Antimicrobially Active Zn(II) Complexes of Reduced Schiff Bases Derived from Cyclohexane-1,2-diamine and Fluorinated Benzaldehydes—Synthesis, Crystal Structure and Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Schiff Bases and Reduced Schiff Bases
2.2.1. General Synthetic Procedure for the Preparation of Schiff Bases
2.2.2. General Synthetic Procedure for the Preparation of Reduced Schiff Bases
2.3. Synthesis of the Zinc Complexes
2.4. X-ray Structure Determination
2.5. Antimicrobial Activity
3. Results and Discussion
3.1. Synthesis
3.2. Analytical Characterization
3.3. X-ray Crystallography
3.4. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Dąbrowski, J.M.; Mazuryk, O.; Śpiewak, K.; Kyzioł, A.; Pucelik, B.; Brindell, M.; Stochel, G. Bioinorganic antimicrobial strategies in the resistance era. Coord. Chem. Rev. 2017, 351, 76–117. [Google Scholar] [CrossRef]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff bases and their metal complexes: A review on the history, synthesis, and applications. Inorg. Chem. Comm. 2023, 150, 110451. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef] [PubMed]
- Jeevadason, A.W.; Murugavel, K.K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- De, S.; Jain, A.; Barman, P. Recent advances in the catalytic applications of chiral Schiff-base ligands and metal complexes in asymmetric organic transformations. ChemistrySelect 2022, 7, e202104334. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Habala, L.; Devínsky, F.; Egger, A.E. Metal complexes as urease inhibitors. J. Coord. Chem. 2018, 71, 907–940. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Zafar, W.; Imran, M.; Chohan, Z.H. A review on the biomedical efficacy of transition metal triazole compounds. J. Coord. Chem. 2022, 75, 293–334. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Sahrish, I.; Raza, M.A.; Ahmad, Z.; Zafar, M.N.; Chohan, Z.H.; Khalid, M.; Ahmed, S. Efficient synthesis, characterization, and in vitro bactericidal studies of unsymmetrically substituted triazole-derived Schiff base ligand and its transition metal complexes. Monatsh. Chem. 2020, 151, 549–557. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Alsantali, R.I.; Mughal, E.U.; Naeem, N.; Alsharif, M.A.; Sadiq, A.; Ali, A.; Jassas, R.S.; Javed, Q.; Javid, A.; Sumrra, S.H.; et al. Flavone-based hydrazones as new tyrosinase inhibitors: Synthetic imines with emerging biological potential, SAR, molecular docking and drug-likeness studies. J. Mol. Struct. 2022, 1251, 131933. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Qassem, S.A.; Rabee, M.M.; Bräse, S. Transition metal complexes of thiosemicarbazides, thiocarbohydrazides, and their corresponding carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A review. Molecules 2023, 28, 1808. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The new wave in cancer treatment. Future Med. Chem. 2009, 1, 1143–1151. [Google Scholar] [CrossRef]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Nutting, C.M.; van Herpen, C.M.; Miah, A.B.; Bhide, S.A.; Machiels, J.P.; Buter, J.; Kelly, C.; de Raucourt, D.; Harrington, K.J. Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2009, 20, 1275–1279. [Google Scholar] [CrossRef]
