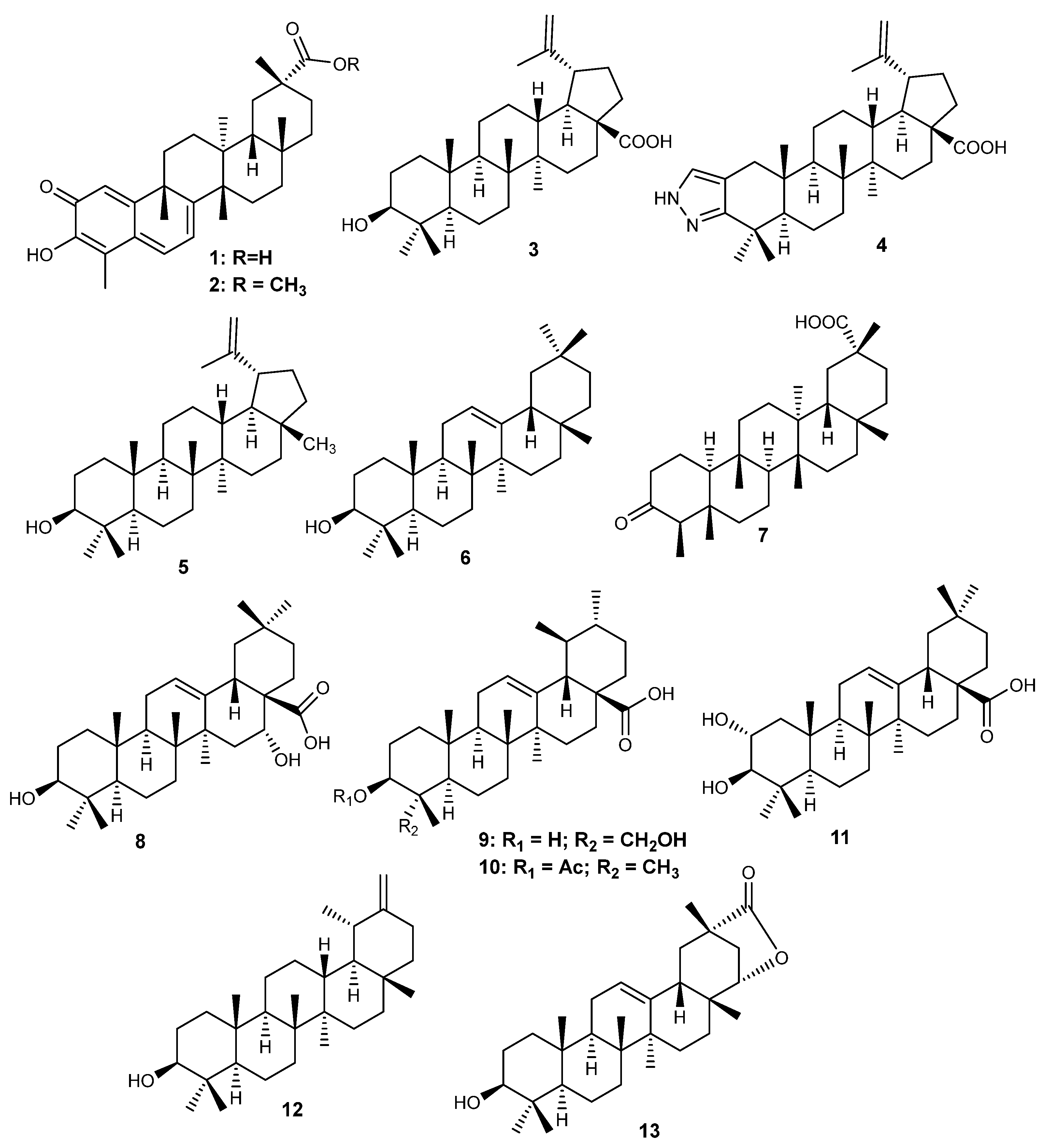
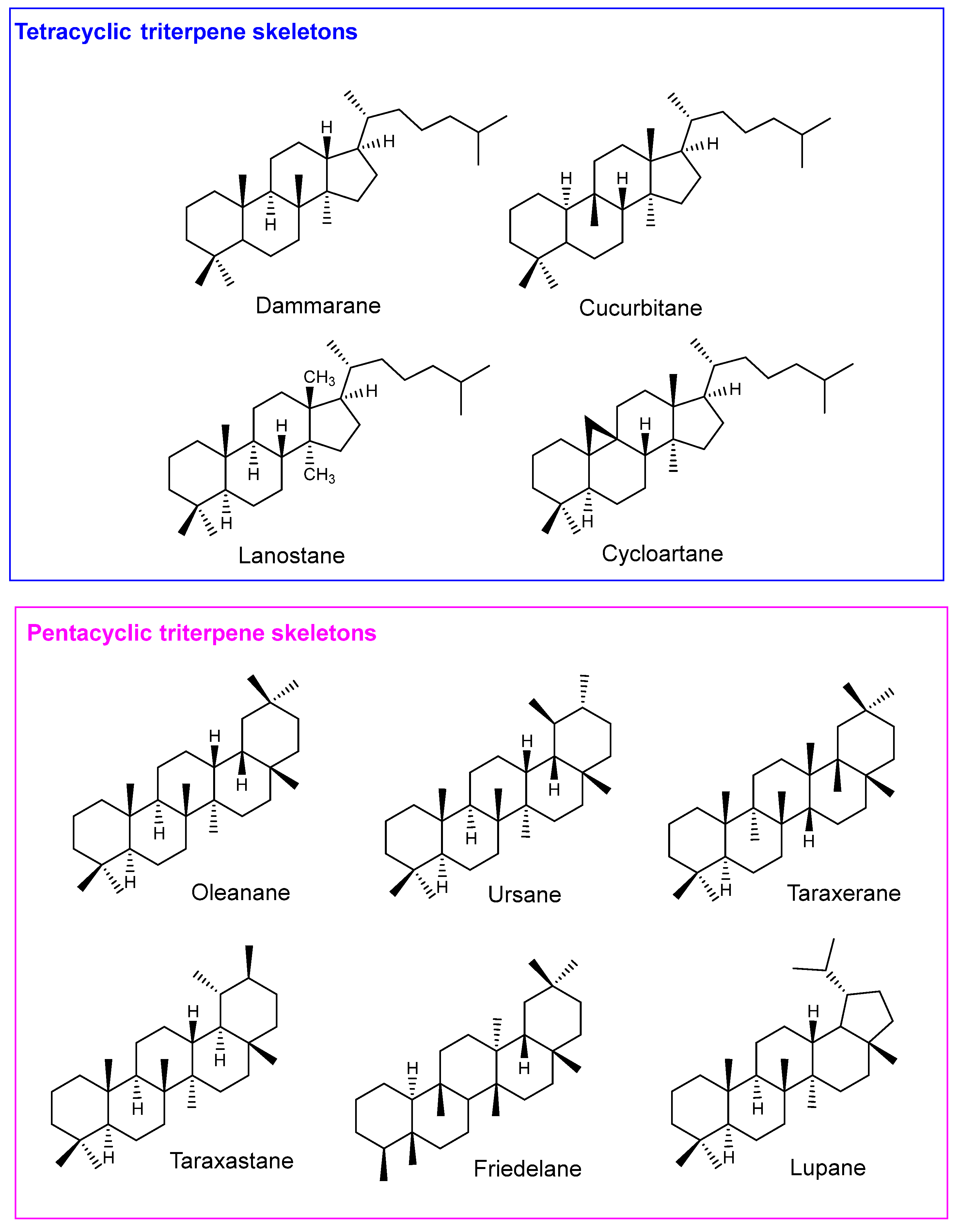
6.1. Pentacyclic Triterpenes
Celastrol (
1), also known as tripterine, is a nor-triterpene quinone methide with the friedelane skeleton found in
Tripterygium wilfordii, known as “Thunder God Vine”, a vine commonly grown is southeast China and used in traditional Chinese medicine for the treatment of RA and other autoimmune and inflammatory diseases [
63]. Recent studies suggest that NLRP3 inflammasome-induced inflammation is involved in the pathogenesis of RA [
47]. Celastrol (
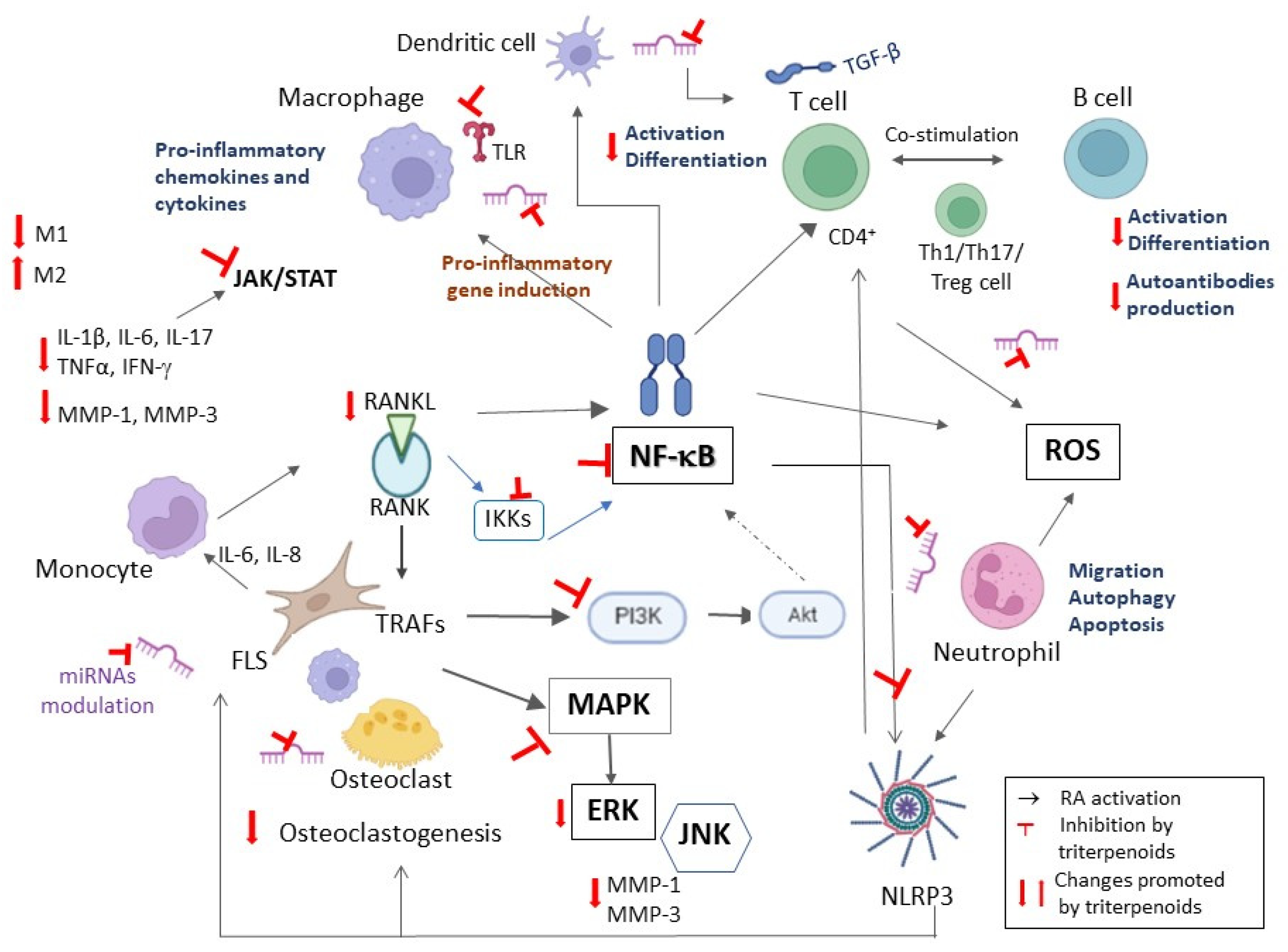
1) treatment significantly reduced the secretion of IL-1β and IL-18 in the serum of CFA-induced rats and in supernatants of human mononuclear macrophages (THP-1 cells) due to inhibition of the NF-κB pathway and hindering of NLRP3 inflammasome activation [
63].
