Agonal Thrombus at Necropsy—A Third Category of Blood Coagulation in Domestic Carnivores
Abstract
1. Introduction
2. Materials and Methods
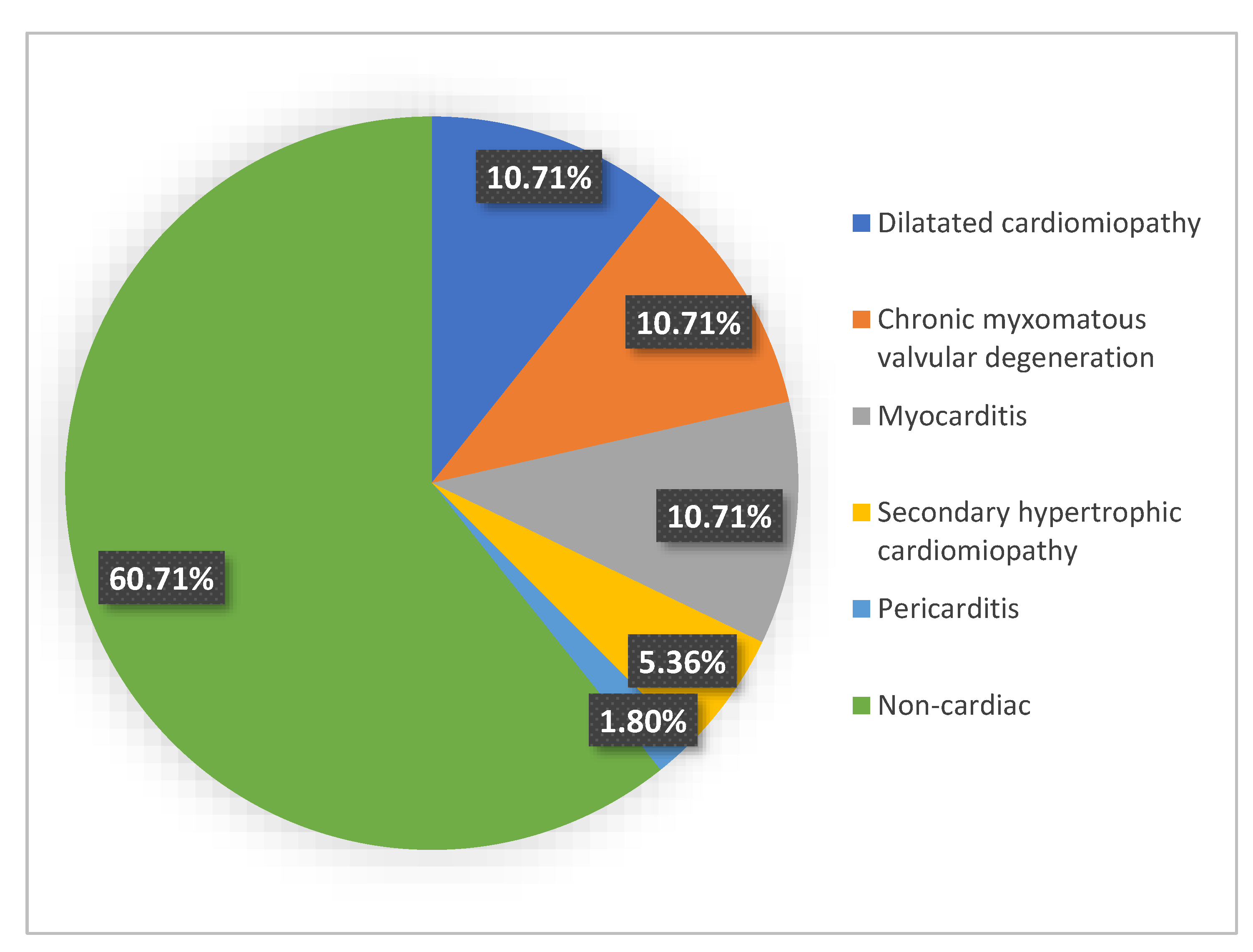
3. Results
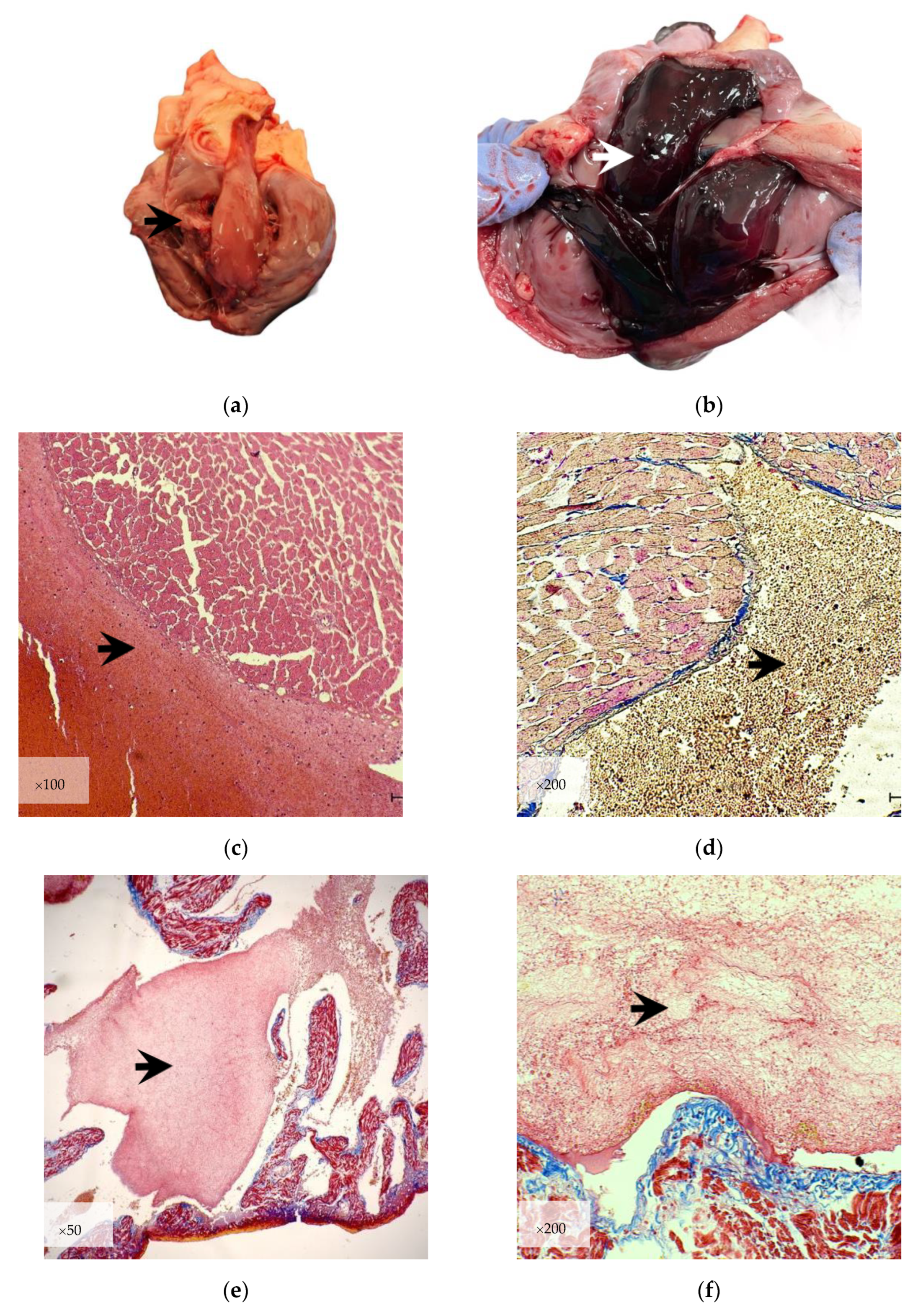
3.1. Gross Examination
3.2. Microscopic Examination
3.2.1. Histological Evaluation
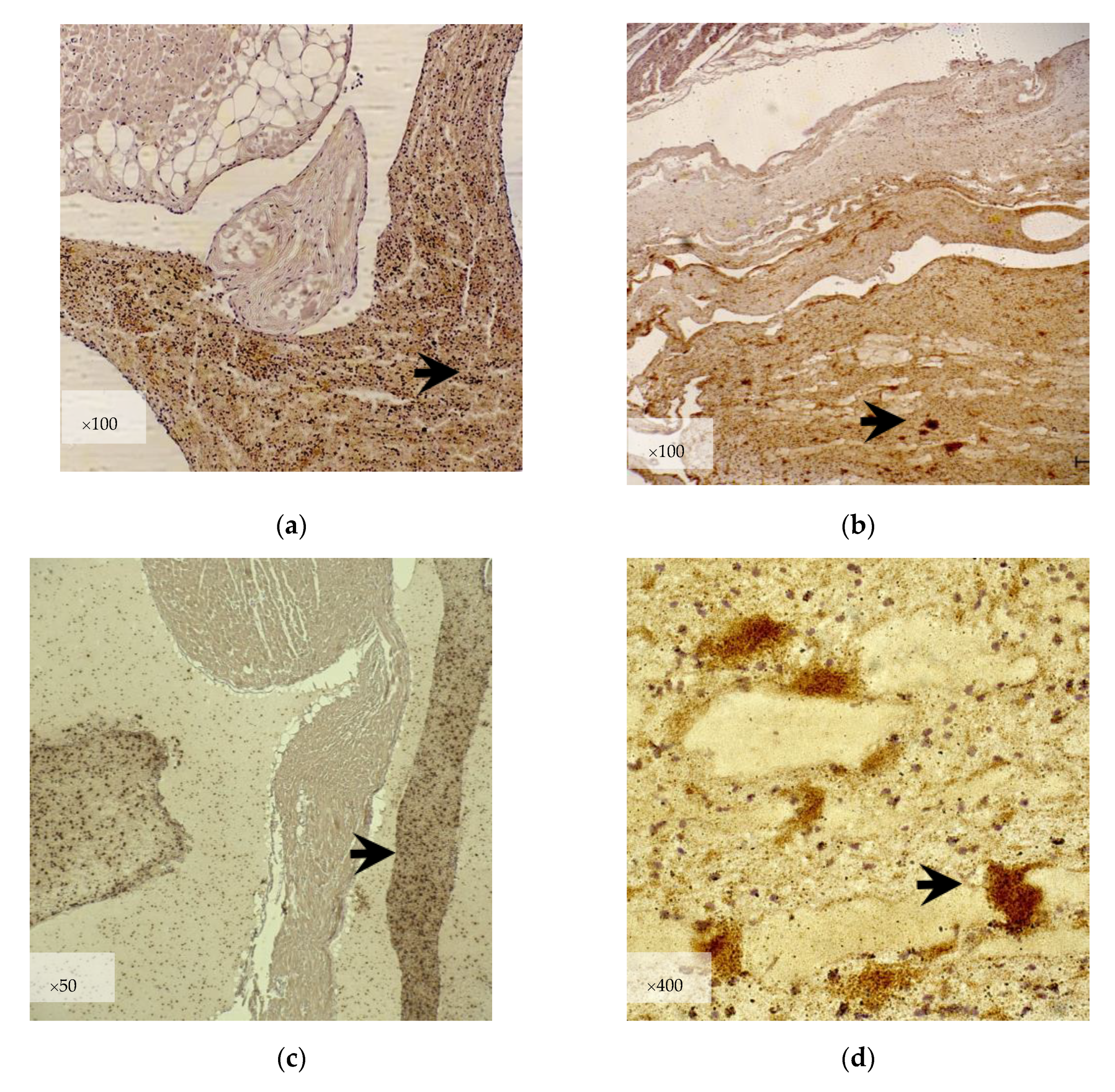
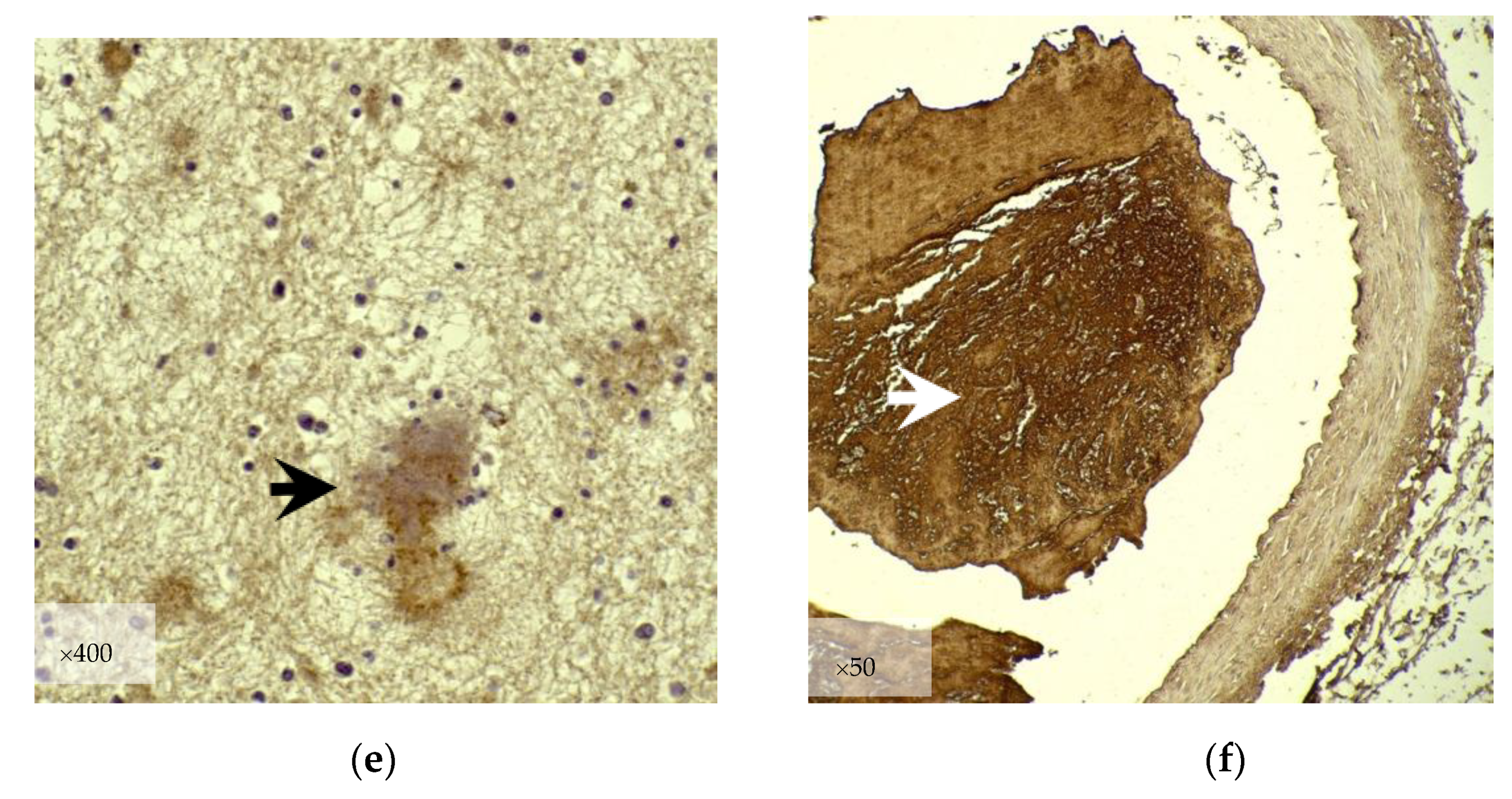
3.2.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMichael, M. New Models of Hemostasis. Top. Companion Anim. Med. 2012, 27, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zachary, J.F. Pathologic Basis of Veterinary Disease, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Di Fazio, N.; Delogu, G.; Ciallella, C.; Padovano, M.; Spadazzi, F.; Frati, P.; Fineschi, V. State-of-Art in the Age Determination of Venous Thromboembolism: A Systematic Review. Diagnostics 2021, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- Jubb, K.P. Pathology of Domestic Animals, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3. [Google Scholar]
- Maffeis, V.; Nicole, L.; Rago, C.; Fassina, A. Histological criteria for age determination of fatal venous thromboembolism. Int. J. Legal. Med. 2018, 132, 775–780. [Google Scholar] [CrossRef]
- Kushner, A.; West, W.P.; Khan Suheb, M.Z.; Pillarisetty, L.S. Virchow Triad; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nosaka, M.; Ishida, Y.; Kuninaka, Y.; Taruya, A.; Kimura, A.; Shimada, E.; Yamamoto, H.; Michiue, T.; Furukawa, F.; Kondo, T. The application of autophagy to thrombus age estimation in murine deep vein thrombosis model. Int. J. Legal. Med. 2020, 134, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kondo, T. Time-dependent organic changes of intravenous thrombi in stasis-induced deep vein thrombosis model and its application to thrombus age determination. Forensic Sci. Int. 2010, 195, 143–147. [Google Scholar] [CrossRef]
