Lupenone-Rich Fraction Derived from Cissus quadrangularis L. Suppresses Lipid Accumulation in 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of CQ Hexane Extract by Fractionation
2.3. Flash Column Chromatography
2.4. Purification of Lupenone from 2H
2.5. Cell Culture
2.6. Adipogenic Differentiation
2.7. Oil Red O Staining
2.8. Cell Viability Assay
- Eox570 = E of oxidized alamarBlue Reagent at 570 nm = 80,586
- Eox600 = E of oxidized alamarBlue Reagent at 600 nm = 117,216
- Ered570 = E of reduced alamarBlue Reagent at 570 nm = 155,677
- Ered600 = E of reduced alamarBlue Reagent at 600 nm = 14,652
- A570 = absorbance of test wells at 570 nm
- A600 = absorbance of test wells at 600 nm
- A570 t0 = absorbance of negative control well at 570 nm
- A600 t0 = absorbance of negative control well at 600 nm
2.9. Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity Assay
2.10. BODIPY and DAPI Staining
2.11. Mitochondrial Staining
2.12. Gene Expression Analysis
2.13. Glucose Consumption
2.14. Statistical Analysis
3. Results
3.1. Fractionation of CQ-H
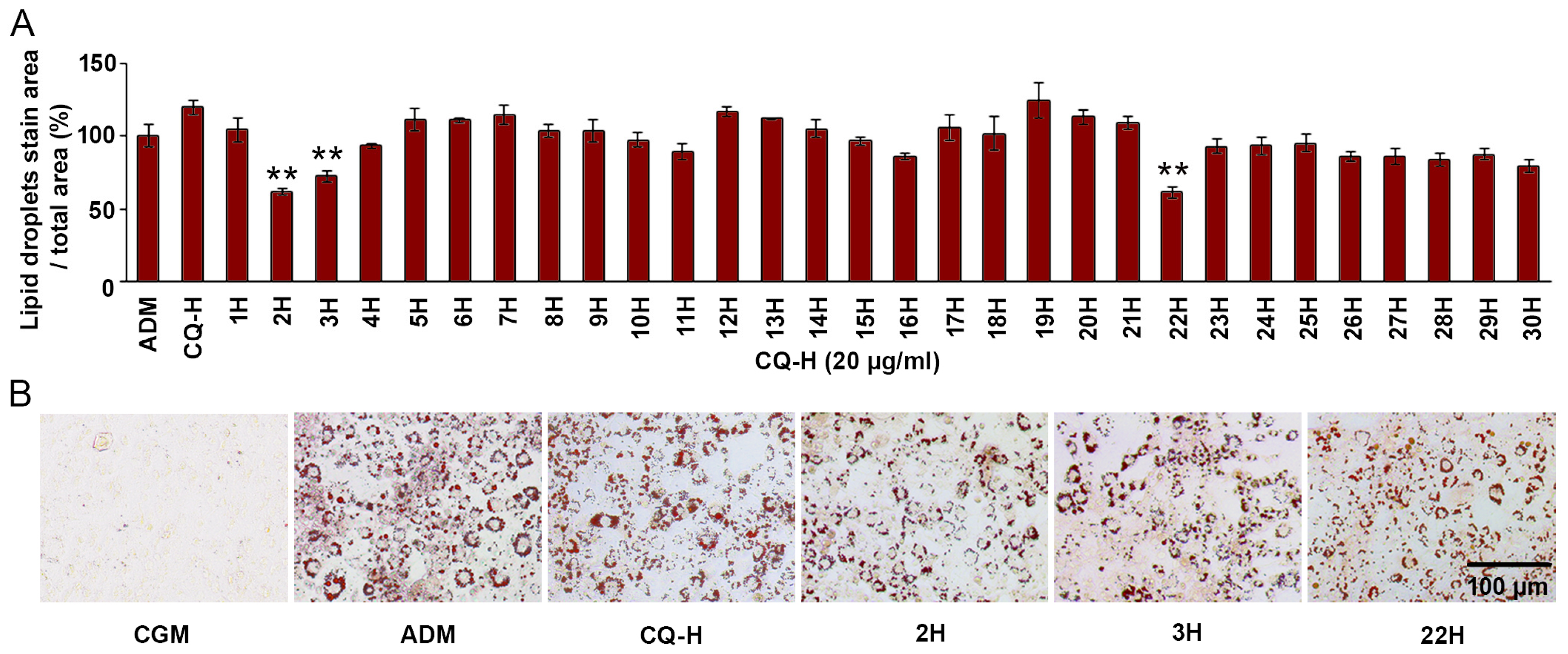
3.2. Antiadipogenesis of CQ-H Fractions
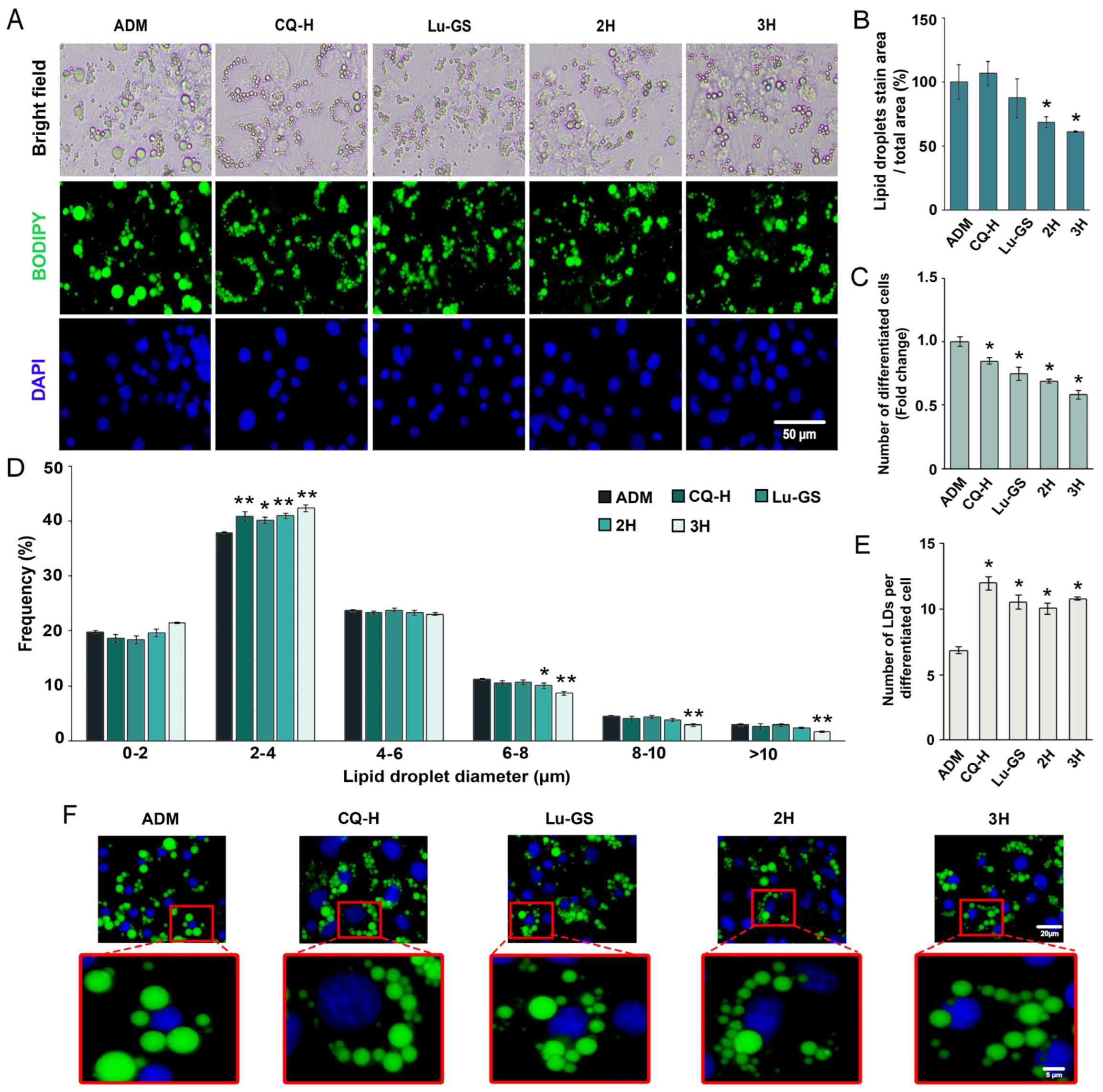
3.3. Lupenone-Rich Fractions Reduced Adipogenesis
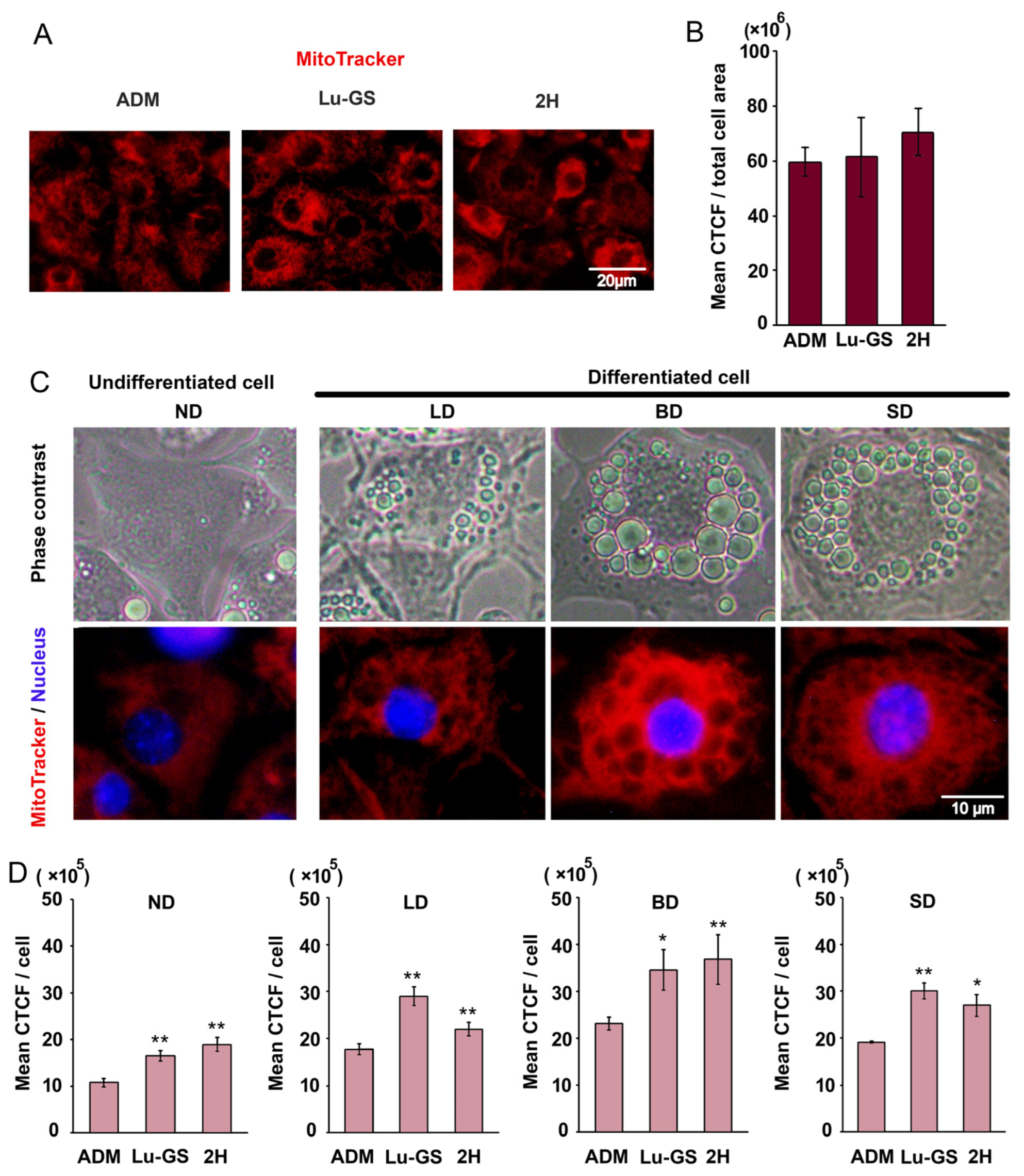
3.4. Lupenone and Lupenone-Rich CQ-H Fraction Increased Mitochondrial Density in 3T3-L1 Cells
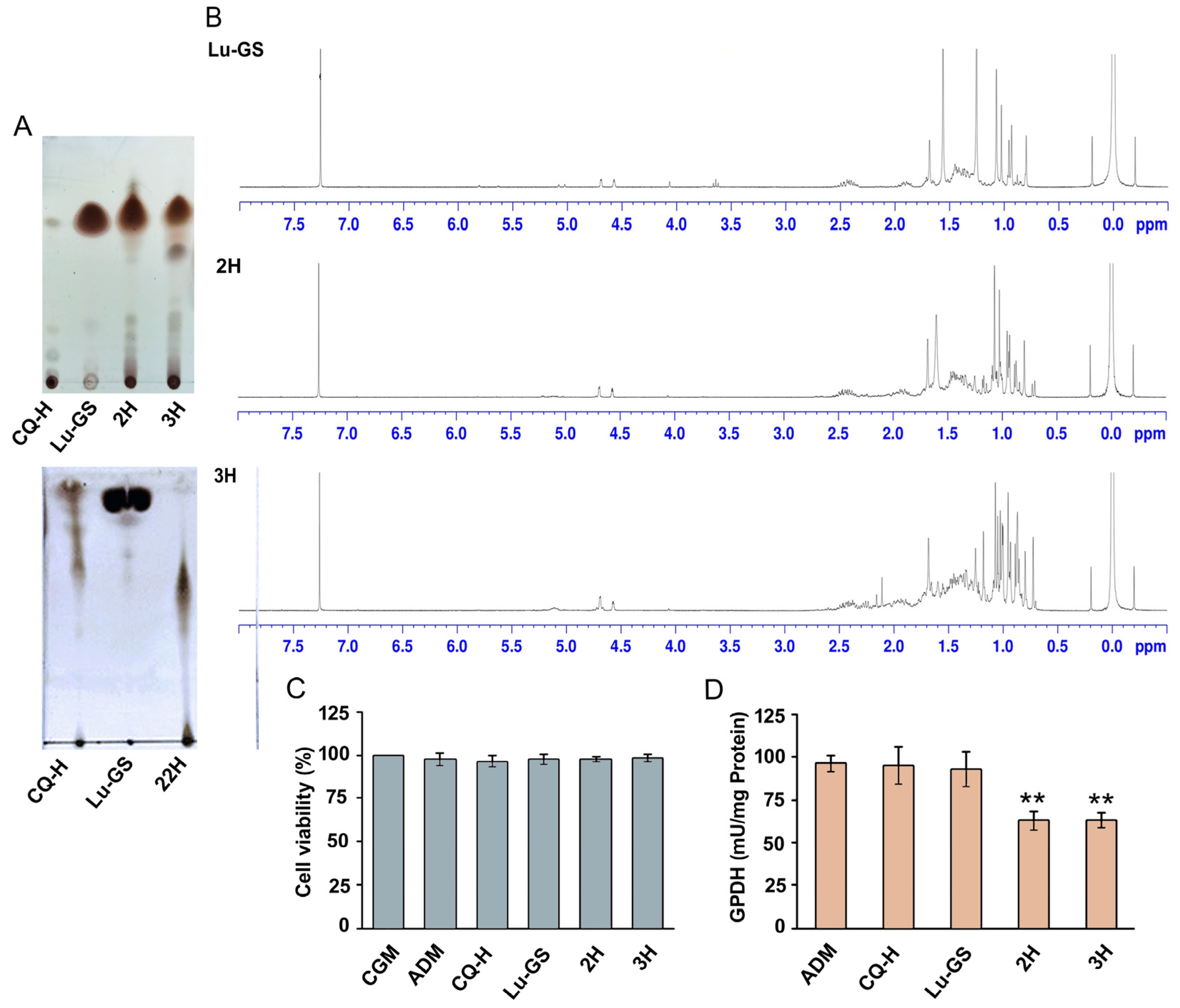
3.5. Extraction and Identification of Lupenone from 2H Fraction
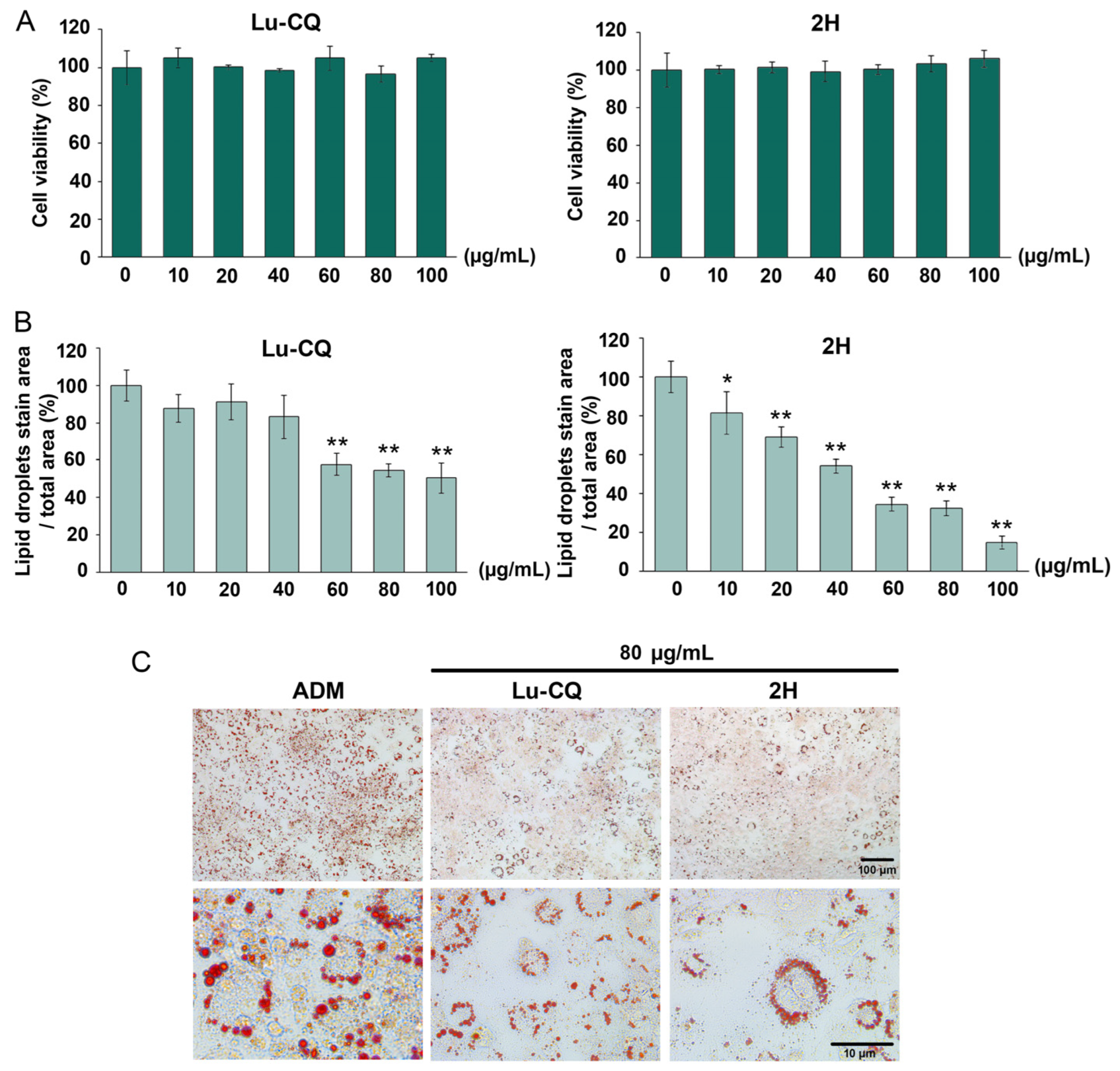
3.6. Higher Adipogenic Inhibitory Effect of the 2H Fraction Compared with Lu-CQ
3.7. Downregulation of Expression of Adipogenic Master Gene with Increased Expression of Glucose Transporter Genes Induced by Lu-CQ and 2H
