Contemporary Review of Transcatheter Mitral Valve Interventions for Mitral Regurgitation
Abstract
1. Introduction
2. Transcatheter Edge-to-Edge Repair
2.1. MitraClip for Primary Mitral Regurgitation
2.2. MitraClip for Secondary MR
2.3. Edwards PASCAL System
2.4. PASCAL vs. MitraClip
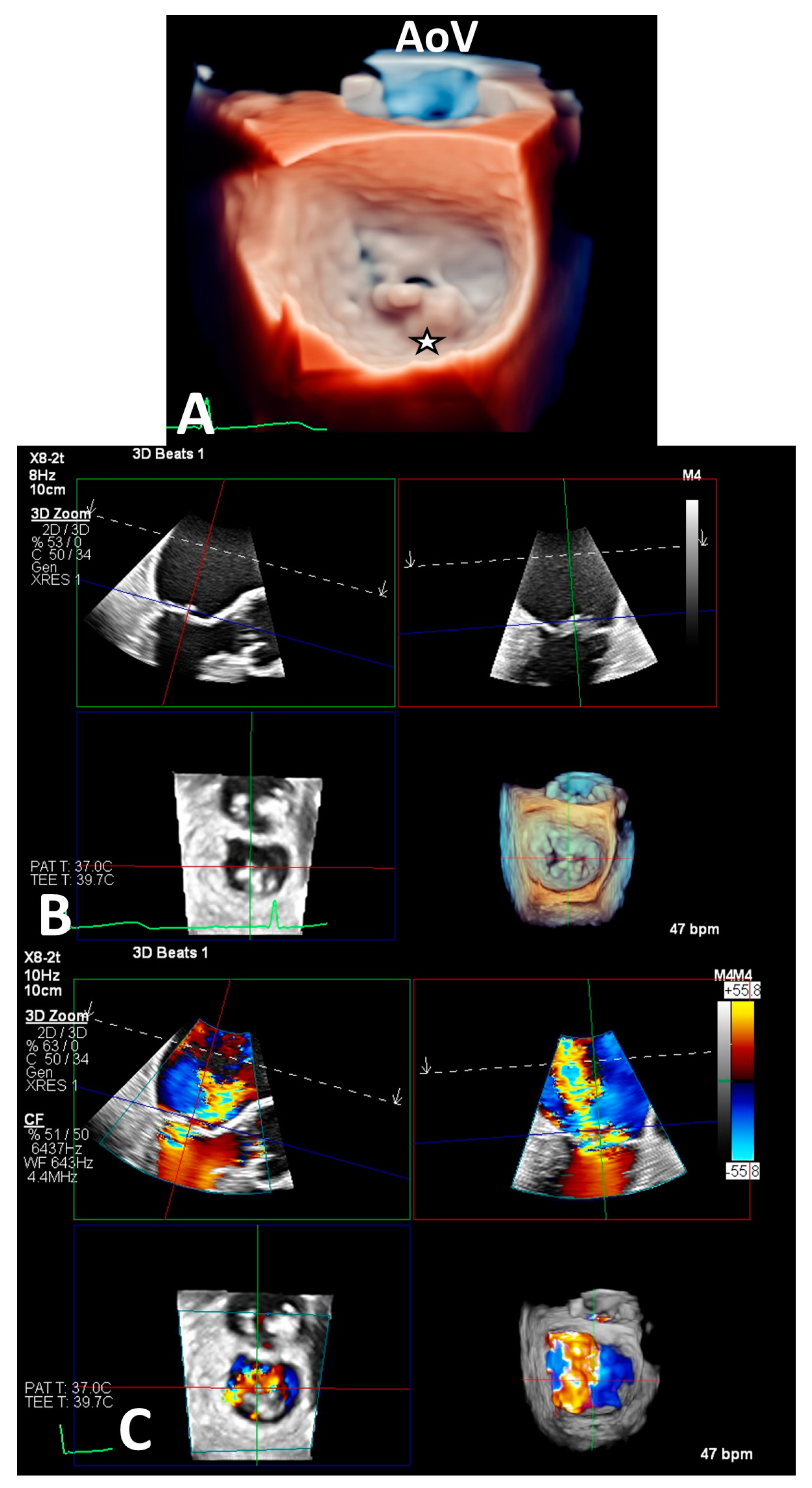
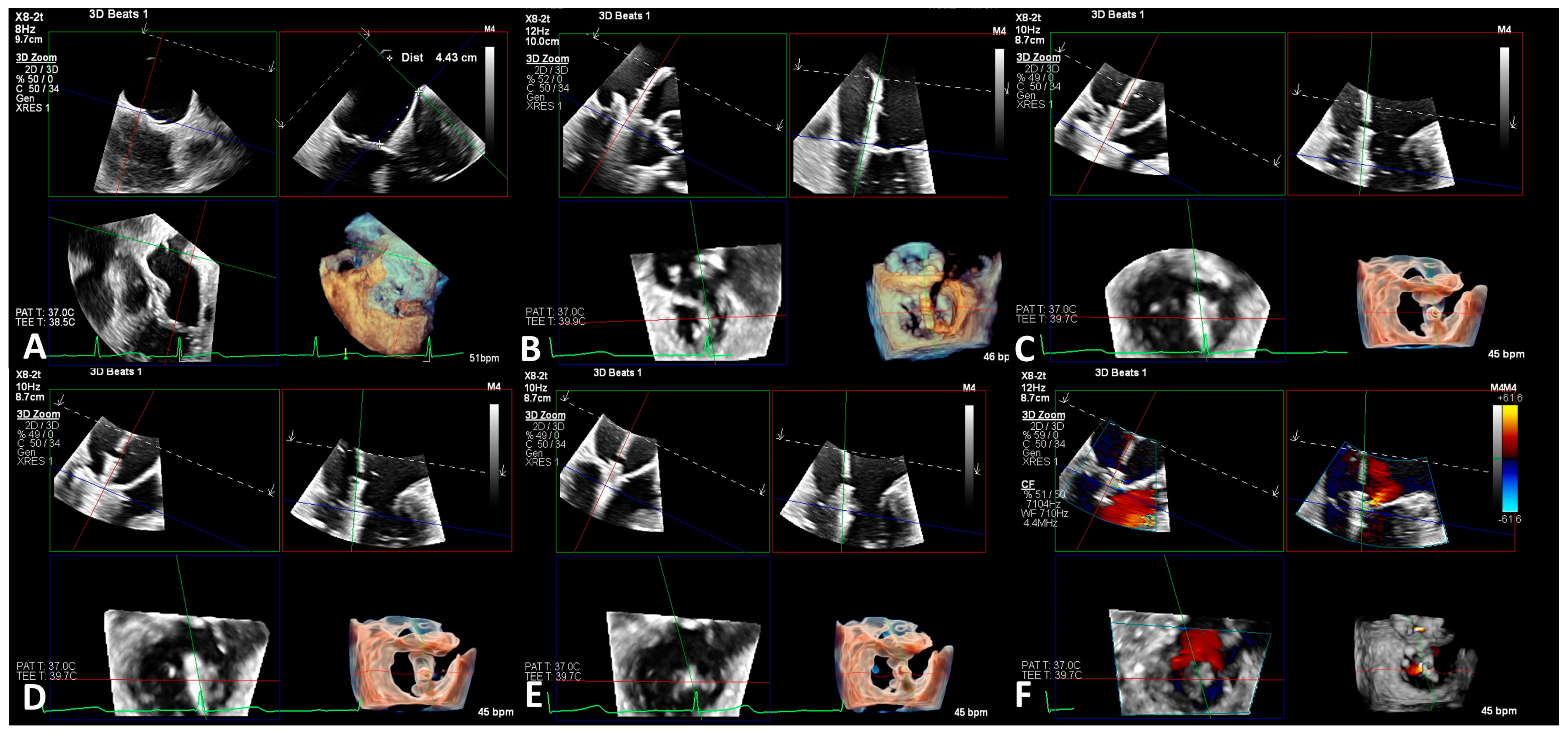
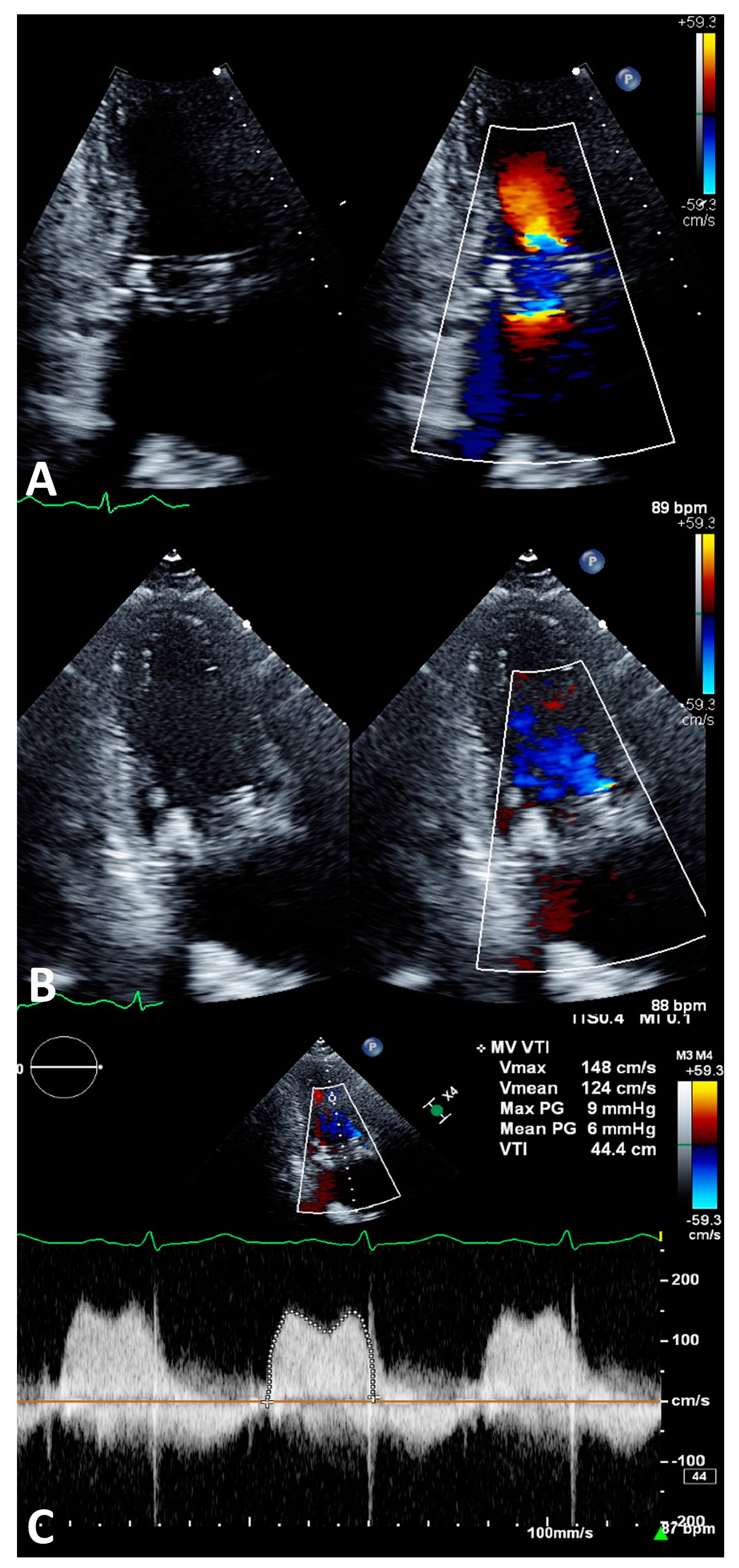
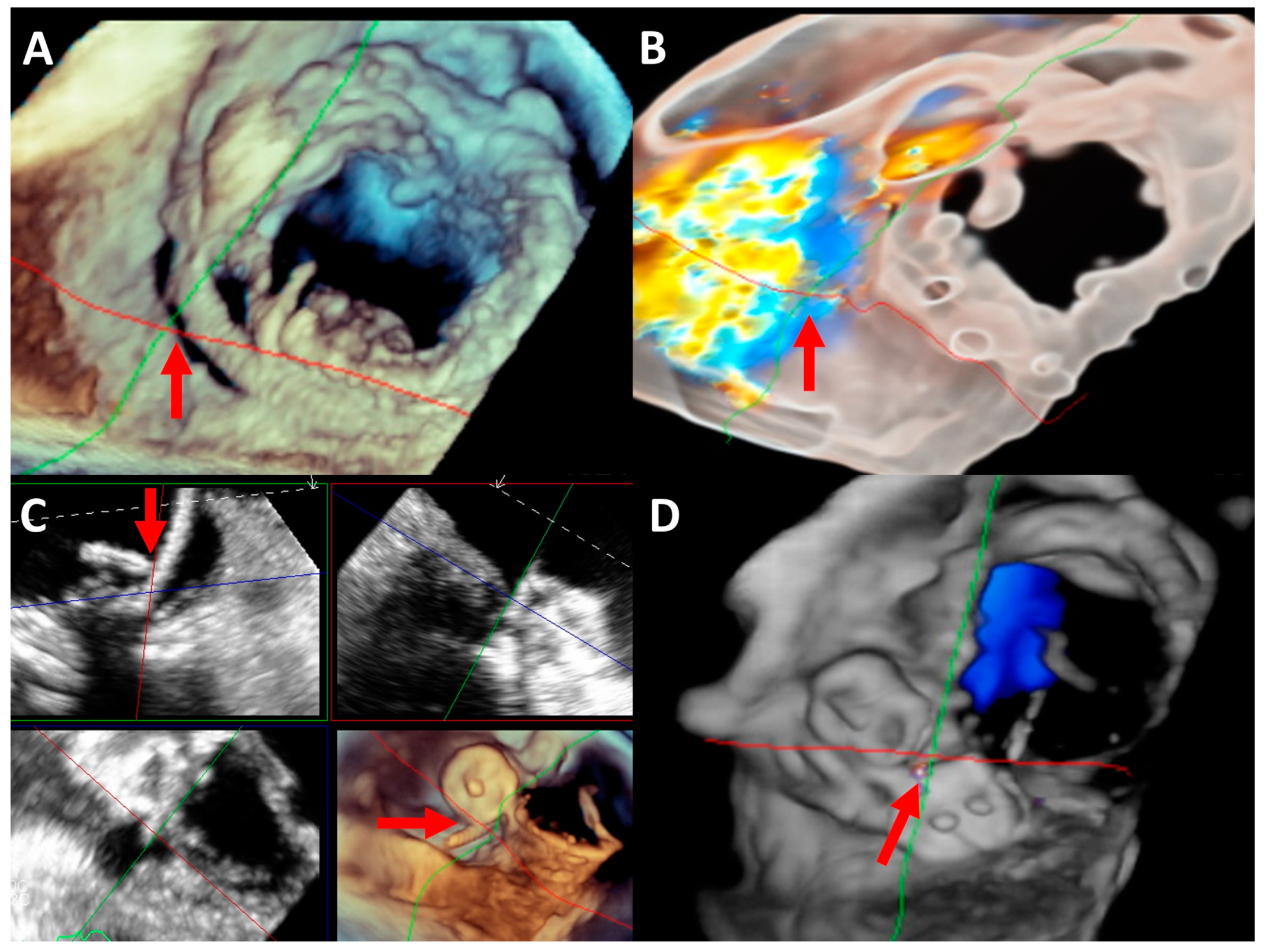
2.5. Imaging Evaluation
2.6. Procedure
3. Percutaneous Mitral Annuloplasty
3.1. Direct Annuloplasty
3.2. Indirect Annuloplasty
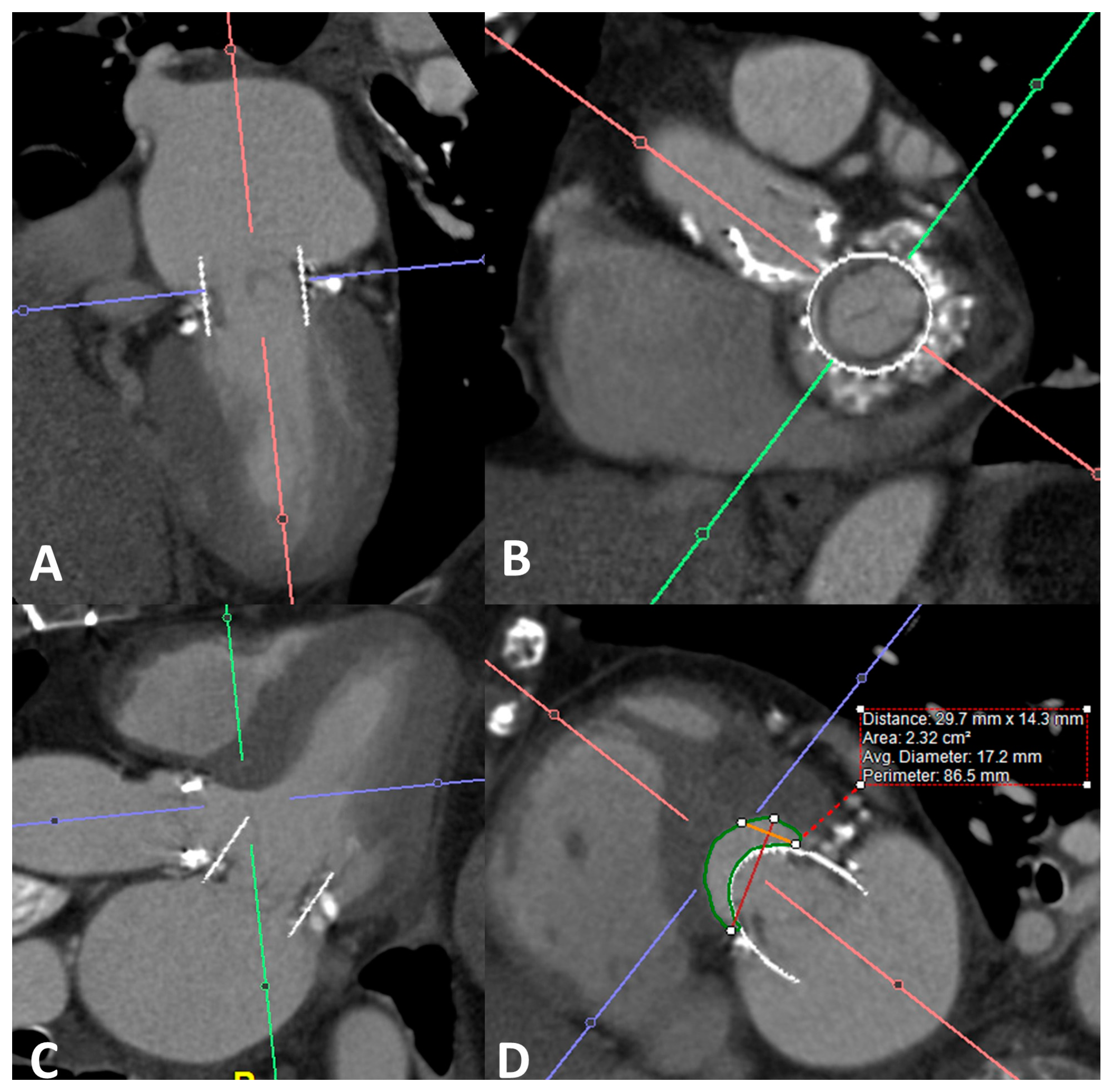
4. Transcatheter Mitral Valve Replacement
4.1. Valve in Valve
4.2. Valve in Ring
4.3. Valve in MAC
4.4. Differences in Outcomes of Valve in Valve vs. Valve in Ring vs. Valve in MAC
4.5. Valve in Native Noncalcified Valve
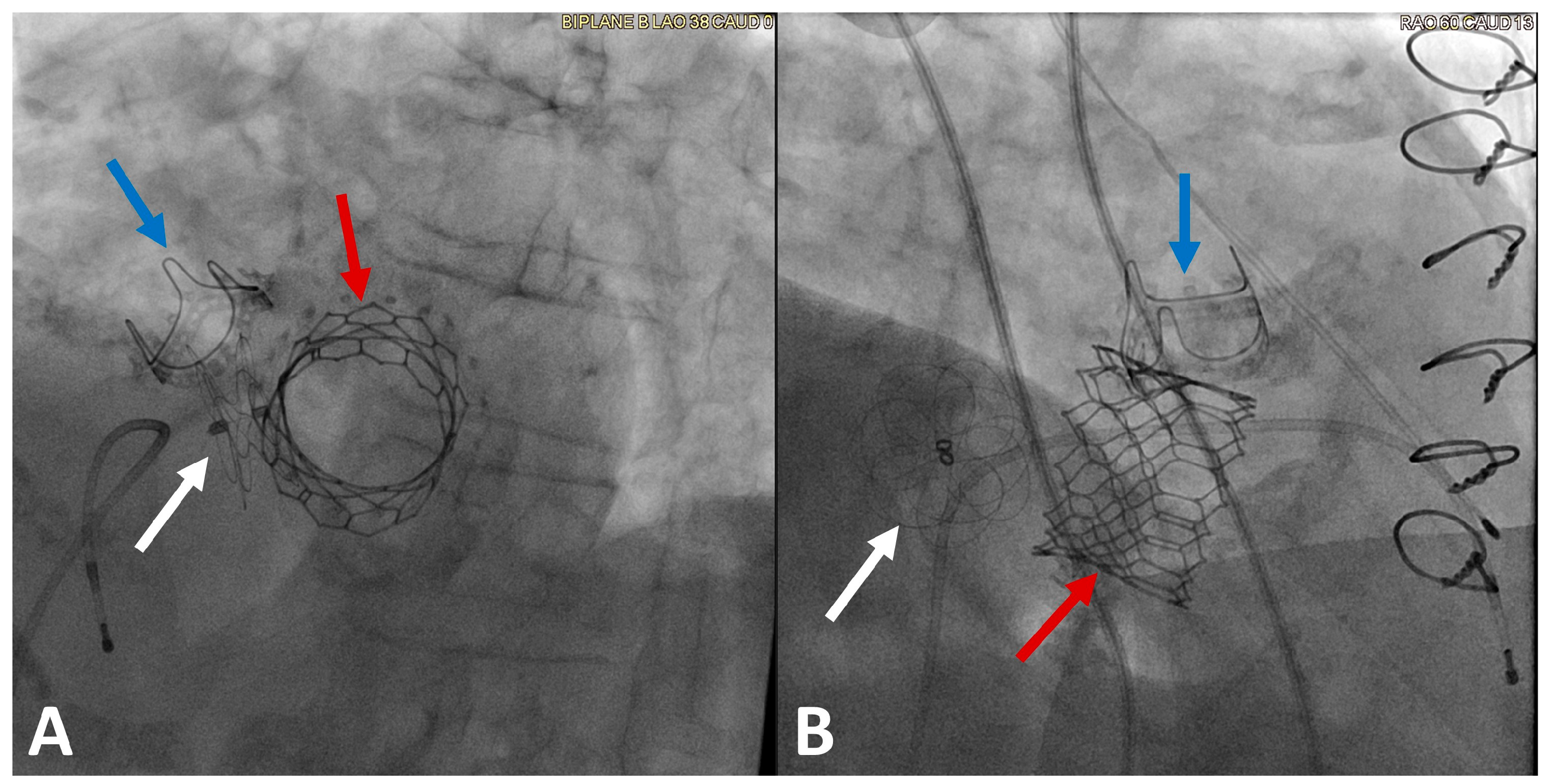
5. Mitral Paravalvular Leak Closure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 2015, 65, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- de Marchena, E.; Badiye, A.; Robalino, G.; Junttila, J.; Atapattu, S.; Nakamura, M.; De Canniere, D.; Salerno, T. Respective Prevalence of the Different Carpentier Classes of Mitral Regurgitation: A Stepping Stone for Future Therapeutic Research and Development. J. Card. Surg. 2011, 26, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Delahaye, J.P.; Gare, J.P.; Viguier, E.; Delahaye, F.; De Gevigney, G.; Milon, H. Natural history of severe mitral regurgitation. Eur. Heart J. 1991, 12, 5–9. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Avierinos, J.-F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative Determinants of the Outcome of Asymptomatic Mitral Regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Sundt, T.M., 3rd. Early surgery is recommended for mitral regurgitation. Circulation 2010, 121, 804–811, discussion 812. [Google Scholar] [CrossRef]
- Head, S.J.; van Leeuwen, W.J.; Van Mieghem, N.M.; Kappetein, A.P. Surgical or transcatheter mitral valve intervention: Complex disease requires complex decisions. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2014, 9, 1133–1135. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Zhou, S.; Egorova, N.; Moskowitz, G.; Giustino, G.; Ailawadi, G.; Acker, M.A.; Gillinov, M.; Moskowitz, A.; Gelijns, A. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J. Thorac. Cardiovasc. Surg. 2021, 162, 551–562.e4. [Google Scholar] [CrossRef]