1 also suppressed ROS production induced by LPS and adenosine triphosphate (ATP) in THP-1 cells [
63] and prevented NLRP3 inflammasome activation
in vitro by inhibiting complex formation between NLRP3 and ASC adaptor protein [
64], essential for recruitment of caspase-1 and maturation of IL-1β.
1 also inhibited TNF-α-induced proliferation of FLSs, enhanced autophagosome levels and expression of autophagy-related proteins (LC3, p62 and Beclin-1) and increased the LC3-II/LC3-I ratio [
65]. Furthermore, the autophagy inhibitor 3-methyladenine significantly reversed effects of
1 on the expression of autophagy-related proteins [
65]. In CIA mice,
1 attenuated disease severity via upregulation of autophagy through inhibition of the PI3K/Akt/mTOR axis [
65]. Autophagy dysregulation has been implicated in several autoimmune diseases, including RA. Enhanced autophagy contributes to RA FLS hyperplasia and apoptosis resistance, production of citrullinated peptides, osteoclastogenesis and bone resorption, resulting in severe bone and cartilage damage [
66].
Figure 2.
Structures of pentacyclic triterpenes (1–13) with activity on RA.
Figure 2.
Structures of pentacyclic triterpenes (1–13) with activity on RA.
The co-administration of
1 and diclofenac has been routinely used in Chinese medicine for the treatment of RA. In order to shed light on the possible interaction potential of the two drugs, Wang et al. studied the
in vivo effects of diclofenac on the pharmacokinetic profiles of
1 in rats [
67]. When co-administered, several pharmacokinetic parameters significantly change, in particular, the
Cmax and the AUC
0 of
1 decreased from 66.93 ± 10.28 to 41.25 ± 8.06 μg/L and 765.84 ± 163.61 to 451.33 ± 110.88 (μg × h/L), respectively. On the other hand,
Tmax increased from 6.05 ± 1.12 to 7.82 ± 1.15 h, and oral clearance increased from 1.29 ± 0.15 to 2.27 ± 0.31 L/h/kg. Moreover, it was found that the efflux ratio of
1 across the Caco-2 cell model increased when co-administered with diclofenac. In this way, the authors concluded that diclofenac could decrease the exposure of
1 in rats. It was also suggested that this effect could be carried out by decreasing the intestinal absorption of celastrol (
1) through induction of P-glycoprotein (P-gp) activity [
67].
To evaluate the progression of the disease and the response of RA patients to treatment, several biomarkers have been used, such as RF and ACPAs, although they can also be found in other autoimmune diseases. In this way, Dudics et al. studied the micro-RNA profile of immune (lymphoid) cells of arthritic Lewis rats and celastrol (
1)-treated arthritic rats, in order to evaluate its ability as a novel RA biomarker [
68]. Using combined miRNA–microarray technology and bioinformatics-based analysis, it was found that eight specific miRNAs (miR-22, miR-27a, miR-96, miR-142, miR-223, miR-296, miR-298 and miR-451) and their target genes are crucially involved in functional pathways for RA pathogenesis. In particular, miR-22, miR-27a, miR-96, miR-142, miR-223, miR-296, miR-298 and miR-451 were modulated by celastrol (
1) treatment. Through the quantitation of these miRNAs in serum samples of control, arthritic and celastrol (
1)-treated rats, in the peak phase of adjuvant-induced arthritis, it was found that miR-142, miR-155, miR-212 and miR-223 levels were higher in arthritic
vs. control rats, further validating their value as circulating biomarkers to assess arthritis progression and response to therapy [
68].
Fang et al. aimed at studying the effect of
1 on activated RA FLSs obtained from synovial biopsies of human RA patients [
69]. Several assays were carried out in order to assess proliferation, invasion and expression of pro-inflammatory cytokines and to screen for differentially expressed genes. The authors found that
1 significantly modulated the RA–FLS activation status by reducing the proliferation and invasion of the cells. Moreover, a change in the expression of several chemokine genes, including CCL2, CXCL10, CXCL12, CCR2 and CXCR4, was also observed. This finding could be useful for therapy since chemokines could be responsible for the arthritis pain by promoting leukocyte infiltration and synoviocyte proliferation and activation. In particular, the release of CCL2 and CXCL12 proteins from RA FLS cells was significantly downregulated by celastrol (
1) treatment. Celastrol (
1) treatment also diminished the activation and translocation of NF-κB p65, which is known to participate in the regulation of many cytokines, adhesion molecules, chemokines, receptors and adaptive enzymes in arthritis [
69].
Inhibition of oxidative stress underlies the improvement observed in CIA rats treated with
1 (1 mg/kg) in a study carried out by Gao et al. [
70].
1 enhanced the superoxide dismutase activity and significantly inhibited the levels of malondialdehyde, superoxide anions and NADPH oxidase activity [
70]. Reduction of arthritis scores and spleen and thymus indexes was also observed, as well as the suppression of serum levels of TNF-α, IL-1β, IL-6 and interferon gamma (IFN-γ), which could be attributed to the downregulation of inflammatory mediators [
70].
The mechanistic complexity of
1, due to its multiple targets, was analyzed by Song et al. by employing a network pharmacological approach. The authors identified probable molecular targets of the compound and the interaction pathways related to their roles, investigating the networks formed by those pathways [
71]. Using a web-based bioinformatics application (ingenuity pathway analysis), pathways and networks were built grounded in the functions of the human genes appertaining to RA and the selected potential targets. The networks comprised cell movement, immune cell trafficking, hematological system development and function, inflammatory response, connective tissue disorders, organismal injury and abnormalities and cell-to-cell signaling and interactions. Results indicated that MMP-9, COX-2, c-Myc, TGF-β, c-JUN, JAK-1, JAK-3, IKK-β, SYK, MMP-3, JNK and MEK1 were the direct targets of
1 in RA. Being high-degree nodes in RA-associated networks probably affected by
1, COX-2, IKK-β, JNK and MEK1 were selected for docking studies [
71]. Results of the pathway analysis obtained by Song et al. suggested that
1 can regulate the functions of Th1 and Th2 cells, fibroblasts, macrophages and endothelial cells, which would explain its therapeutic effects against RA [
71].
Pristimerin (
2) is the celastrol methyl ester, a natural triterpene found in plants of the Celastraceae and Hippocrateaceae families. In TNF-α-stimulated human RA FLSs, treatment with
2 decreases cell viability and migration in a dose-dependent manner [
72]. According to cell metabolomics analysis, the effects involved phospholipid and fatty acid biosynthesis, glutathione metabolism and amino acid metabolic pathways [
72].
In vivo, compound
2 ameliorated arthritis symptoms and reduced serum levels of TNF-α and NO and synovial expressions of p-Akt and p-ERK in the CFA-induced arthritis rat model. Network pharmacology analysis showed that the effects were mediated through the MAPK/ERK1/2 and PI3K/Akt pathways and direct binding to TNF-α [
72].
The effects of betulinic acid (
3) on the proliferation, migration and inflammatory response of RA FLSs were studied by Wang and Zhao [
73]. Compound
3 inhibited the proliferation, migration and invasion of RA FLSs in a dose- and time-dependent manner at non-cytotoxic concentrations (5–20 μM). It also decreased MMP expression and inhibited the production of TNF-α-induced inflammatory cytokines, namely of IL-6 and IL-8. The PI3K/Akt signaling pathway plays a significant role in regulating inflammation, proliferation and migration of RA FLSs and in signal activation of NF-κB, being highly expressed in the synovial tissues of RA patients. Betulinic acid (
3) also avoided activation of the Akt/NF-κB pathway and can be considered a potential therapeutic agent for the treatment of RA [
73].
Huimin et al. explored the protective effects of
3 on CFA-induced rats, observing the significant inhibition activity of the drug regarding the arthritis index, toe swelling, joint pathology and hemorheology [
74]. Serum and synovial levels of Il-6, IL-1β and TNF-α were improved following treatment with
3. Since Rho and Rho-associated protein kinase (ROCK) control the production of inflammatory cytokines, the anti-inflammatory mechanism of
3 was investigated through the Rho/ROCK/NF-κB activation by treating rats with fasudil (a ROCK inhibitor). Protein levels of RhoA, ROCK1 and ROCK2 were downregulated, leading to the blockage of phosphorylation of IKKα, IKKβ, lκB and NF-κB. The results provided information about the mechanism of compound
3 on RA, which may be related to the downregulation of ROCK/NF-κB signaling pathways [
74].
Since RA FLSs display an aggressive phenotype, which is linked to cartilage and bone destruction, Li et al. examined the effects of
3 on the migration and invasion of RA FLSs (prepared from synovial tissue specimens of diagnosed RA patients), seeking a mechanistic understanding of the therapeutic potential of
3 [
75]. Treatment with
3 restrained the migratory and invasion capacity of RA FLSs and decreased the formation of actin stress fibers and actin cytoskeleton score [
75]. Considering the TNF-α-induced RA FLSs, treatment with
3 led to a significant decrease in the mRNA expression of IL-1β, IL-6, IL-8 and IL-17A, as well as to a decrease in phosphorylated IKK, IκBα and NF-κB and to a reduction of the NF-κB accumulation. These results suggest that the inhibition of NF-κB signaling pathways by
3 causes the inhibition of migration, invasion, actin cytoskeleton reorganization and interleukin expression of RA FLSs [
75].
RA is highly associated with increased risk of cardiovascular disease, with RA patients being almost twice as likely to develop heart disease as compared with the general population [
76]. Besides the traditional risk factors, chronic inflammation associated with RA appears to promote atherosclerosis, and in both diseases similar pathophysiologic processes are recognized, including increased expression of cellular adhesion molecules, pronounced infiltration by macrophages and Th1 cells, neovascularization and collagen degradation mediated by MMPs [
77]. Statins are HMG CoA reductase inhibitors widely used for treatment of hyperlipidemia and prevention of cardiovascular disease, and their anti-inflammatory properties have been proved to be associated with several molecular mechanisms such as suppression of chemokine and pro-inflammatory cytokine synthesis, MMP inhibition, reduced MHC-II expression induced by IFN-γ and reduced expression of CD40 on macrophages and other smooth muscle cells [
78,
79]. The synergist effect of oral co-administration of
3 (2 mg/kg) and fluvastatin (5 mg/kg) was studied
in vivo using a CIA rat model, and several physical, morphological and biochemical parameters were collected [
80]. Combined treatment with
3 and fluvastatin showed a decrease in the severity of arthritic index values and inhibition of paw edema (89%) after 60 days when compared with the single administration of the drugs (80% and 74%, respectively) or the control group without treatment. A reduction of RF, C-reactive protein, total lipids and ACPAs, as well as an increased activity of catalase, superoxide dismutase and glutathione peroxidase enzymes, in the different tissues was also observed in the rats treated with a combination of both drugs. Moreover, it was also found that the expression of the anti-inflammatory cytokine IL-10 was increased in the co-treated group, while the expression of Toll-like receptor (TLR) 2 and TLR4, IL-1β, TNF-α, IFN-γ, cell adhesion molecules and nuclear translocation of NF-κB in the aorta decreased, when compared to the single-treated groups [
80].