3.8. Lu-CQ and 2H Increased Glucose Consumption during Adipogenesis of 3T3-L1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suárez-Cuenca, J.A.; De La Peña-Sosa, G.; De La Vega-Moreno, K.; Banderas-Lares, D.Z.; Salamanca-García, M.; Martínez-Hernández, J.E.; Vera-Gómez, E.; Hernández-Patricio, A.; Zamora-Alemán, C.R.; Domínguez-Pérez, G.A. Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity. Sci. Rep. 2021, 11, 1831. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory microenvironment and adipogenic differentiation in obesity: The inhibitory effect of theobromine in a model of human obesity in vitro. Mediat. Inflamm. 2019, 2019, 1515621. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Halpern, B.; Oliveira, E.S.; Faria, A.M.; Halpern, A.; de Melo, M.E.; Cercato, C.; Mancini, M.C. Combinations of drugs in the treatment of obesity. Pharmaceuticals 2010, 3, 2398–2415. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lu, J.; Peng, C.; Lin, L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. Nutr. 2021, 61, 1947–1965. [Google Scholar] [CrossRef]
- Andersen, E.; Ingerslev, L.R.; Fabre, O.; Donkin, I.; Altıntaş, A.; Versteyhe, S.; Bisgaard, T.; Kristiansen, V.B.; Simar, D.; Barrès, R. Preadipocytes from obese humans with type 2 diabetes are epigenetically reprogrammed at genes controlling adipose tissue function. Int. J. Obes. 2019, 43, 306–318. [Google Scholar] [CrossRef]
- Bafna, P.S.; Patil, P.H.; Maru, S.K.; Mutha, R.E. Cissus quadrangularis L: A comprehensive multidisciplinary review. J. Ethnopharmacol. 2021, 279, 114355. [Google Scholar] [CrossRef]
- Kavitha, A.; Babu, A.N.; Nadendla, R.R. Acute Toxicity Study of Cissus Quadrangularis in Swiss Albino Mice. Panacea J. Pharm. Pharm. Sci. 2018, 7, 748–756. [Google Scholar]
- Chaudhari, R.; Patil, P.; Chaudhari, R.; Bhangale, J. Antihyperglycaemic activity of ethanolic extract of cissus quadrangularis (l.) Leaves in alloxan induced diabetic rats. J. Appl. Pharm. Sci. 2013, 3, 073–077. [Google Scholar] [CrossRef]
