Abstract
Although immunotherapy has proved to be a very efficient therapeutic strategy for many types of tumors, the results for pancreatic cancer (PC) have been very poor. Indeed, chemotherapy remains the standard treatment for this tumor in the advanced stage. Clinical data showed that only a small portion of PC patients with high microsatellite instability/mismatch repair deficiency benefit from immunotherapy. However, the low prevalence of these alterations was not sufficient to lead to a practice change in the treatment strategy of this tumor. The main reasons for the poor efficacy of immunotherapy probably lie in the peculiar features of the pancreatic tumor microenvironment in comparison with other malignancies. In addition, the biomarkers usually evaluated to define immunotherapy efficacy in other cancers appear to be useless in PC. This review aims to describe the main features of the pancreatic tumor microenvironment from an immunological point of view and to summarize the current data on immunotherapy efficacy and immune biomarkers in PC.
1. Introduction
Although pancreatic cancer (PC) has a lower incidence with respect to other tumors, it corresponds to one of the leading causes of cancer death in the world [1]. In fact, it is the third highest cause of cancer-related death with a constantly increasing number of cases. The poor prognosis is mainly due to the late diagnosis, at an advanced stage, with only 3% of patients that survive after 5 years from diagnosis [2,3], hence the need to test new drugs for improving therapeutic strategies. Nowadays, despite biological therapies that have revolutionized the prognosis of several types of tumors, chemotherapy still represents the gold standard treatment for patients affected by PC [4,5]. In fact, targeted therapies and immunotherapy have been unable to provide a significant survival improvement to PC patients [6]. This ineffectiveness could be explained by the specific features of the tumor microenvironment (TME) in PC. The knowledge about the roles of all involved elements in the TME, their complex interactions, and the mechanisms that lead to treatment resistance and cancer immune escape mechanisms is a fundamental requisite to develop new immunological therapeutic strategies against pancreatic tumor cells [7]. In addition, it is important to identify predictive factors of the response to immunotherapy with the aim of selecting those patients that might benefit from these therapies. In this review, we focused on the immunological aspects of the pancreatic TME and their role in therapeutic strategies. Moreover, we also discussed the potential value of predictive and prognostic immune factors. Finally, we provided an up-to-date overview of immunotherapy for PC.
2. The Pancreatic Tumor Microenvironment
PC has a rich and dense stroma, in which several types of cells are present such as fibroblasts, immune cells, stellate cells, and endothelial cells [8]. The complex signals between these cells and tumor cells are responsible for tumor proliferation and survival and/or the response or resistance to drugs. Various immune cells are present in the pancreatic TME in different proportions. These cells can interact with each other, leading to various effects (Figure 1) [9,10].
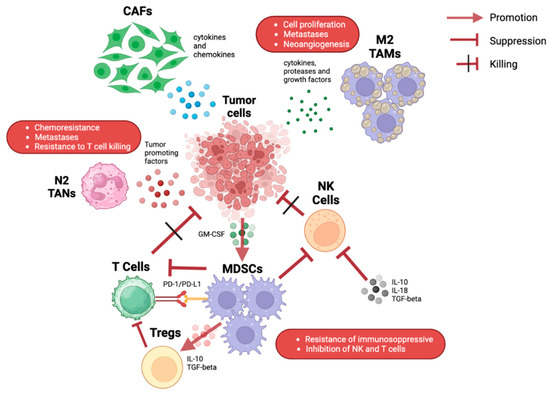
Figure 1.
Illustration of the various possible interactions among immune cells in the pancreatic tumor microenvironment. CAFs: cancer-associated fibroblasts; GM-CSF: granulocyte-macrophage colony-stimulating factor; MDSC: myeloid-derived suppressor cells; NK: natural killer; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; TAMs: tumor-associated macrophages; TANs: tumor-associated neutrophils.
2.1. Tumor-Associated Macrophages
Myeloid cells correspond to the most represented cellular component in the stroma. Literature data correlate the high number of tumor-associated macrophages (TAMs) with poor prognosis in PC patients [11,12]. TAMs might exist in two different phenotypes with opposite functions: M1-like macrophages release pro-inflammatory cytokines with anti-tumor effects while M2-like macrophages have anti-inflammatory effects. M2-like macrophages produce several proteases, cytokines, and growth factors leading to cancer cell proliferation, neo-angiogenesis, and metastases [13,14,15,16].
2.2. Myeloid-Derived Suppressive Cells
Pancreatic tumor cells can recruit Myeloid-derived suppressive cells (MDSCs) in the pancreatic TME by means of the production of the granulocyte-macrophage colony-stimulating factor (GM-CSF) [17,18]. MDSCs exert an important anti-inflammatory action through the inhibition of both innate and adaptive immunity systems. In particular, MDSCs may employ a direct-contact mechanism for blocking natural killer cells (NK cells) and favoring the suppression of T-cell activation through the upregulation expression of programmed death-1 (PD-1) on its surface [19,20,21]. In addition, MDSCs can recruit immunosuppressive regulatory T cells, called Tregs, by producing transforming growth factor-beta (TGF-beta) and interleukin-10 (IL-10) [22].