- Ratner, E.S.; Zhu, Y.L.; Penketh, P.G.; Berenblum, J.; Whicker, M.E.; Huang, P.H.; Lee, Y.; Ishiguro, K.; Zhu, R.; Sartorelli, A.C.; et al. Triapine potentiates platinum-based combination therapy by disruption of homologous recombination repair. Br. J. Cancer 2016, 114, 777–786. [Google Scholar] [CrossRef]
- Pósa, V.; Hajdu, B.; Tóth, G.; Dömötör, O.; Kowol, C.R.; Keppler, B.K.; Spengler, G.; Gyurcsik, B.; Enyedy, É.A. The coordination modes of (thio)semicarbazone copper(II) complexes strongly modulate the solution chemical properties and mechanism of anticancer activity. J. Inorg. Biochem. 2022, 231, 111786. [Google Scholar] [CrossRef] [PubMed]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal complexes with Schiff bases: Data collection and recent studies on biological activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C.; Correia, I. Salan vs. salen metal complexes in catalysis and medicinal applications: Virtues and pitfalls. Coord. Chem. Rev. 2019, 388, 227–247. [Google Scholar] [CrossRef]
- Habala, L.; Varényi, S.; Bilková, A.; Herich, P.; Valentová, J.; Kožíšek, J.; Devínsky, F. Antimicrobial activity and urease inhibition of Schiff bases derived from isoniazid and fluorinated benzaldehydes and of their copper(II) complexes. Molecules 2016, 21, 1742. [Google Scholar] [CrossRef] [PubMed]
- Valentová, J.; Varényi, S.; Herich, P.; Baran, P.; Bilková, A.; Kožíšek, J.; Habala, L. Synthesis, structures and biological activity of copper(II) and zinc(II) Schiff base complexes derived from aminocyclohexane-1-carboxylic acid. New type of geometrical isomerism in polynuclear complexes. Inorg. Chim. Acta 2018, 480, 16–26. [Google Scholar] [CrossRef]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Mohamad Zain, N. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Liverani, L.; Kurtuldu, F.; Galusek, D.; Boccaccini, A.R. Zinc improves antibacterial, anti-inflammatory and cell motility activity of chitosan for wound healing applications. Int. J. Biol. Macromol. 2022, 213, 845–857. [Google Scholar] [CrossRef]
- Schwartz, J.R. Zinc pyrithione: A topical antimicrobial with complex pharmaceutics. J. Drugs Dermatol. 2016, 15, 140–144. [Google Scholar]
- Böhm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Jeong, J.H. Syntheses and X-ray structures of Cu(II) and Zn(II) complexes of N,N′-dibenzyl-(R,R)-1,2-diaminocyclohexane and application to nitroaldol reaction. Polyhedron 2008, 27, 3227–3230. [Google Scholar] [CrossRef]
- Roh, S.G.; Yoon, J.U.; Jeong, J.H. Synthesis and characterization of a chiral Zn(II) complex based on a trans-1,2-diaminocyclohexane derivative and catalytic reduction of acetophenone. Polyhedron 2004, 23, 2063–2067. [Google Scholar] [CrossRef]
- Nayab, S.; Lee, H.; Jeong, J.H. Synthesis and structural characterization of a dichloro zinc complex of N,N′-bis-(2,6-dichloro-benzyl)-(R,R)-1,2-diaminocyclohexane: Application to ring opening polymerization of rac-lactide. Polyhedron 2012, 31, 682–687. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Jeong, J.H. Synthesis and X-ray structure of Zn(II) complex of N,N′-bis(2-fluorobenzyl)-(R,R)-1,2-diaminocyclohexane and application to nitroaldol reaction. Bull. Korean Chem. Soc. 2008, 29, 483–486. [Google Scholar] [CrossRef]
- Cho, J.; Jeong, J.H.; Shin, H.J.; Min, K.S. Synthesis, structure and photoluminescence properties of naphthalene-based chiral zinc(II) complexes. Polyhedron 2020, 187, 114643. [Google Scholar] [CrossRef]