- Nash, R.; Azantsa, B.; Kuate, D.; Singh, H.; Oben, J. The use of a stem and leaf aqueous extract of Cissus quadrangularis (CQR-300) to reduce body fat and other components of metabolic syndrome in overweight participants. J. Altern. Complement. Med. 2019, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Le, B.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Cissus quadrangularis extract (CQR-300) inhibits lipid accumulation by downregulating adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol. Rep. 2018, 5, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Park, S.-B.; Jin, Y.-Y.; Yang, S.H.; Suh, J.-W. Cissus quadrangularis extracts decreases body fat through regulation of fatty acid synthesis in high-fat diet-induced obese mice. J. Appl. Biol. Chem. 2016, 59, 49–56. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Cissus Quadrangularis enhances UCP1 mRNA, indicative of white adipocyte browning and decreases central obesity in humans in a randomized trial. Sci. Rep. 2021, 11, 2008. [Google Scholar] [CrossRef]
- Anwar, P.Z.; Sezhian, U.G.; Narasingam, A. Bioactive compound analysis and bioactivities of endophytic bacteria from Cissus quadrangularis. Int. J. Pharm. Sci. Res 2020, 11, 5553–5560. [Google Scholar]
- Teware, K.; Singh, P.; Mehta, R. Phytochemical Extraction and Analysis of Medicinally Important Plant Cissus qudrangularis L. (Hadjod). Biomed. Pharmacol. J. 2011, 4, 175. [Google Scholar] [CrossRef][Green Version]
- Vandana, J.; Achal, T.; Lal, H.; Laddha, K. Lipid constituents from Cissus quadrangularis leaves. Pharmacogn. Res. 2009, 1, 231–233. [Google Scholar]
- Pathomwichaiwat, T.; Ochareon, P.; Soonthornchareonnon, N.; Ali, Z.; Khan, I.A.; Prathanturarug, S. Alkaline phosphatase activity-guided isolation of active compounds and new dammarane-type triterpenes from Cissus quadrangularis hexane extract. J. Ethnopharmacol. 2015, 160, 52–60. [Google Scholar] [CrossRef]