- Rashidi, A.; Gilles, S.; Linden, M.A. Lines of Zahn in the Splenic Vein. Thromb. Haemost. 2018, 118, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Darie, L.; Mihai, A.S.; Ion, C.; Gagniuc, E.; Ciobotaru-Pirvu, E. Case report: Aortic thromboembolism related to plycistic kidney disease in a cat. Sci. Work. Ser. C Vet. Med. 2022, 68, 56–63. [Google Scholar]
- Hansma, P.; Powers, S.; Diaz, F.; Li, W. Agonal Thrombi at Autopsy. Am. J. Forensic Med. Pathol. 2015, 36, 141–144. [Google Scholar] [CrossRef]
- Arbustini, E.; Dal Bello, B.; Morbini, P.; Gavazzi, A.; Specchia, G.; Vigano, M. Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation 2000, 69, 1095–1101. [Google Scholar] [CrossRef]
- Mansueto, G.; Costa, D.; Capasso, E.; Varavallo, F.; Brunitto, G.; Caserta, R.; Esposito, S.; Niola, M.; Sardu, C.; Marfella, R.; et al. The dating of thrombus organization in cases of pulmonary embolism: An autopsy study. BMC Cardiovasc. Disord. 2019, 19, 250. [Google Scholar] [CrossRef]
- Krywanczyk, A.R.; Tan, C.D.; Rodriguez, E.R. Histologic and Immunohistochemical Features of Antemortem Thrombus Compared to Postmortem Clot: Updating the Definition of Lines of Zahn. Arch. Pathol. Lab. Med. 2023. ahead of print. [Google Scholar] [CrossRef]
- Malone, P.C.; Agutter, P.S. Cadaver clots or agonal thrombi. In The Aetiology of Deep Venous Thrombosis; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Ciobotaru, E. Medicina Legala Veterinara; Editura Ceres: Bucharest, Romania, 2013. [Google Scholar]