2.3. Natural Killer Cells
NK cells play a pivotal role through direct killing action on cancer cells. This function is not related to antigen stimulation, but it is based on several types of cell receptors, including natural cytotoxicity receptors (NCRs), natural killer group 2 membrane D (NKG2D), 90 CD16, and DNAM-1 [23,24].
However, NK cells have a low and impaired activity in the pancreatic TME due to complex interactions with tumor cells and other immune cells. These NK cells produce a very low number of proteases such as perforin and granzyme B, and they are characterized by a lower surface expression of the chemokine receptor CXCR2 [25,26].
The main causes of this low-function state of NK cells correspond to the over-production of IL-18, IL-10, and TGF-beta, and to the downregulation of activating receptors [27,28].
2.4. Tumor-Associated Neutrophils
Neutrophils in the TME are called tumor-associated neutrophils (TANs). In the pancreatic TME, they may be present in two different states: N1 neutrophils that are polarized by TGF and N2 neutrophils by IFN [29]. The first ones have pro-inflammatory functions; indeed, they promote the chemotaxis and activation of CD8+ T cells [30]; the second ones have a pro-tumor action through the release of several types of proteases including neutrophil elastase (NE), metalloproteinase (MMPs), and other tumor-promoting factors such as reactive oxygen and nitrogen species [31,32]. Neutrophils can also produce a type of adipokine called lipocalin-2, which is involved in cancer cell activation and stromal remodeling [33].
Tregs can produce IL-17 that can indirectly induce the production of neutrophil extracellular traps (NETs) [34]. The formation of NETs may block CD8+ T cells and favor the occurrence of immune checkpoint inhibitor (ICI) resistance and liver metastasis.
2.5. T-Regs
T regs have numerous anti-inflammatory functions, mainly due to the suppression of T-112 cell function. Moreover, they are involved in the early phases of carcinogenesis [35]. In this regard, a lot of Th-17 cells and Treg are present in premalignant lesions, including pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN) [36,37,38]. In addition, an elevated Th2/Th1 tumor-infiltrating lymphocyte ratio has been discovered in the pancreatic TME [38,39,40]. Various stimulating factors produced by different immune cells such as B cells, dendritic cells, TAMs, cancer-associated fibroblasts (CAFs), dendritic cells, and TAMs favor Th2 enrichment in the pancreatic TME [41,42,43]. Th2 lymphocytes exert their role through GATA3, which stimulates M2 macrophage activation and induces tumor cell proliferation. The pro-tumor function is also strictly correlated with the elevated activation of AKT, STAT3, and MAPK pathways [44].
2.6. CD8+ T Lymphocytes
Although CD8+ T cells lymphocytes usually exert a direct and cytotoxic activity on tumor cells, the TME may lead to CD8+ T exhaustion of this type of lymphocyte, with subsequent impaired cytotoxic actions and shorter cell survival [45]. T cell exhaustion corresponds to a progressive loss of effector activity (loss of TNF-α, IL-2, and IFN-γ production) and increased expression of inhibitory receptors such as CD160, the T cell immunoglobulin domain, lymphocyte-activation gene 3 (LAG-3), PD-1, and CTLA-4 [46]. The interactions between immune system and tumor cells in the TME led to a change in differentiation of CD8+T lymphocytes [47]. A lot of tumor cells express self-antigens, depleting many partial tumor-specific T lymphocytes during thymic maturation, while the other tumor-specific T cells have a low affinity for antigen recognition [47,48]. Moreover, the TME is characterized by the lack of innate stimulators, so antigen-presenting cells (APCs) are weakly activated, leading to a suboptimal activation of tumor-specific T lymphocytes [49]. In addition, on the one hand, immune cells kill tumor cells but, on the other hand, tumor cells recruit immunosuppressive cells, generating the immunosuppressive TME [50,51].