- Toor, R.H.; Khan, Z.N.; Tariq, M.; Tassaduq, R.; Gardner, Q.A.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Shakoori, A.R. Bioactivity-guided isolation and identification of anti-adipogenic constituents from the n-butanol fraction of Cissus quadrangularis. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 519–541. [Google Scholar] [CrossRef]
- Puapairoj, P.; Naengchomnong, W.; Kijjoa, A.; Pinto, M.M.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.; Herz, W. Cytotoxic activity of lupane-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Medica 2005, 71, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Liu, C.L.; Shiu, H.T.; Wong, H.L.; Siu, W.S.; Zhang, C.; Han, X.Q.; Ye, C.X.; Leung, P.C.; Ko, C.H. Cocoa tea (Camellia ptilophylla) water extract inhibits adipocyte differentiation in mouse 3T3-L1 preadipocytes. Sci. Rep. 2016, 6, 20172. [Google Scholar] [CrossRef] [PubMed]
- Chinchu, J.; Mohan, M.C. Downregulation of adipogenic genes in 3T3-L1 Pre adipocytes-a possible mechanism of anti-obesity activity of herbal decoction Varanadi Kashayam. J. Herb. Med. 2020, 19, 100309. [Google Scholar]
- Hu, Y.; Yu, J.; Cui, X.; Zhang, Z.; Li, Q.; Guo, W.; Zhao, C.; Chen, X.; Meng, M.; Li, Y. Combination usage of adipocount and image-pro plus/imagej software for quantification of adipocyte sizes. Front. Endocrinol. 2021, 12, 642000. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Jain, A. Measuring Cell-Viability by Resazurin (Alamarblue®) Assay Using Photopette® Cell. Application Note, Tip Biosystems, Cell Viability Life Sciences. 2017. Available online: http://tipbiosystems.com/tbs/wpcontent/uploads/2017/06/AN016-Cell-Viability-using-Resazurin-Dye_2017_05_29.pdf (accessed on 15 May 2020).