- Tiu, R.E.; Rizac, I.R.; Turbatu, R.M.; Pirvu, A.M.; MIlitaru, M.; Ciobotaru-Pirvu, E. Histopathological aspects of agonal thrombus and its role in agonal death diagnosis-preliminary study. Sci. Works Ser. C Vet. Med. 2022, 68, 129–133. [Google Scholar]
- Rizac, R.I.; Tiu, R.E.; Turbatu, R.M.; Ciobotaru-Pirvu, E. Thrombi, Post-Mortem Clots and Agonal Thrombi: How to Tell the Difference. Rev. Romana Med. Vet. 2021, 31, 36–40. [Google Scholar]
- Uekita, I.; Ijiri, I.; Nagasaki, Y.; Haba, R.; Funamoto, Y.; Matsunaga, T.; Jamal, M.; Wang, W.; Kumihashi, M.; Ameno, K. Medico-legal investigation of chicken fat clot in forensic cases: Immunohistochemical and retrospective studies. Leg. Med. 2008, 10, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Konecný, F. Thromboembolic conditions, aetiology diagnosis and treatment in dogs and cats. Acta Vet. Brno 2010, 79, 497–508. [Google Scholar] [CrossRef]
- Supeanu, T.D.; Supeanu, A.; Cobzariu, D.; Baraitareanu, S.; Danes, D. Clinical and paraclinical peculiarities of effusive feline infectious peritonitis under non-specific IGY treatment: A case study. AgroLife Sci. J. 2017, 6, 257–261. [Google Scholar]
- Tiu, R.E.; Rizac, R.I.; Turbatu, R.M.; Gagniuc, E.; Ciobotaru-Pirvu, E. A case report: Immunohistochemical evaluation of agonal thrombus in a horse. In Proceedings of the International Conference Life Sciences for Sustainable Development, Cluj, Romania, 24–26 September 2022. [Google Scholar]
- Takashima, Y.; Onoda, I.; Chiou, S.P.; Kitoh, K. In vitro canine platelet aggregation caused by Dirofilaria immitis extract. J. Vet. Med. Sci. 2017, 79, 387–392. [Google Scholar] [CrossRef]