- Pacini, D.; Murana, G. Commentary: Trends in mitral valve interventions: The good, the bad, and the ugly. J. Thorac. Cardiovasc. Surg. 2021, 162, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.; Satpathy, R.; et al. Multisociety expert consensus systems of care document 2019 AATS/ACC/SCAI/STS expert consensus systems of care document: Operator and institutional recommendations and requirements for transcatheter mitral valve intervention: A Joint Report of the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and The Society of Thoracic Surgeons. Catheter. Cardiovasc. Interv. 2020, 95, 866–884. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Foster, E.; Glower, D.D.; Apruzzese, P.; Massaro, J.M.; Herrmann, H.C.; Hermiller, J.; Gray, W.; Wang, A.; Pedersen, W.R.; et al. 4-Year Results of a Randomized Controlled Trial of Percutaneous Repair Versus Surgery for Mitral Regurgitation. J. Am. Coll. Cardiol. 2013, 62, 317–328. [Google Scholar] [CrossRef]
- Sorajja, P.; Vemulapalli, S.; Feldman, T.; Mack, M.; Holmes, D.R.; Stebbins, A.; Kar, S.; Thourani, V.; Ailawadi, G. Outcomes with Transcatheter Mitral Valve Repair in the United States: An STS/ACC TVT Registry Report. J. Am. Coll. Cardiol. 2017, 70, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Kalbacher, D.; Schäfer, U.; Bardeleben, R.S.V.; Eggebrecht, H.; Sievert, H.; Nickenig, G.; Butter, C.; May, A.E.; Bekeredjian, R.; Ouarrak, T.; et al. Long-term outcome, survival and predictors of mortality after MitraClip therapy: Results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int. J. Cardiol. 2019, 277, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Melica, B.; Braga, P.; Ribeiro, J.; Pires-Morais, G.; Boa, A.F.; Guerreiro, C.; Caeiro, D.; Pereira, R.; Meerkin, D.; Fontes-Carvalho, R. Transseptal Mitral Annuloplasty with the AMEND System. JACC Cardiovasc. Interv. 2022, 15, e3–e5. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Praz, F.; Spargias, K.; Chrissoheris, M.; Büllesfeld, L.; Nickenig, G.; Deuschl, F.; Schueler, R.; Fam, N.P.; Moss, R.; Makar, M.; et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: A multicentre, prospective, observational, first-in-man study. Lancet 2017, 390, 773–780. [Google Scholar] [CrossRef]
