Abstract
Objectives: An analysis of the weight and blood glucose management associated with fecal microbiota transplantation (FMT) as well as metabolic diseases associated with FMT was conducted by the authors in order to provide clinical recommendations regarding the treatment of nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM). Methods: We searched PubMed, Embase, and the Cochrane Library for papers that were published between the creation of the database and October 2022. We reviewed research that investigated how FMT affected weight and glycemic management in cases of obesity and metabolic conditions that are related to obesity. Studies that were published more than once, lacked the entire text, included insufficient information, or were impossible to extract data from were excluded. Additionally, case reports, reviews, and systematic reviews were excluded from the analysis. In order to analyze the data, STATA 15.1 was used. Outcomes: When we combined all of our findings, we discovered that pooled outcomes showed that weight levels (WMD equals −4.77, 95%CI: −7.40~−2.14), BMI levels (WMD equals −1.59, 95%CI: −2.21~−0.97), HOMA-IR (WMD equals −0.79, 95%CI: −1.57~−0.00), and HbA1c (WMD equals −0.65, 95%CI: −0.75~−0.55) after FMT treatment were significantly lower than before treatment. However, FMT treatment may have no effect on glucose and insulin levels in obese patients at fasting and related metabolic diseases. Additionally, subgroup analysis outcomes found that FMT significantly reduced fasting blood glucose in people with diabetes. Conclusions: As a weight loss and glycemic control therapy, FMT helps to prevent and treat metabolic problems linked to obesity, and is a viable alternative to bariatric surgery for patients who do not wish to undergo the procedure.
1. Introduction
Obesity refers to a certain degree of being obviously overweight and where the fat layer is too thick, in terms of a state of body fat, especially where there is too much triglyceride accumulation. It is not simply due to weight gain but also fat tissue that accumulates in the body as a result of obesity. Obesity is on the rise globally, according to the World Health Organization Statistics, which show that by 2017, nearly 2.1 billion adults in the world were overweight, accounting for 43.10% of the total population, and more than 700 million were obese [1]. Obesity is not simply a matter of body size management. More importantly, obesity not only leads to a decreased quality of life but also poses serious health risks [2]. In recent decades, the number of obese individuals has increased significantly [1]. It is currently very difficult to find effective medication therapies for metabolic illnesses, such as type 2 diabetes, heart disease, and nonalcoholic fatty liver disease, which are associated with obesity [2,3]. At present, there are not many weight-loss methods applied in clinical practice, and people have sought a variety of weight-loss methods. For people with extreme obesity, surgery can be used to lose weight in clinic [4]. As a result of bariatric surgery, weight loss can be achieved effectively and metabolic diseases associated with obesity can be significantly reduced [3,5]. While bariatric surgery aids in weight loss and reduces health issues, it is invasive and associated with a high rate of illness and death [4]. In addition, bariatric surgery alters how much food the body eats by altering the secretion of ghrelin, bile acids, gut hormones, and so on. This process also affects the central prefrontal cortex and dopaminergic signaling pathways, which may increase the body’s sensitivity to other rewards, such as alcohol [6]. Therefore, these side effects also limit the widespread use of bariatric surgery in the treatment of obesity. Experts recommend adjuvant drug therapy for obese persons with a body mass index (BMI) of 30 kg/m2 or higher with obesity-related diseases [7]. Weight-loss drugs used in obesity are primarily appetite suppressants that target the center and other drugs that target peripheral tissues to a lesser extent, reducing body weight as a result of a change in the balance between intake and the expenditure of energy [8]. However, diet pills can also cause pain, insomnia, anxiety, and gastrointestinal adverse reactions, and are prone to relapse after withdrawal [9,10,11,12]. The discovery of new, effective treatment options for obesity and related metabolic disorders is of vital importance [13,14,15].
At present, the international implementation of FMT is still not standardized [16]. Donors are usually strictly screened before transplantation of FMT, and the selection of child donors is usually made from their mothers, siblings, or healthy children [17]. Then, according to the recipient’s own condition and disease type, the corresponding intestinal preparation is made, and antibiotics or laxatives are used to clean the intestinal tract before transplantation [18,19,20]. The transplanted materials are divided into fresh feces and frozen feces [21]. Transplantation routes can be divided into oral coprofecal capsules, upper GI tract (gastroscopy, nasogastric tube, nasojejunal tube, or percutaneous gastro-jejunostomy tube infusion), and lower GI tract diameter (colonoscopy, colonic tube insertion, or enema) [22]. Colonoscopy is the current first-line approach, which is usually performed in a single FMT and can be repeated in initial non-responders [23]. The first successful use of FMT in children with Clostridioides and separate difficile infection (CDI) was reported in 2010 [24]. Subsequently, there is more experience of using FMT in treating children with CDI. In 2013, the Food and Drug Administration included FMT in its treatment guidelines for recurrent CDI [25]. Other scholars have found that FMT can be used to treat children with metabolic diseases, allergic diseases, autism spectrum disorders, and other extra-intestinal diseases. For metabolic diseases, the transplantation of fecal microbiota is an innovative method for restoring gut microbiota diversity and balance [26,27,28,29,30]. Microbiome dysbiosis has been linked to gut flora control in studies, which contributes to type 2 diabetes, nonalcoholic fatty liver disease, and obesity. In recent years, researchers have explored the potential use of FTM in the treatment of metabolic conditions such as obesity. As of yet, there is no scientific evidence to support the hypothesis that FTM affects glycemic control and weight in individuals suffering from obesity and other metabolic diseases. As part of the study, this research employed a thorough investigation and meta-analysis of how individuals with FTM, NAFLD, T2DM, and other related metabolic disorders manage their weight and blood glucose levels in order to enhance clinical therapy [6,7,8,9,10].
2. Approaches
2.1. Criteria for Including and Excluding Literature
Inclusion criteria: This study is a single-arm study; it reports on fat mass and glycemic control in metabolic illnesses including obesity. The sole language used for the research is English.
There are a number of exclusion criteria, including repetition of publication; studies without full texts, incomplete information, or difficulty extracting data; animal experiments; case studies; as well as review articles and systematic reviews.
2.2. Search Technique
For this meta-assessment, we investigated PubMed, Embase, as well as the Cochrane Library from time the databases were created until October 2022. The search terms are “FMT”, “obesity”, “faecal microbiota transplantation”, “faecal bacteria transplantation”, “faecal transplantation”, and “faecal bacteriotherapy”. This disorder is also known as “obesity”, “type-2 diabetes mellitus”, “type 2 diabetes”, “nonalcoholic liver problems”, or “NAFLD”.
2.3. Data Extraction and Literature Screening
The literature was searched, screened, and collected independently by two researchers. If there is a dispute or disagreement, a decision is reached after consulting with a third party or bargaining with them. All of the above information was gathered from the papers, including the author, publication year, country, sample size, age, gender, and outcomes including weight, body mass index, fasting blood sugar (mmol/L), fasting insulin, assessment of insulin resistance using the homeostatic model, and hemoglobin A1C.
2.4. Literature Quality Assessment
To evaluate the strength of the evidence in each study, we used the methodological indicator for non-randomized studies (MINORS) scale. Two independent researchers conducted the study. A score between 0 and 2 is assigned to each of the 12 items for a total of 24 points. From the age of 9 to 16, the quality of the study was rated moderate, and from the age of 17 to 24, the study was rated excellent.
2.5. Synthesis of Data and Statistical Evaluation
In order to determine whether there is heterogeneity in the data, STATA 15.1 was used (Stata Crop LP, College Station, TX, USA). [14] I2 and Q tests were used to check for heterogeneity. When the heterogeneity test outcomes in p ≥ 0.1 and I2 ≤ 50%, then both studies are equivalent, and a fixed effects model is used in the combined analysis. Sensitivity analysis was conducted to determine why the outcomes differed if p < 0.1 or I2 > 50 percent. The random effects model is used if the groups still differ significantly, or quit if trying to combine the findings and turn to descriptive analysis. The assessment of the publishing bias employed a funnel plot.
3. Outcomes
3.1. Outcomes of Literature Study
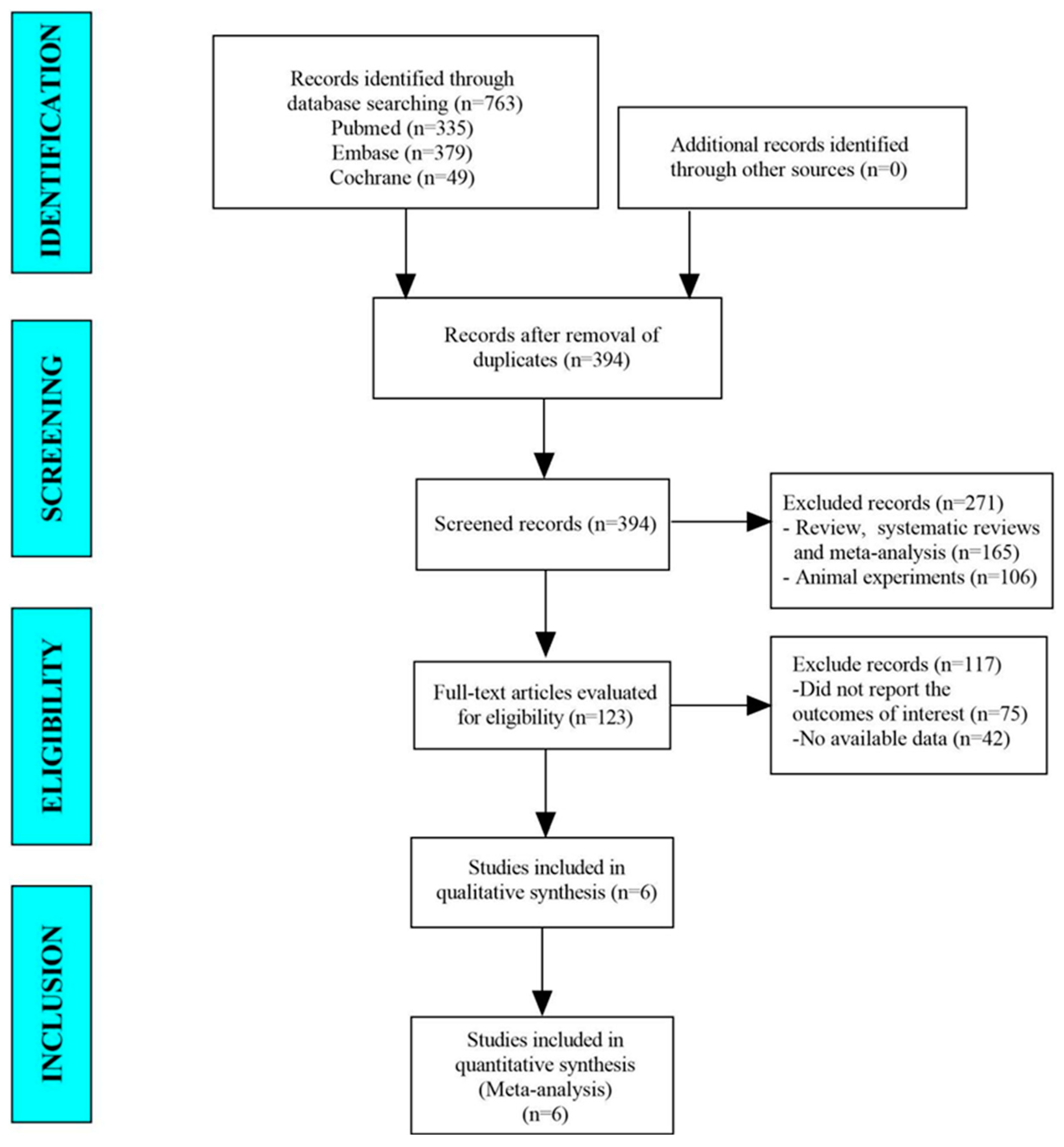
PubMed, Embase, and the Cochrane Library’s databases, which included 763 papers, were examined in this meta-analysis. There were 394 studies remaining after the duplicates were removed. In total, 123 studies were found thanks to the titles and abstracts. In the end, the meta-analysis looked at 6 papers (Figure 1).