- Cortes, G.A.; Moore, M.J.; El-Nakeep, S. Physiology, Von Willebrand Factor; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chaudhry, R.; Usama, S.M.; Babiker, H.M. Physiology, Coagulation Pathways; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Landing, B.H. A suggestion that “agonal” thrombosis in right heart chambers indicates limitation of thoracic motion. Am. J. Clin. Pathol. 1960, 34, 439–441. [Google Scholar] [CrossRef]
- Evans, C.E. Hypoxia and HIF activation as a possible link between sepsis and thrombosis. Thromb. J. 2019, 17, 16. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 2007, 120, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, T.J.; Bruce, E. Thrombosis of the portal vein in eleven dogs. Vet. Pathol. 1993, 30, 28–35. [Google Scholar] [CrossRef]
- Fineschi, V.; Bafunno, V.; Bello, S.; De Stefano, F.; Margaglione, M.; Neri, M.; Riezzo, I.; Turillazzi, E.; Bonsignore, A.; Vecchione, G.; et al. Fatal pulmonary thromboembolism. A retrospective autopsy study: Searching for genetic thrombophilias (Factor V Leiden (G1691A) and FII (G20210A) gene variants) and dating the thrombus. Forensic Sci. Int. 2012, 214, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Turillazzi, E.; Neri, M.; Pomara, C.; Riezzo, I. Histological age determination of venous thrombosis: A neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci. Int. 2009, 186, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.M.; Gordon, A.E.; Henke, P.K. Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. Int. J. Mol. Sci. 2020, 21, 2080. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A. Fundamentals of Veterinary Clinical Pathology, 2nd ed.; Blackwell Publication: Ames, IA, USA, 2008; Volume 9, 908p, 16p. of plates. [Google Scholar]
