Spore Evidence for the Origin of Isoetalean Lycopsids?
Abstract
1. Introduction
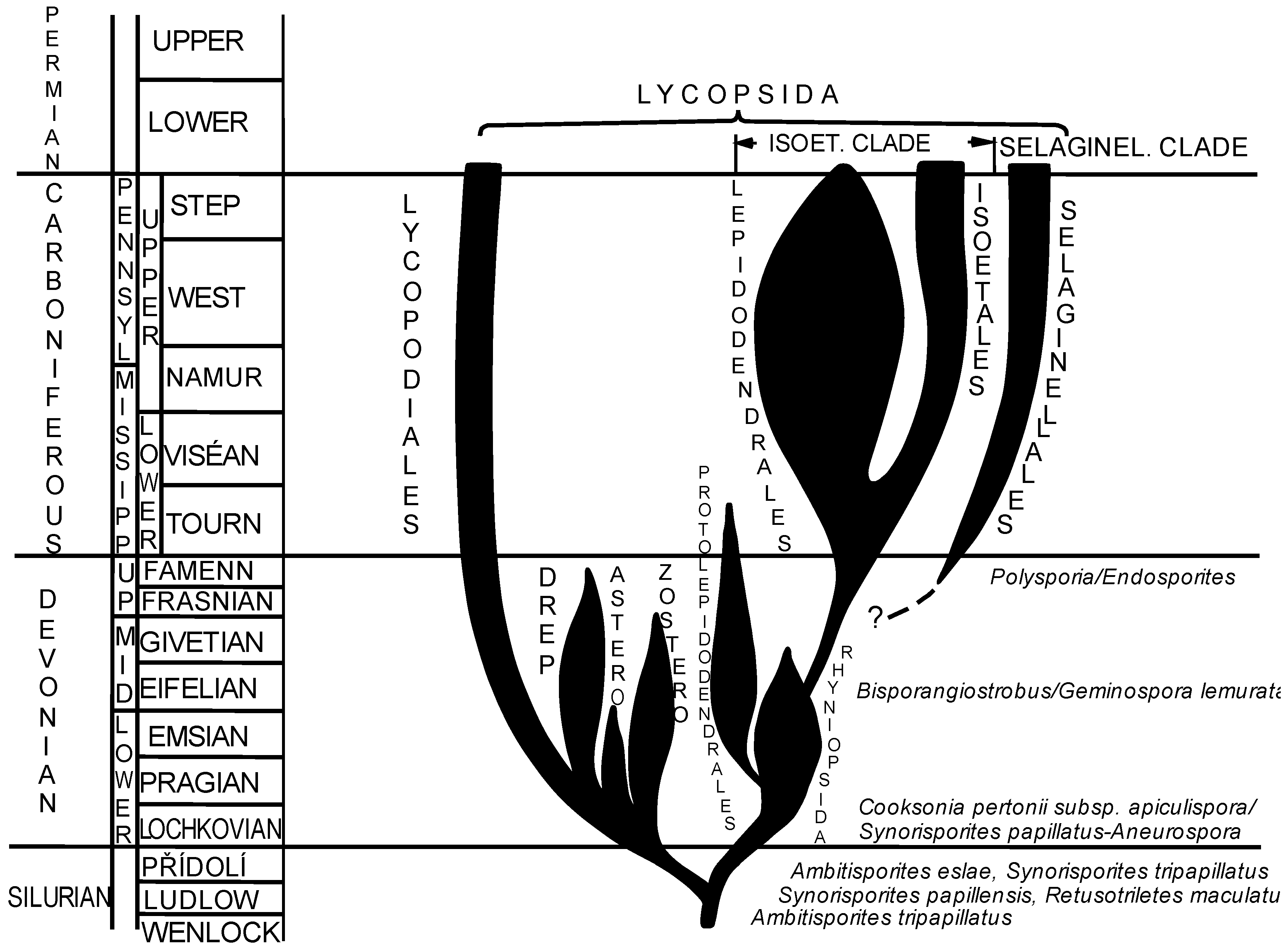
1.1. Isoetalean Clade
1.2. Selaginellalean Clade
1.3. Lycopodialean Clade
1.4. Protolepidodendrales
1.5. Rhyniopsida
2. Material and Methods
3. Results
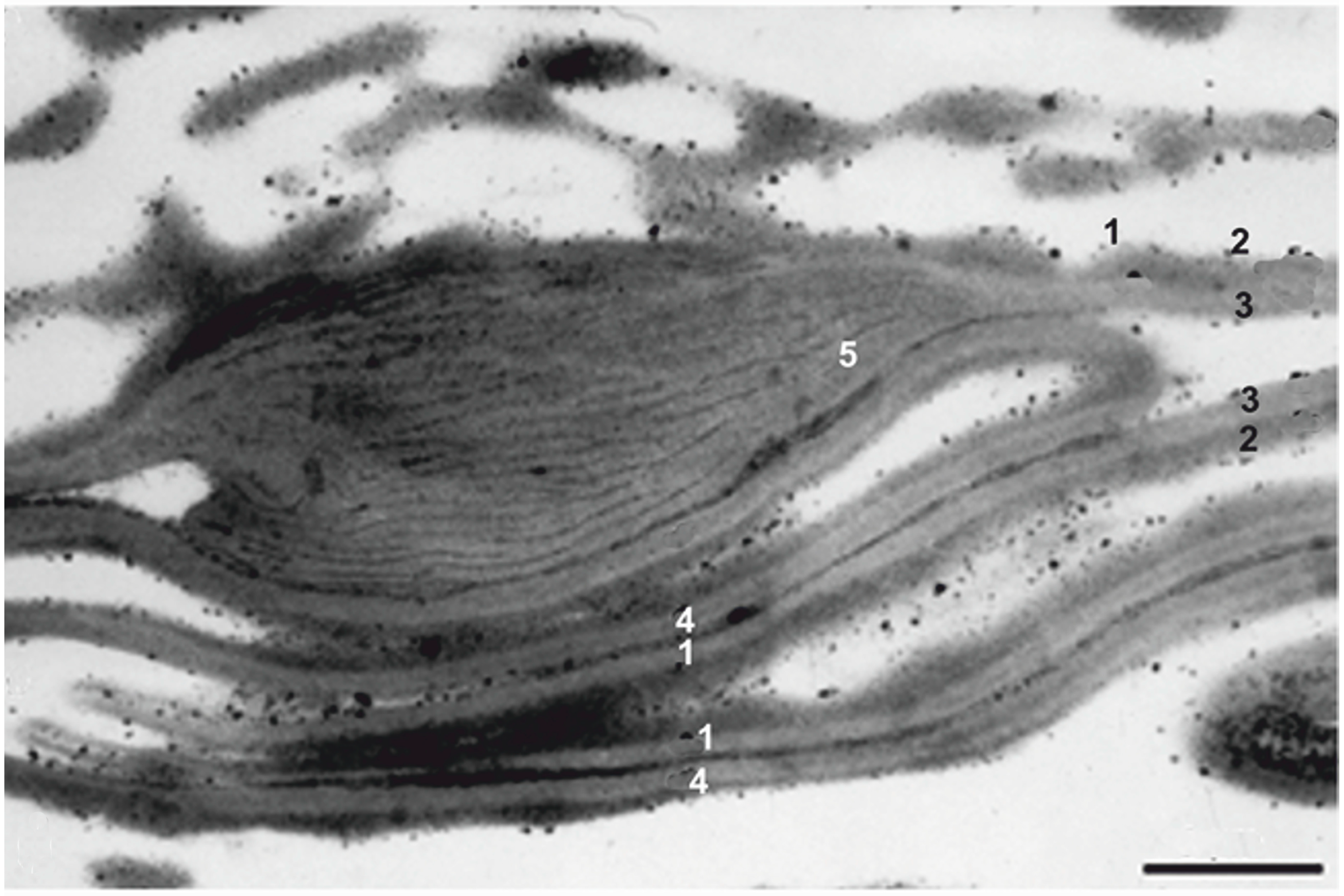
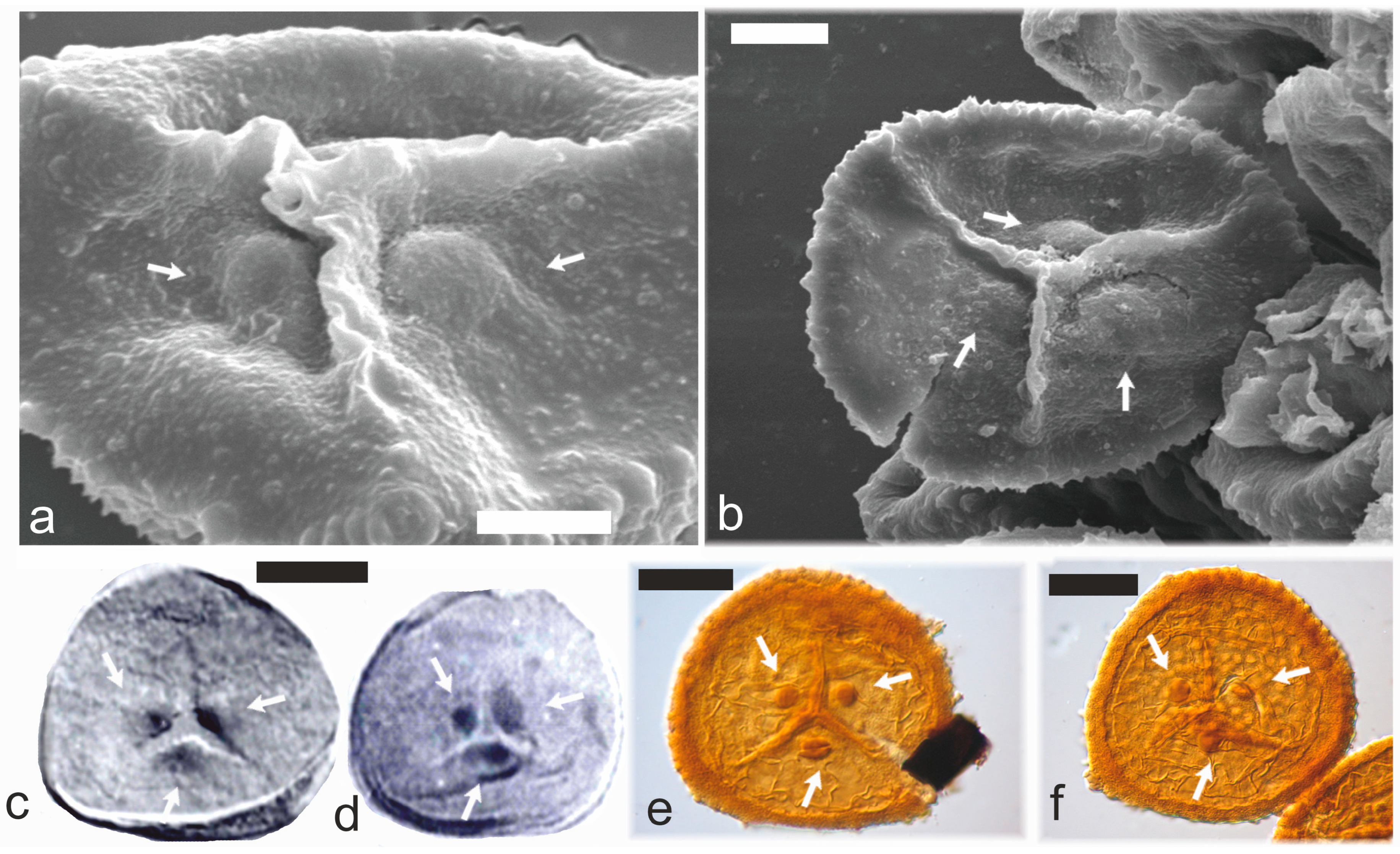
3.1. Apical Papillae/Laminated Zones (TAP/LZ)
3.2. Dispersed TAP Spores
3.3. Botanical Affinity of TAP Spores
3.4. New Approach to Phylogeny of Paleozoic Isoetalean Lycopsids Based on Palynological Evidence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, W.N.; Rothwell, G.R. Paleobotany and the Evolution of Plants, 2nd ed.; Cambridge University Press: Singapore, 2010; 536p. [Google Scholar]
- Taylor, T.N.; Taylor, E.L.; Krings, M. Paleobotany: The Biology and Evolution of Fossil Plants; Academic Press: New York, NY, USA, 2009; 1230p. [Google Scholar]
- Thomas, B.A.; Cleal, C.J. Arborescent lycophyte growth in the late Carboniferous coal swamps. New Phytol. 2018, 218, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, C.S.; Geng, B.Y.; Chitaley, S. A new species of Lepidostrobus from the Upper Devonian of Xinjiang, China and its bearing on the phylogenetic significance of the order Isoëtales. Bot. J. Linn. Soc. 2003, 143, 55–67. [Google Scholar] [CrossRef]
- Thomas, B.A. Paleozoic herbaceous lycopsids and the beginning of the extant genera Lycopodium and Selaginella. Ann. Miss. Bot. Gard. 1992, 79, 129–153. [Google Scholar] [CrossRef]
- Simpson, M.G. Evolution and diversity of vascular plants. In Plant Systematics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; 744p. [Google Scholar]
- Bek, J. Paleozoic in situ spores and pollen. Lycopsida. Palaeontographica B 2017, 296, 1–199. [Google Scholar] [CrossRef]
- Libertín, M.; Kvaček, J.; Bek, J.; Žárský, V.; Štorch, P. Sporophytes of polysporangiate land plants from the early Silurian period may have been photosynthetically autonomous. Nat. Plants 2018, 4, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gonez, P.; Gerrienne, P. A new definition and a lectotypification of the genus Cooksonia Lang 1937. Int. J. Pl. Sci. 2010, 171, 199–215. [Google Scholar] [CrossRef]
- Fanning, U.; Richardson, J.B.; Edwards, D. Cryptic evolution in an early land plant. Evol. Trends Pl. 1988, 2, 13–24. [Google Scholar]
- Edwards, D.; Davies, K.L.; Richardson, J.B.; Axe, L. The ultrasctucture of of spores of Cooksonia pertonii. Palaeontologia 1995, 38, 153–168. [Google Scholar]