- STOE & Cie GmbH. X-Area, version 1.84; Software Package for Collecting Single-Crystal Data on STOE Area-Detector Diffractometers, for Image Processing, Scaling Reflection Intensities and for Outlier Rejection; STOE & Cie GmbH: Darmstadt, Germany, 2018. [Google Scholar]
- Rigaku. CrysAlisPRO, version 1.0.43; Rigaku Oxford Diffraction: Yarnton, UK, 2022. [Google Scholar]
- Dolomanov, H.; Bouhris, O.V.; Gildea, L.J.; Howard, R.J.; Puschmann, J.A.K. OLEX 2. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Bergerhoff, G.; Berndt, M.; Brandenburg, K. Evaluation of Crystallographic Data with the Program DIAMOND. J. Res. Natl. Inst. Stand. Technol. 1996, 101, 221–225. [Google Scholar] [CrossRef]
- Lukáč, M.; Lacko, I.; Bukovský, M.; Kyselová, Z.; Karlovská, J.; Horváth, B.; Devínsky, F. Synthesis and Antimicrobial Activity of a Series of Optically Active Quaternary Ammonium Salts Derived from Phenylalanine. Open Chem. 2010, 8, 194–201. [Google Scholar] [CrossRef]
- Sreenivasulu, B. Schiff Base and Reduced Schiff Base Ligands. In Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P., Steed, J., Eds.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Smith, B. Organic Nitrogen Compounds. In Infrared Spectral Interpretation; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Sharma, M.; Joshi, P.; Kumar, N.; Joshi, S.; Rohilla, R.K.; Roy, N.; Rawat, D.S. Synthesis, Antimicrobial Activity and Structure–Activity Relationship Study of N,N-Dibenzyl-Cyclohexane-1,2-Diamine Derivatives. Eur. J. Med. Chem. 2011, 46, 480–487. [Google Scholar] [CrossRef]