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Sottile, V.; Seuwen, K. A high-capacity screen for adipogenic differentiation. Anal. Biochem. 2001, 293, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Adomshick, V.; Pu, Y.; Veiga-Lopez, A. Automated lipid droplet quantification system for phenotypic analysis of adipocytes using CellProfiler. Toxicol. Mech. Methods 2020, 30, 378–387. [Google Scholar] [CrossRef]
- Bora, P.; Gahurova, L.; Mašek, T.; Hauserova, A.; Potěšil, D.; Jansova, D.; Susor, A.; Zdráhal, Z.; Ajduk, A.; Pospíšek, M. p38-MAPK-mediated translation regulation during early blastocyst development is required for primitive endoderm differentiation in mice. Commun. Biol. 2021, 4, 788. [Google Scholar] [CrossRef]
- Ribeiro Filho, H.V.; Bernardi Videira, N.; Bridi, A.V.; Tittanegro, T.H.; Helena Batista, F.A.; de Carvalho Pereira, J.G.; De Oliveira, P.S.L.; Bajgelman, M.C.; Le Maire, A.; Figueira, A.C.M. Screening for PPAR non-agonist ligands followed by characterization of a hit, AM-879, with additional no-adipogenic and cdk5-mediated phosphorylation inhibition properties. Front. Endocrinol. 2018, 9, 11. [Google Scholar] [CrossRef]
- Kim, H.-I.; Moon, S.-H.; Lee, W.-C.; Lee, H.-J.; Shivakumar, S.B.; Lee, S.-H.; Park, B.-W.; Rho, G.-J.; Jeon, B.-G. Inhibition of cell growth by cellular differentiation into adipocyte-like cells in dexamethasone sensitive cancer cell lines. Anim. Cells Syst. 2018, 22, 178–188. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Tanachatchairatana, T.; Kanokmedhakul, S. Antiplasmodial triterpenes from twigs of Gardenia saxatilis. J. Ethnopharmacol. 2003, 88, 275–277. [Google Scholar] [CrossRef]
- Dhanasekaran, S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi J. Biol. Sci. 2020, 27, 1302–1309. [Google Scholar] [CrossRef]
- Kuate, D.; Nash, R.J.; Bartholomew, B.; Penkova, Y. The use of Cissus quadrangularis (CQR-300) in the management of components of metabolic syndrome in overweight and obese participants. Nat. Prod. Commun. 2015, 10, 1934578X1501000737. [Google Scholar] [CrossRef]
- Pluemjai, T.; Saifah, E. Constituents of Cissus quadrangularis Linn. Thai J. Pharm. Sci. 1986, 11, 205–211. [Google Scholar]
- Xu, F.; Wu, H.; Wang, X.; Yang, Y.; Wang, Y.; Qian, H.; Zhang, Y. RP-HPLC characterization of lupenone and β-sitosterol in Rhizoma Musae and evaluation of the anti-diabetic activity of lupenone in diabetic Sprague-Dawley rats. Molecules 2014, 19, 14114–14127. [Google Scholar] [CrossRef]