- Fanning, U.; Richardson, J.B.; Edwards, D. A review of in situ spores in Silurian land plants. In Pollen and Spores: Patterns of Diversification, Systematics Association; Blackmore, S., Barnes, S.H., Eds.; The Systematics Association (Clarendon Press): Oxford, UK, 1991; Volume, 44, pp. 25–47. [Google Scholar]
- Gensel, P.G. Devonian in situ spores: A survey and discussion. Rev. Palaeobot. Palynol. 1980, 30, 101–132. [Google Scholar] [CrossRef]
- Allen, K.C. A review of in situ late Silurian and Devonian spores. Rev. Palaeobot. Palynol. 1980, 29, 253–270. [Google Scholar] [CrossRef]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; LeThomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Neuburg, M.F. Recent data on the morphology of Pleuromeia Corda from the Lower Triassic of the Russian Platform. Dokl. Akad. Nauk S.S.S.R. 1961, 136, 200–203. [Google Scholar]
- Magdefrau, K. Zur Morfologie und phylogenetischen Bedeutung der fossilen Pflanzengattung Pleuromeia. Beih. Bot. Zbl. 1931, 48, 119–140. [Google Scholar]
- Grauvogel-Stamm, L.; Lugardon, B. The Triassic lycopsids Pleuromeia and Annalepis: Relationships, evolution, and origin. Amer. Fern J. 2001, 91, 115–149. [Google Scholar] [CrossRef]
- Grauvogel-Stamm, L.; Lugardon, B. The spores of the Triassic lycopsid Pleuromeia sternbergii (Munster) Corda: Morphology, ultrastructure, phylogenetic implications and chronostratigraphic inferences. Int. J. Pl. Sci. 2004, 165, 631–650. [Google Scholar] [CrossRef]
- Bek, J.; Chitaley, S.; Grauvogel-Stamm, L. Occurrence of spores from an isoetalean lycopsid of the Polysporia-type in the Late Devonian of Ohio, USA. Rev. Palaeobot. Palynol. 2009, 156, 34–50. [Google Scholar] [CrossRef]
- Lugardon, B.; Grauvogel-Stamm, L.; Dobrushkina, I. The microspores of Pleuromeia rossica Neuburg (Lycopsida, Triassic): Comparative ultrastructure and phylogenetic implications. C. R. Acad. Sci. Paris 1999, 329, 435–444. [Google Scholar] [CrossRef]
- Lugardon, B.; Grauvogel-Stamm, L.; Dobrushkina, I. Comparative ultrastructure of the megaspores of the Triassic lycopsid Pleuromeia rossica Neuburg. C. R. Acad. Sci. 2000, 330, 505–508. [Google Scholar] [CrossRef]
- Grauvogel-Stamm, L.; Lugardon, B.; Zavialova, N. Microspores of the Middle Triassic lycopsid Lepacyclotes (syn. Annalepis) zeilleri: Morphology, ultrastructure, laminated zones and comments about the lycopsid evolution. Rev. Palaeobot. Palynol. 2022, 301, 104642. [Google Scholar] [CrossRef]
- Naugolnykh, S.V.; Zavialova, N. Densosporites polaznaensis sp. nov. with comments on its relation to Viatcheslavia vorcutensis Zalessky. Palaeobotanist 2004, 53, 21–33. [Google Scholar]