- Ruggeri, Z.M.; Orje, J.N.; Habermann, R.; Federici, A.B.; Reininger, A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006, 108, 1903–1910. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
| Species | Breed | Age | Gender | Cardiac Lesions | Cause of Death |
|---|---|---|---|---|---|
| Canine | Golden Retriever | 1 year old | M | No | Congenital renal dysplasia |
| Canine | Half breed | 14 years old | F | No | Metastazised mammary carcinoma |
| Canine | Half breed | 12 years old | F | No | Peritonitis |
| Canine | Siberian Husky | 10 years old | M | No | Splenic torsion |
| Canine | Presa Canario | 6 months old | F | No | Gastric and splenic torsion |
| Canine | Half breed | 4 months old | F | No | Purulent meningoencephalitis |
| Canine | Half breed | 13 years old | M | No | Gastric torsion |
| Canine | Half breed | 10 years old | F | No | Metastazised mammary carcinoma |
| Canine | Half breed | 9 months old | F | No | Canine Parvovirus |
| Canine | Half breed | 5 years old | M | No | Pulmonary edema |
| Canine | French Bulldog | 5 years old | F | No | Traumatic subdural hematoma |
| Canine | Half breed | 8 years old | F | No | Metastazised mammary carcinoma |
| Feline | Domestic shorthair | 2 years old | M | No | Pulmonary edema |
| Feline | Domestic shorthair | 2 years old | M | No | Feline Coronavirus |
| Feline | Domestic shorthair | 3 months old | M | No | Pulmonary edema |
| Feline | Persian | 9 months old | M | No | Pulmonary edema |
| Feline | Main Coon | 6 months old | F | No | Feline panleukopenia virus |