- Ahn, E.K.; Oh, J.S. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 cells. Phytother. Res. 2013, 27, 761–766. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Tolstikova, T.; Sorokina, I.; Tolstikov, G.; Tolstikov, A.; Flekhter, O. Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives. Russ. J. Bioorg. Chem. 2006, 32, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Brusotti, G.; Montanari, R.; Capelli, D.; Cattaneo, G.; Laghezza, A.; Tortorella, P.; Loiodice, F.; Peiretti, F.; Bonardo, B.; Paiardini, A. Betulinic acid is a PPARγ antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci. Rep. 2017, 7, 5777. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Hiwatashi, K.; Itoh, M.; Suzuki, N.; Watanabe, T.; Takahashi, J.; Sasaki, H. Inhibitory effects of lupeol on 3T3-L1 preadipocyte differentiation. Phytochem. Lett. 2008, 1, 191–194. [Google Scholar] [CrossRef]
- Senamontree, S.; Lakthan, T.; Charoenpanich, P.; Chanchao, C.; Charoenpanich, A. Betulinic acid decreases lipid accumulation in adipogenesis-induced human mesenchymal stem cells with upregulation of PGC-1α and UCP-1 and post-transcriptional downregulation of adiponectin and leptin secretion. PeerJ 2021, 9, e12321. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Minakshi, G.C.; Velu, K.; Priya, T.; Kumar, R.M.; Kaliappan, I.; Dubey, G.P. Anti-adipogenic β-sitosterol and lupeol from Moringa oleifera suppress adipocyte differentiation through regulation of cell cycle progression. J. Food Biochem. 2022, 46, e14170. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Reza, M.I.; Singh, P.; Husain, A.; Dadge, S.; Gayen, J.R. Polyphenolic-rich Cissus quadrangularis extract ameliorates insulin resistance by activating AdipoR1 in peri-/post-menopausal rats. Exp. Gerontol. 2022, 159, 111681. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, M.; Wu, H.; Wang, Y.; Yang, Y.; Wang, X. Study on the mechanism of lupenone for treating type 2 diabetes by integrating pharmacological evaluation and network pharmacology. Pharm. Biol. 2022, 60, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Hoener, P.A.; Jow, L.; Bilakovics, J.; Klausing, K.; Mais, D.E.; Faulkner, A.; Croston, G.E.; Paterniti Jr, J.R. A selective peroxisome proliferator-activated receptor-γ (PPARγ) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol. Endocrinol. 2000, 14, 1425–1433. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Rajagopal, R.; Sun-Ju, K.; Kim, H.-J.; Kim, Y.O.; Mothana, R.A.; Kadaikunnan, S.; Khaled, J.M.; Siddiqui, N.A.; Al-Rehaily, A.J. Betulinic acid lowers lipid accumulation in adipocytes through enhanced NCoA1–PPARγ interaction. J. Infect. Public Health 2019, 12, 726–732. [Google Scholar]

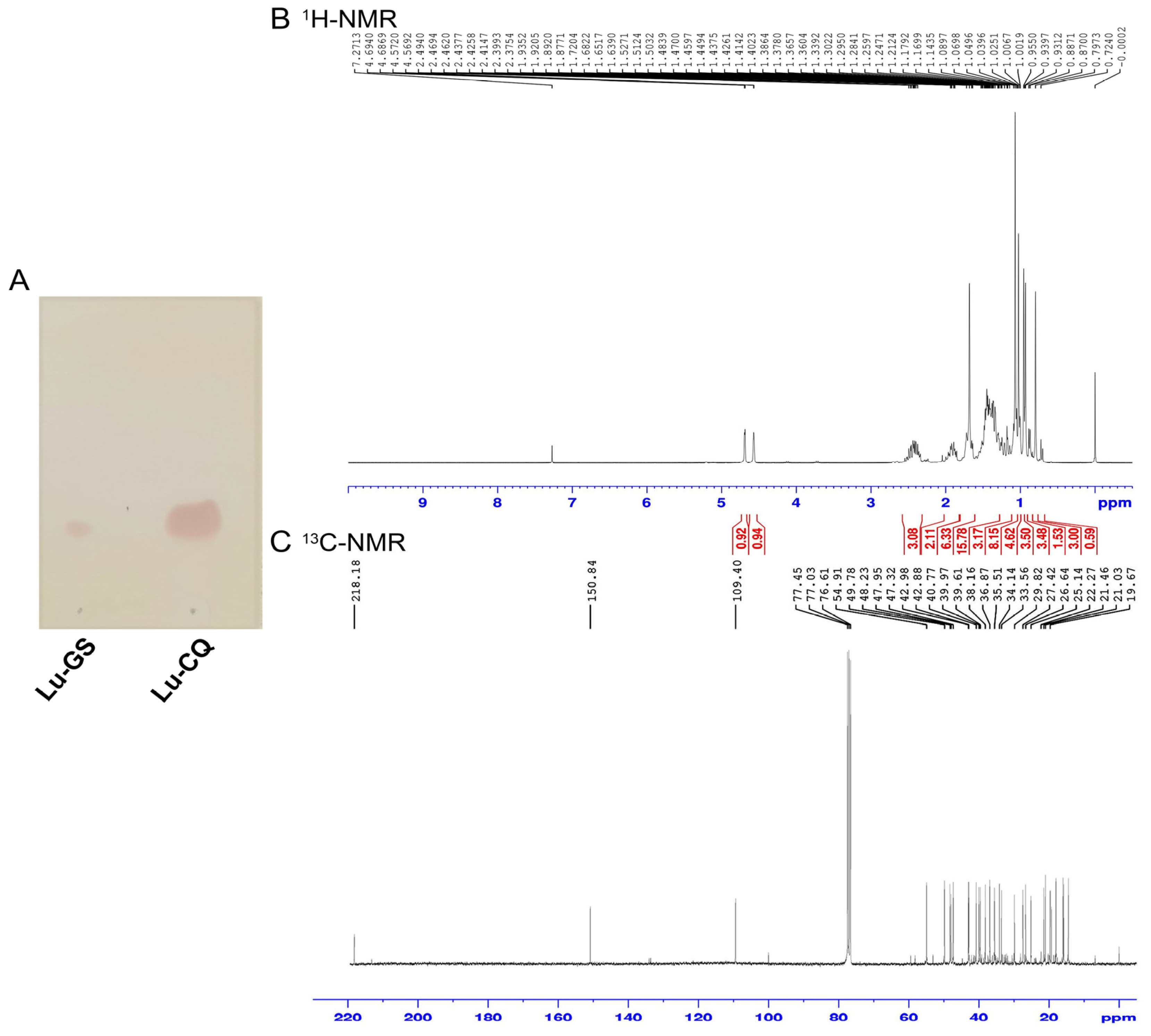
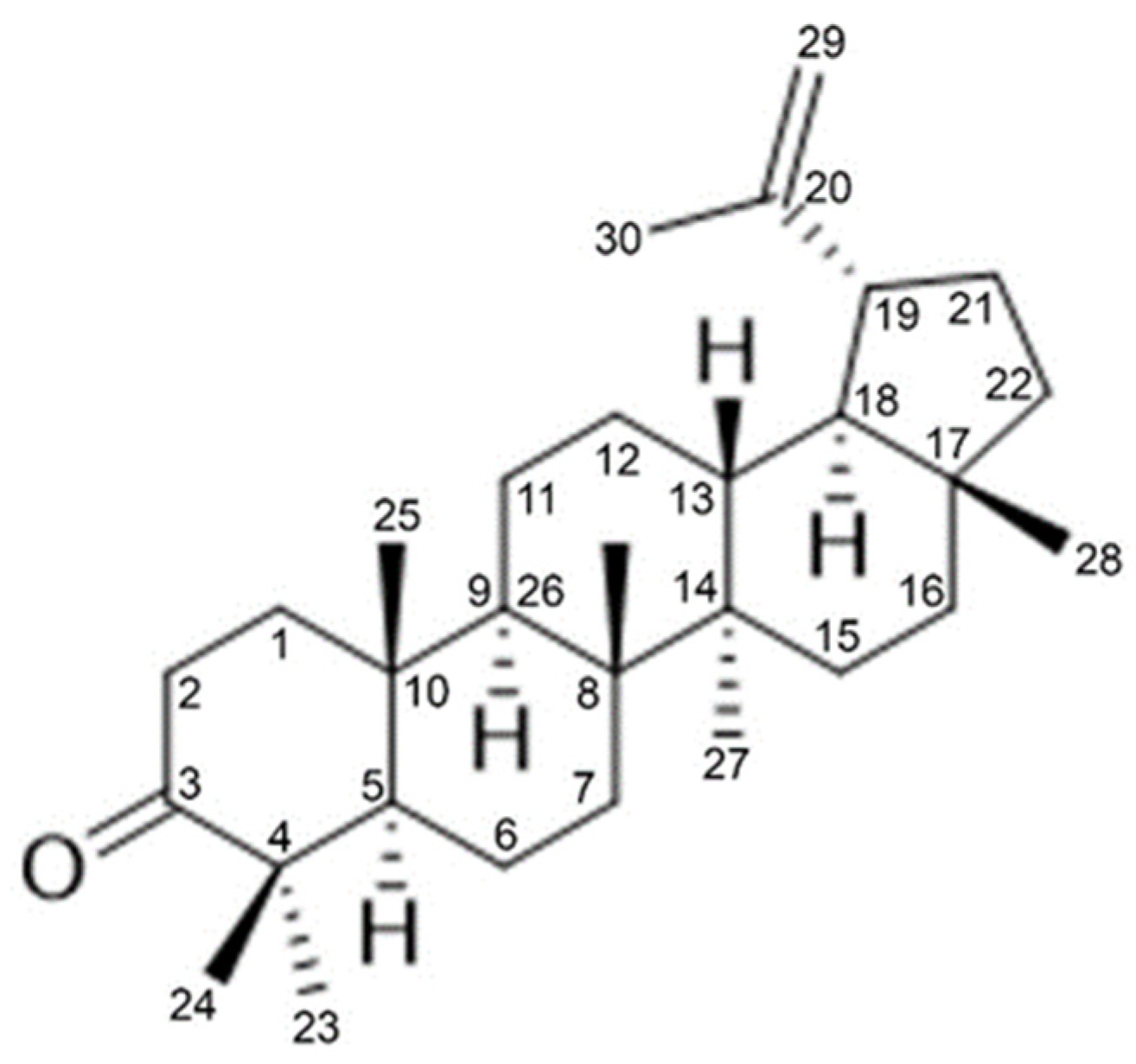


| No. | Solvent | Times | Volume (mL) | Fractions |
|---|---|---|---|---|
| 1. | 100% Hexane | 5 | 2500 | 1–5 |
| 2. | 1% Ethyl acetate/Hexane | 5 | 2500 | 6 –10 |
| 3. | 2% Ethyl acetate/Hexane | 5 | 2500 | 11–15 |
| 4. | 3% Ethyl acetate/Hexane | 6 | 3000 | 16–21 |
| 5. | 4% Ethyl acetate/Hexane | 7 | 3500 | 22–28 |
| 6. | 5% Ethyl acetate/Hexane | 7 | 3500 | 29–35 |
| 7. | 6% Ethyl acetate/Hexane | 6 | 3000 | 36–41 |
| 8. | 7% Ethyl acetate/Hexane | 5 | 2500 | 42–46 |