- Lopez, G.; Perreira, Z.; Fernandez, P.; Picarra, M.; Oliveira, T.J. Silurian to Lower Devonian palynomorphs from the Barrancos region, Ossa Morena zone, Portugal. In Proceedings of the CIMP Faro’09 II Joint Meeting of Spores/Pollen and Acritarch Subcommissions, Faro, Portugal, 20–24 September 2009; pp. 63–68. [Google Scholar]
- Wellman, C.; Steemans, P.; Vecoli, M. Paleophytogeography of Ordovician–Silurian land plants. Geol. Soc. London Mem. 2015, 38, 461–476. [Google Scholar] [CrossRef]
- Mehlqvist, K.; Steemans, P.; Vajda, V. First evidence of Devonian strata in Sweden—A palynological investigation of Övedskloster drillcores 1 and 2, Skåne, Sweden. Rev. Palaeobot. Palynol. 2015, 221, 144–159. [Google Scholar] [CrossRef]
- Rubinstein, C.; Steemans, P. New palynological data from the Devonian Villavicencio Formation, Precordillera of Mendoza, Argentina. Amegh. 2007, 44, 3–9. [Google Scholar]
- Kermandji, A.M.H. Silurian-Devonian miospores from the western and central Algeria. Rev. Micropaléontol. 2007, 50, 109–128. [Google Scholar] [CrossRef]
- Rubinstein, C.; Steemans, P. Spore assemblages from the Silurian–Devonian boundary, in A1-61 borehole, Ghadames Basin, Libya. Rev. Palaeobot. Palynol. 2002, 118, 397–421. [Google Scholar] [CrossRef]
- Steemans, P.; Rubinstein, C.; Mélo, J.H. Siluro–Devonian spore biostratigraphy of the Urubu River area, western Amazon Basin, northern Brazil. Geobios 2008, 41, 263–282. [Google Scholar] [CrossRef]
- Cramer, F.H.; Diez, J.B. Earliest Devonian spores from the Province of Leon, Spain. Pollen Spores 1975, 17, 331–344. [Google Scholar]
- Rodriguez, R.M. Miosporas de la Formacion San Pedro/Furada, (Silurico superior–Devonico Inferior), Cordillera Cantabrica, NO de Espana. Palinology 1978, 1, 407–433. [Google Scholar]
- Richardson, J.B.; Rodriguez, R.M.; Sutherland, S.J. Palynological zonation of Mid-Palaeozoic sequences from the Cantabrian Mountains, NW Spain: Implications for inter-regional and interfacies correlation of the Ludford/Prıdolı and Silurian/Devonian boundaries, and plant dispersal patterns. Bull. Nat. Hist. Mus. Geol. 2001, 57, 115–162. [Google Scholar]
- Le Herissé, A. Les spores du Dévonien inférieur du Synclinorium de Laval (Massif Armoricain). Palaeontographica B 1983, 188, 1–81. [Google Scholar]
- Breuer, P.; Steemans, P. Devonian Spore Assemblages from Northwestern Gondwana: Taxonomy and Biostratigraphy. Spec. Pap. Palaeontol. 2013, 89, 5–163. [Google Scholar]