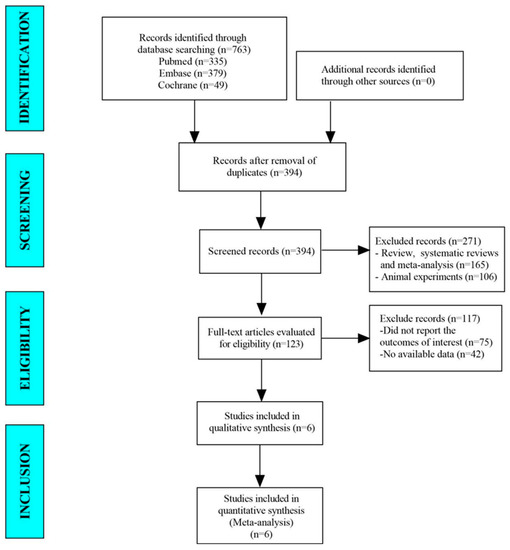
Figure 1.
The flow diagram for selecting studies.
3.2. Baseline Attributes and Evaluation of the Listed Studies’ Quality
This meta-analysis comprised a total of six publications. There were 130 patients in the sample, of which a total of 92 patients were males and 38 patients were females. Patients from the United States participated in three trials, whereas participants in the other three studies were from China. The range of ages was 42.5 to 57.3 on average. Average fasting blood glucose concentrations ranged from 5.19 to 8.5. Since every MINORS score was higher than 16, the included material was of a moderate or high quality (Table 1).

Table 1.
Qualitative assessment of the included studies based on their baseline characteristics.
3.3. Meta-Analysis Outcomes
3.3.1. Weight
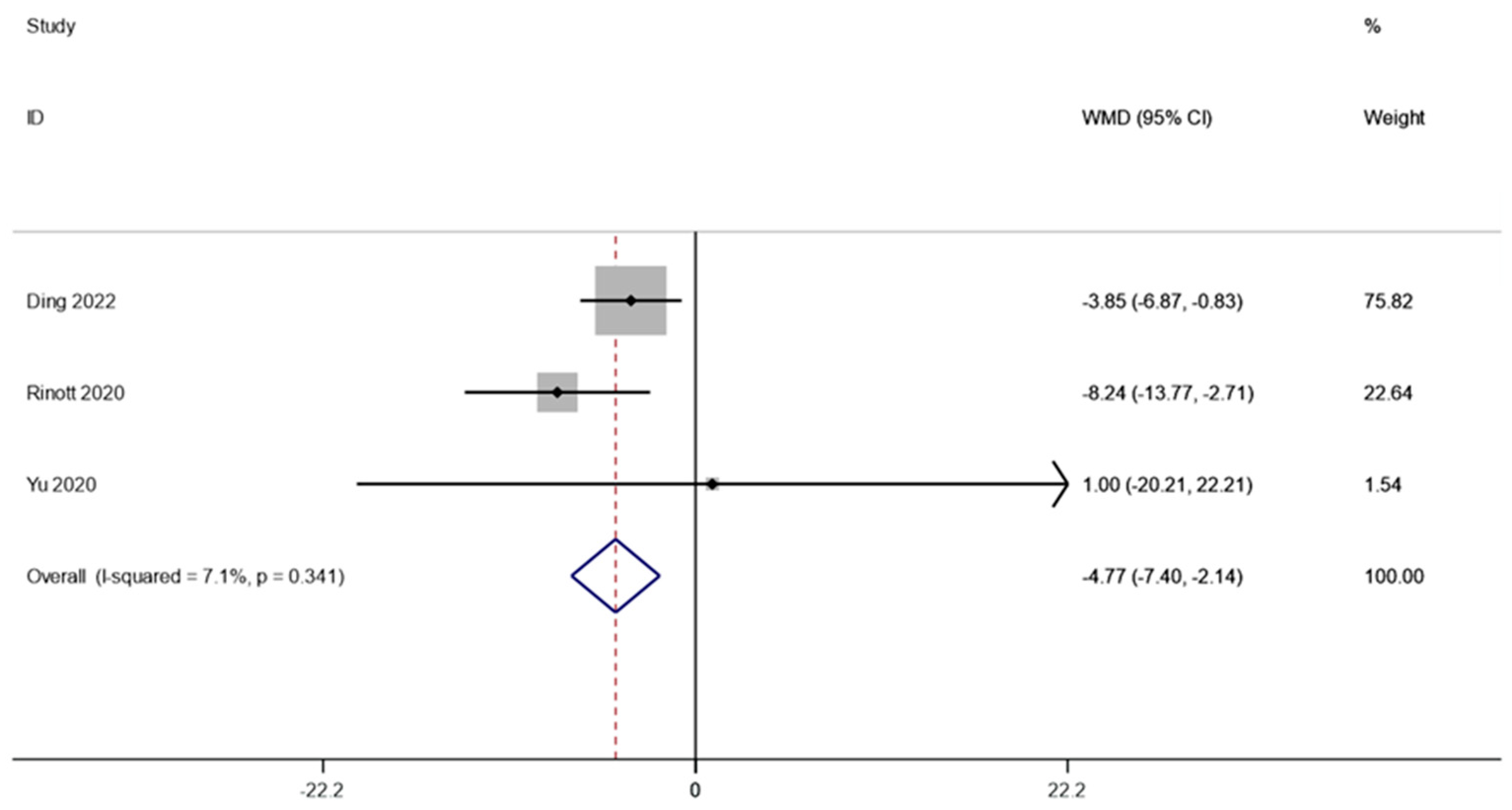
Three studies involving 67 patients examined the weight of individuals with obesity and metabolic disorders. A fixed effects model was employed to do a meta-analysis since there was not much of a difference between the groups (I2 is equal to 7.1 percent, p is equal to 0.341). The combined data demonstrated that weight levels were significantly lower following FMT therapy compared to the baseline (WMD is equal to −4.77, 95 percent CI: −7.40 ~−2.14, p is equal to 0.000; Figure 2), indicating that FMT therapy helps reduce body weight in obese patients and those with metabolic diseases related to obesity.
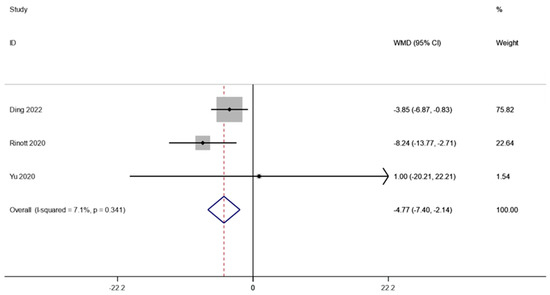
Figure 2.
Weight in obesity and associated metabolic diseases following FMT therapy.
3.3.2. BMI
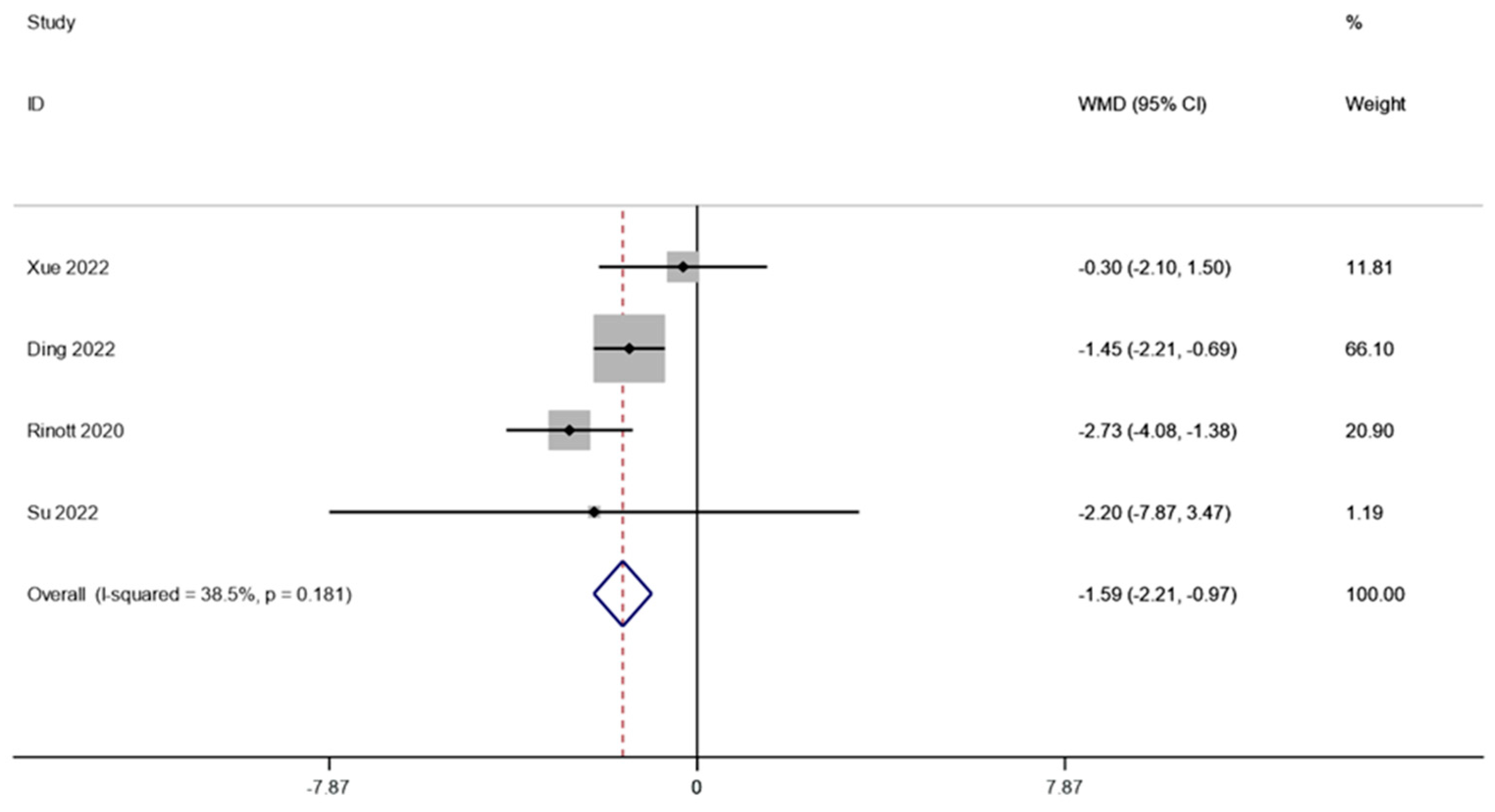
The BMI of obese individuals and the associated metabolic illnesses were examined in four investigations (including 105 patients). A fixed effects model was employed to perform a meta-analysis since there was not much of distinction between the cohorts (I2 is equal to 38.5 percent, p is equal to 0.181). The combined data demonstrated that BMI levels were significantly lower after FMT therapy than they were before therapy (WMD is equal to −1.59, 95 percent CI: −2.21~−0.97, p is equal to 0.000; Figure 3), indicating that FMT therapy helps reduce BMI levels in obese patients and those with metabolic diseases related to obesity.