| Feline | Domestic shorthair | 7 years old | M | No | Feline panleukopenia virus |
| Feline | Domestic shorthair | 7 years old | M | No | Pulmonary edema |
| Feline | Main Coon | 6 months old | F | No | Feline panleukopenia virus |
| Feline | Domestic shorthair | 6 years old | F | No | Feline Coronavirus |
| Feline | Domestic shorthair | 3 years old | M | No | Stercoral peritonitis |
| Feline | Domestic shorthair | 2 years old | M | No | Feline Coronavirus |
| Feline | Domestic shorthair | 3 years old | M | No | Traumatic— trans—diaphragmatic hernia |
| Feline | Domestic shorthair | 5 years old | M | No | Traumatic hepatic rupture |
| Feline | Domestic shorthair | 4 years old | M | No | Traumatic trans—diaphragmatic hernia |
| Feline | Domestic shorthair | 4 years old | M | No | Esophagian rupture |
| Feline | Domestic shorthair | 2 years old | F | No | Pulmonary edema |
| Feline | Domestic shorthair | 6 months old | F | No | Traumatic hepatic rupture |
| Feline | Ragdoll | 4 months old | M | No | Feline panleukopenia virus |
| Feline | Domestic shorthair | 10 years old | F | No | Metastazised osteosarcoma |
| Feline | Domestic shorthair | 10 years old | F | No | Metastazised mammary carcinoma |
| Feline | Domestic shorthair | 1 year old | F | No | Idiopathic chilothorax |
| Feline | Domestic shorthair | 5 years old | F | No | Traumatic— trans—diaphragmatic hernia |
| Species | Breed | Age | Gender | Cardiac Lesions | Cause of Death |
|---|---|---|---|---|---|
| Feline | Domestic shorthair | 10 years old | F | Yes | Secondary hypertrophic cardiomyopathy |
| Feline | Domestic shorthair | 10 years old | F | Yes | Secondary hypertrophic cardiomyopathy |