- Muro, G.V.J.; Rubinstein, C.V.; Steemans, P. Silurian miospores from the Precordillera Basin, Argentina: Biostratigraphic, palaeonvironmental and palaeogeographic implications. Geol. Mag. 2014, 151, 472–490. [Google Scholar] [CrossRef]
- Holland, C.B.; Richardson, J.B. The British Isles. In The Silurian–Devonian Boundary; Martinsson, A., Ed.; International Union of Geophysical Sciences Series A No. 5. E; Schweitzerbarthische Verlagsbuchhandlung: Stuttgart, Germany, 1977; pp. 35–44. [Google Scholar]
- Steemans, P. Palynostratigraphie de l’Dévonien dans l’ouest de l’Europe. Service Géologique de Belgique. Mém. Eplicat. Cart. Géol. Min. Belg. 1989, 27, 453. [Google Scholar]
- Rubinstein, C.; Melo, J.H.; Steemans, P. Lochkovian (earliest Devonian) spores from the Solimoes Basin, northwestern Brazil. Rev. Palaeobot. Palynol. 2005, 133, 91–113. [Google Scholar] [CrossRef]
- Mendlowicz, P.; Machado-Cardoso, K.B.; Pereira, T.R.; Steemans, P. Resultados Palinoestratigráficos do Devoniano da Sub-Bacia de Alto Garcías (Bacia do Paranza–Brasil). In Paleontologia: Cenários de Vida; Carvalho, I.S., Cassa, B.R.C.T., Schwanke, C., Carvalho, M.A., Fernandes, A.C., Eds.; Interciência: Rio de Janeiro, Brazil, 2007; pp. 607–619. [Google Scholar]
- McGregor, C.D.; Owens, B. Devonian spores of eastern and northern Canada. Geol. Surv. Can. Pap. 1996, 66, 1–66. [Google Scholar]
- McGregor, C.D. Lower and Middle Devonian spores of Eastern Gaspé, Canada. I. Systematics. Palaeontographica B 1973, 142, 1–77. [Google Scholar]
- McGregor, C.D.; Camfield, M.M. Upper Silurian to Middle Devonian spores of the Moose River Basin, Ontario. Geol. Surv. Can. Bull. 1976, 263, 1–63. [Google Scholar]
- Richardson, J.B.; Lister, R.T. Upper Silurian and Lower Devonian spore assemblages from the Welsh Borderland and South Wales. Palaeontol. 1969, 12, 201–252. [Google Scholar]
- Al-Ghazí, A. New evidence for the Early Devonian age of the Jauf Formation in northern Saudi Arabia. Rev. Micropaléontol. 2007, 50, 59–72. [Google Scholar] [CrossRef]
- Moreau-Benoit, A. Les spores du Dévonien de Libye. Premiére partie. Cah. Micropaléontol. 1979, 4, 1–58. [Google Scholar]
- Turnau, E.; Milaczewski, L.I.; Wood, G.D. Spore stratigraphy of Lower Devonian and Eifelian, alluvial and marginal marine deposits of the Radom-Lublin area (central Poland). Ann. Soc. Geol. Pol. 2005, 75, 121–137. [Google Scholar]
- McGregor, C.D. Late Silurian and Devonian spores from Bolivia. Acad. Nac. Cien. 1984, 69, 1–75. [Google Scholar]
- Perez-Leyton, M.A. Spores du Dévonien Moyen et Supérieur de la coupe de Bermejo-La Angostura (Sud-Est de la Bolivie). Ann. Soc. Géol. Belg. 1990, 113, 373–389. [Google Scholar]