| 9. | 9% Ethyl acetate/Hexane | 3 | 1500 | 47–49 |
| 10. | 12% Ethyl acetate/Hexane | 3 | 1500 | 50–52 |
| 11. | 20% Ethyl acetate/Hexane | 3 | 1500 | 53–55 |
| 12. | 25% Ethyl acetate/Hexane | 3 | 1500 | 56–58 |
| 13. | 30% Ethyl acetate/Hexane | 3 | 1500 | 59–61 |
| 14. | 35% Ethyl acetate/Hexane | 3 | 1500 | 62–64 |
| 15. | 40% Ethyl acetate/Hexane | 2 | 1000 | 65–66 |
| 16. | 50% Ethyl acetate/Hexane | 3 | 1500 | 67–69 |
| 17. | 60% Ethyl acetate/Hexane | 3 | 1500 | 70–72 |
| 18. | 80% Ethyl acetate/Hexane | 3 | 1500 | 73–75 |
| 19. | 90% Acetate/Hexane | 3 | 1500 | 76–78 |
| 20. | 100% Ethyl acetate | 2 | 1000 | 79–80 |
| 21. | 2% Methanol/Ethyl acetate | 1 | 500 | 81 |
| 22. | 5% Methanol/Ethyl acetate | 1 | 500 | 82 |
| 23. | 10% Methanol/Ethyl acetate | 3 | 1500 | 83–85 |
| 24. | 100% Methanol | 2 | 1000 | 86–87 |
| Gene Symbol | Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| 18S | 18S ribosomal RNA | 5′-ACCGCAGCTAGGAATAATGGA-3′ | 5′-GCCTCAGTTCCGAAAACCA-3′ |
| Adipoq | Adiponectin | 5′-GATGCAGGTCTTCTTGGTCCTAA-3′ | 5′-GGCCCTTCAGCTCCTGTC-3′ |
| Cebpb | CCAAT/enhancer-binding protein beta | 5′-GCGCACCGGGTTTCG-3′ | 5′-GCGCTCAGCCACGTTTG-3′ |
| Cebpa | CCAAT/enhancer-binding protein alpha | 5′-GAGCCGAGATAAAGCCAAACA-3′ | 5′-CGGTCATTGTCACTGGTCAACT-3′ |
| Lep | Leptin | 5′-TCCCTGCCTCAGACCAGTG-3′ | 5′-TAGAGTGAGGCTTCCAGGACG-3′ |
| Nrf1 | Nuclear respiratory factor 1 | 5′-AGCACGGAGTGACCCAAAC -3′ | 5′-TGTACGTGGCTACATGGACCT -3′ |
| Pparg2 | Peroxisome proliferator activated receptor gramma 2 | 5′-TGTCGGTTTCAGAAGTGCCTTG-3′ | 5′-TTCAGCTGGTCGATATCACTGGAG-3′ |
| Ppargc1a | Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | 5′-ACAGCTTTCTGGGTGGATT-3′ | 5′-TGAGGACCGCTAGCAAGTTT-3′ |
| Ptgs1 | Prostaglandin-endoperoxide synthase 1 | 5′-GTGCTGGGGCAGTGCTGGAG-3′ | 5′-TGGGGCCTGAGTAGCCCGTG-3′ |
| Slc2a1 | Solute carrier family 2 (facilitated glucose transporter), member 1 | 5′-CCGCTTCCTGCTCATCAATC-3′ | 5′-CGACCCTCTTCTTTCATCTCC-3′ |
| Slc2a4 | Solute carrier family 2 (facilitated glucose transporter), member 4 | 5′-GTGACTGGAACACTGGTCCTA-3′ | 5′-CCAGCCAGTTGCATTGTAG-3′ |
| Tfam | Transcription factor A mitochondrial | 5′-GAGGCCAGTGTGAACCAGTG -3′ | 5′-GTAGTGCCTGCTGCTCCTGA -3′ |
| Ucp1 | Uncoupling protein 1 | 5′-GGCATTCAGAGGCAAATCAGCT-3′ | 5′-CAATGAACACTGCCACACCTC-3′ |
| Fractions | Weight (g) | % Yield |
|---|---|---|
| 1H | 1.6752 | 0.28 |
| 2H | 0.7629 | 0.13 |
| 3H | 0.2637 | 0.04 |
| 4H | 0.0830 | 0.01 |
| 5H | 0.7585 | 0.13 |
| 6H | 0.3403 | 0.06 |
| 7H | 0.2472 | 0.04 |
| 8H | 0.5413 | 0.09 |
| 9H | 0.0840 | 0.01 |
| 10H | 0.1143 | 0.02 |
| 11H | 0.0864 | 0.01 |
| 12H | 0.2380 | 0.04 |
| 13H | 0.2762 | 0.05 |
| 14H | 0.8879 | 0.15 |
| 15H | 0.2510 | 0.04 |
| 16H | 0.3697 | 0.06 |
| 17H | 1.8605 | 0.31 |
| 18H | 0.4872 | 0.08 |
| 19H | 0.0799 | 0.01 |
| 20H | 0.7666 | 0.13 |
| 21H | 0.1261 | 0.02 |
| 22H | 0.0888 | 0.01 |