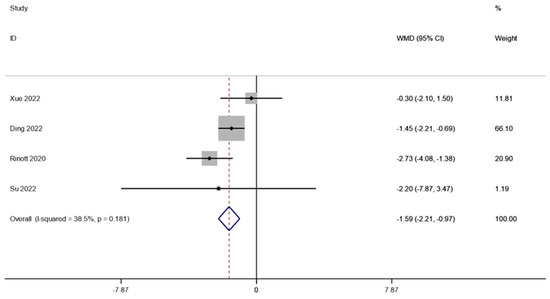
Figure 3.
BMI in obesity and associated metabolic diseases following FMT.
3.3.3. Fasting Blood Glucose
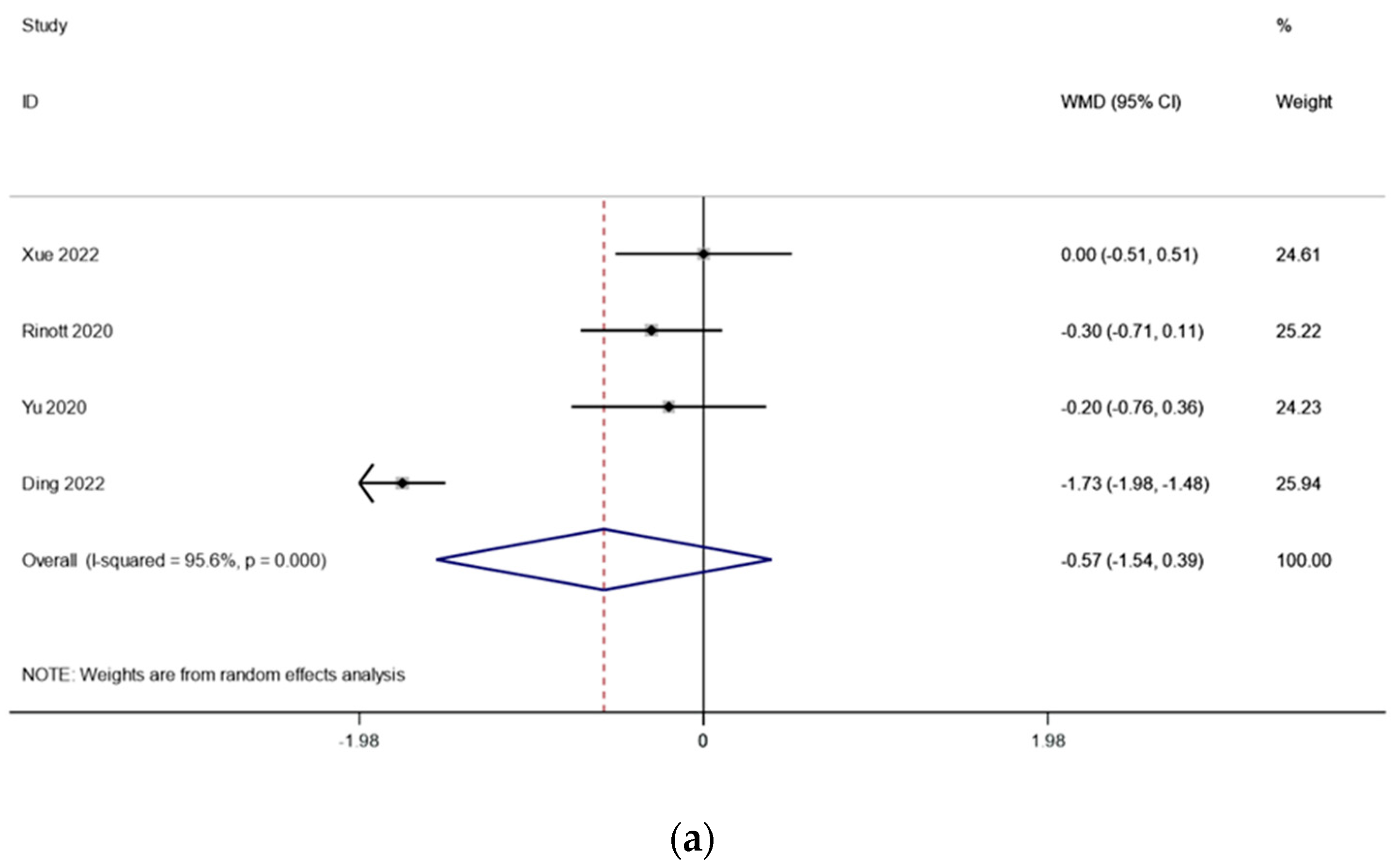
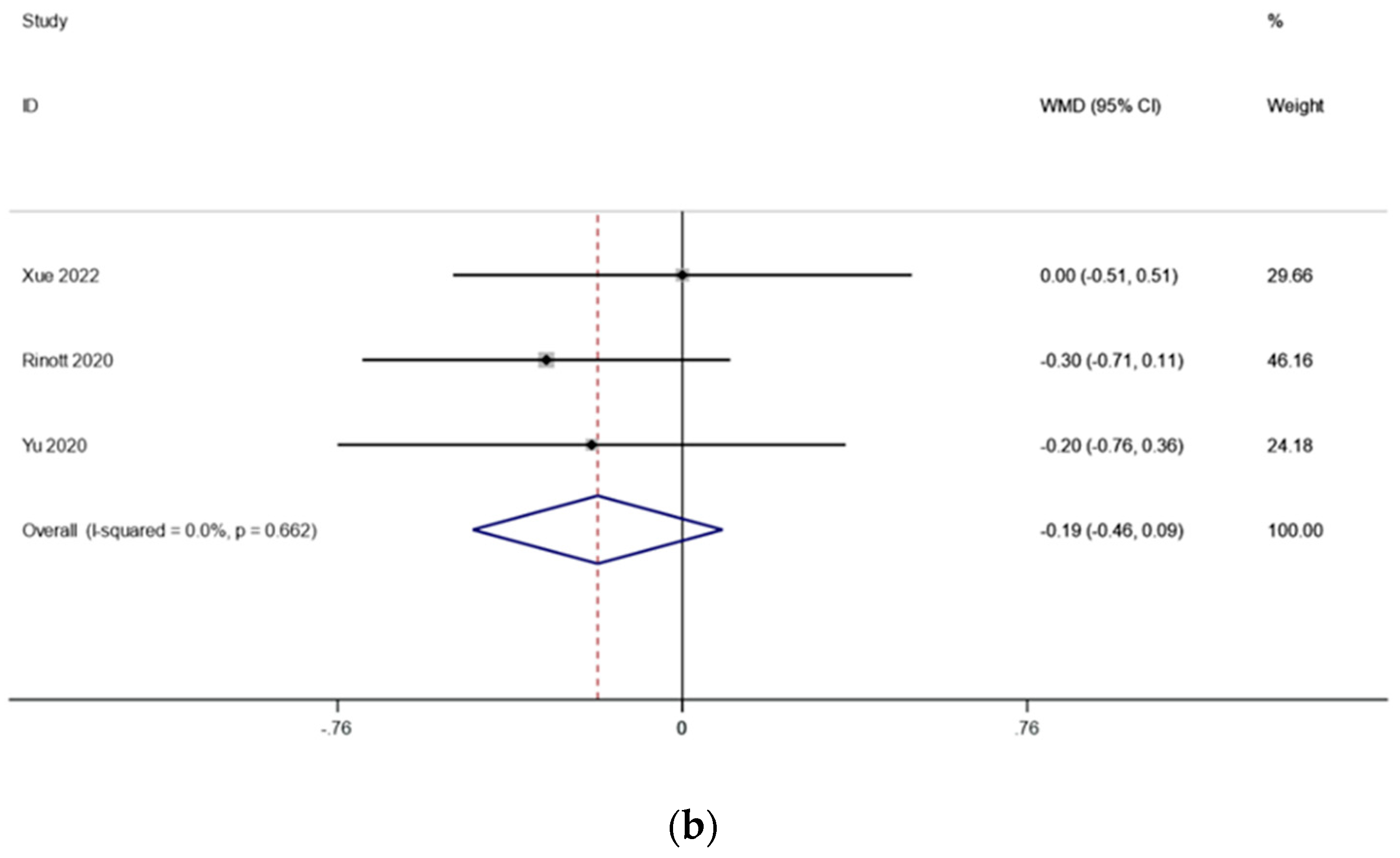
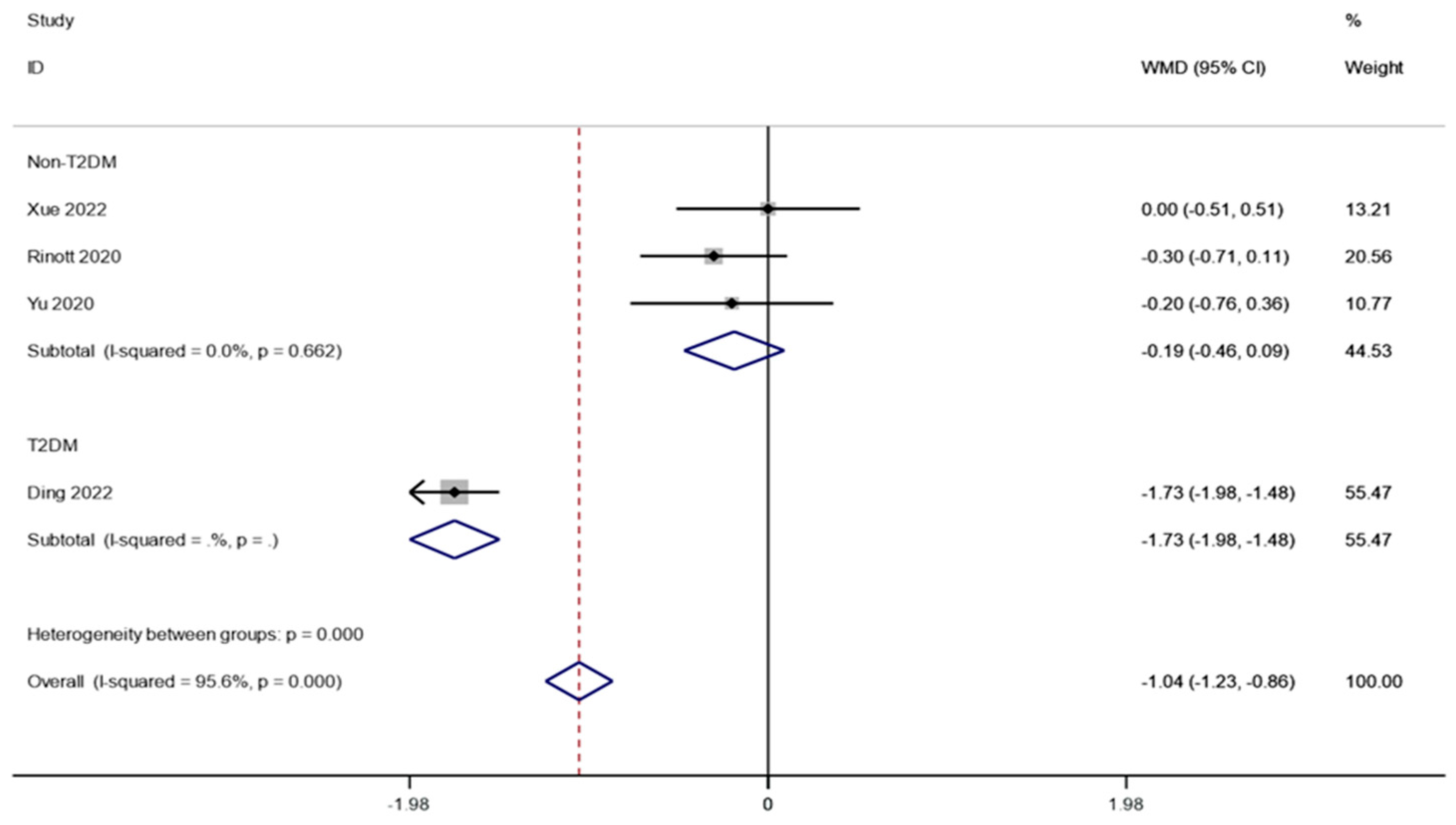
There were three studies (including 114 patients) that looked at the fasting blood sugar levels in people with obesity and metabolic diseases related to obesity. The outcomes changed because of what Ding et al. found. Because there was very little heterogeneity (I2 is equal to 95.6 percent, p is equal to 0.000) (Figure 4a), we conducted a sensitivity analysis and found that the study by Ding et al. [17] had an effect on the outcomes. There was significantly reduced heterogeneity (I2 is equal to 0.0 percent, p is equal to 0.662) when this literature was excluded, and the meta-analysis was carried out using a fixed effects model. the fasting blood glucose levels before and after FMT treatment, according to the pooled outcomes, did not change substantially from one another (WMD is equal to −0.19, 95 percent CI: −0.46~0.36, p is equal to 0.184; Figure 4b), suggesting that FMT treatment may have no effect on fasting blood glucose in obese patients and those with metabolic diseases related to obesity.