Taking into consideration that betulinic acid (
3) can be regarded as a lead compound for further development of potential anti-inflammatory agents, several derivatives having heterocyclic rings fused at C-2 and C-3 were synthesized and assayed as inhibitors of osteoclast differentiation and bone resorption [
81]. The most potent compound, pyrazole derivative
4, exhibited potent inhibitory activity on RANKL-induced osteoclast formation (IC
50 = 0.09 μM), being 200-fold more active than the parent triterpene
3. In a later work, Chen et al. studied the modulation activity of
4 on T cell differentiation and proliferation and potential anti-rheumatic effects in a CIA mouse model [
82]. When compared to the control group that received no treatment, the severity of symptoms was significantly attenuated in treated mice, that showed a mean arthritis score of 2.63 on day 41 (control group: 6.88). Further radiological and histopathological analysis corroborated these findings, since considerably less articular damage was observed and arthritis cartilage destruction and inflammatory cell infiltration were highly decreased, possibly due to inhibition of Th1 and Th17 differentiation, enhanced IL-4, IL-10, IL-13 expression and increased CD4
+ Foxp3
+ cells [
82].
Lupeol (
5), a lupane triterpenoid with antioxidant and anti-inflammatory properties found in many edible fruits and vegetables, inhibited PI3K/Akt signaling in CIA rats [
83]. Lupeol significantly reduced paw edema, reverted the high levels of biochemical markers (RF, C-reactive protein and ceruloplasmin) and pro-inflammatory mediators (TNF-α, IL-6 and PGE2) in the rat serum and enhanced apoptosis by downregulating Bcl-2 protein expression while upregulating Bax, caspase-3 and caspase-9 [
83]. However, the overall effects were inferior to those of indomethacin, the NSAID used as positive control [
83].
β-amyrin (
6) and polpunonic acid (
7) are found in the root bark of
Ziziphus abyssinica (Hochst Ex A. Rich), a recognized medicinal plant widely distributed in the tropical regions of the world, showing antioxidant, anti-bacterial and anti-plasmodial activities, among others [
84]. Henneh et al. were able to isolate them as pure chemical entities and determine their absolute configuration, examining possible therapeutic effects in RA in a CFA-induced arthritis rat model [
84]. Compounds
6 and
7 (at equal doses) reversed the changes induced in the RA model (considering body weight, paw thickness, erythema and arthritic index). Histopathological examinations of rat hind paws showed a significant reduction of cartilage erosion and subchondral cyst and Weichselbaum’s lacunae formation, with an effect dependent on the type of compound and the doses of administration. There was also evidence of bone remodeling and decreased bone cavitation after treatment with both compounds, most pronounced for
6 [
84].
Echinocystic acid (
8) isolated from the bark of
Albizia julibrissin Durazz was able to ameliorate arthritic symptoms induced in transgenic SKG mice after a single intraperitoneal injection of zymosan. The treatment with
8 reduced inflammatory cell infiltration, pro-inflammatory cytokine levels, synovial hyperplasia and bone loss in mouse paw tissues [
85]. These effects have been attributed to inhibition of both IL-6- and TGF-β-induced Th17 cell differentiation, namely by suppression of phosphorylation of STAT3. In TNF-α-activated human RA FLSs (MH7A cells), administration of
8 reduced both protein and mRNA expression of inflammatory cytokines (IL-6 and IL-1β) by downregulating MAPK and NF-κB signaling pathways [
85].
Bone homeostasis depends on the balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Excessive osteoclast activity has been associated with RA, osteoarthritis, osteoporosis and other bone-related diseases [
36,
52,
86]. 23-Hydroxyursolic acid (
9) isolated from
Viburnum lutescens was found to inhibit RANKL-induced osteoclastogenesis
in vitro by decreasing the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts and F-actin ring formation [
87]. Actin ring formation is a characteristic marker of bone resorption activity of mature osteoclasts. Compound
9 also inhibited RANKL-induced phosphorylation of ERK and JNK, IκBα degradation, c-Fos expression, activation of the nuclear factor NFATc1 and expression of its target genes [
87]. Oral administration of
9 to mice conferred protection against LPS-induced osteoclast formation and bone loss [
87].
The study conducted by Lee et al. compared the
in vitro and
in vivo effects of ursolic acid-3-acetate (
10) and dexamethasone, using TNF-α-stimulated human FLSs and a murine model of RA [
88]. The treated rats showed a decrease in clinical symptoms, including clinical arthritis score, disease incidence and paw thickness, which were confirmed by microPET imaging. A decrease in serum IgG1 and IgG2a levels was also observed. Characteristic RA histological and radiological changes, such as hyperplasia, pannus formation, cartilage destruction and bone erosion in the joint, were improved, with results comparable to the anti-inflammatory drug dexamethasone. On the other hand, the
in vitro studies revealed a reduction of Th1/Th17 phenotype CD4
+ T lymphocyte expansion, pro-inflammatory cytokines (IL-1β, IL-6, IFN-γ and IL-17) and MMP-1/3 production in the knee joint tissue and RA synovial fibroblasts, through the downregulation of IKKα/β, ΙκBα and NF-κB [
88].
Maslinic acid (
11), a pentacyclic triterpenoid found in olive (
Olea europaea) fruit, displays a vast number of therapeutic properties, including preventing and mitigating arthritis in animals and humans, particularly in relation to knee joint arthritis symptoms [
89]. Using the CAIA mouse model of RA, Shimazu et al. clarified the molecular mechanisms implicated in the anti-arthritic properties of
11. Arthritis symptoms were mitigated, and the gene expression of inflammatory cytokines in synovial membranes was inhibited downstream of NF-κB signaling, with
11 also inactivating the TLR signaling pathway. Treatment of CAIA mice with
11 (200 mg/kg) downregulated the expression of the mRNA encoding LTA4 hydrolase, which catalyzes the hydrolysis of LTA4 to LTB4, a chemotactic factor whose overproduction is involved in RA.
11 suppressed the production of LTB4 by acting through the glucocorticoid receptor, as expression levels of several genes controlled by this receptor were altered by
11 [
89]. Upregulation of the mRNAs encoding MMP-2 and MMP-9 was observed, along with the upregulation of the expression levels of transcripts encoding tissue inhibitor of metalloproteinases (TIMP)-1, TIMP-2 and TIMP-4, where the proteinase/inhibitor imbalance can facilitate proteolysis in the cartilage of arthritis [
89]. The anti-arthritis efficacy of compound
11 thus appears to be grounded in the suppression of synovial inflammation through the inactivation of TLRs, the downregulation of leukotrienes via the glucocorticoid receptor and the promotion of tissue formation with the repair of damaged cartilage [
89].
Taraxasterol (
12) is a taraxastane-type triterpenoid mostly isolated from Chinese medicinal
Taraxacum officinale, exhibiting anti-inflammatory and antioxidant activities in several disorders [
90]. Literature reports have been pointing to its ability to lower pro-inflammatory cytokines and mediators in LPS-induced RAW 264.7 cells
in vitro and in the ovalbumin-induced asthma mouse model [
90].
In vitro and
in vivo studies of
12 in IL-1β-stimulated human RA FLSs and CIA mice, respectively, allowed Chen et al. to investigate the anti-inflammatory effects and subjacent mechanisms of
12 on RA [
90]. Since the inflammatory responses in RA FLSs are mostly modulated by NF-κB and the NLRP3 inflammasome [
90], the inhibition of NF-κB/NLRP3 pathways is therefore a potential therapeutic approach in RA management. In fact,
12 suppressed NF-κB activation in human RA FLSs, inhibiting the IL-1β-induced IκB degradation and nuclear translocation of p65 in the studied cell line. Results showed that
12 can modulate TGF-β-activated kinase 1 (TAK1) activation (which in turn regulates NF-κB activation), probably exerting its anti-inflammatory activity by modulating the TAK1/IκB/IKK pathway in human RA FLSs [
90]. Compound
12 suppressed the expression of NLRP3 inflammasome (reported to be well associated with NF-κB signal transduction) and its modulators, such as TXNIP and ACS, both in human RA FLSs and CIA mice, thereby decreasing cleaved caspase-1 levels; thus, anti-inflammatory effects of
12 could be related to the inhibition of NLRP3 inflammasome signaling. Treatment of CIA mice with
12 mitigated joint destruction and other clinical RA manifestations, downregulated NF-κB and reduced the IL-1β-induced expressions of TNF-α, IL-6, IL-8, MMP-1 and MMP-3 [
90].
Macrophage plasticity produces different functional phenotypes in reaction to specific stimuli. Macrophages can be polarized into the classical M1 or the alternative M2 phenotypes. Classically activated (M1) macrophages, induced by LPS or Th1 cytokines IFN-γ and granulocyte–macrophage colony-stimulating factor (GM-CSF), express MHC-II, inducible nitric oxide synthase (iNOS) and co-stimulation molecules like CD80 and CD86 for effective T cell antigen presentation and secrete pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, IL-12 and IL-23) as well as NO and ROS which are essential for killing intracellular pathogens [
36,
91]. Alternatively, activated (M2) macrophages, stimulated mainly by Th2 cytokines IL-4 and IL-13 and by macrophage colony-stimulating factor (M-CSF), express mannose receptor CD206, IL-4 receptor, arginase 1 and peroxisome proliferator-activated receptor gamma (PPARγ) and produce anti-inflammatory cytokines (e.g., IL-10 and TGF-β) and trophic polyamines involved in tissue repair [
36,
91]. The M1/M2 polarization is imbalanced in RA, with higher expression of M1 macrophages in the synovial fluid of RA patients, which promotes osteoclastogenesis [
86,
91]. ACPAs in the RA synovial fluid can induce interferon regulatory factor 5 (IRF5), leading to increased polarization of peripheral blood monocytes into the M1-like phenotype and thus increasing the M1/M2 ratio [
91]. Glucocorticoids and some DMARDs like MTX act by repolarizing M1-like macrophages of RA patients into the M2-like state [
86,
91]. Wilforlide A (
13), a pentacyclic triterpenoid from
Tripterygium wilfordii Hook F, delays the development of RA in CIA mice, inhibiting iNOS production (an M1 surface marker), pro-inflammatory M1 cytokines and chemokines in the mouse synovium [
92]. Similarly,
in vitro results showed that
13 hindered macrophage chemotaxis and M1 polarization in LPS/IFN-γ-stimulated THP-1 cells presumably through inactivation of the TLR4/NF-κB signaling pathway [
92].
Table 4.
Pentacyclic triterpenes with in vitro/in vivo RA-related effects.
Table 4.
Pentacyclic triterpenes with in vitro/in vivo RA-related effects.