- Mortimer, M.G. Some lower Devonian microfloras from southern Britain. Rev. Palaeobot. Palynol. 1967, 1, 95–109. [Google Scholar] [CrossRef]
- LeHerisse, A. Lower Devonian spores of the Laval Synclinorium (Massif Armoricain). Palaeontographica A 1983, 188, 1–81. [Google Scholar]
- Delsate, D.; Blieck, A.; Steemans, P. Preliminary report of Lower to Middle Emsian (Lower Devonian) flora and fauna from Consthum and Merkholtz (Grand Duchy of Luxembourg) with Porolepid (Sarcopterygii) and Heterostracan Fish remains. Geol. Belg. 2003, 7, 21–26. [Google Scholar]
- McGregor, D.C.; Playford, G. Canadian and Australian Devonian Spores: Zonation and Correlation; Geological Survey of Canada: St. Ottawa, ON, Canada, 1992; Volume 125. [Google Scholar]
- Loboziak, S.; Streel, M. Late Lower and Middle Devonian miospores from Saudi Arabia. Rev. Palaeobot. Palynol. 1992, 89, 105–113. [Google Scholar] [CrossRef]
- Moreau-Benoit, A. Palynological datation the beds enclosing the plomb-zinc mineralization in the Weiss and Luderich Mines, (Lower Devonian, Bergishes Land, Rhine Schist Massif). C. R. Acad. Sci. 1985, 300, 295–300. [Google Scholar]
- Arkhangelskaya, A.D. Spores from Lower and Middle Devonian deposits of the Russian Plate. In Atlas of Spores and Pollen of Phanerozoic Oil- and Gas-Bearing Strata of the Russian and Turanian; Menner, V.V., Byvsheva, T.V., Eds.; VTrudy Vsesoiuznogo Nauchno-Issledovatel’skogo Geologorazvedochnogo Neftianogo Instituta (VNIGNI): Moscow, Russia, 1985; Volume 253, pp. 32–80. (In Russian) [Google Scholar]
- Richardson, J.B.; McGregor, D.C. Silurian and Devonian spore zones of the Old Red Sandstone Continent and adjacent regions. Geol. Surv. Can. Bull. 1986, 364, 1–79. [Google Scholar]
- Ravn, R.L.; Benson, D.G. Devonian miospores and reworked acritarchs from southeastern Georgia, USA. Palynology 1988, 12, 179–200. [Google Scholar] [CrossRef]
- Loboziak, S.; Streel, M.; Caputo, M.V.; Melo, J.H.G. Middle Devonian to Lower Carboniferous miospore stratigraphy in the Central Parnaiba Basin (Brazil). Ann. Soc. Géol. Belg. 1988, 115, 215–226. [Google Scholar]
- McGregor, D.C. Devonian spores from the Barrandian Region of Czechoslovakia and their significance for Interfacies correlation. Geol. Surv. Can. Pap. 1979, 79, 189–197. [Google Scholar]
- Ouyang, S. Microfossils from the Devonian Heitai Formation of Mishan County, Heilungjiang Province. Acta Palaeontol. Sin. 1984, 23, 69–84, (In Chinese with English 1324 summary). [Google Scholar]