| 23H | 0.0729 | 0.01 |
| 24H | 0.0503 | 0.01 |
| 25H | 0.0700 | 0.01 |
| 26H | 0.1846 | 0.03 |
| 27H | 0.2121 | 0.04 |
| 28H | 0.2961 | 0.05 |
| 29H | 1.4841 | 0.25 |
| 30H | 0.2945 | 0.05 |
| Position | Lupenone [21] | Lu-CQ | ||
|---|---|---|---|---|
| δH (ppm) (CDCl3, 300 MHz) | δC (ppm) (CDCl3, 75 MHz) | δH (ppm) (CDCl3, 300 MHz) | δC (ppm) (CDCl3, 75 MHz) | |
| 1 | - | 34.15 | - | 34.14 |
| 2 | - | 39.58 | - | 39.61 |
| 3 | - | 218.32 | - | 218.18 |
| 4 | - | 47.3 | - | 47.32 |
| 5 | - | 54.87 | - | 54.91 |
| 6 | - | 19.65 | - | 19.67 |
| 7 | - | 33.52 | - | 33.56 |
| 8 | - | 40.74 | - | 40.77 |
| 9 | - | 49.75 | - | 49.78 |
| 10 | - | 36.85 | - | 36.87 |
| 11 | - | 21.43 | - | 21.46 |
| 12 | - | 25.09 | - | 25.14 |
| 13 | - | 38.12 | - | 38.16 |
| 14 | - | 42.97 | - | 42.98 |
| 15 | - | 27.39 | - | 27.42 |
| 16 | - | 35.48 | - | 35.51 |
| 17 | - | 42.87 | - | 42.88 |
| 18 | - | 48.19 | - | 48.23 |
| 19 | - | 47.93 | - | 47.95 |
| 20 | - | 150.89 | - | 150.84 |
| 21 | - | 29.81 | - | 29.82 |
| 22 | - | 39.94 | - | 39.97 |
| 23 | 1.03 (3H, s, H-23) | 26.6 | 1.03 (3H, s, H-23) | 26.64 |
| 24 | 1.07 (6H, s, H-24) | 21.02 | 1.07 (6H, s, H-24) | 21.03 |
| 25 | 0.93 (3H, s, H-25) | 15.96 | 0.93 (3H, s, H-25) | 15.97 |
| 26 | 1.07 (6H, s, H-26) | 15.75 | 1.07 (6H, s, H-26) | 15.78 |
| 27 | 0.96 (3H, s, H-27) | 14.45 | 0.96 (3H, s, H-27) | 14.47 |
| 28 | 0.80 (3H, s, H-28) | 17.99 | 0.80 (3H, s, H-28) | 18.01 |
| 29 | 4.70 (1H, brs, H-29a), 4.58 (1H, brs, H-29b) | 109.38 | 4.69 (1H, d, J = 2.1 Hz, H-29a), 4.57 (1H, d, J = 0.84 Hz, H-29b) | 109.40 |
| 30 | 1.68 (3H, brs, H-30) | 19.19 | 1.69 (3H, brs, H-30) | 19.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakthan, T.; Limpachayaporn, P.; Rayanil, K.-o.; Charoenpanich, P.; Phuangbubpha, P.; Charoenpanich, A. Lupenone-Rich Fraction Derived from Cissus quadrangularis L. Suppresses Lipid Accumulation in 3T3-L1 Adipocytes. Life 2023, 13, 1724. https://doi.org/10.3390/life13081724
Lakthan T, Limpachayaporn P, Rayanil K-o, Charoenpanich P, Phuangbubpha P, Charoenpanich A. Lupenone-Rich Fraction Derived from Cissus quadrangularis L. Suppresses Lipid Accumulation in 3T3-L1 Adipocytes. Life. 2023; 13(8):1724. https://doi.org/10.3390/life13081724
Chicago/Turabian StyleLakthan, Thitiporn, Panupun Limpachayaporn, Kanok-on Rayanil, Pornsri Charoenpanich, Pornwipa Phuangbubpha, and Adisri Charoenpanich. 2023. "Lupenone-Rich Fraction Derived from Cissus quadrangularis L. Suppresses Lipid Accumulation in 3T3-L1 Adipocytes" Life 13, no. 8: 1724. https://doi.org/10.3390/life13081724
APA StyleLakthan, T., Limpachayaporn, P., Rayanil, K.-o., Charoenpanich, P., Phuangbubpha, P., & Charoenpanich, A. (2023). Lupenone-Rich Fraction Derived from Cissus quadrangularis L. Suppresses Lipid Accumulation in 3T3-L1 Adipocytes. Life, 13(8), 1724. https://doi.org/10.3390/life13081724






