellos. 2023. "Evaluation of the Effects of Radiation Therapy on Muscle Contractibility and Skin Healing: An Experimental Study of the Cancer Treatment Implications" Life 13, no. 9: 1838. https://doi.org/10.3390/life13091838
APA StyleAvelino, S. O. M., Neves, R. M., Sobral-Silva, L. A., Tango, R. N., Federico, C. A., Vegian, M. R. C., de Almeida-Silva, L. A., Kaminagakura, E., Amorim, J. B. O., & Vasconcellos, L. M. R. (2023). Evaluation of the Effects of Radiation Therapy on Muscle Contractibility and Skin Healing: An Experimental Study of the Cancer Treatment Implications. Life, 13(9), 1838. https://doi.org/10.3390/life13091838