- Marshall, J.E.A.; Fletcher, T.P. Middle Devonian (Eifelian) spores from a fluvial dominated lake margin in the Orcadian Basin, Scotland. Rev. Palaeobot. Palynol. 2002, 118, 195–209. [Google Scholar] [CrossRef]
- Loboziak, S.; Caputo, M.V.; Melo, J.H.G. Middle Devonian—Tournaisian miospores biostratigrahy in the southwestern outcrop belt of the Parnaiba Basin, north-central Brazil. Rev. Micropaléontol. 2000, 43, 301–318. [Google Scholar] [CrossRef]
- Blieck, A.; Gagnier, P.I.; Bigey, F.P.; Edgecombe, G.D.; Janvier, P.; Loboziak, S.; Rachebeuf, P.R.; Sempere, T.; Steemans, P. New Devonian fossil localities in Bolivia. J. South Amer. Earth Sci. 1996, 9, 295–308. [Google Scholar] [CrossRef]
- Turnau, E. Miospore stratigraphy of Middle Devonian deposits from Western Pomerania. Rev. Palaeobot. Palynol. 1996, 93, 107–125. [Google Scholar] [CrossRef]
- Uyeno, T.T. Biostratigraphy and Conodont Faunas of Upper Ordovician through Middle Devonian Rocks, Eastern Arctic Archipelagon; Geological Survey of Canada: St. Ottawa, ON, Canada, 1990; Volume 210. [Google Scholar]
- Loboziak, S.; Streel, M. Some new data on the Devonian miospores of the Parana Basin (Brazil). Sci. Géol. Bull. 1987, 44, 381–391. [Google Scholar]
- Burjack, M.I.A.; Loboziak, S.; Streel, M. Quelques donne´es nouvelles sur les miospores de´voniennes du basin Paraná (Brasil). Sci. Geol. 1987, 40, 381–391. [Google Scholar]
- McGregor, D.C.; Uyeno, T.T. Devonian spores and conodonts of Melville and Bathurst Island, District of Franklin. Geol. Surv. Can. Pap. 1972, 71, 1–37. [Google Scholar]
- McGregor, D.C. Devonian miospores of North America. Palynology 1979, 3, 31–52. [Google Scholar] [CrossRef]
- Marshall, J.E.A.; Allen, K.C. Devonian miospore assemblages from Fair Isle, Shetland. Palaeontology 1992, 25, 277–312. [Google Scholar]
- Su, Y. A second time study of the Devonian “Heitai Formation” in the type locality of eastern Heilongjiang Province. Bull. Shen. Inst. Geol. Miner. Res. 1983, 6, 1–10. [Google Scholar]
- Grey, K.A. A mid-Givetian miospore age for the onset of reef development on the Lennard Shelf, Canning Basin, Western Australia. Rev. Palaeobot. Palynol. 1991, 68, 37–48. [Google Scholar] [CrossRef]
- Bek, J.; Drábková, J.; Dašková, J.; Libertín, M. The sub-arborescent lycopsid genus Polysporia Newberry and its spores from the Pennsylvanian (Bolsovian–Stephanian B) continental basins of the Czech Republic. Rev. Palaeobot. Palynol. 2008, 152, 176–199. [Google Scholar] [CrossRef]