- Webb, J.G.; Hensey, M.; Szerlip, M.; Shafer, U.; Cohen, G.N.; Kar, S.; Makkar, R.; Kipperman, R.M.; Spargias, K.; O’Neill, W.W.; et al. 1-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2020, 13, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Hausleiter, J.; Lim, D.S.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Rassi, A.N.; Makkar, R.; Goldman, S.; Rudolph, V.; Hermiller, J.; et al. Transcatheter Edge-to-Edge Repair in Patients with Anatomically Complex Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2023, 81, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Geis, N.A.; Schlegel, P.; Heckmann, M.B.; Katus, H.A.; Frey, N.; Crespo López, P.; Raake, P.W. One-year results following PASCAL-based or MitraClip-based mitral valve transcatheter edge-to-edge repair. ESC Heart Fail. 2022, 9, 853–865. [Google Scholar] [CrossRef]
- Schneider, L.; Markovic, S.; Mueller, K.; Felbel, D.; Gerçek, M.; Friedrichs, K.; Stolz, L.; Rudolph, V.; Hausleiter, J.; Rottbauer, W.; et al. Mitral Valve Transcatheter Edge-to-Edge Repair Using MitraClip or PASCAL: A Multicenter Propensity Score-Matched Comparison. JACC Cardiovasc. Interv. 2022, 15, 2554–2567. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- El Sabbagh, A.; Reddy, Y.N.V.; Nishimura, R.A. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Lesevic, H.; Karl, M.; Braun, D.; Barthel, P.; Orban, M.; Pache, J.; Hadamitzky, M.; Mehilli, J.; Stecher, L.; Massberg, S.; et al. Long-Term Outcomes after MitraClip Implantation According to the Presence or Absence of EVEREST Inclusion Criteria. Am. J. Cardiol. 2017, 119, 1255–1261. [Google Scholar] [CrossRef]
- Sherif, M.A.; Paranskaya, L.; Yuecel, S.; Kische, S.; Thiele, O.; D’Ancona, G.; Neuhausen-Abramkina, A.; Ortak, J.; Ince, H.; Öner, A. MitraClip step by step; how to simplify the procedure. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2017, 25, 125–130. [Google Scholar] [CrossRef]