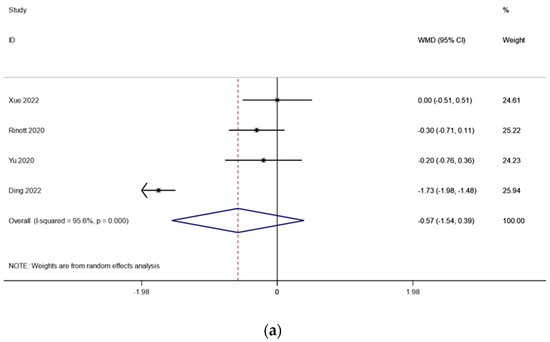
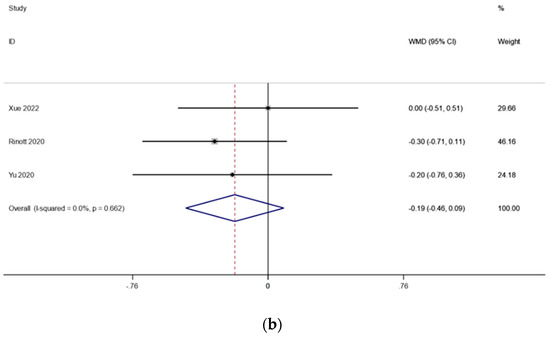
Figure 4.
(a) Fasting blood glucose in obesity and associated metabolic diseases after FMT (before sensitivity analysis); (b) fasting blood glucose in obesity and associated metabolic diseases following FMT (after sensitivity analysis).
3.3.4. Fasting Insulin
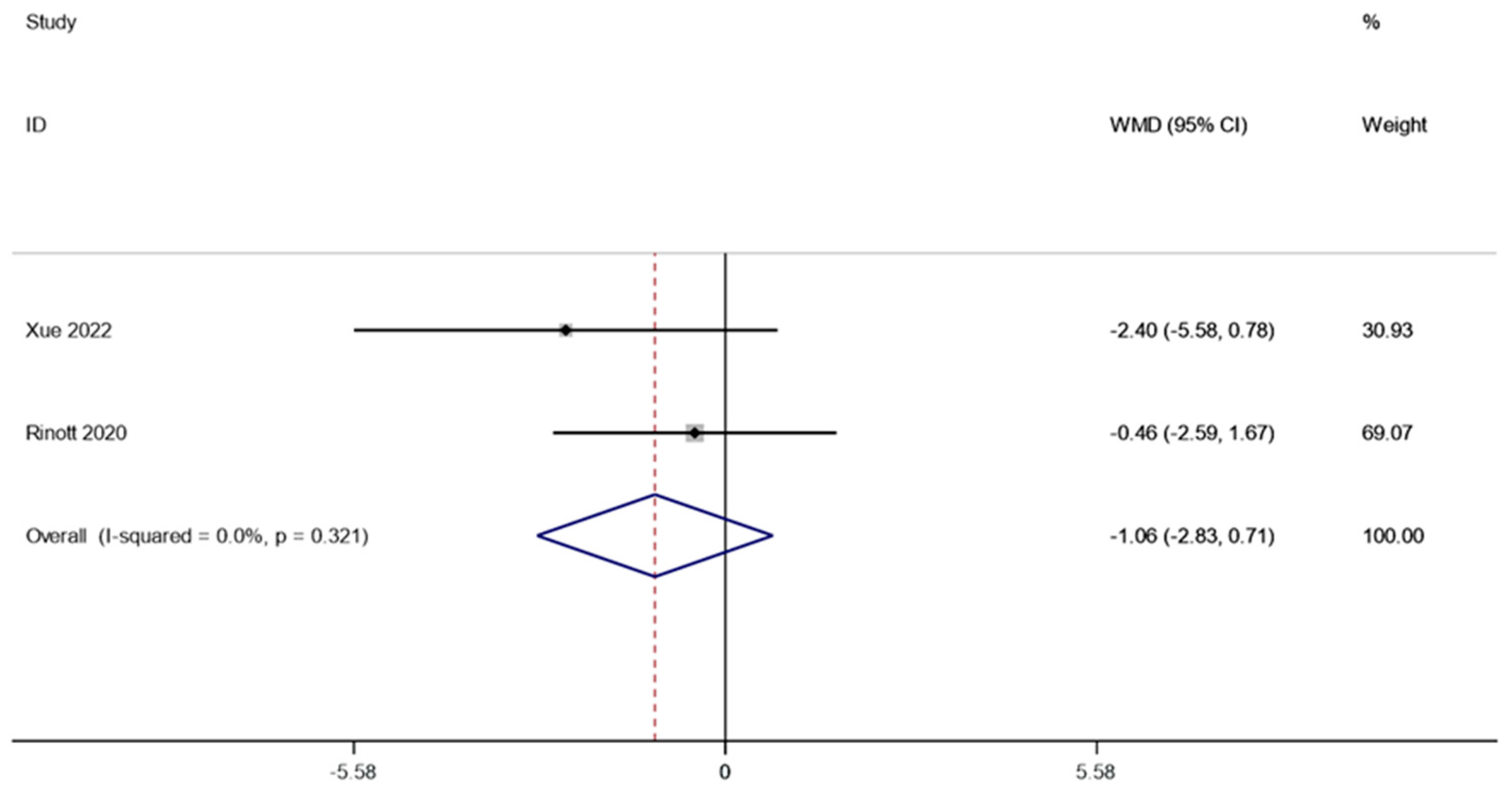
Following FMT, two research studies (involving 91 patients) examined how fasting insulin affected obesity and other metabolic illnesses. A fixed effect model was utilized to perform a meta-analysis since there was no discernible heterogeneity (I2 is equal to 0.0 percent, p is equal to 0.321). According to the combined data, the level of fasting insulin after FMT therapy did not vary substantially from the level before therapy (WMD is equal to −1.06, 95 percent CI: −2.83~0.71, p is equal to 0.241; Figure 5), suggesting that patients suffering from obesity and related metabolic diseases may not benefit from FMT treatment in terms of fasting insulin levels.
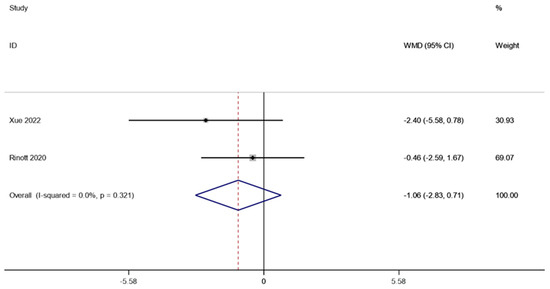
Figure 5.