| Pentacyclic Triterpene | Cell Model/Animal Model/Dosage | Effects and Mode of Action | Ref. |
|---|
| Celastrol (1) | TNF-α-stimulated FLSs; pre-treated with 1 (0, 25, 50 or 100 nM) for 2 h and stimulated with TNF-α (10 ng/mL) for 48 h CIA in male DBA/1 SPF grade mice; intragastric administration of 1 (0, 0.5, 1 or 2 mg/kg/day), vehicle (0.5% CMC-Na) or MTX (2 mg/kg/day), on days 28 to 56 post-immunization
| In vitro inhibition of TNF-α-induced proliferation of FLSs Decrease in p-mTOR, PI3K and p-AKT levels Increase in autophagosome levels, LC3-II/LC-I ratio and Beclin-1 expression, in vitro and in vivo In vivo inhibition of the production of pro-inflammatory cytokines TNF-α and IL-1β Reduction of protein levels of PI3K, p-AKT, p-mTOR and p62 in joint tissue, thus ameliorating paw swelling and hind paw bone damage in CIA mice
| [65] |
| | LPS/ATP-stimulated human macrophages (THP-1 cells); incubation with PMA (100 nM) for 48 h and treated with 1 (0, 12.5, 25 or 50 nM) or dexamethasone (50 nM) for 1 h prior to incubation with LPS (1 µg/mL) for 24 h followed by ATP (5 mM) stimulation for 30 min AA in male SD rats; injected with CFA in the left hind joint on day 1 and treated with 1 (0.5 or 1 mg/kg) or vehicle (0.9% saline), i.p., daily, from day 9 up to day 30
| Reduction of joint swelling, arthritis index score, inflammatory cell infiltration and synovial hyperplasia in CFA-induced rats Decrease in levels of IL-1β and IL-18 in the rat serum and supernatants of THP-1 cells exposed to 1 Inhibition of ROS production, blocking of NF-κB signaling and hindering the activation of the NLRP3 inflammasome
| [63] |
| | | Significant reduction of paw edema and arthritis scores. Improvement of the spleen and thymus indexes Reduction of TNF-α, IL-1β, IL-6, IFN-γ levels in CIA rats Increase in superoxide dismutase activity; reduction of malondialdehyde and superoxide anions levels and NADPH oxidase activity Potential therapeutic effects on RA may be ascribed to downregulation of inflammatory cytokine levels and attenuation of oxidative stress
| [70] |
| | Caco-2 cell line; treated with increasing concentrations of 1 (1–10 μM for viability assays; 2 μM for P-gp efflux) Male Sprague Dawley rats administered with 1 (1 mg/kg, control group) or both 1 (1 mg/kg) and diclofenac (10 mg/kg)
| | [67] |
| | | miRNAs (miR-22, miR-27a, miR-96, miR-142, miR-223, miR-296, miR-298 and miR-451) and their target genes in functional pathways important for RA pathogenesis miR-22, miR-27a, miR-96, miR-142, miR-223 and miR-296 were modulated by 1 Higher levels of serum miR-142, miR-155, miR-212 and miR-223 in arthritic vs. control rats
| [68] |
| | | Impaired cell proliferation and cell cycle arrest and inhibition of RA FLS invasion Reduction of secretion of IL-6, IL-8 and MCP-1 in a dose-dependent manner; no change in the secretion of IL-10 Expression of some chemokines and chemokine receptors was altered significantly after treatment
| [69] |
| Pristimerin (2) | TNF-α-stimulated human RA FLSs (MH7A cells) at 20 ng/mL and treated with 2 (0, 0.5, 1 or 2 μM) for 24 h AA male Wistar rats; intragastric administration of 2 (0.8 mg/kg/day), vehicle (0.3% CMC-Na) or MTX (0.6 mg/kg/day), for 28 days, starting the next day after CFA immunization
| Inhibition of viability and migration of TNF-α-stimulated MH7A cells (IC50 1.408 μM) Reduction of paw swelling, TNF-α and NO serum levels as well as p-Akt and p-ERK levels Alteration of phospholipid and fatty acid biosynthesis, glutathione metabolism and amino acid metabolic pathways Network pharmacology analysis and molecular docking studies showed that effects were mediated through the MAPK/ERK1/2, PI3K/Akt pathways and direct binding to TNF-α
| [72] |
| Betulinic acid (3) | | Inhibition of proliferation and migration of RA FLSs Attenuation of TNF-α-enhanced MMP expression in RA FLSs Inhibition of inflammatory response in RA FLSs exposed to TNF-α and prevention of the activation of Akt/NF-κB pathway
| [73] |
| | RA FLSs treated with DMSO or 3 (0, 2.5, 5, 10 μM) for 24 h. Stimulation with TNF-α (0 or 10 ng/mL) CIA male DBA/1 mice; injected i.d. on day 0 with emulsion of BTIIC (100 mg) in CFA (1:1, v/v) and on day 21 with emulsion of BTIIC (100 mg) in IFA (1:1, v/v). CIA mice injected i.p. with 3 (20 mg/kg/day) or DMSO, for 21 days
| Suppression of the migratory capacity of RA FLSs Downregulation of the mRNA expression of IL-1β, IL-6, IL-8 and IL-17A in TNF-α-induced RA FLSs Decrease in TNF-α-induced activation of NF-κB signal pathway (phosphorylated NF-κB, IκBα and IKK) and the NF-κB nuclear accumulation Inhibitory effect of NF-κB PDTC on the formation of actin stress fibers and actin cytoskeleton score of RA FLSs Attenuation of synovitis, synovial hyperplasia and invasion into calcified cartilage and bone in CIA mice
| [75] |
| | CIA male rats twice immunized with BTIIC:CFA (1:1) injection into the right hind paw, back and tail (7 days, 2 weeks). On day 15, 3 (20 and 40 mg/kg/day, orally) or diclofenac sodium (5 mg/kg/day, orally) or ROCK inhibitor fasudil (5 mg/kg/day, i.p.) was administered for 4 weeks
| Inhibition of arthritis index, amelioration of joint pathology, diminished hind paw swelling, enhanced blood rheology and synovial cell apoptosis and re-establishment of cytokine negative regulation of ROCK/NF-κB signaling pathways Decreased secretion of IL-6, IL-1β and TNF-α, inhibition of proliferation of synovial tissue, reduction of monocytes and lymphocytes Decreased levels of RhoA, ROCK1, ROCK2, p-NF-κBp65 and p-IκBα levels. Mechanistically, 3 downregulated ROCK/NF-κB signaling pathways
| [74] |
| | | Decrease in the severity of arthritic index values and inhibition of paw edema on combined treatment Reduction of RF, C-reactive protein, total lipids and ACPAs; increased activity of catalase, superoxide dismutase and glutathione peroxidase enzymes and expression of the anti-inflammatory cytokine IL-10 Decreased expression of TLR2 and TLR4, IL-1β, TNF-α, IFN-γ, cell adhesion molecules and nuclear translocation of NF-κB in aorta decreased, when compared to the single-treated groups
| [80] |
| Betulinic acid derivative SH479 (4) | | In vivo inhibition of CD4+ T cell infiltration and cytokine production; inhibition of Th1 and Th17 differentiation as well as antigen-specific T cell proliferation Decrease in arthritis scores as well as bone destruction and cartilage depletion in the CIA mouse model
| [82] |
| Lupeol (5) | | Reduction of paw edema Inhibition of COX-2 and 5-LOX enzymes and reversion of the high serum levels of pro-inflammatory mediators (PGE2, TNF-α and IL-6), RF, C-reactive protein and ceruloplasmin Downregulation of Bcl-2 protein expression and upregulation of Bax, caspase-3 and -9 through PI3K/Akt inhibition
| [83] |
| β-amyrin (6) and Polpunonic acid (7) | Sprague Dawley rats; intraplantar injection of CFA (100 μL) in AA rats and IFA (100 μL) in non-AA rats; rats treated with 6 (3, 10, 30 mg/kg, p.o.), 7 (3, 10, 30 mg/kg, p.o.) and dexamethasone (3 mg/kg, p.o.) or distilled water (10 mL/kg) once every day, for 14 days
| Reduction of the primary and secondary paw swelling and the arthritis score in the later stage of the adjuvant-induced arthritis (from 14.25 to 6.5) Reversion of cartilage erosion and subchondral cyst and Weichselbaum’s lacunae formation Non-marked impact on general hematological and serum biochemical parameters due to treatment with 6, 7 or dexamethasone
| [84] |
| Echinocystic acid (8) | TNF-α-stimulated human RA FLSs at 10 ng/mL for 24 h and treated with 8 (0, 5 or 10 µM) for additional 24 h ZIA in female SKG/Jcl mice; oral administration of 8 (10 or 25 mg/kg) or vehicle (90% glyceryl trioctanoate and 10% DMSO) or MTX (10 mg/kg), i.p., daily, for 3 consecutive weeks, starting on the 21st day after single i.p. injection of zymosan A (2 mg/mice)
| Reduction of synovial hyperplasia, inflammatory cell infiltration and cartilage damage on ankle joints Attenuated levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-17A, IFN-γ and GM-CSF) and sustained reduction in joint swelling of arthritic hind paws, similar to MTX at the highest EA dose Cellular reduction of both protein and mRNA expression of IL-6 and IL-1β by downregulating MAPK and NF-κB pathways The effects were attributed to phosphorylation inhibition of STAT3 (but not JAK2) and subsequent suppression of IL-6- and TGF-β-induced Th17 cell differentiation
| [85] |
| 23-Hydroxyursolic acid (9) | RAW264.7 cells and primary mouse BMDMs; incubation with 9 (0, 1, 3 or 10 µM) in the presence of RANKL (100 ng/mL) and M-CSF (30 ng/mL) for 4 days (RAW264.7) or 6 days (BMDMs) LPS-stimulated ICR mice: oral administration of 9 (25 or 50 mg/kg) or vehicle (corn oil), 1 h before LPS (5 mg/kg, i.p.) injection and thereafter every other day for 8 days
| Inhibition of RANKL-induced osteoclastogenesis in RAW264.7 (IC50 = 1.9 ± 0.2 μM) and BMDMs (IC50 = 2.1 ± 0.3 μM) without affecting cell viability and protected mice against LPS-induced bone loss Attenuation of osteoclast formation by inhibiting RANKL-mediated ERK and JNF phosphorylation, NF-κB signaling, c-Fos expression, NFATc1 activation and expression of osteoclast-specific marker genes (OSCAR, MMP-9, TRAP, DC-STAMP and CtsK), both in vitro and in vivo
| [87] |
| Ursolic acid-3-acetate (10) | | Decrease in clinical arthritis symptoms, paw thickness, histological and radiological changes and serum IgG1 and IgG2a levels Reduction of Th1/Th17 phenotype CD4+ T lymphocyte expansion and inflammatory cytokine production Decreased expression and production of inflammatory mediators, in the knee joint tissue and RA synovial fibroblasts, through the downregulation of IKKα/β, ΙκBα and NF-κB
| [88] |
| Maslinic acid (11) | CAIA male DBA/1J mice treated with 11 (200 mg/kg) by daily oral administration, from day 1 to day 11 Mice injected i.p. with 1 mg of a CII monoclonal antibody on day 8 and 25 μg of LPS on day 11
| Lowering of arthritis score, paw thickness and front paw swelling on day 12 Suppression of the gene expression of inflammatory cytokines downstream of NF-κB signaling and inactivation of the TLR signaling pathway Downregulation of the expression levels of the genes encoding TNF-α, IL-1β, IL-6 and IL-12, and upregulation of IκBα transcript and protein expression Decrease in the production of LTB4 and alteration of the gene expression of glucocorticoids
| [89] |
| Taraxasterol (12) | | Downregulation of IL-1β, increase in TNF-α, IL-6, IL-8, MMP-1 and MMP-3 levels in human RA FLSs and in joint tissues of CIA mice, in a dose-dependent manner Inhibition of NF-κB activations and modulation of the TAK-1/IKK/IκB regulators in human RA FLSs and joint tissues of CIA mice, in a dose-dependent manner NLRP3, TXNIP and ASC expressions were blocked and the maturation of caspase-1 was decreased, in vitro and in vivo Reduction of clinical arthritis score and cartilage destruction in ankle joints of CIA mice Potential therapeutic action of 12 by modulation of NF-κB/NLRP3 inflammasome pathways
| [90] |
| Wilforlide A (13) | LPS/IFN-γ-stimulated macrophages (THP-1 cells) treated with PMA (200 nM) for 3 days, then stimulated with LPS (1 μg/mL) and IFN-γ (100 ng/mL) and treated with 13 (0, 1, 5, 10, 20, 40, 80, 160 and 300 ng/mL) for 48 h
| Reduction of inflammatory infiltration, joint swelling and histological damage in the ankle joints of CIA mice Inhibition of iNOS expression in activated macrophages of arthritic synovial joints, reduction of the high levels of pro-inflammatory cytokines (MCP1, GM-CSF and M-CSF) in joint synovium and enhanced expression of anti-inflammatory cytokines (IL-10 and TGF-β) in mouse serum In vitro inhibition of M1 macrophage polarization by suppressing LPS/IFN-γ-induced TLR4 upregulation, IκBα degradation and NF-κB p65 activation
| [92] |
6.2. Tetracyclic and Rearranged Triterpenes
Antcin K (
14) is a tetracyclic ergostane-type triterpenoid isolated from
Antrodia cinnamomea, a mushroom endemic to Taiwan and used in folk medicine due to its antioxidant, anti-inflammatory and immunomodulatory activities [
93]. Antcin K (
14) decreased pro-inflammatory cytokine production in human RA FLSs by inhibiting the phosphorylation of focal adhesion kinase (FAK), PI3K, Akt and NF-κB. Moreover,
14 also ameliorated paw swelling, cartilage degeneration and bone erosion in the CIA mouse model [
93].