- Rogers, J.H.; Bolling, S.F. Transseptal direct complete annuloplasty: Early experience. Ann. Cardiothorac. Surg. 2021, 10, 57–65. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Nickenig, G.; Latib, A.; Kuck, K.-H.; Baldus, S.; Schueler, R.; La Canna, G.; Agricola, E.; Kreidel, F.; Huntgeburth, M.; et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur. Heart J. 2019, 40, 466–472. [Google Scholar] [CrossRef]
- Rogers, J.H.; Boyd, W.D.; Smith, T.W.R.; Ebner, A.A.; Grube, E.; Bolling, S.F. Transcatheter Annuloplasty for Mitral Regurgitation with an Adjustable Semi-Rigid Complete Ring: Initial Experience with the Millipede IRIS Device. Struct. Heart 2018, 2, 43–50. [Google Scholar] [CrossRef]
- Khatib, D.; Neuburger, P.J.; Pan, S.; Rong, L.Q. Transcatheter Mitral Valve Interventions for Mitral Regurgitation: A Review of Mitral Annuloplasty, Valve Replacement, and Chordal Repair Devices. J. Cardiothorac. Vasc. Anesthesia 2022, 36, 3887–3903. [Google Scholar] [CrossRef] [PubMed]
- Siminiak, T.; Wu, J.C.; Haude, M.; Hoppe, U.C.; Sadowski, J.; Lipiecki, J.; Fajadet, J.; Shah, A.M.; Feldman, T.; Kaye, D.M.; et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: Results of the TITAN Trial. Eur. J. Heart Fail. 2012, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lipiecki, J.; Siminiak, T.; Sievert, H.; Müller-Ehmsen, J.; Degen, H.; Wu, J.C.; Schandrin, C.; Kalmucki, P.; Hofmann, I.; Reuter, D.; et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: The TITAN II trial. Open Heart 2016, 3, e000411. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef]
- Guerrero, M.; Pursnani, A.; Narang, A.; Salinger, M.; Wang, D.D.; Eleid, M.; Kodali, S.K.; George, I.; Satler, L.; Waksman, R.; et al. Prospective Evaluation of Transseptal TMVR for Failed Surgical Bioprostheses: MITRAL Trial Valve-in-Valve Arm 1-Year Outcomes. JACC Cardiovasc. Interv. 2021, 14, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, B.; Kapadia, S.R.; Eleid, M.F.; Kodali, S.K.; McCabe, J.M.; Krishnaswamy, A.; Morse, M.; Smalling, R.W.; Reisman, M.; Mack, M.; et al. One-Year Outcomes of Mitral Valve-in-Valve Using the SAPIEN 3 Transcatheter Heart Valve. JAMA Cardiol. 2020, 5, 1245–1252. [Google Scholar] [CrossRef]
- Resor, C.D. Transcatheter mitral valve interventions. Prog. Cardiovasc. Dis. 2021, 69, 84–88. [Google Scholar] [CrossRef]
- Edwards, L. PARTNER 3 Trial—Mitral Valve in Valve. 2022. Available online: https://www.clinicaltrials.gov/study/NCT03193801 (accessed on 31 May 2023).