Fasting insulin in obesity and associated metabolic diseases following FMT.
3.3.5. HOMA-IR
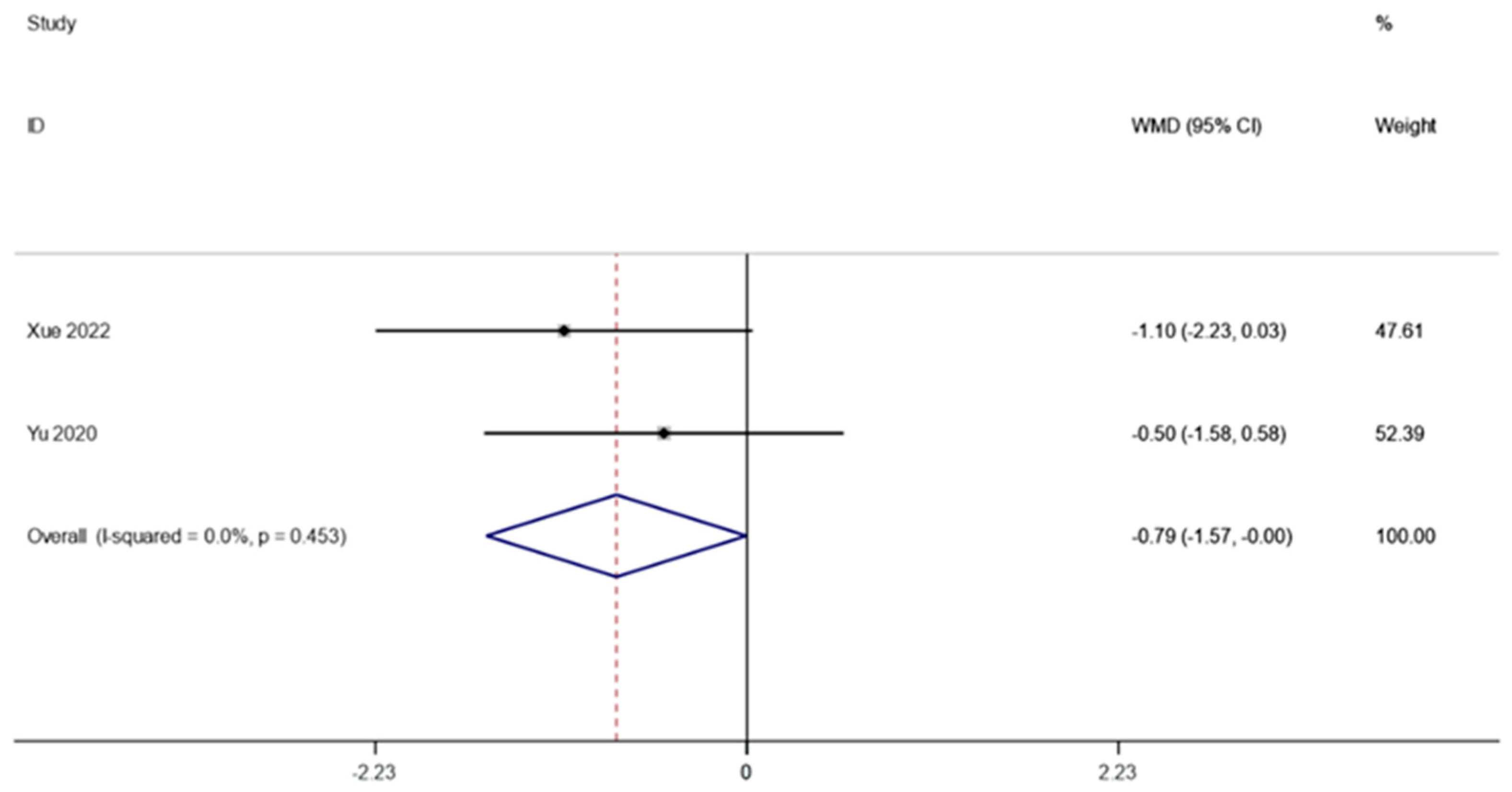
Following FMT, the HOMA-IR in obesity and associated metabolic disorders was observed in two investigations (including 59 patients). A fixed effects model was employed to perform a meta-analysis since there was not much of a difference between the groups (I2 is equal to 0.0 percent, p is equal to 0.453). HOMA-IR was significantly lower after FMT therapy than it was before, according to the pooled data (WMD is equal to −0.79, 95 percent CI: −1.57~−0.00, p is equal to 0.049; Figure 6), indicating that FMT therapy helps reduce HOMA-IR in obese patients and those with metabolic diseases related to obesity.
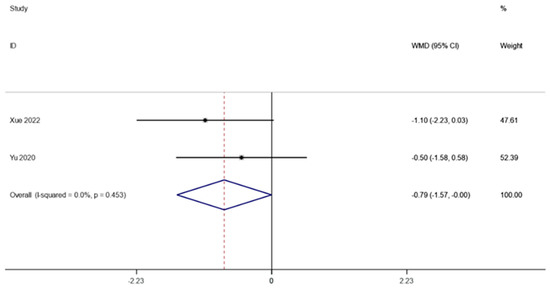
Figure 6.
HOMA-IR in obesity and associated metabolic diseases following FMT.
3.3.6. HbA1c
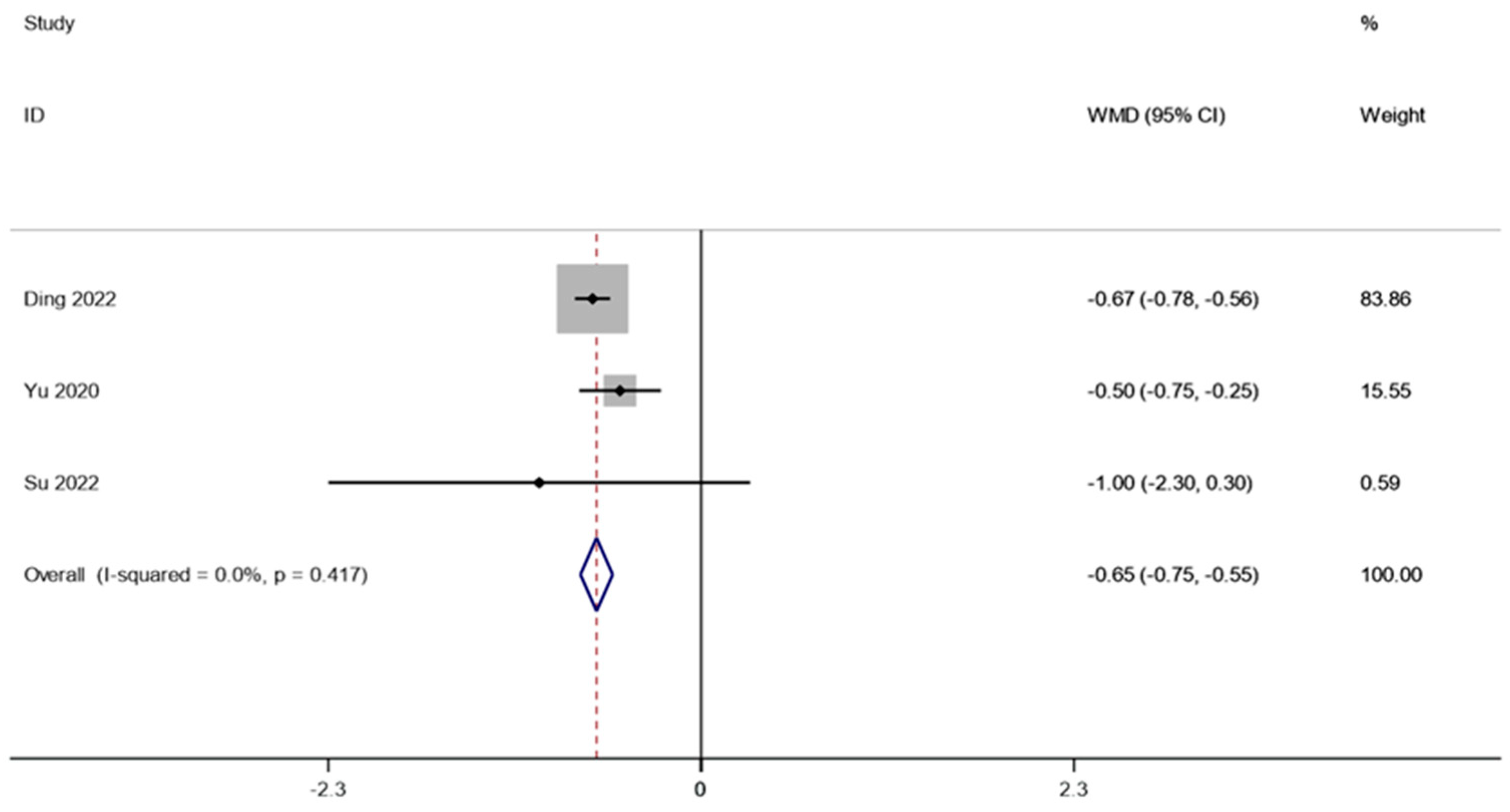
There were two studies (involving 28 patients) that reported the HbA1c in obesity and related metabolic diseases after FMT. A fixed effects model was employed to conduct a meta-analysis since there was not much of a difference between the groups (I2 is equal to 0.0 percent, p is equal to 0.417). A1c was considerably lower after FMT therapy than it was before (WMD is equal to −0.65, 95 percent CI: −0.75~−0.55, p is equal to 0.000; Figure 7), indicating that FMT therapy helps reduce HbA1c in obese patients and those with metabolic diseases related to obesity.
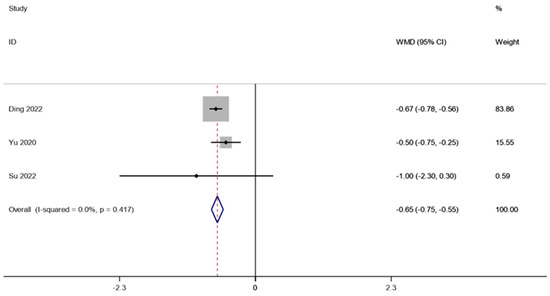
Figure 7.
HbA1c in obesity and associated metabolic diseases following FMT.
3.4. Analysis of Subgroups
Even though there were significant differences in the blood glucose measurement, sensitivity analysis revealed that research from Ding et al. had a significant impact on the outcomes. However, looking at baseline characteristics, we found that Ding et al.’s study included diabetic populations, while other studies included samples of normal glycemic populations. Therefore, we further based our subgroup analyses on whether or not people with diabetes were identified. It was found that FMT significantly reduced fasting blood glucose in people with diabetes (WMD is equal to −1.73, 95 percent CI:−1.98~−1.48, p is equal to 0.000; Figure 8).