Ganoderic acid A (
15), a lanostane triterpenoid extracted from
Ganoderma lucidum (an edible mushroom), has been traditionally used in East Asia to treat inflammatory, proliferative and immunological diseases without side effects, making it a potential therapeutic agent for RA [
94,
95]. Cao et al. evaluated the protective effects of
15 in CIA rats to explore its therapeutic role in RA [
95]. A reduction in toe swelling and arthritis index was observed, as well as an improvement in joint pathological changes and hemorheology. Serum and synovium levels of IL-1β, IL-6 and TNF-α were markedly reduced in CIA rats, and oxidative stress was regulated.
15 substantially reduced p-STAT3 and suppressor of cytokine signaling 1 (SOCS1); these results indicate a downregulation of protein expression of p-JAK3 and p-STAT3, which may lead to the regulation of the JAK/STAT signaling pathway [
95]. Furthermore, protein expression levels of p-NF-κB p65 and p-IκBα in joint synovial tissue of CIA rats were reduced by
15. The therapeutic role may also be related to the regulation of the NF-κB signaling pathway [
95].
Gedunin (
16), a limonoid-type triterpenoid isolated from several genera of the Meliaceae family, such as the Indian neem tree (
Azadirachta indica), antagonized ROS production and reduced pro-inflammatory cytokine levels and iNOS expression in LPS-stimulated macrophages (RAW264.7 cells), TNF-α-stimulated FLSs (MH7A cells) and IL-1β-stimulated primary RA FLSs [
96]. Furthermore,
16 was able to reduce paw swelling, arthritis score and cytokine production in CIA mice [
96]. The
in vitro and
in vivo anti-inflammatory and anti-arthritic effects of
16 were due to activation of the Nrf2 signaling through inhibition of Keap1, a key oxidative stress sensor protein, by inducing p62 expression and upregulation of anti-oxidative enzymes, including heme oxygenase (HO)-1 [
96].
Other studies showed that 7-deacetyl-gedunin (
17) isolated from the fruits of
Toona sinensis (A. Juss.) Roem suppressed ROS production and inhibited proliferation of human RA FLSs isolated from the joint synovium cave of RA patients submitted to knee surgery [
97]. Compound
17 also decreased pro-inflammatory cytokine release in human FLSs (MH7A cells) but with significant inhibition of cell viability [
97]. Mechanistic studies revealed that
17 exerted anti-inflammatory effects by regulating antioxidative enzymes through Nrf2 activation by inhibiting Keap1 via inducing p62 expression and antioxidant response element (ARE)-driven gene transcription [
97].
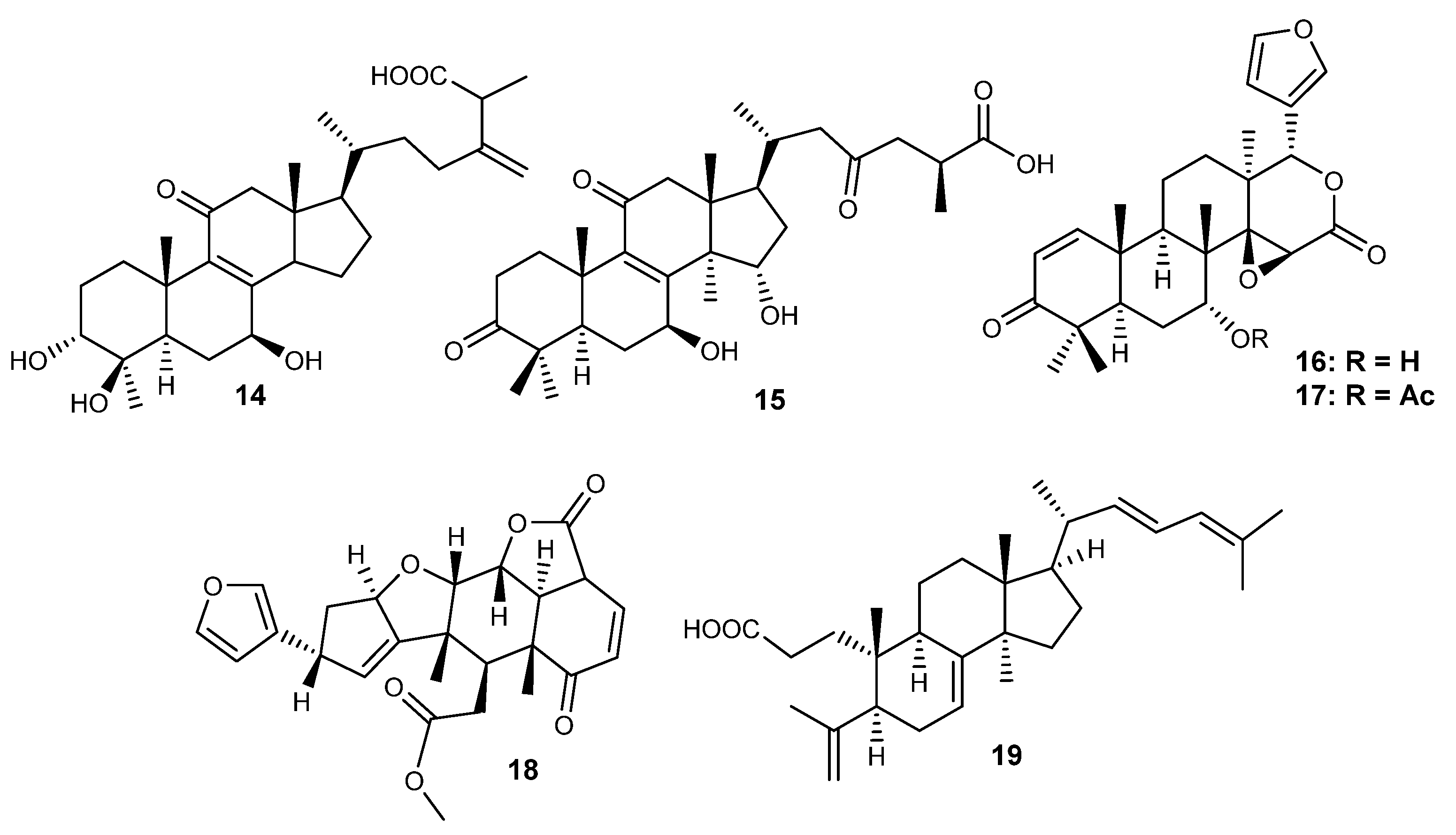
Figure 3.
Structures of tetracyclic (14–15) and rearranged triterpenes (16–19) with activity on RA.
Figure 3.
Structures of tetracyclic (14–15) and rearranged triterpenes (16–19) with activity on RA.
Nimbolide (
18), a major limonoid from
Azadirachta indica, dose-dependently reduced the expression of p38 MAPK and inhibited the phosphorylation of NF-κB in IL-β-stimulated rabbit FLSs (HIG-82 cells) [
98]. In a rat model of inflammatory arthritis,
18 significantly reduced STAT3 phosphorylation, attenuating STAT3 signaling with simultaneous inhibition of Notch-1 transmembrane protein receptors and NF-κB activation, thus reducing oxidative stress and pro-inflammatory cytokine levels in synovial tissue of arthritic rats [
98]. Furthermore, combination therapy with both
18 (3 mg/kg/day) and MTX (2 mg/kg/week) potentiated the anti-arthritic effects of MTX while reducing its hepato-renal toxicity in a rat model of RA, presumably through antioxidant and anti-inflammatory effects [
98]. The efficiency of nimbolide (
18) was also examined by Cui et al. against joint inflammation in CIA male albino rats [
99]. Treatment with
18 (20 mg/kg) resulted in a substantial increase in body weight and a pronounced reduction in arthritic index score, thymus and spleen indices, hind paw volume and edema formation, comparable to diclofenac [
99]. Serum levels of IL-1β, IL-6, IL-10 and TNF-α showed a marked reduction in arthritic rats, and the activities of antioxidant enzymes were significantly improved. Supplementation with
18 downregulated the protein expression of iNOS, NF-κB, p-IκBα, IKKα and COX-2, reinforcing the contribution of nimbolide to the therapeutic strategy against RA [
99].
Heilaohuacid G (
19) is a new 3,4-seco-lanostane type triterpenoid isolated from the roots of
Kadsura coccinea, a medicinal plant distributed in South China and used in Tujia ethnomedicine to treat RA [
100]. Biological activity screening tests revealed that
19 inhibited the proliferation of RA FLSs in a concentration-dependent manner, with IC
50 values of 8.16 ± 0.47 μM [
100]. Further studies showed that
19 induced RA FLS apoptosis and suppressed inflammatory responses in LPS-induced RA FLSs and macrophages (RAW264.7 cells) by inhibiting NF-κB signaling [
100].
Table 5.
Tetracyclic and rearranged triterpenes with in vitro/in vivo RA-related effects.
Table 5.
Tetracyclic and rearranged triterpenes with in vitro/in vivo RA-related effects.