- Kalińczuk, Ł.; Mintz, G.S.; Chmielak, Z.; Rudziński, P.N.; Witkowski, A. Intraprocedural assessment of valve geometry during transcatheter mitral valve replacement by large field-of-view intravascular ultrasound: A case report. Eur. Heart J. Case Rep. 2021, 5, 1–3. [Google Scholar] [CrossRef]
- Kalinczuk, L.; Skotarczak, W.; Chmielak, Z.; Dabrowski, M.; Wolny, R.; Stoklosa, P.; Wozniak, O.; Hoffman, P.; Michalowska, I.; Demkow, M.; et al. Abstract 12383: Large Field-of-View Intravascular Ultrasound for Peri-Procedural Assessment of Stent Frame Expansion after Transcatheter Heart Valve-in-Valve. Circulation 2022, 146, A12383. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Pursnani, A.; Salinger, M.; Russell, H.M.; Eleid, M.; Chakravarty, T.; Ng, M.H.; Kodali, S.K.; Meduri, C.U.; et al. Prospective Evaluation of TMVR for Failed Surgical Annuloplasty Rings: MITRAL Trial Valve-in-Ring Arm 1-Year Outcomes. JACC Cardiovasc. Interv. 2021, 14, 846–858. [Google Scholar] [CrossRef]
- Xu, B.; Kocyigit, D.; Wang, T.K.M.; Tan, C.D.; Rodriguez, E.R.; Pettersson, G.B.; Unai, S.; Griffin, B.P. Mitral annular calcification and valvular dysfunction: Multimodality imaging evaluation, grading, and management. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e111–e122. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Saijo, Y.; Reyaldeen, R.M.; Brizneda, M.V.; Chan, N.; Gillinov, A.M.; Pettersson, G.B.; Unai, S.; Flamm, S.D.; Schoenhagen, P.; et al. Novel Multi-Parametric Mitral Annular Calcification Score Predicts Outcomes in Mitral Valve Dysfunction. Curr. Probl. Cardiol. 2023, 48, 101456. [Google Scholar] [CrossRef]
- Fox, C.S.; Vasan, R.S.; Parise, H.; Levy, D.; O’Donnell, C.J.; D’Agostino, R.B.; Benjamin, E.J. Mitral annular calcification predicts cardiovascular morbidity and mortality: The Framingham Heart Study. Circulation 2003, 107, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Guerrero, M.; Padang, R.; Amadio, J.M.; Eleid, M.F.; Scott, C.G.; Lee, A.T.; Pislaru, S.V.; Nkomo, V.T.; Pellikka, P.A. Prevalence and Natural History of Mitral Annulus Calcification and Related Valve Dysfunction. Mayo Clin. Proc. 2022, 97, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Pellikka, P.A.; Scott, C.G.; Lee, A.T.; Jain, V.; Eleid, M.F.; Alkhouli, M.A.; Reeder, G.S.; Michelena, H.I.; Pislaru, S.V.; et al. Impact of mitral intervention on outcomes of patients with mitral valve dysfunction and annulus calcification. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 99, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Chan, N.; Brizneda, M.V.; Lak, H.M.; Reyaldeen, R.M.; Gillinov, A.M.; Pettersson, G.B.; Unai, S.; Jellis, C.; Grimm, R.A.; et al. Impact of Frailty and Mitral Valve Surgery on Outcomes of Severe Mitral Stenosis Due to Mitral Annular Calcification. Am. J. Cardiol. 2021, 160, 83–90. [Google Scholar] [CrossRef]
- Guerrero, M.; Urena, M.; Himbert, D.; Wang, D.D.; Eleid, M.; Kodali, S.; George, I.; Chakravarty, T.; Mathur, M.; Holzhey, D.; et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients with Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2018, 71, 1841–1853. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2018, 40, 441–451. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Himbert, D.; Urena, M.; Pursnani, A.; Kaddissi, G.; Iyer, V.; Salinger, M.; Chakravarty, T.; Greenbaum, A.; et al. Short-term results of alcohol septal ablation as a bail-out strategy to treat severe left ventricular outflow tract obstruction after transcatheter mitral valve replacement in patients with severe mitral annular calcification. Catheter. Cardiovasc. Interv. 2017, 90, 1220–1226. [Google Scholar] [CrossRef]
- Wang, D.D.; Guerrero, M.; Eng, M.H.; Eleid, M.F.; Meduri, C.U.; Rajagopal, V.; Yadav, P.K.; Fifer, M.A.; Palacios, I.F.; Rihal, C.S.; et al. Alcohol Septal Ablation to Prevent Left Ventricular Outflow Tract Obstruction during Transcatheter Mitral Valve Replacement: First-in-Man Study. JACC Cardiovasc. Interv. 2019, 12, 1268–1279. [Google Scholar] [CrossRef]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction during Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2521–2534. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Eleid, M.F.; Pursnani, A.; Salinger, M.; Russell, H.M.; Kodali, S.K.; George, I.; Bapat, V.N.; Dangas, G.D.; et al. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC: MITRAL Trial 1-Year Outcomes. JACC Cardiovasc. Interv. 