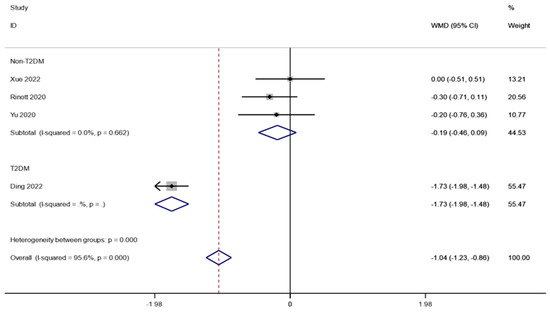
Figure 8.
Subgroup analysis for fasting blood glucose in obesity and associated metabolic diseases following FMT.
3.5. Sensitivity Evaluation
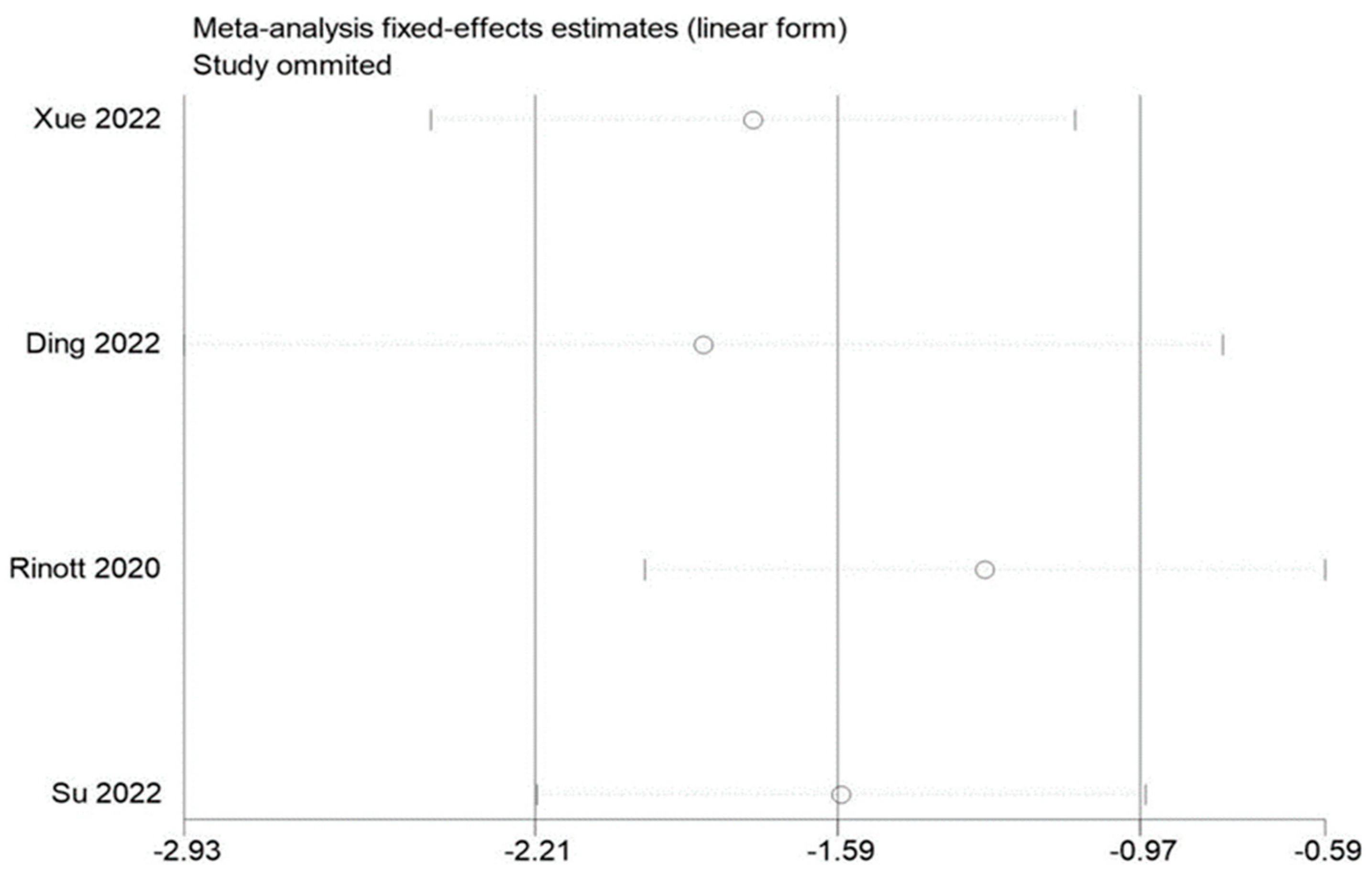
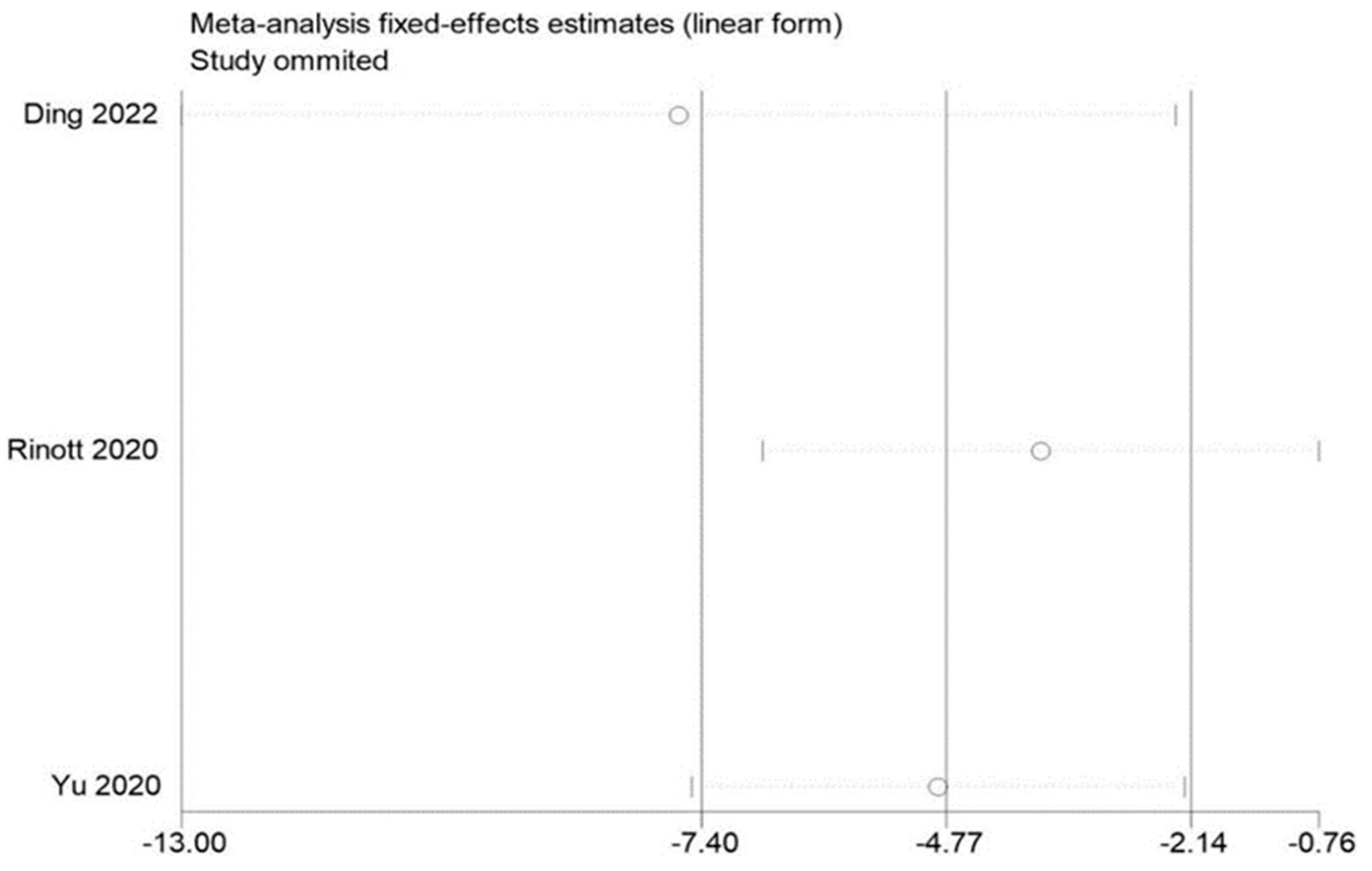
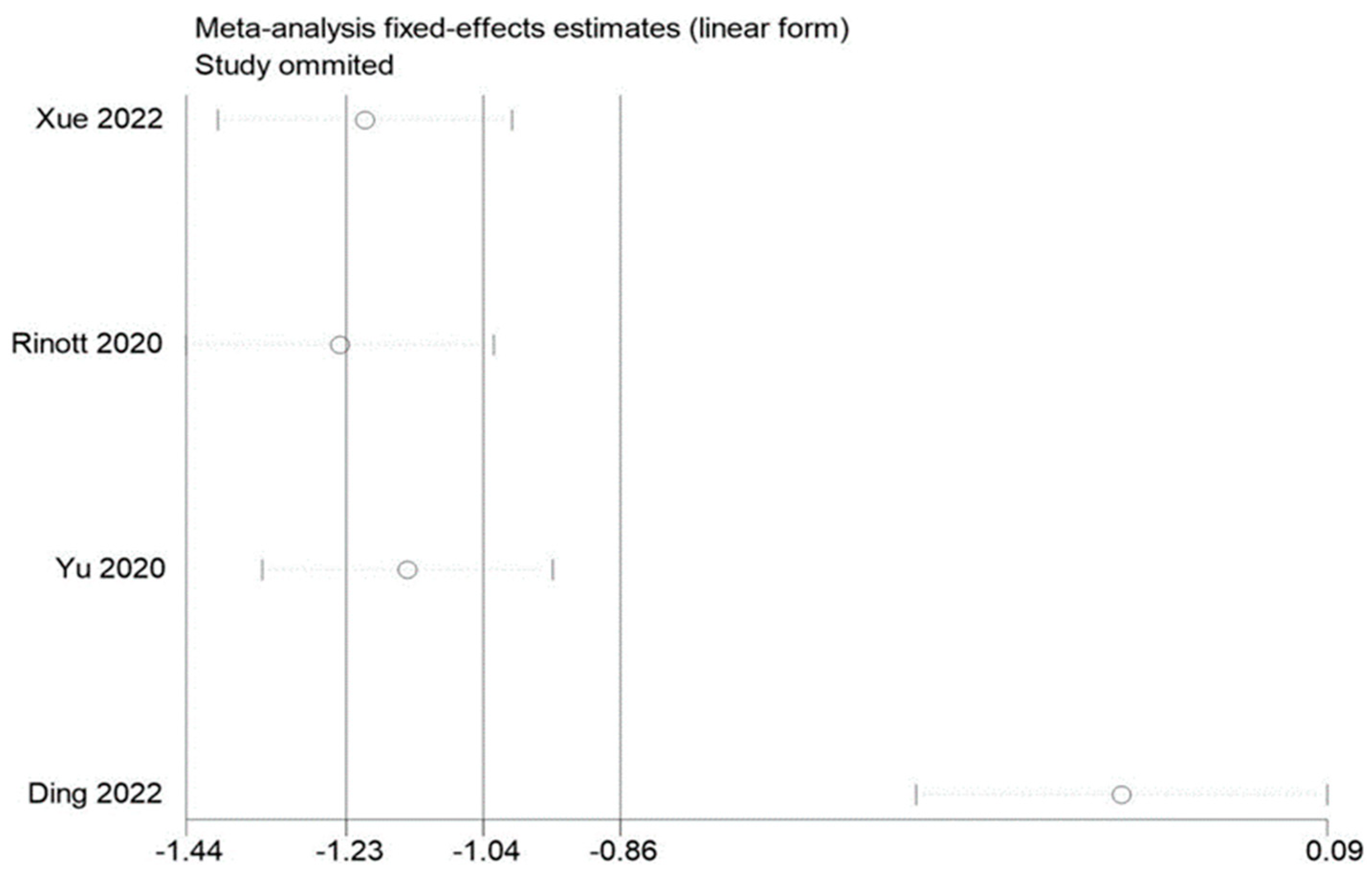
By removing each of the included studies one at a time and performing a summary assessment of those that were still available, we performed a sensitivity analysis. Our investigation revealed that, although the other findings were mostly constant, the study by Ding et al. had an effect on the analysis of fasting blood glucose. In addition, no significant influence on the outcomes was found in the indicators of other studies, indicating that there is a good deal of stability and reliability in the outcomes of other research. The findings of the sensitivity analysis are displayed in Figure 9, Figure 10 and Figure 11.