| Tetracyclic and Rearranged Triterpenes | Cell Model/Animal Model/Dosage | Effects and Mode of Action | Ref. |
|---|
| Antcin K (14) | Human RA FLSs (MH7A cells) treated with 14 (0, 0.3, 1, 3 or 10 μM) for 24 h CIA C57BL/6J mice treated with 14 (0, 10 or 30 mg/kg), i.p., on alternated days for 4 weeks
| Inhibition of pro-inflammatory cytokines (TNF-α, IL-1β and IL-8) in human RA FLSs through downregulation of FAK, PI3K, Akt and NF-κB signaling pathways Amelioration of paw swelling, cartilage damage and bone erosion in CIA mice and decreased serum levels of TNF-α, IL-1β, IL-6 and IL-8
| [93] |
| Ganoderic acid A (15) | Rats twice immunized with BTIIC:CFA (1:1) s.c. injection into the right hind paw, back and tail root (7 days, 2 weeks); on day 15, oral administration of 15 (20 and 40 mg/kg/day) or diclofenac sodium (5 mg/kg/day) or physiological saline, for 4 weeks
| Improvement of glossiness, food intake and body weight of rats Reduction of swelling and limping of the hind feet, degree of toe swelling and joint inflammation Decrease in TNF-α, IL-6 and IL-1β serum and synovium levels was observed. p-JAK3, p-STAT3, SOCS1, p-NF-κB p65 and p-IκBα protein expression levels were significantly reduced The mechanism may lie in the downregulation of JAK/STAT and NF-κB signaling pathways
| [95] |
| Gedunin (16) | LPS-induced macrophages (RAW264.7 cells), TNF-α-stimulated FLSs (MH7A cells) and IL-1β-stimulated primary RA FLSs; cells pre-treated with 16 (0, 1, 5, 10, 25 or 50 μM) for 1 h and incubated with 100 ng/mL LPS (RAW264.7 cells), 10 ng/mL TNF-α (MH7A cells) or 2.5 ng/mL IL-1β (RA FLSs) for 24 h CIA DBA/1 male mice; daily i.p. administration of 16 (2.5 or 5 mg/kg) or vehicle (saline, PEG400 and DMSO 6:3:1 v/v) or MTX (10 mg/kg), intragastrically, for 20 days
| Reduction of iNOS expression, inhibition of IL-1β, IL-6 and TNF-α secretion and antagonization of ROS production in vitro Reduction of arthritis incidence, suppression of mRNA expression of IL-1β and amelioration of arthritis score, paw edema and bone erosion in CIA mice Mechanistic in vitro studies showed that 16 downregulated Keap1 protein expression and upregulated that of Nrf2, HO-1, NQO1 and p62, in time- and dose-dependent manners
| [96] |
| 7-Deacetyl-gedunin (17) | TNF-α- stimulated MH7A cells and IL-1β-stimulated human RA FLSs from the joints of RA patients; cells treated with 17 (0, 1, 2.5, 5, 10, 25, 50 75, 100 or 150 μM) for 24, 48 or 72 h after incubation with 10 ng/mL TNF-α (MH7A cells) or 2.5 ng/mL IL-1β (RA FLSs)
| Suppressed cell proliferation, inhibited ROS production and downregulated MMP-1, -3, -9 and -13 without cytotoxicity (IL-1β-treated cells) Downregulation of IL-6 and IL-33 with inhibition of cell viability (TNF-α-treated cells) Mechanistically, 17 increased the expression of anti-oxidative enzymes (HO-1 and NQO1) and p62, thus downregulating Keap1 and activating Nrf2
| [97] |
| Nimbolide (18) | IL-1β stimulated rabbit FLSs (HIG-82 cells) pre-treated with 18 (0, 0.5 or 1 μM) for 24 h and stimulated with IL-1β (10 ng/mL) for next 6 h AA Wistar rats injected with CFA (100 μL, i.a.) in the knee joint and treated with 18 (1 or 3 mg/kg) or vehicle (1% DMSO), i.p., daily, for 21 days
| Inhibition of the migration of FLSs in vitro (IC50 3.29 ± 0.15 μM) and decreased expression levels of iNOS, COX-2, MMP-2 and p38. Suppressed nitroso-oxidative stress and reduced the levels of iNOS, COX-2, IL-6 and MMP-2, both in vitro and in vivo Decrease in synovial hyperplasia, prevention of cartilage destruction, pain attenuation and amelioration of arthritis progression in vivo by abrogating the STAT3/NF-κB/Notch-1 signaling pathway in synovial tissue of arthritic rats
| [98] |
| | Rats injected with CFA (100 μL, i.d.) into the right hind footpad and treated with vehicle (DMSO), 18 (20 mg/kg/day) or diclofenac sodium (5 mg/kg/day), by oral gavage, for 25 days
| Significant body weight increase Decrease in paw volume and arthritic index score and in activities of liver marker serum enzymes (SGOT, SGPT, ALP) Reduction of serum levels of TNF-α, IL-6, IL-1β and IL-10. Decrease in MDA levels and enhancement in activities of antioxidant enzymes; outcomes were comparable to diclofenac sodium 18 reduced the higher protein levels of COX-2, iNOS, NF-κB, p-IκBα and IKKα in CFA-induced RA rats
| [99] |
| Heilaohuacid G (19) | LPS-stimulated human RA FLSs and macrophages (RAW264.7 cells). Cells treated with 19 (0, 2.5, 5, 10 or 20 μM) for 24 h (RAW264.7) or 48 h (RA FLSs) and then incubated with LPS (100 ng/mL) for another 4 h
| Inhibition of RA FLS proliferation with IC50 value of 8.16 ± 0.47 μM Induction of RA FLS apoptosis and inhibition of the secretion of pro-inflammatory cytokines Reduced TNF-α and IL-6 in LPS-induced RA FLSs and RAW264.7 cells by suppressing NF-κB signaling
| [100] |
6.3. Triterpenic Saponins
Astragaloside (
20), a saponin found in
Astragalus membranaceus, suppressed excessive FLS proliferation in the AA rat model of RA through the inhibition of the expression of the long non-coding RNA (lncRNA) LOC100912373 and increased release of miR-17-5p, which binds to 3-phosphoinositide-dependent protein kinase 1 (PDK1) and prevents activation of the PDK1/Akt pathway [
101]. Abnormal expression of non-coding RNAs, such as miRNAs and lncRNAs, has been implicated in the pathogenesis of autoimmune diseases, including RA [
102]. lncRNAs are expressed by many immune system cells, including T and B lymphocytes, monocytes, macrophages and dendritic cells, and lncRNA dysregulation has been associated with autoimmunity onset [
102]. Additionally, lncRNAs can act as molecular sponges, sequestering miRNAs and RNA-binding proteins, hindering interactions with their target RNAs [
102]. The lncRNA LOC100912373 is a critical gene involved in RA pathogenesis since it can induce FLS proliferation by competing with miR-17-5p and thus promoting activation of the PDK1/Akt signaling pathway that contributes to RA development [
103].
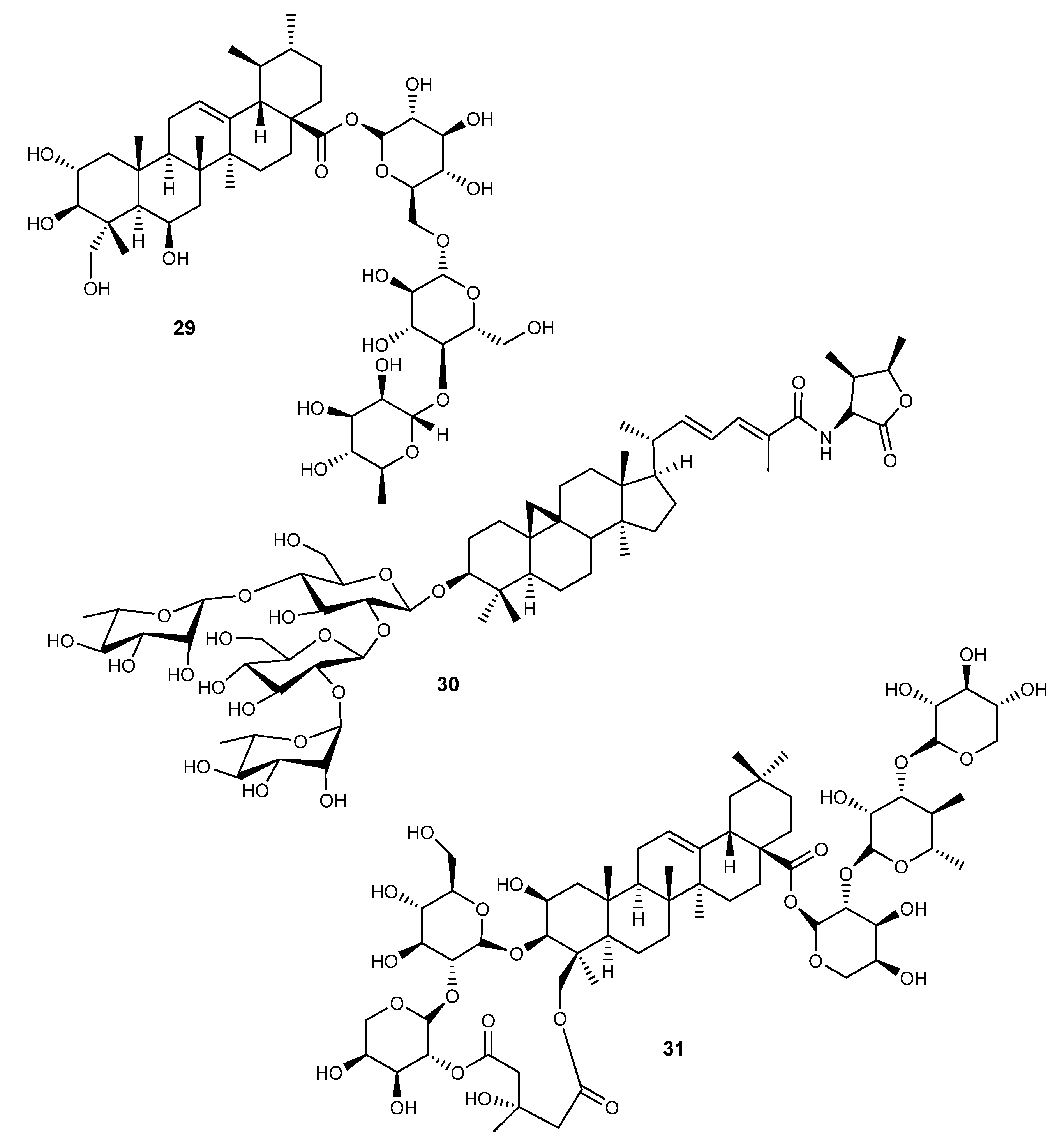
Figure 4.
Structures of triterpenic saponins (20–31) with activity on RA.
Figure 4.
Structures of triterpenic saponins (20–31) with activity on RA.
Chikusetsusaponin IVa (
21), an oleanane-type saponin from
Panax japonicus C.A. Mey, alleviated RA symptoms in CIA mice [
104]. Molecular docking and molecular dynamics simulations revealed that
21 can bind to RA core targets IFN-γ and IL-1β. The study results suggest that the
in vivo anti-inflammatory and osteoprotective effects of
21 were due to inhibition of the JAK/STAT signaling pathway [
104].
Immunopathology in RA is driven by a predominance of arthritogenic Th1 cells (secreting IFN-γ) and Th17 cells (secreting IL-17) over T
reg cells [
105]. Cytokine IL-6 is critical for the differentiation of Th17 cells and the balance between pathogenic Th17 and protective T
reg [
105]. Biologic DMARDs targeting the IL-6 receptor have been shown to improve signs and symptoms of RA. Chikusetsusaponin IVa butyl ester (
22) is a triterpenoid saponin extracted from
Acanthopanas gracilistylus and a small-molecule IL-6R inhibitor. IL-6R blockade by
22 inhibited Th17 cell differentiation, IL-17A secretion and STAT3 phosphorylation in mouse CD4
+ cells (under Th17 polarization conditions)
in vitro and ameliorated RA symptoms in the CIA mouse model [
106]. Thus, saponin
22 represents a promising agent for RA therapy.
Circular RNAs (circRNAs), which are endogenous non-coding RNAs forming stable covalently closed-loop structures, act as miRNA sponges and participate in the regulation of several cellular signaling pathways [
102,
107]. circRNAs are important epigenetic modulators of gene expression in inflammation and autoimmune regulation, closely associated with RA pathogenesis [
102,
107]. Clematichinenoside AR (
23) is a triterpenoid saponin isolated from the roots of
Clematis chinensis Osbeck. Saponin
23 inhibited proliferation and inflammatory response in FLSs from RA patients
in vitro and ameliorated RA pathology in CIA mice by combining with frizzled class receptor 4 (FZD4) and blocking the circular pleiotrophin (circPTN)/miR-145-5p/FZD4 signal axis [
108]. The authors demonstrated that circPTN promoted FZD4 expression through sponging miR-145-5p with subsequent activation of the Wnt/β-catenin pathway [
108]. Confocal microscopy showed that
23 downregulated the expression of β-catenin and its nuclear entry in FLSs by binding FZD4, thus inhibiting the Wnt pathway [
108]. Compound (
23) was also the focus of Xiong et al., who explored its protective action against human TNF-α-induced inflammation and cytotoxicity based on the accumulated evidence about the correlation between RA therapeutic effects and the antagonist effects against TNF-α in RA mouse models [
109].
23 markedly inhibited IL-6 and IL-8 release from recombinant human (rh) TNF-α-stimulated MH7A cells. Cartilage and bone destruction were reversed, probably through downregulation of MMP-1 expression and downregulation of p38 and ERK MAPK signal activation by
23 in rhTNF-α-induced MH7A cells [
109]. Treatment of TNF-α-sensitive murine fibroblast L929 cells with
23 reduced the proliferation inhibition ratio caused by rhTNF-α/actinomycin D (ActD) and antagonized rhTNF-α-induced cytotoxicity. Morphological changes in apoptosis (including chromatin condensation, nuclear fragmentation and cell shrinkage) stimulated by rhTNF-α/ActD in L929 cells were attenuated after pre-treatment with
23 [
109]. The antagonistic effect of
23 upon cytotoxicity might be ascribed to the degeneration of ROS and the raising of mitochondrial membrane potential, together with the inhibition of prolonged JNK activation following pre-treatment.
Entadaosides
24–
28, oleanane-type triterpene saponins isolated from the stems of
Entada phaseolides (L.) Merr, possess anti-inflammatory properties and are used in traditional Chinese medicine for the treatment of arthritis [
110]. All entadaoside saponins
24–
28 were able to prevent RA progression and ameliorate hyperalgesia, paw swelling and joint destruction in CIA rats by reducing pro-inflammatory cytokine levels, upregulating ubiquitin-editing enzyme A20 expression, inhibiting p38 and ERK1/2 in the periphery and phosphorylation of p38 in the spinal cord [
110].