2021, 14, 830–845. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H.; Kapadia, S.; Pichard, A.D.; Douglas, P.S.; Thourani, V.H.; Babaliaros, V.C.; Webb, J.G.; Herrmann, H.C.; et al. Faculty Opinions recommendation of Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Vemulapalli, S.; Xiang, Q.; Wang, D.D.; Eleid, M.; Cabalka, A.K.; Sandhu, G.; Salinger, M.; Russell, H.; Greenbaum, A.; et al. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2020, 13, e008425. [Google Scholar]
- Urena, M.; Brochet, E.; LeComte, M.; Kerneis, C.; Carrasco, J.L.; Ghodbane, W.; Abtan, J.; Alkhoder, S.; Raffoul, R.; Iung, B.; et al. Clinical and haemodynamic outcomes of balloon-expandable transcatheter mitral valve implantation: A 7-year experience. Eur. Heart J. 2018, 39, 2679–2689. [Google Scholar] [CrossRef]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Faculty Opinions recommendation of Initial feasibility study of a new transcatheter mitral prosthesis: The first 100 patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Dahle, G. Current Devices in TMVI and Their Limitations: Focus on Tendyne. Front. Cardiovasc. Med. 2020, 7, 592909. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.W.; Sorajja, P.; Duncan, A.; Bethea, B.; Dahle, G.; Grayburn, P.; Babaliaros, V.; Guerrero, M.; Thourani, V.H.; Bedogni, F.; et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J. Am. Coll. Cardiol. 2021, 78, 1847–1859. [Google Scholar] [CrossRef]
- Sorajja, P.; Gössl, M.; Babaliaros, V.; Rizik, D.; Conradi, L.; Bae, R.; Burke, R.F.; Schäfer, U.; Lisko, J.C.; Riley, R.D.; et al. Novel Transcatheter Mitral Valve Prosthesis for Patients with Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2019, 74, 1431–1440. [Google Scholar] [CrossRef]
- Gössl, M.; Thourani, V.; Babaliaros, V.; Conradi, L.; Chehab, B.; Dumonteil, N.; Badhwar, V.; Rizik, D.; Sun, B.; Bae, R.; et al. Early outcomes of transcatheter mitral valve replacement with the Tendyne system in severe mitral annular calcification. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 17, 1523–1531. [Google Scholar] [CrossRef]
- Medical, D.A. Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Transcatheter Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03433274 (accessed on 31 May 2023).