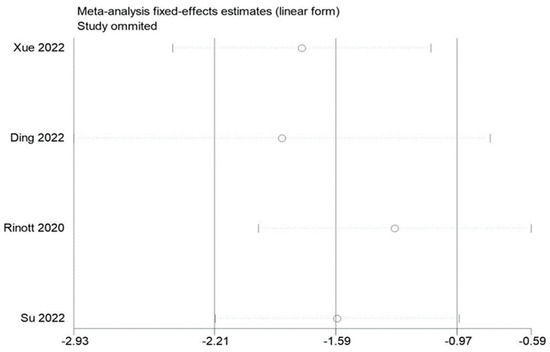
Figure 9.
Sensitivity analysis of BMI in obesity and associated metabolic diseases following FMT.
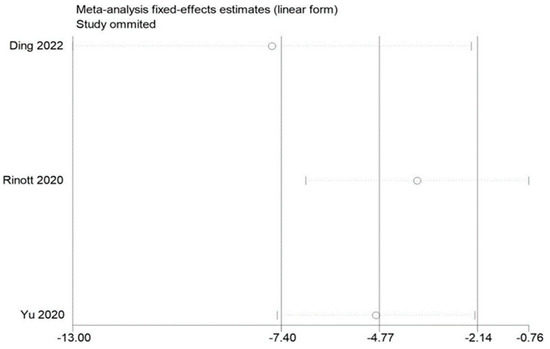
Figure 10.
Sensitivity analysis of weight in obesity and associated metabolic diseases following FMT.
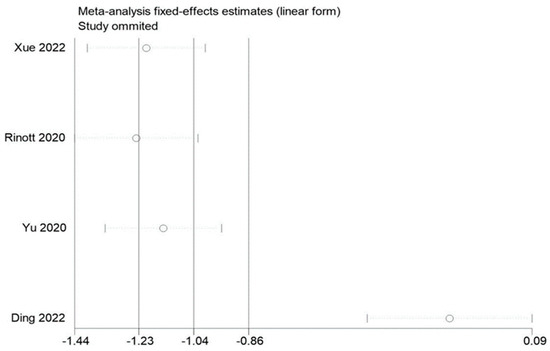
Figure 11.
Sensitivity analysis of fasting blood glucose in obesity and associated metabolic diseases following FMT.
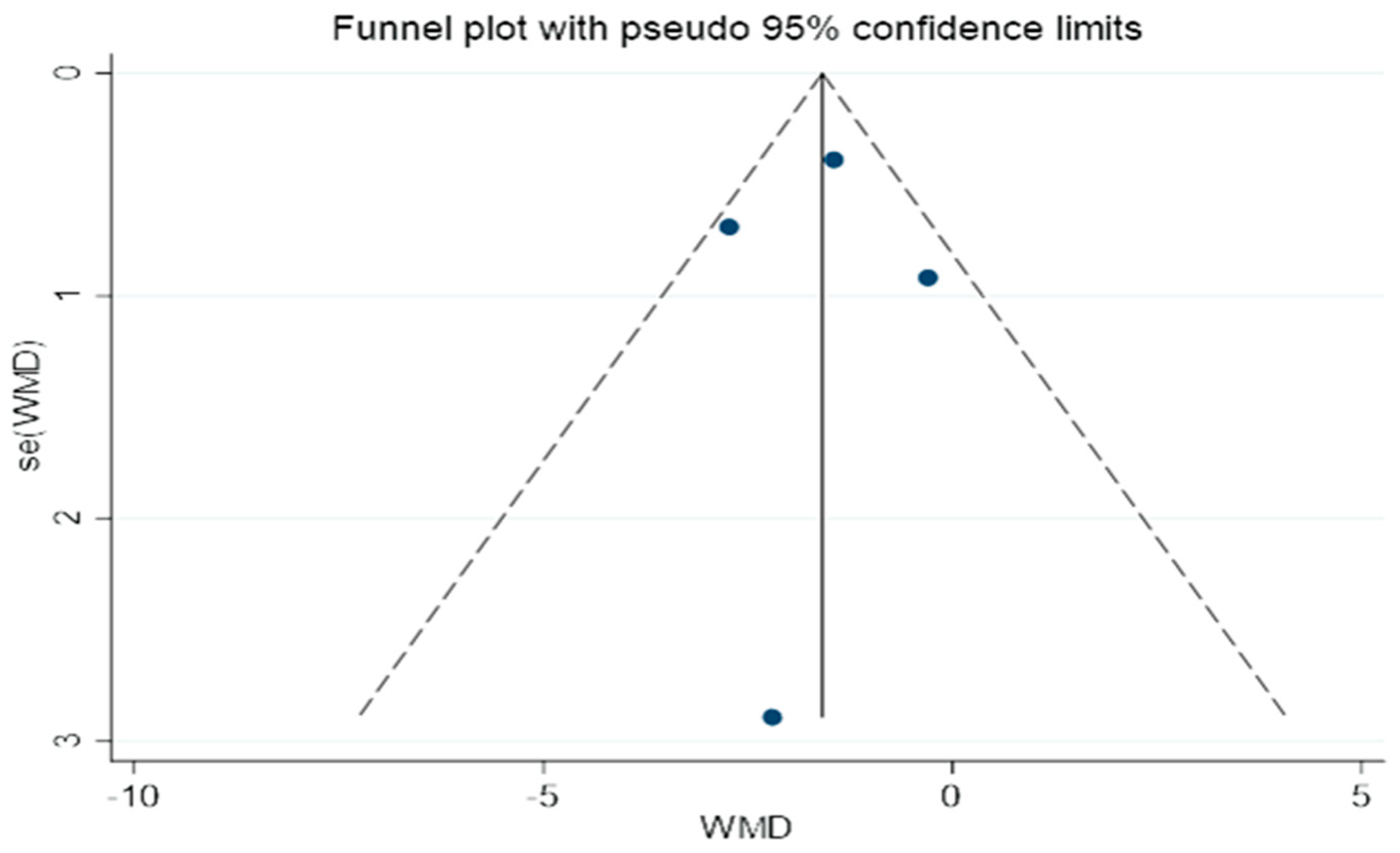
3.6. Publication Bias
An illustration of the funnel plots can be found in Figure 12. A bias in publication is not evident in this study, as shown by the fact that the two funnel plots all had the same shape.
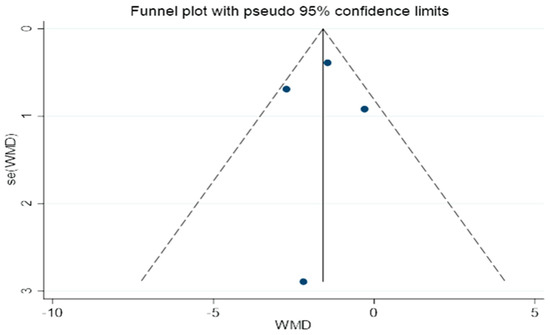
Figure 12.
A funnel plot for determining publication bias.
4. Discussion
Over the past 50 years, throughout the world, the amount of obese individuals has continued to rise, making obesity a major public health issue. Obesity is not simply a matter of body size management [31]. More importantly, obesity not only leads to a decreased quality of life but also poses serious health risks. Overweight and obesity are associated with several metabolic diseases (including type 2 diabetes and nonalcoholic fatty liver disease), as well as cardiovascular diseases (such as hypertension, myocardial infarction, and stroke), as well as certain cancers (such as liver, colon, breast, etc.) [32]. As a result, obesity imposes a heavy burden on the health of individuals, the public health system, and the social economy. As overweight and obesity are on the rise, the epidemic level is high, and the whole population is affected by the situation; preventing obesity and controlling it is still faced with great challenges [33]. However, existing treatments for obesity are still limited and come with health risks. Bariatric surgery and weight-loss drugs help people get rid of the trouble of obesity by reducing appetite, reducing the body’s absorption of lipids, and other means, at the same time; they also bury dangerous hidden dangers in people’s body, such as increased blood pressure, rapid heart rate, anorexia, insomnia, abnormal liver function, depression, diarrhea, and other harmful side effects [34]. Although changing one’s lifestyle in terms of diet and rhythm is considered to be the safest and most effective way to prevent the occurrence of obesity, the effect is limited and difficult to maintain [35]. Obesity and obesity-related metabolic diseases require the development of new, effective treatment strategies. FMT, which involves replenishing the gut microbiota with healthy human feces, is a successful way to treat diseases brought on by intestinal dysbiosis. It is presently the established standard of care for the therapy of recurrent Clostridioides difficile infection [36]. The gut microbiota and metabolic processes have been linked in many research papers, including FMT from thin persons being found to lower obesity and undesirable metabolic characteristics [37]. We evaluated participants’ body weight, BMI, fasting blood glucose, fasting insulin, and HOMA-IR, as well as HbA1C in six studies that looked at the effects of FMT on weight and glycemic management in obesity and other metabolic disorders.