Madecassoside (
29) is a pentacyclic triterpenoid saponin present in
Centella asiatica, with previously reported anti-inflammatory and anti-arthritis potential, among other important biological activities. It was also found to induce apoptosis of keloid fibroblasts and keratinocytes and to inhibit LPS-induced TNF-α production, as well as the migration of keloid fibroblasts [
111]. Yu et al. used IL-1β stimulation to induce the invasion of FLSs, aiming at exploring the anti-arthritis mechanism of saponin
29 [
112]. It was found that oral administration of the triterpenoid exerted a significant therapeutic effect, reducing the articular and bone tissue damage and decreasing hyperemia in the synovial tissue. A dose-dependent
in vitro inhibitory effect on FLS invasion mediated by IL-1β was also observed, as well as a decrease in MMP-13 activity and mRNA level expression, possibly by preventing NF-κB translocation and phosphorylation.
Qiao et al. compared the anti-arthritis effect of madecassoside (
29) and its metabolite madecassic acid in pseudo-germ-free CIA rats, discussing the influence of gut microbiota and the mechanism of
29 to stimulate T
reg cells [
113]. Previous studies revealed the potential of
29 to increase the number of T
reg cells in the small intestine, improving the release of IL-10 through the increase in Foxp3
+ T lymphocytes in the intestinal lamina propria. However, neither
29 nor its metabolite was able to foment the differentiation of T
reg cells and the expression of IL-10 in CD4
+T cells of CIA rats [
113]. In the comparison study, oral administration of
29 was shown to mitigate the arthritis symptoms and the histological alterations in CIA rats, unlike intestinal madecassic acid, suggesting its functionality in the parent form. The increased number of T
reg cells by oral administration of
29 was observed mainly in the ileum but without a significant effect concerning T
reg cell differentiation and Foxp3 and IL-10 expression
in vitro. The anti-arthritis effect of compound
29 was strongly influenced by gut microbiota; the sequencing of the 16S rRNA gene indicated that
29 antagonized the richness and diversity of gut microbiota in CIA rats, enhancing the level of n-butyric acid (which increased the immunosuppressive function of T
reg cells
in vitro). The co-administration of heptanoyl CoA (a competitive inhibitor of butyrate synthase) confirmed the contribution of madecassoside-induced butyrate to the anti-arthritis action, as it caused the downregulation of ileum T
reg cell number and expression of Foxp3 and IL-10 [
113].
Mussaendoside O (
30), a
N-triterpene cycloartane saponin isolated from
Mussaenda pubescens, inhibits RANKL-induced osteoclastogenesis
in vitro in a concentration-dependent manner [
114]. Moreover, 30 attenuates LPS-induced bone resorption and osteoclast formation in mice by repressing RANKL-induced activation of p38 MAPK and JNK, preventing c-Fos activation and subsequent expression of NFATc1. Saponin
30 also diminished RANKL-induced increase in mRNA expression of NFATc1 target genes, including OSCAR, TRAP, DC-STAMP and cathepsin K [
114].
Tubeimoside I (
31) is a triterpenoid saponin previously isolated from
Bolbostemma paniculatum tubers and found in several Chinese medicine preparations, with anti-inflammatory, anti-tumor and anti-viral activities [
115]. The effect of this compound on RA was studied
in vivo using a CIA rat model and
in vitro using cultured FLSs [
116]. The treatment with
31 suppressed the synovial inflammation and bone destruction in CIA rats in a dose-dependent manner, decreasing erythema and swelling at the doses of 5 and 10 mg/kg. These results were further confirmed by histopathological assays. Moreover, when compared with the control group, an important decrease in pro-inflammatory cytokine production was observed in the joint tissues of tubeimoside I-treated rats, including IL-1β, IL-6, IL-8 and TNF-α, and downregulation of MMP-9 expression.
In vitro studies also showed that the compound suppressed the proliferation and migration of FLS cells, which are the main causes of synovial hyperplasia, contributing to the cartilage destruction and exacerbating joint damage. The authors suggested that the observed effects may be due to the inhibition of TNF-α-induced activation of NF-κB and MAPKs (p38 and JNK) [
116].
Ginsenosides are glycosylated dammarane-type triterpenoids unique to ginseng species. Ginseng is a drug derived from the roots of
Panax ginseng, used in traditional medicine to treat several diseases, including anemia, diabetes, gastritis and insomnia. It is also used as a general restorative, promoting health and longevity [
21]. Based on the location and number of glycoside residues, ginsenosides can be further subdivided into protopanaxadiols (e.g., Rb1, Rb2, Rg3, Rg5 and Rh2), with glycoside residues attached to C-3 and/or C-20 positions, or protopanaxatriols (e.g., Rg1, Rg2 and Rh1), with an additional glycoside residue at C-6 (
Figure 4).
Zhang et al. compared several ginsenosides (CK, Rg1, Rg3, Rg5 and Rb1;
32–
36) from
Panax ginseng Meyer for their therapeutic effect on RA [
117]. Ginsenoside CK (
32) is the major metabolite of natural diol ginsenosides in the intestinal tract [
117]. Among the tested ginsenosides,
32 was the most effective, showing strong anti-inflammatory and immunomodulating properties [
117]. Ginsenoside CK (
32) significantly inhibited cell proliferation and enhanced apoptosis of LPS-activated RAW264.7 and TNF-α-stimulated human umbilical vein endothelial cells (HUVECs) [
117].
In vivo,
32 ameliorated swelling and joint functional impairment in CIA mice [
117]. Moreover,
32 was able to increase CD8
+ T cells to downregulate the immune response and decrease the number of activated CD4
+ T cells and M1 macrophages, thus inhibiting pro-inflammatory cytokine secretion [
117]. In an attempt to explore the mechanism of macrophage polarization and phagocytosis by compound
32, Wang et al. concluded that, through β-arrestin2 regulation in peritoneal macrophages, the compound inhibited TLR4 coupling with Gαi and stimulated TLR4-Gαs coupling [
118]. Due to the significant decrease in colocalization of β-arrestin2 and Gαi, owing to
32 treatment, their combined interaction foments the regulation of immune inflammation and the polarization of macrophages to M1. Potential therapeutic properties of
32 for RA therapy seem to be related to the reduction of M1 polarization and secretion of inflammatory cytokines, while overexpressing M2 and IL-10 levels to alleviate inflammation and repair bone tissue in CIA mice. Compound
32 also appears to restore B cell function, in addition to alleviating clinical manifestations of RA (such as the polyarthritis index, spleen and joint pathological scores and spleen index) and the level of serum antibodies in CIA mice [
119]. Although IgD B cell receptor (BCR) endocytosis was promoted, it should be noted that the expression level of IgD-BCR did not change.
32 facilitated the co-localization between β-arrestin1 and IgD and between adaptor protein 2 (AP2) and IgD. Mechanistically, the IgD-BCR internalization, in a β-arrestin-AP2-dependent manner, led to the inhibition of B cell activation, which may explain the improvement observed in CIA model mice [
119].
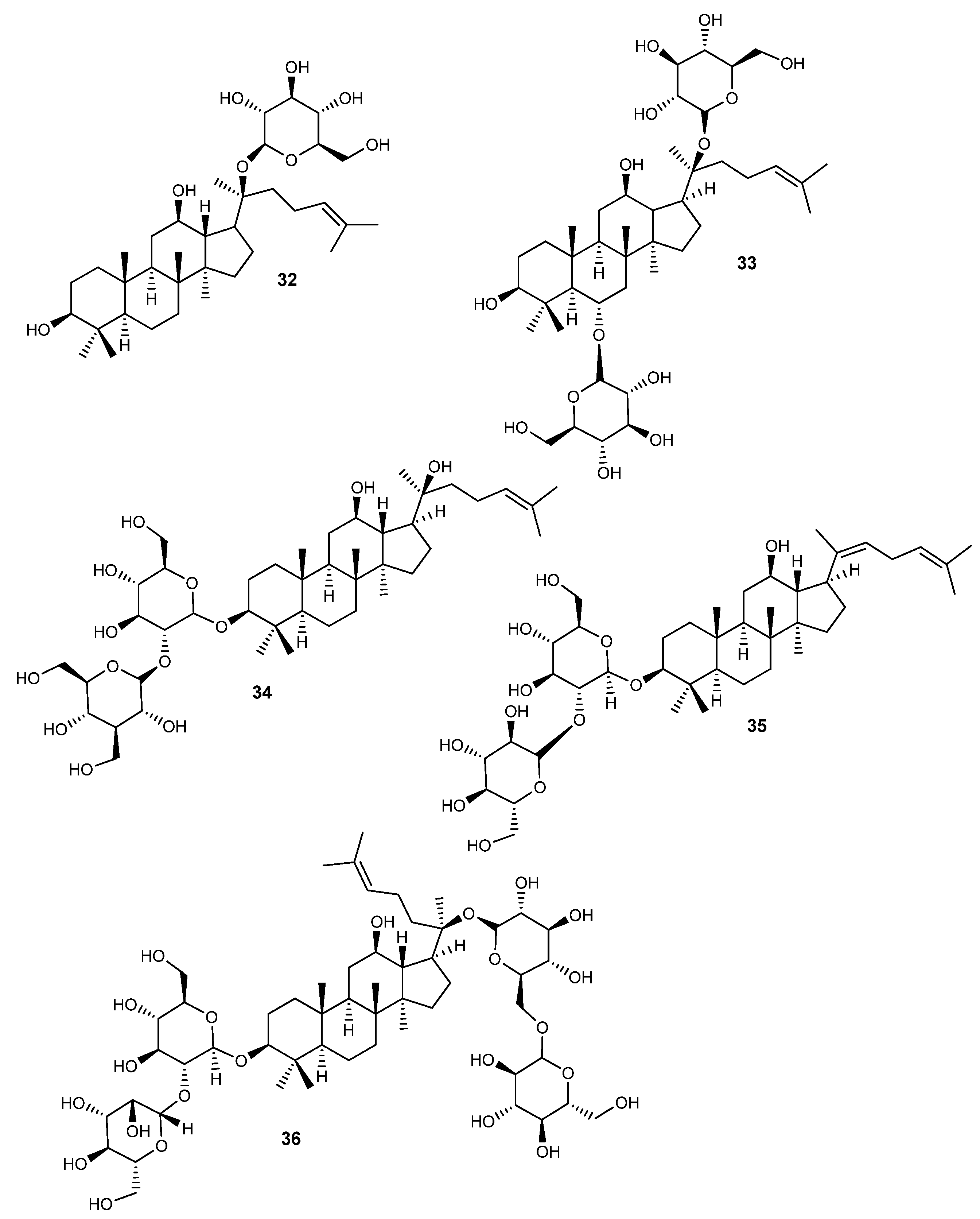
Figure 5.
Structures of ginsenosides (32–36) with activity on RA.
Figure 5.
Structures of ginsenosides (32–36) with activity on RA.
Ginsenoside CK (
32) is thus a potential candidate for RA therapy and is currently being tested as an anti-RA drug in China. Phase 1 clinical trials in healthy Chinese volunteers to evaluate the pharmacokinetics and safety of
32 showed that a single oral dose of a 200 mg tablet was well tolerated, reaching a maximum plasma concentration (
Cmax) of 796.8 ng/mL in 3.6 h (
Tmax) with a terminal half-life (
t1/2) of 27.7 h [
120]. High-fat food was found to accelerate and increase absorption of
32 while plasma levels were slightly higher in women compared to men [
120]. A double-blind, phase 2 study (NCT03755258) to evaluate the safety, efficacy and pharmacokinetics of ginsenoside CK (
32) tablets in RA patients started in China in March 2017. RA patients (
n = 128) were randomly assigned ginsenoside CK tablets (100, 200 or 300 mg) or placebo once daily, orally, for 12 weeks. However, the study was suspended after two years due to the high cost associated with manufacturing of
32, essentially dependent on
Panax plants, extraction and biotransformation of ginsenosides. Therefore, development of alternative production methods, such as microbial fermentation processes suitable for scale-up, is an attractive solution.