- Bernard, S.; Yucel, E. Paravalvular Leaks-From Diagnosis to Management. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 67. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Rama-Merchan, J.C.; Rodríguez-Collado, J.; Martín-Moreiras, J.; Diego-Nieto, A.; Barreiro-Pérez, M.; Sánchez, P.L. Transcatheter closure of paravalvular leaks: State of the art. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2017, 25, 116–124. [Google Scholar] [CrossRef]
- Dávila-Román, V.G.; Waggoner, A.D.; Kennard, E.D.; Holubkov, R.; Jamieson, W.; Englberger, L.; Carrel, T.P.; Schaff, H.V. Prevalence and severity of paravalvular regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) echocardiography study. J. Am. Coll. Cardiol. 2004, 44, 1467–1472. [Google Scholar] [CrossRef]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [CrossRef]
- Ionescu, A.; Fraser, A.G.; Butchart, E.G. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: A prospective study using transoesophageal echocardiography. Heart 2003, 89, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Genoni, M.; Franzen, D.; Vogt, P.; Seifert, B.; Jenni, R.; Künzli, A.; Niederhäuser, U.; Turina, M. Paravalvular leakage after mitral valve replacement: Improved long-term survival with aggressive surgery? Eur. J. Cardio-Thorac. Surg. 2000, 17, 14–19. [Google Scholar] [CrossRef]
- Gafoor, S.; Franke, J.; Bertog, S.; Lam, S.; Vaskelyte, L.; Hofmann, I.; Sievert, H.; Matic, P. A Quick Guide to Paravalvular Leak Closure. Interv. Cardiol. 2015, 10, 112–117. [Google Scholar]
- Gafoor, S.; Steinberg, D.H.; Franke, J.; Bertog, S.; Vaskelyte, L.; Hofmann, I.; Sievert, H. Tools and Techniques—Clinical: Paravalvular leak closure. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2014, 9, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- García, E.; Sandoval, J.; Unzue, L.; Hernandez-Antolin, R.; Almeria, C.; Macaya, C. Paravalvular leaks: Mechanisms, diagnosis and management. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2012, 8, Q41–Q52. [Google Scholar] [CrossRef]
- Sorajja, P.; Cabalka, A.K.; Hagler, D.J.; Rihal, C.S. Percutaneous repair of paravalvular prosthetic regurgitation: Acute and 30-day outcomes in 115 patients. Circ. Cardiovasc. Interv. 2011, 4, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.E.; Jelnin, V.; Kronzon, I.; Dudiy, Y.; Del Valle-Fernandez, R.; Einhorn, B.N.; Chiam, P.T.; Martinez, C.; Eiros, R.; Roubin, G.; et al. Clinical Outcomes in Patients Undergoing Percutaneous Closure of Periprosthetic Paravalvular Leaks. J. Am. Coll. Cardiol. 2011, 58, 2210–2217. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Tuzcu, E.M.; Kapadia, S.R. Three-dimensional computed tomography in the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2011, 77, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jelnin, V.; Kliger, C.; Ruiz, C.E. Percutaneous Paravalvular Leak Closure. Cardiol. Clin. 2013, 31, 431–440. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Antunez-Muiños, P.; Lopez-Tejero, S.; Sanchez, P.L. Mitral Paravalvular Leak: Clinical Implications, Diagnosis and Management. J. Clin. Med. 2022, 11, 1245. [Google Scholar] [CrossRef]
- García, E.; Arzamendi, D.; Jimenez-Quevedo, P.; Sarnago, F.; Martí, G.; Sanchez-Recalde, A.; Lasa-Larraya, G.; Sancho, M.; Iñiguez, A.; Goicolea, J.; et al. Outcomes and predictors of success and complications for paravalvular leak closure: An analysis of the SpanisH real-wOrld paravalvular LEaks closure (HOLE) registry. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 12, 1962–1968. [Google Scholar] [CrossRef]
- Calvert, P.A.; Northridge, D.B.; Malik, I.S.; Shapiro, L.; Ludman, P.; Qureshi, S.A.; Mullen, M.; Henderson, R.; Turner, M.; Been, M.; et al. Percutaneous Device Closure of Paravalvular Leak: Combined Experience from the United Kingdom and Ireland. Circulation 2016, 134, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Onorato, E.M.; Muratori, M.; Smolka, G.; Zakarkaite, D.; Mussayev, A.; Christos, C.P.; Bauer, F.; Gandet, T.; Luca, G.; Martinelli, M.D.; et al. Midterm procedural and clinical outcomes of percutaneous paravalvular leak closure with the Occlutech Paravalvular Leak Device. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2020, 15, 1251–1259. [Google Scholar] [CrossRef]

| Technique | Transcatheter Edge-to-Edge Repair | Direct Annuloplasty | Indirect Annuloplasty | Transcatheter Mitral Valve Replacement a |
|---|---|---|---|---|
| Device(s) | MitraClip (Abbot) Pascal (Edwards) | Cardioband (Edwards) | Carillon (Cardiac Dimensions) | AltaValve (4C Medical Technologies) Caisson (Caisson Interventional) Evoque (Edwards) HighLife (HighLife Medical Inc.) Intrepid (Medtronic) NAVI (NaviGate) Sapien 3 (Edwards) Tendyne (Abbott) Tiara (Neovasc) |
| Mechanism | Apposition of leaflets to reduce malcoaptation gap | Anchors are placed along posterior MV annulus and the band is then “cinched” | Cinching with Nitinol wire and anchors from the coronary sinus | Deploying a prosthetic valve within a prior MVR, MVr or MAC |
| Indications | High-risk or prohibitive-risk surgical candidates with primary > 3+ MR or secondary > 3+ MR | Secondary MR | Secondary MR | Primary (including MVR, MVr, MAC) and secondary MR |
| Cautions |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, N.; Dong, T.; Sabbak, N.; Xu, B.; Wang, T.K.M. Contemporary Review of Transcatheter Mitral Valve Interventions for Mitral Regurgitation. Life 2023, 13, 1511. https://doi.org/10.3390/life13071511
Chan N, Dong T, Sabbak N, Xu B, Wang TKM. Contemporary Review of Transcatheter Mitral Valve Interventions for Mitral Regurgitation. Life. 2023; 13(7):1511. https://doi.org/10.3390/life13071511
Chicago/Turabian StyleChan, Nicholas, Tiffany Dong, Nabil Sabbak, Bo Xu, and Tom Kai Ming Wang. 2023. "Contemporary Review of Transcatheter Mitral Valve Interventions for Mitral Regurgitation" Life 13, no. 7: 1511. https://doi.org/10.3390/life13071511
APA StyleChan, N., Dong, T., Sabbak, N., Xu, B., & Wang, T. K. M. (2023). Contemporary Review of Transcatheter Mitral Valve Interventions for Mitral Regurgitation. Life, 13(7), 1511. https://doi.org/10.3390/life13071511