| Canine | Half breed | 10 years old | F | Yes | Dirofilariasis |
| Canine | Half breed | Adult | M | Yes | Dirofilariasis |
| Canine | Half breed | 14 years old | F | Yes | Dirofilariasis |
| Canine | Half breed | 5 years old | M | Yes | Dirofilariasis |
| Canine | Half breed | 8 years old | M | Yes | Mitral valve disease |
| Canine | Half breed | 8 years old | M | Yes | Dirofilariasis |
| Canine | French Bulldog | 5 years old | F | Yes | Mitral valve disease |
| Canine | Half breed | Adult | M | Yes | Mitral valve disease |
| Canine | Half breed | 12 years old | F | Yes | Mitral valve disease |
| Canine | German Shepard | 5 years old | M | Yes | Dirofilariasis |
| Canine | Half breed | 12 years old | M | Yes | Mitral valve disease |
| Canine | Bullmastiff | 6 years old | F | Yes | Acute myocarditis |
| Canine | German Shepard | 1 month old | F | Yes | Acute myocarditis— Canine Parvovirus |
| Canine | Half breed | 14 years old | F | Yes | Mitral valve disease |
| Feline | Domestic shorthair | 3 years old | M | Yes | Secondary hypertrophic cardiomyopathy |
| Feline | Domestic shorthair | 5 years old | F | Yes | Acute myocarditis |
| Feline | Domestic shorthair | 5 years old | M | Yes | Myocarditis |
| Feline | Domestic shorthair | 2 months old | F | Yes | Myocarditis |
| Feline | Domestic shorthair | 6 years old | F | Yes | Acute myocarditis |
| Feline | Domestic shorthair | 2 years old | F | Yes | Pericarditis |
| Feline * | Domestic shorthair | 9 years old | F | Yes | Aortic thromboembolism |
| Canine ** | Half breed | 13 years old | M | Yes | Dilated cardiomyopathy |
| Thrombi | Cruors | Agonal Thrombi | |