Many studies on the anti-inflammatory effect of ginsenoside Rg3 (
34) have been described, emphasizing its ability to regulate NF-κB activity, causing the reduction of cytokine levels, to promote M2 macrophage polarization and to inhibit the inflammation process in the liver through the activation of the PI3K/AKT signaling pathway [
53]. Considering the lack of mechanistic robustness regarding the effect of ginsenoside Rg3 in RA, Zhang et al. evaluated the anti-inflammatory effect of the compound
34 through a set of clinical features, pathological alterations and cytokine levels observed in RA mice. CD4
+CD25
+Foxp3
+ T
reg cell percentage was analyzed and a metabolomic analysis (GC-MS/MS) was performed, aiming to provide information on immunosuppressive activity and related mechanisms [
53]. Treatment with
34 (25 mg/kg) led to a decrease in IL-6 and TNF-α levels and an increase in TGF-β and IL-10 levels, mirroring its anti-inflammatory potential.
34 regulated the pathways of oxidative phosphorylation and maintained peripheral immune tolerance in RA mice, enhancing the function of CD4
+CD25
+Foxp3
+ T
reg cells [
53].
Table 6.
Triterpenic saponins with in vitro/in vivo RA-related effects.
Table 6.
Triterpenic saponins with in vitro/in vivo RA-related effects.
| Triterpenic Saponins | Cell Model/Animal Model/Dosage | Effects and Mode of Action | Ref. |
|---|
| Astragaloside (20) | AA rat FLSs incubated with 20 (0, 7.8, 15.6, 31.25, 62.5, 125, 250 or 500 mg/L) for 24, 48 or 72 h at 37 °C AA in male SD SPF grade rats; rats were immunized with single CFA injection into the left hind foot and studied for 20 days
| Inhibition of FLS proliferation, reduction of lncRNA LOC100912373 expression, increased miR-17-5p expression and decreased PDK1 and p-AKT levels Reversion of the effects of LOC100912373 overexpression on FLS proliferation and cell cycle progression by regulating the expression of LOC100912373 and the miR-17-5p/PDK1 axis
| [101] |
| Chikusetsusaponin IVa (21) | | Reduction of arthritis index, joint synovial inflammation, paw edema and bone loss in CIA mice Decrease in both rat serum levels and mRNA expression of inflammatory cytokines (IL-1β, IL-6, IFN-γ and TNF-α) and inhibition of protein expression levels of JAK1, JAK2, STAT3 and p-STAT3 in the rat synovial tissue Molecular docking and molecular dynamics simulations revealed that 21 binds to IFN-γ and IL-1β
| [104] |
| Chikusetsusaponin IVa butyl ester (22) | Naïve CD4+ cells from C57BL/6 mouse spleens, incubated with 22 (0, 2.5, 5.0, 7.5 or 10 μM) for 24 h before stimulation under Th17 polarizing conditions for 3 days CIA DBA/1J mice treated with 22 (20 or 40 mg/kg) or vehicle (5% Solutol® HS), 6 days weekly for 8 weeks from first immunization
| Decrease in arthritis scores, inflammation scores of the ankle joints, hind paw swelling and ankle joint bone erosion in CIA mice Reduction of Th17 cells and increased Treg cells, reversing the abnormal Th17/Treg ratio in the spleens of CIA mice In vitro inhibition of Th17 cell differentiation, IL-17A secretion and STAT3 phosphorylation and decrease in mRNA levels of IRF4 and RORγT in splenic CD4+ cells under Th17 polarization conditions
| [106] |
| Clematichinenoside AR (23) | FLSs from RA patients and CIA mice incubated with 23 (0.187 mg/L) for 36 h CIA DBA/1 SPF grade mice administered 23 (0, 0.18, 0.37, 0.75 or 1.5 mg/kg) or MTX (0.75 mg/kg), by oral gavage, on the 28th day after first immunization
| Inhibition of the arthritis score of CIA mice, reduction of paw swelling and restored mouse body weight Suppression of FLS proliferation, secretion inhibition of IL-1β, IL-6 and IL-8 and reduction of the expression of β-catenin, fibronectin and MMP-3 in vitro Inhibition of the Wnt/β-catenin pathway by binding to FZD4 and blocking the circPTN/miR-145-5p/FZD4 signal axis
| [108] |
| | Human RA FLSs (MH7A cells) incubated with 23 (3, 10 or 30 μM) for 1 h, followed by exposure to rhTNF-α (10 ng/mL) for 24 h TNF-α-sensitive mouse fibroblast (L929) cells pre-incubated for 1 h with 23 (1 10 or 100 μM), followed by rhTNF-α (5 ng/mL) stimulation in the presence of ActD (0.5 μg/mL) for 24 h
| Significant reduction of IL-6 secretion and IL-8 production in a concentration-dependent mode, in MH7A cells stimulated by recombinant human TNF-α Decrease in rhTNF-α-induced MMP-1. Suppression of phosphorylated levels of p38 and ERK1/2 produced by rhTNF-α. Abolition of rhTNF-α-induced L929 cell cytotoxicity Attenuation of L929 cells’ morphological induced modifications (increase in cell density and decrease in apoptotic morphology levels) Mechanistically, anti-destructive effects of 23 caused by rhTNF-α may be through the downregulation of MMP-1 expression, and the protective effects of murine L929 cells may lie in the suppression of JNK continuous phosphorylation
| [109] |
| Entadaosides (24–28) | | Reduction of mRNA levels and production of pro-inflammatory cytokines (TNF-α, IL-17) in synovial tissues and hind paw joint Upregulation of A20 and inhibition of ERK1/2 activation in hind paw joints as well as p38, both in the periphery and spinal cord
| [110] |
| Madecassoside (29) | CIA-induced Wistar rats twice injected i.d. at the base of tail with emulsion CII in CFA (1 mg/mL), on day 0 (200 μL) and day 7 (100 μL). From day 14 to day 30, oral administration of 29 (30 mg/kg) or madecassic acid (15 mg/kg) or vehicle (CMC-Na). Co-administration of heptanoyl CoA (0.3 mg/kg) with MAD, through insertion of a Teflon canula into the anus (8 cm), from day 14 to day 34.
| Decrease in the maximum paw swelling and arthritis index score; improvement of body weight loss and histological changes (joints’ synovial hyperplasia, inflammatory cell infiltration and cartilage and bone destruction) Reversion of changes in gut microbiota, rise in acetic acid and butyric acid levels Selective promotion of the production of Treg cells in the parent form (29), although in vitro the effects on Treg cell differentiation and the expression of Foxp3 and IL-10 were not so significant Increase in the expression of Treg cells and promotion of the expression of Foxp3 and IL-10 in rat ileum (rather than duodenum and jejunum), fomented by sodium butyrate (in a concentration-dependent mode)
| [113] |
| | | | [112] |
| Mussaendoside O (30) | Mouse BMDMs and RAW264.7 cells incubated with 30 (0, 0.3, 1 or 3 μM) in the presence of RANKL (100 ng/mL) and M-CSF (30 ng/mL) for 4 days (RAW264.7) or 7 days (BMDMs) LPS-stimulated ICR mice treated with 30 (10 or 20 mg/kg) or vehicle (corn oil), orally, 1 h before the first injection of LPS (5 mg/kg, i.p.) and thereafter every other day for 8 days
| Inhibition of RANKL-induced osteoclast differentiation in BMDMs (IC50 0.75 ± 0.15 μM) and RAW264.7 (IC50 0.75 ± 0.15 μM), without decreasing cell viability 30 failed to inhibit LPS-induced production of pro-inflammatory mediators (NO, iNOS, COX-2 and TNF-α) in RAW264.7 cells Inhibition of RANKL-induced osteoclastogenesis in vitro was attributed to the impairing of c-Fos and subsequent NFATc1 expression At 20 mg/kg, 30 significantly protected mice against LPS-induced bone loss presumably by suppressing c-Fos expression through inhibition of JNK and p38 MAPK pathways
| [114] |
| Tubeimoside I (31) | | | [116] |
| Ginsenosides CK, Rg1, Rg3, Rg5 and Rb1 (32–36) | LPS-activated RAW264.7 cells and TNF-α-stimulated HUVECs; cells treated with 100 ng/mL LPS (RAW264.7) or 10 ng/mL TNF-α (HUVECs) for 24 h followed by incubation with each ginsenoside (1.5625, 3.125, 6.25, 12.5, 25, 50, 100 or 200 μg/mL), MTX (positive control) or DMSO (negative control) CIA male DBA/1 mice treated with 15 mg/kg ginsenosides (32–36) or vehicle (0.5% Tween-80), i.v., once every 2 days, 15 times, after onset of joint swelling
| All ginsenosides 32–36 showed good therapeutic effect on acute arthritis. Among the tested ginsenosides, 32 was the most effective In vitro, 32 inhibited cell proliferation and enhanced apoptosis In vivo, 32 reduced swelling and joint functional impairment in CIA mice 32 increased CD8+ T cells to downregulate the immune response and decreased the number of activated CD4+ T cells and pro-inflammatory M1 macrophages, inhibiting the secretion of TNF-α and IL-6
| [117] |
| Ginsenoside CK (32) | CIA DBA/1 mice; CIA induced by two i.d. injections in the tail root with 100 μL emulsion of CII (1 mg/mL) and Calmette’s vaccine (2 mg/mL), on days 0 and 21. On day 28, mice treated with intragastric administration of 32 (112 mg/kg/day) or MTX (2 mg/kg/day), for 24 days.
| 32 restored mouse body weight and alleviated symptoms of arthritis Spleen index was attenuated, and proliferation of splenic and thymic lymphocytes was inhibited Secretion of IL-1β, IL-17 and TNF-α was decreased. IL-10 level was promoted in serum and macrophage culture supernatants. M1 and M2 macrophages were diminished and augmented, respectively Inhibition of the expression of Gαi, TLR4 and NF-κB, increasing Gαs level. The performance of 32 was similar to MTX. But unlike MTX, 32 inhibited the expression of β-arrestin2. Through β-arrestin2 regulation in macrophages, 32 inhibited TLR4–GαI coupling and promoted TLR4–Gαs coupling
| [121] |
| | CIA male DBA/1 mice; CIA induced by two i.d. injections in the tail root with 100 μL emulsion of CFA and CCII (at equal volumes), on days 0 and 21. Mice treated with 32 (28, 56 or 112 mg/kg) or MTX (2 mg/kg) or vehicle (CMC-Na), from day 28 to day 51
| Improvement of the polyarthritis index, swollen joint count, spleen and joint pathological scores and spleen index Abnormal B cell spreading was inhibited. Production of serum antibodies (IgG1, IgG2a, anti-CII) was prevented, and the pathogenesis of CIA was improved. These outcomes were more pronounced with 32 (112 mg/kg) and in a similar trend to MTX Homeostasis of B cell subsets (regulatory B cells, plasma cells, memory B cells, mature B and FO B cells) was restored in CIA mice 32 promoted co-localizations between IgD and β-arrestin1 and between IgD and AP2. Although 32 did not alter IgD-BCR expression, it seemed to foment IgD-BCR internalization in a β-arrestin1-AP2-dependent manner
| [119] |
| Ginsenoside Rg3 (34) | | 34 reduced the swelling rates of RA mice, decreased the degree of cartilage destruction and vasodilation, diminished protein expression of TNF-α and IL-6 and raised the protein expression of IL-10 and TGF-β in the ankle joint Enhancement of oxidative phosphorylation and reinforcement of the TCA cycle and the respiration of ETC. CD4+CD25+Foxp3+ Treg cell percentage was increased; lipids played a crucial role in the proliferation and differentiation of these cells
| [53] |