The first findings of this research indicated that weight and BMI were much lower following FMT therapy than they had been. Glucagon-like peptide 1 (GLP1) may be released by the gut microbiota via a route involving SCFA and receptors that are G protein-coupled, which makes you feel full, aids in weight loss, and promotes the emptying of the stomach [38,39]. Additionally, the gut microbiota alters the routes used by bile acids, which impacts how lipids are broken down [40]. This implies that FMT aids in weight loss in obese individuals and the treatment of obesity may be possible with this method.
In addition, the study found that blood glucose levels were not statistically significantly affected by FMT. This may be due to the fact that the people included in the study were all individuals with normal blood glucose. Therefore, we further conducted subgroup analyses of people with diabetes. It was revealed that FMT greatly reduced fasting blood glucose in people with diabetes. However, because Ding et al. only conducted one research [41,42,43,44], which explored the impact of FMT on blood glucose among people suffering from T2DM, the objectivity of the outcomes was challenged. The combined data also revealed that the level of fasting insulin before and after FMT treatment did not change substantially from one another. This may possibly be because FMT only affects insulin levels in diabetic patients; more clinical studies are required to examine how FMT affects insulin levels in both healthy individuals and diabetic patients. Notably, the pooled outcomes showed that HOMA-IR and HbA1c after FMT therapy were significantly lower than before therapy. This outcome is consistent with that of a clinical experiment conducted by Vrieze et al. [9] in which the participants either obtained FMT from their own waste or that of a thin donor (BMI 23 kg/m2). The insulin sensitivity of individuals who finished the lean FMT rose. The outcomes suggest that FMT may help control the level of glucose in people with obesity and the metabolic diseases that come with it. FMT was most commonly associated with nausea, and serious adverse events were rare [45,46,47,48]. A small number of children had fever, allergy, and other adverse events, but they were mild and transient [49,50,51]. Of concern, on 13 June 2019, the FDA issued an important warning that FMT may present a serious or life-threatening risk of infection, which may be associated with multidrug-resistant bacteria. Medical workers can avoid risks by standardizing FMT treatment and strictly managing procedures. FMT is a challenging new technique, and its safety needs to be repeatedly demonstrated in future F, M, and T therapies [52].
There are several limitations to this study. Firstly, concerns regarding the impartiality of the combined findings are raised by the limited number of research studies that were included in this analysis, and for the validity of our findings to continue to be validated; new available literature will need to be considered in the future. Secondly, there may be differences between healthy and diabetic people in the analysis of blood glucose and insulin, which may lead to heterogeneity. In the future, there is a need for clinical trials to determine whether FMT has a differential effect on blood glucose levels between healthy individuals and diabetics.
5. Conclusions
As a weight loss and glycemic control therapy, FMT helps to prevent and treat metabolic problems linked to obesity, and it is a viable alternative to bariatric surgery for patients who do not wish to undergo the procedure.
Author Contributions
Methodology and conceptualization, Z.G. and G.W.; research, D.H.; drafting the original draft, D.H.; manuscript preparation, review, and editing, J.Z., H.Z., Z.G. and G.W.; supervision, Z.G. and G.W.; project administration, G.W. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the support of the National Nature Science Foundation of China (No.31972052, 31820103010, 32021005), the Fundamental Reach Funds for Central Universities (JUSRP22006, JUSRP51501), and Jiangsu Province’s Collaborative Innovation Centre for Food Safety and Quality Control.
Informed Consent Statement
It was not necessary to obtain ethical approval for the research since it does not involve patients.
Data Availability Statement
Datasets will be made available upon reasonable request by the corresponding author.
Acknowledgments
A great deal of support and assistance has been provided to me throughout the writing of this Systematic Review. The first thing I would like to do is to express my gratitude to my supervisor, Gang W, whose expertise was instrumental in formulating the research questions and methodology for this study. In response to your insightful feedback, I was able to sharpen my thinking and take my work to a higher level. I am particularly grateful for the support and collaboration of my team members, Zhen G, Jian Z, and Hao Z. Throughout my studies, I have received valuable guidance from my tutor, Zhennan G. Through your guidance, I was able to choose the right direction and successfully complete my dissertation. A special thank you must also be extended to my parents for their wise advice and sympathetic ear. It is always a pleasure to have you by my side.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franzago, M.; Pilenzi, L.; Di Rado, S.; Vitacolonna, E.; Stuppia, L. The epigenetic aging, obesity, and lifestyle. Front. Cell Dev. Biol. 2022, 10, 985274. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA J. Am. Med. Assoc. 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Longitudinal Assessment of Bariatric Surgery (LABS) Consortium; Flum, D.R.; Belle, S.H.; King, W.C.; Wahed, A.S.; Berk, P.; Chapman, W.; Pories, W.; Courcoulas, A.; McCloskey, C.; et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 2009, 361, 445–454. [Google Scholar] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J. Hosp. Infect. 2018, 100, S1–S31. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Rs, K.; El, J.S.; Lp, S.; Holst, J.J.; Av, H.; Sd, U.; G, H.; Ke, B.; Am, K.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Ajmera, V.; Loomba, R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin. Gastroenterol. Hepatol. 2019, 17, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS ): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, S.; Jiang, X.; Luo, K.; Huang, X.; Hua, F. Association Of Blood Lipocalin-2 Levels with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2022, 54, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863. [Google Scholar] [CrossRef]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef]
- Ding, D.; Yong, H.; You, N.; Lu, W.; Yang, X.; Ye, X.; Wang, Y.; Cai, T.; Zheng, X.; Chen, H.; et al. Prospective Study Reveals Host Microbial Determinants of Clinical Response to Fecal Microbiota Transplant Therapy in Type 2 Diabetes Patients. Front. Cell Infect. Microbiol. 2022, 12, 820367. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e10. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Glucagon-like peptide-1 receptors in the brain: Controlling food intake and body weight. J. Clin. Investig. 2014, 124, 4223–4226. [Google Scholar] [CrossRef]
- Mullish, B.H.; Pechlivanis, A.; Barker, G.F.; Thursz, M.R.; Marchesi, J.R.; McDonald, J.A. Functional microbiomics: Evaluation of gut microbiota-bile acid metabolism interactions in health and disease. Methods 2018, 149, 49–58. [Google Scholar] [CrossRef]
- Mullish, B.H.; McDonald, J.A.K.; Pechlivanis, A.; Allegretti, J.R.; Kao, D.; Barker, G.F.; Kapila, D.; O Petrof, E.; A Joyce, S.; Gahan, C.G.M.; et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut 2019, 68, 1791–1800. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who will Win? Adv. Nutr. 2019, 10 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, N.; Pan, X.-F.; Chen, L.; Pan, A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 393–405. [Google Scholar] [CrossRef]
- Telles, S.; Gangadhar, B.N.; Chandwani, K.D. Lifestyle Modification in the Prevention and Management of Obesity. J. Obes. 2016, 2016, 5818601. [Google Scholar] [CrossRef]
- Aminian, A.; Zelisko, A.; Kirwan, J.P.; Brethauer, S.A.; Schauer, P.R. Exploring the impact of bariatric surgery on high density lipoprotein. Surg. Obes. Relat. Dis. 2015, 11, 238–247. [Google Scholar] [CrossRef]
- Lefere, S.; Onghena, L.; Vanlander, A.; van Nieuwenhove, Y.; Devisscher, L.; Geerts, A. Bariatric surgery and the liver-Mechanisms, benefits, and risks. Obes. Rev. 2021, 22, e13294. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef]
- Crowley, V.E.F.; Yeo, G.S.H.; O’Rahilly, S. Obesity therapy: Altering the energy intake-and-expenditure balance sheet. Nat. Rev. Drug Discov. 2002, 1, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.W.; Arhire, L.; Covasa, M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity. Obesity 2018, 26, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.; Nieuwdorp, M. The environment within: How gut microbiota may influence metabolism and body composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Zven, S.E.; Susi, A.; Mitre, E.; Nylund, C.M. Association Between Use of Multiple Classes of Antibiotic in Infancy and Allergic Disease in Childhood. JAMA Pediatr. 2020, 174, 199–200. [Google Scholar] [CrossRef]
- Sheykhsaran, E.; Abbasi, A.; Leylabadlo, H.E.; Sadeghi, J.; Mehri, S.; Mazraeh, F.N.; Feizi, H.; Baghi, H.B. Gut microbiota and obesity: An overview of microbiota to microbial-based therapies. Postgrad. Med. J. 2022, 99, 384–402. [Google Scholar] [CrossRef]
- Jamal, R.; Messaoudene, M.; de Figuieredo, M.; Routy, B. Future indications and clinical management for fecal microbiota transplantation (FMT) in immuno-oncology. Semin. Immunol. 2023, 67, 101754. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Salonen, A.; Salojärvi, J.; Ritari, J.; Immonen, O.; Marciani, L.; Gowland, P.; Hoad, C.; Garsed, K.; Lam, C.; et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2014, 64, 1562–1568. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Kaplan, J.; Ferraro, M.; Michelow, I.C. Fecal Bacteriotherapy for Relapsing Clostridium difficile Infection in a Child: A Proposed Treatment Protocol. Pediatrics 2010, 126, e239–e242. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498; quiz 499. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Xu, J.-Y.; Wang, W.; Zeng, Y.; Jiang, J. Obesity and cancer: Mouse models used in studies. Front. Oncol. 2023, 13, 1125178. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. Eclinicalmedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Mandoh, M.; Redfern, J.; Mihrshahi, S.; Cheng, H.L.; Phongsavan, P.; Partridge, S.R. How are adolescents engaged in obesity and chronic disease prevention policy and guideline development? A scoping review. Glob. Heal. Res. Policy 2023, 8, 9. [Google Scholar] [CrossRef]
- Lee, Y.; Samarasinghe, Y.; Chen, L.H.; Hapugall, A.; Javidan, A.; McKechnie, T.; Doumouras, A.; Hong, D. Fragility of statistically significant results in randomized trials comparing bariatric surgeries. Int. J. Obes. 2023, 47, 546–553. [Google Scholar] [CrossRef]
- Rozanski, A.; Sakul, S.; Narula, J.; Uretsky, S.; Lavie, C.J.; Berman, D. Assessment of lifestyle-related risk factors enhances the effectiveness of cardiac stress testing. Prog. Cardiovasc. Dis. 2023, 77, 95–106. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Outcomes from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).