- Aslantaş, M.; Kendi, E.; Demir, N.; Şabik, A.E.; Tümer, M.; Kertmen, M. Synthesis, spectroscopic, structural characterization, electrochemical and antimicrobial activity studies of the Schiff base ligand and its transition metal complexes. Spectrochim. Acta A 2009, 74, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Bahaffi, S.O.; Abdel Aziz, A.A.; El-Naggar, M.M. Synthesis, spectral characterization, DNA binding ability and antibacterial screening of copper(II) complexes of symmetrical NOON tetradentate Schiff bases bearing different bridges. J. Mol. Struct. 2012, 1020, 188–196. [Google Scholar] [CrossRef]

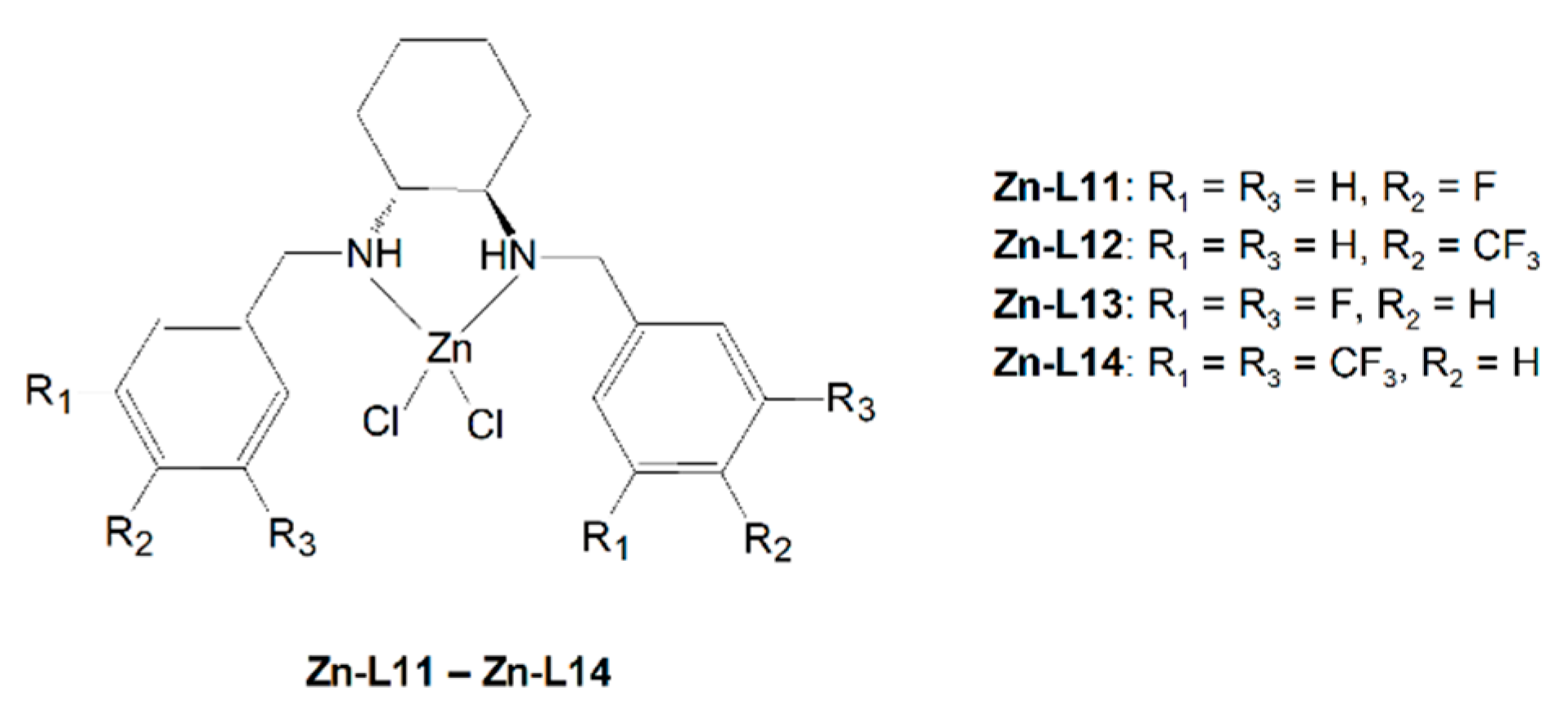

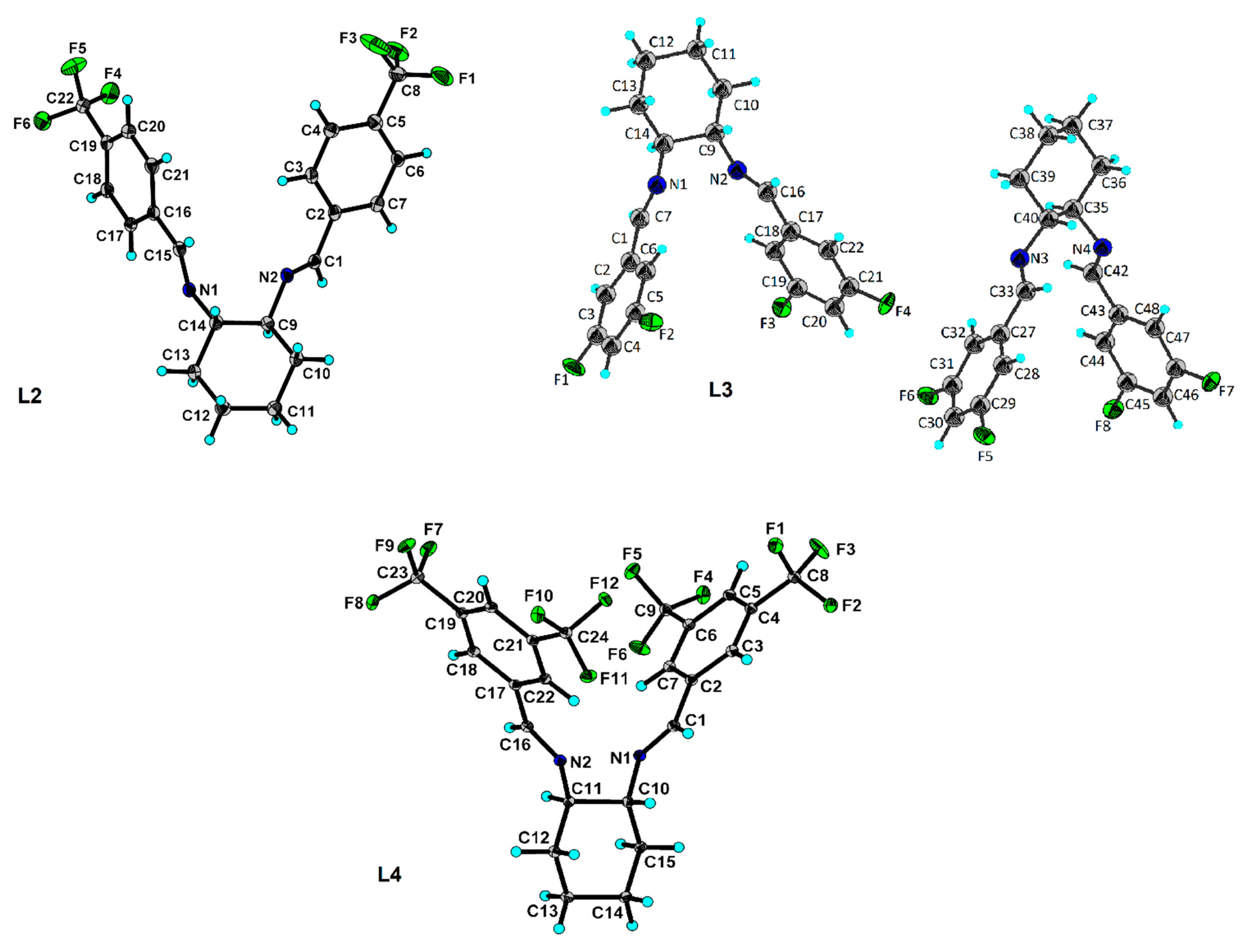
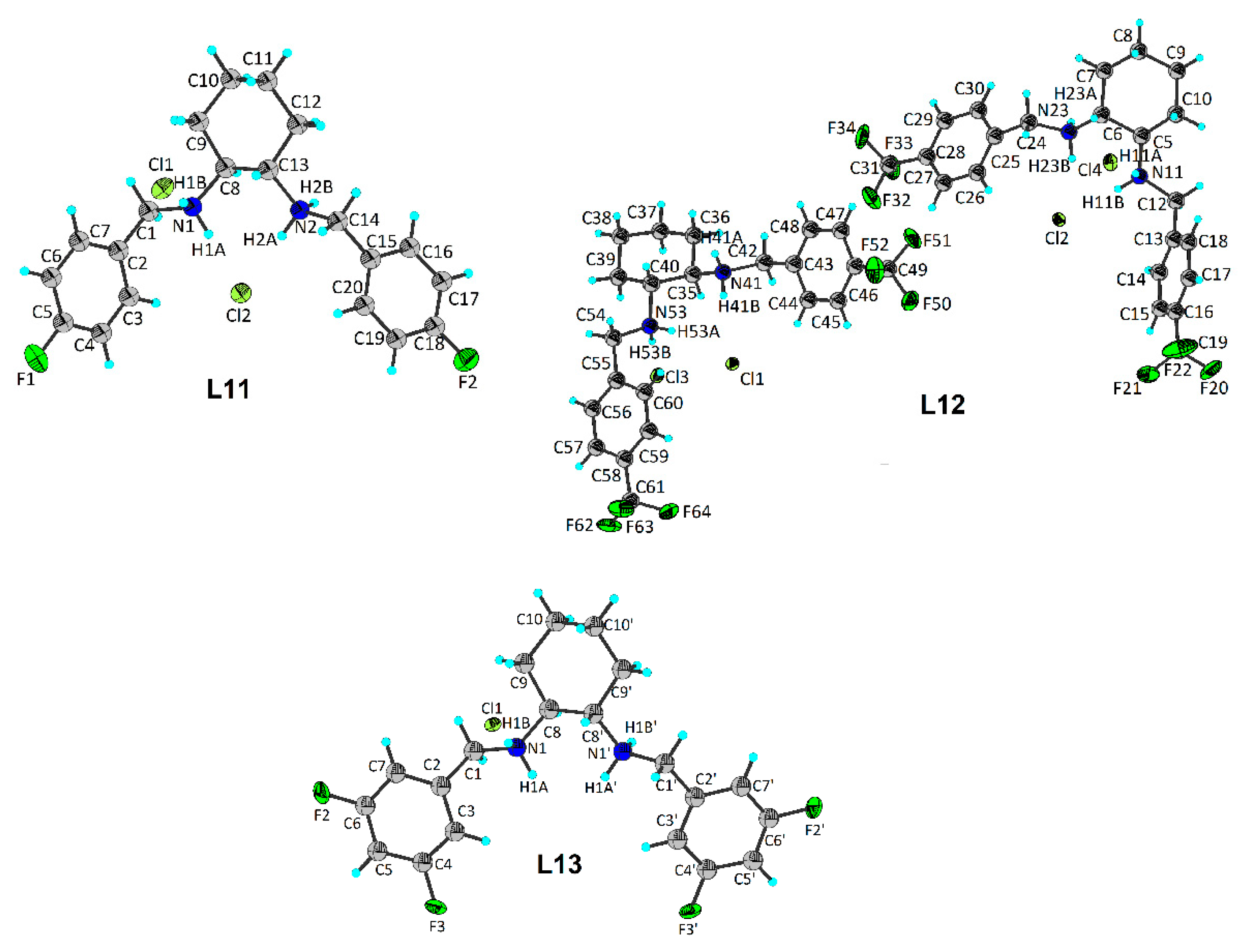
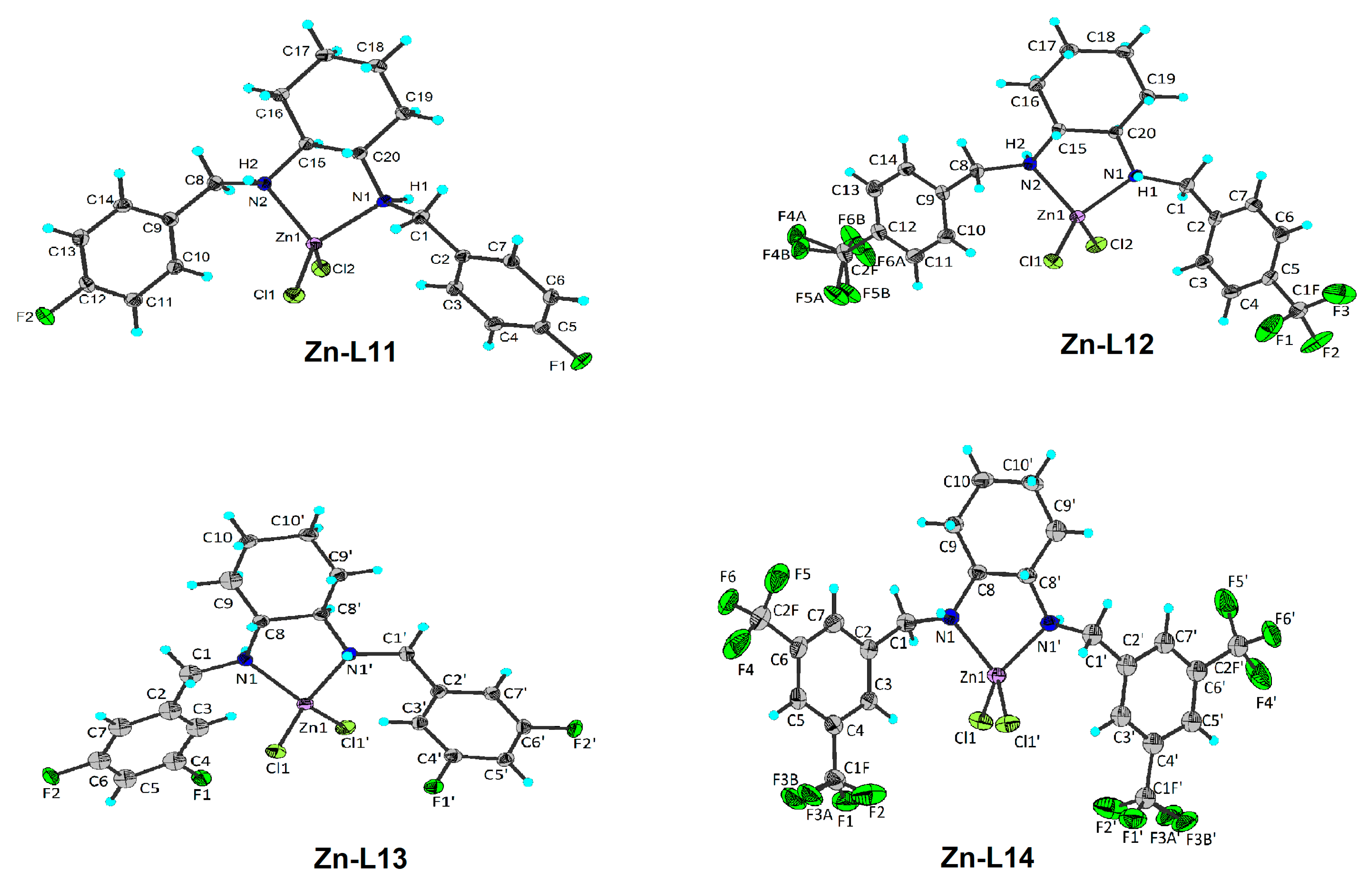
| Compound (Chemical Formula) | MW (g/mol) | Color | M.p. °C | Yield (%) | Elemental Analysis Found (Calcd) (%) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| L1 (C20H20F2N2) | 326.38 | white | 128–129 | 59.5 | 72.86 (73.60) | 5.77 (6.18) | 8.66 (8.58) |
| L2 (C22H20F6N2) | 426.40 | white | 100–101 | 86.4 | 61.05 (61.97) | 3.31 (4.73) | 6.39 (6.57) |
| L3 (C20H18F4N2) | 362.36 | white | 103–105 | 95.0 | 65.43 (66.29) | 4.26 (5.01) | 7.57 (7.73) |
| L4 (C24H18F12N2) | 562.39 | white | 112–113 | 66.5 | 53.64 (51.26) | 2.72 (3.23) | 4.81 (4.98) |
| L11 (C20H26Cl2F2N2) | 403.34 | white | 232–235 | 56.2 | 58.99 (59.56) | 6.30 (6.50) | 7.02 (6.95) |
| L12 (C22H26Cl2F6N2) | 503.35 | white | 240–245 | 52.6 | 50.21 (52.50) | 4.81 (5.21) | 5.91 (5.57) |
| L13 (C20H24Cl2F4N2) | 439.32 | white | 202–207 | 53.3 | 52.84 (54.68) | 4.77 (5.51) | 6.55 (6.38) |
| L14 (C24H24Cl2F12N2) | 639.35 | white | 239–242 | 44.3 | 44.81 (45.09) | 2.93 (3.78) | 4.43 (4.38) |