- Somers, Y.; Alpern, B.; Doubinger, J.; Grebe, H. Revision du genre Lycospora Schopf, Wilson & Bentall. In Les Spores; Alpern, B., Streel, M., Eds.; Microfossiles organiques du Paléozoïque; Centre National de la Recherche Scientifique (CNRS): Paris, France, 1972; Volume 5, pp. 9–110. [Google Scholar]
- Ravn, R.L. An Introduction to the Stratigraphic Palynology of the Cherokee Group (Pennsylvanian) Coals of Iowa; Iowa Geological Survey Technical Paper; Iowa Geological Survey: Iowa City, IA, USA, 1986; Volume 6, pp. 1–117. [Google Scholar]
- Bek, J. Spore Populations of Some Plants of Groups Lycophyta, Sphenophyta, Pteridophyta and Progymnospermophyta from Carboniferous Limnic Basins of the Czech Republic. PhD Thesis, Geological Institute of the Academy of Sciences of the Czech Republic, Prague, Czech Republic, 1998; pp. 1–505, (In Czech with English summary). [Google Scholar]
- Molyneux, S.G.; Manger, W.L.; Owens, B. Preliminary account of Late Devonian palynomorph assemblages from the Bedford Shale and Berea Sandstone Formations of central Ohio, USA. J. Micropalaeontol. 1994, 3, 41–51. [Google Scholar] [CrossRef]
- Amenabar, C.M.; di Pasquo, M.; Carrizo, H.A.; Azcuy, C.S. Palynology of the Chigua (Devonian) and Malimán (Carboniferous) formations in the Volcán Range, San Juan Province, Argentina. Part II. Cavate, pseudosaccate and cingulizonate spores. Ameghiniana 2007, 44, 547–564. [Google Scholar]
- Smith, A.H.V.; Butterworth, M.A. Miospores in the coal seams of the Carboniferous of Great Britain. Spec. Pap. Palaeontol. 1967, 1, 1–324. [Google Scholar]
- Bharadwaj, D.C. On Porostrobus zeilleri Nathorst and its spores with remarks on the systematic position of P. benholdii Bode and the phylogeny of Densosporites Berry. Palaeobotanist 1957, 7, 67–75. [Google Scholar]
- Potonié, R.; Kremp, W. Die Sporae dispersae des Ruhrkarbons ihre Morphographie and Stratigraphie mit Ausblicken auf Arten anderer Gebiete und Zeitabschnitte. Teil I. Palaeontographica B 1955, 98, 65–121. [Google Scholar]
- Peppers, R.A. Spores in Strata of Late Pennsylvanian cyclothems in the Illinois Basin. Ill. St. Geol. Surv. Bull. 1964, 90, 1–89. [Google Scholar]
- Bek, J.; Pšenička, J.; Drábková, J.; Zhou, W.M.; Wang, J. Thomasites gen. nov. a new herbaceous lycophyte and its spores from late Duckmantian of the Radnice Basin, Czech Republic and palynological grouping of Palaeozoic herbaceous lycophytes. Rev. Palaeobot. Palynol. 2023, 310, 104842. [Google Scholar] [CrossRef]