|---|---|---|---|
| Consistency | Firm to crumble | Soft | Soft to mild |
| Color | Variable | Dark red, black colored | Variable |
| Adherence | STRONG adherence | NO adherence | MILD adherence |
| Low to Absent Reaction | Positive Reaction | Strong Reaction | |
|---|---|---|---|
| CD45 | Yes | No | No |
| CD61 | No | Yes | Yes |
| CD68 | Yes | No | No |
| Von Willebrand factor | Yes | No | No |
| Fibrinogen | No | Yes | Yes |
| Fibrin | No | Yes | Yes |
| Recent Thrombus | Agonal Thrombus | |
|---|---|---|
| CD45 | Yes | No |
| CD61 | Yes | Yes |
| CD68 | Yes | No |
| Von Willebrand factor | Yes | No |
| Fibrinogen | Yes | Yes |
| Fibrin | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiu, R.E.; Rizac, R.I.; Nicolae, G.L.; Turbatu, R.M.; Ciobotaru-Pirvu, E. Agonal Thrombus at Necropsy—A Third Category of Blood Coagulation in Domestic Carnivores. Life 2023, 13, 1834. https://doi.org/10.3390/life13091834
Tiu RE, Rizac RI, Nicolae GL, Turbatu RM, Ciobotaru-Pirvu E. Agonal Thrombus at Necropsy—A Third Category of Blood Coagulation in Domestic Carnivores. Life. 2023; 13(9):1834. https://doi.org/10.3390/life13091834
Chicago/Turabian StyleTiu, Raluca Elena, Raluca Ioana Rizac, George Laurentiu Nicolae, Raluca Mihaela Turbatu, and Emilia Ciobotaru-Pirvu. 2023. "Agonal Thrombus at Necropsy—A Third Category of Blood Coagulation in Domestic Carnivores" Life 13, no. 9: 1834. https://doi.org/10.3390/life13091834
APA StyleTiu, R. E., Rizac, R. I., Nicolae, G. L., Turbatu, R. M., & Ciobotaru-Pirvu, E. (2023). Agonal Thrombus at Necropsy—A Third Category of Blood Coagulation in Domestic Carnivores. Life, 13(9), 1834. https://doi.org/10.3390/life13091834