| Zn-L11 (C20H24Cl2ZnF2N2) | 466.70 | white | 283–284 | 82.6 | 51.11 (51.47) | 4.70 (5.18) | 5.96 (6.00) |
| Zn-L12 (C22H24Cl2ZnF6N2) | 566.72 | white | 292–293 | 85.7 | 45.77 (46.63) | 3.52 (4.27) | 4.88 (4.94) |
| Zn-L13 (C20H22Cl2ZnF4N2) | 502.68 | white | 285–286 | 78.6 | 44.05 (47.79) | 3.49 (4.41) | 5.02 (5.57) |
| Zn-L14 (C24H22Cl2ZnF12N2) | 702.71 | white | 290–291 | 80.0 | 43.01 (41.02) | 2.50 (3.16) | 5.16 (3.99) |
| Bond Lengths in Å | Bond Angles in ° | ||
|---|---|---|---|
| Zn-L11 | |||
| Zn1–Cl2 | 2.2330(4) | Cl1–Zn1–Cl2 | 120.118(19) |
| Zn1–Cl1 | 2.2093(4) | N1–Zn1–Cl2 | 107.32(4) |
| Zn1–N1 | 2.0723(14) | N1–Zn1–Cl1 | 114.34(4) |
| Zn1–N2 | 2.0594(14) | N2–Zn1–Cl2 | 108.59(4) |
| N2–Zn1–Cl1 | 113.74(4) | ||
| N2–Zn1–N1 | 88.02(5) | ||
| Zn-L12 | |||
| Zn1–Cl1 | 2.2165(6) | Cl1–Zn1–Cl2 | 117.30(2) |
| Zn1–Cl2 | 2.2171(6) | N1–Zn1–Cl2 | 109.66(5) |
| Zn1–N1 | 2.0699(18) | N1–Zn1–Cl1 | 113.28(5) |
| Zn1–N2 | 2.0701(18) | N2–Zn1–Cl2 | 113.70(5) |
| N2–Zn1–Cl1 | 111.80(5) | ||
| N2–Zn1–N1 | 87.31(7) | ||
| Zn-L13 | |||
| Zn1–Cl1 | 2.2278(5) | Cl1–Zn1–Cl1i | 118.83(3) |
| Zn1–Cl1i | 2.2278(5) | N1i–Zn1–Cl1 | 108.83(4) |
| Zn1–N1i | 2.0752(16) | N1–Zn1–Cl1i | 108.83(4) |
| Zn1–N1 | 2.0752(16) | N1–Zn1–Cl1 | 114.41(5) |
| N1i–Zn1–Cl1i | 114.41(5) | ||
| N1i–Zn1–N1 | 87.35(8) | ||
| Zn-L14 | |||
| Zn1–Cl1 | 2.2265(6) | Cl1–Zn1–Cl1 | 109.74(3) |
| Zn1–Cl1ii | 2.2265(6) | N1ii–Zn1–Cl1 | 119.55(5) |
| Zn1–N1ii | 2.0683(18) | N1–Zn1–Cl1ii | 119.55(5) |
| Zn1–N1 | 2.0683(19) | N1–Zn1–Cl1 | 110.12(5) |
| N1ii–Zn1–Cl1ii | 110.12(5) | ||
| N1ii–Zn1–N1 | 86.66(10) | ||
| Compound | S. aureus | E. coli | C. albicans |
|---|---|---|---|
| L1 | 5.7063 | 5.7063 | 2.8531 |