- Chitaley, S.; McGregor, C.D. Bisporangiostrobus harrissii gen. et sp. nov., an eligulate lycopsid cone with Duosporites megaspores and Geminospora microspores from the Upper Devonian of Pennsylvanian, USA. Palaeontographica B 1989, 210, 127–149. [Google Scholar]
- Grauvogel-Stamm, L.; Langiaux, J. Polysporia doubingeri n. sp., un nouvel organe reproducteur de Lycophyte du Stéphanien (Carbonifère supérieur) de Blanzy Montceau (Massif Central, France). Sci. Géol. Bull. 1995, 48, 63–81. [Google Scholar]
- Courvoisier, J.M.; Phillips, T.L. Correlation of spores from Pennsylvanian coal-ball fructifications with dispersed spores. Micropaleontology 1975, 21, 45–49. [Google Scholar] [CrossRef]
- Bharadwaj, D.C. The palynological investigations of the Saar coals. Part I. Monography of sporae dispersae. Palaeontographica B 1957, 101, 73–125. [Google Scholar]
- Balme, B.A. Fossil in situ spores and pollen grains: An annotated catalogue. Rev. Palaeobot. Palynol. 1995, 87, 81–323. [Google Scholar] [CrossRef]
- Naugolnykh, S.V. The heterosporous lycopodiophyte Pleuromeia rossica Neuburg, 1960 from the Lower Triassic of the Volga River basin (Russia): Organography and recontruction according to the “Whole-plant” concept. Wulfenia 2013, 29, 1–16. [Google Scholar]
- Pigg, K.B. Evolution of isoetalean lycopsids. Ann. Miss. Bot. Gard. 1992, 79, 589–612. [Google Scholar] [CrossRef]

| Parent Plant | In Situ Spores | Stratigraphy | References |
|---|---|---|---|
| Cooksonia pertoni subsp. pertoni | Ambitisporites | Lochkovian | [10] |
| C. pertoni subsp. synorispora | Synorisporites verrucatus | Přídolí | [10] |
| C. pertoni subsp. apiculispora | Streelispora newportensis/Aneurospora | Lochkovian | [11] |
| Caia langii | Retusotriletes | Přídolí | [11] |
| Cooksonia cambrensis | Ambitisporites | Přídolí | [12] |
| Pertonella dactylethra | Retusotriletes coronatus | Přídolí | [12] |
| Renalia hueberi | Retusotriletes/Apiculiretusispora | Lochkovian | [13] |
| Salopella allenii | Retusotriletes | Přídolí | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bek, J.; Frojdová, J.V. Spore Evidence for the Origin of Isoetalean Lycopsids? Life 2023, 13, 1546. https://doi.org/10.3390/life13071546
Bek J, Frojdová JV. Spore Evidence for the Origin of Isoetalean Lycopsids? Life. 2023; 13(7):1546. https://doi.org/10.3390/life13071546
Chicago/Turabian StyleBek, Jiří, and Jana Votočková Frojdová. 2023. "Spore Evidence for the Origin of Isoetalean Lycopsids?" Life 13, no. 7: 1546. https://doi.org/10.3390/life13071546
APA StyleBek, J., & Frojdová, J. V. (2023). Spore Evidence for the Origin of Isoetalean Lycopsids? Life, 13(7), 1546. https://doi.org/10.3390/life13071546