| L11 | 0.7083 | 0.3541 | 2.8331 |
| L12 | 0.0028 | 0.0221 | 0.3536 |
| Zn-L11 | 0.1076 | 0.2151 | 1.7206 |
| Zn-L12 | 0.0008 | 0.0134 | 0.8550 |
| Zn-L13 | 0.1054 | 0.2107 | 1.6856 |
| Zn-L14 | 0.1063 | 0.1063 | 1.7006 |
| cipro | 0.00068 | <0.0003 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oboňová, B.; Habala, L.; Litecká, M.; Herich, P.; Bilková, A.; Bilka, F.; Horváth, B. Antimicrobially Active Zn(II) Complexes of Reduced Schiff Bases Derived from Cyclohexane-1,2-diamine and Fluorinated Benzaldehydes—Synthesis, Crystal Structure and Bioactivity. Life 2023, 13, 1516. https://doi.org/10.3390/life13071516
Oboňová B, Habala L, Litecká M, Herich P, Bilková A, Bilka F, Horváth B. Antimicrobially Active Zn(II) Complexes of Reduced Schiff Bases Derived from Cyclohexane-1,2-diamine and Fluorinated Benzaldehydes—Synthesis, Crystal Structure and Bioactivity. Life. 2023; 13(7):1516. https://doi.org/10.3390/life13071516
Chicago/Turabian StyleOboňová, Bianka, Ladislav Habala, Miroslava Litecká, Peter Herich, Andrea Bilková, František Bilka, and Branislav Horváth. 2023. "Antimicrobially Active Zn(II) Complexes of Reduced Schiff Bases Derived from Cyclohexane-1,2-diamine and Fluorinated Benzaldehydes—Synthesis, Crystal Structure and Bioactivity" Life 13, no. 7: 1516. https://doi.org/10.3390/life13071516
APA StyleOboňová, B., Habala, L., Litecká, M., Herich, P., Bilková, A., Bilka, F., & Horváth, B. (2023). Antimicrobially Active Zn(II) Complexes of Reduced Schiff Bases Derived from Cyclohexane-1,2-diamine and Fluorinated Benzaldehydes—Synthesis, Crystal Structure and Bioactivity. Life, 13(7), 1516. https://doi.org/10.3390/life13071516