2.7. No-Immune Cells
Many types of non-immune cells such as CAFs and stellate cells can be found in the TME with very important roles [52,53,54]. In detail, three different CAF subpopulations have been identified. The first one is close to tumor cells and has myofibroblastic and anti-tumor effects. TGF stimulates this subpopulation, polarizes macrophages to M2 subtypes, suppresses T-cell activity, and is involved in the production of extracellular matrix, in the epithelial-to-mesenchymal transition, and in cell growth [55,56,57,58]. On this basis, Galunisertib, a TGF inhibitor, has been evaluated in combination with chemotherapy as the first-line treatment of PC [59]. The second subpopulation favors an immunosuppressive state of the TME following the IL-1-mediated activation [56]. The expression of IL-1 can be increased by gut commensal or intra-tumoral bacteria with the consequent activation of this type of CAF. This last subpopulation can produce several factors such as IL-33, IL-6, IL-8, and CXCL12, which in turn promote tumor angiogenesis and provide chemoresistance and resistance to the T-cell killing effect [60,61,62]. The third ones, called antigen-presenting CAFs, are the third subpopulations and their role has not been well defined yet, although it seems that they probably favor an immune-suppressive state [54]. Finally, the microbiota can significantly influence the immunological state of the pancreatic TME. In vivo studies on murine models reported that bacterial ablation led to a lower level of M2 macrophages, a larger number of CD8+ T-lymphocytes, and a higher expression level of PD-1 in the pancreatic TME. Therefore, it is possible to assume that a therapeutic strategy including ICIs and antibiotics might be effective in this cancer, although a possible limiting factor might be represented by safety. Further clinical studies will be helpful to better evaluate this combination strategy.
3. Prognostic and Predictive Immune Biomarkers
Although the results reported with immunotherapy in PC are unfavorable, there is a small portion of PC patients that seem to benefit very much from this type of treatment [6], hence the importance of identifying specific biomarkers to predict the response to ICIs. The most important predictive factor of immunotherapy efficacy in PC corresponds to the presence of a high tumor mutational burden (TMB) since it is associated with a high level of cancer neoantigens [63]. However, a cut-off to indicate a TMB as “high” has not been defined yet; indeed, it varies according to studies and histology [64,65]; most studies regarding PC identified a cut-off of 20 mutations/Mb. PC is typically characterized by a very low median TMB, approximately 1–4 mutations/Mb, while PC with high TMB corresponds only to 1.1% of cases [66,67,68]. Note that the highest level of TMB has been detected in two very rare histological types (<2%), mucinous-colloid and medullary [69]. Furthermore, high-TMB PC is often (about 60%) associated with a mismatch repair deficiency (dMMR) or high microsatellite instability (MSI-H) and/or mutations in ERBB2, POLE, BRCA2, and BRAF genes [69]. Clinical data show that PC patients with high-TMB and MSI-H/dMMR experienced an important objective response rate (ORR) when treated with anti-PD-1 anti-bodies [69]. These data suggest a potential role of TMB as a potential predictive factor of response to immunotherapy for PC patients.
The expression level of programmed death-ligand 1 (PD-L1) is a well-defined predictive factor of ICI efficacy for several types of cancers. However, the number of cells expressing PD-1 and PD-L1 is lower in PC than those cancers where ICIs showed an important activity [70]. PD-L1 expression is present in around 30–40% of PC and is related to poor prognosis and a low presence of tumor-infiltrating lymphocytes, specifically CD8+ cells [71,72]. In addition, its expression seems to be favored by MLL1, MYC, and RAS mutations [73,74,75]. The literature data describe four PC models according to PD-L1 expression on immune cells (ICs) and tumor cells (TCs) [71]:
- Adaptive-1 (ICs > 1%, TCs: 0);
- Adaptive-2 (ICs > 1%, TCs > 1% to <25%);
- Constitutive (ICs: 0, TCs ≥ 25%);
- Combined (ICs > 1%, TCs ≥ 25%).
Tumors with an adaptive-1 pattern had a T-cell-inflamed TME, a high level of PD1+ T cells and CD3+, CD4+, and CD8+ lymphocytes, and a reduced number of CD68+ cells, including the M2-polarized macrophages. This pattern is correlated with the longest survival. On the other hand, tumors with constitutive patterns are characterized by a low number of ICs, except for CD68+ macrophages, and are associated with poor clinical results. In terms of prognostic role, the immune infiltration of CD163+ M2-polarized macrophages in PC has a negative impact while an elevated infiltration of CD4+ and CD8+ cells is correlated with a longer disease-free survival [72]. Currently, the presence of MSI-H/dMMR is the only factor proved to predict the response to ICIs for PC, although it regards only 3% of PC patients [76,77,78]. As mentioned before, the coexistence of high-TMB and MSI-H/dMMR occurs in 60% of PC and also shares several features, including a high prevalence of mucinous/colloid and medullary histology and a characteristic genomic landscape, with less common KRAS and TP53 mutations and more frequent JAK mutations with respect to microsatellite stable PC [78]. Mutant KRAS has a well-known oncogenic role, but it seems to be involved also in the formation of an immunosuppressed TME. In detail, KRAS mutations led to the downregulation of HLA class I on the cell surface and the overexpression of PD-L1 and CD47 with the consequent prevention of innate and adaptative anti-cancer responses [79,80,81].
In addition, mutant KRAS (mKRAS) promotes a paracrine network favoring TME infiltration with stromal cells and suppressive ICs and leading to a desmoplastic reaction in the TME [80,82]. Moreover, mKRAS favors TME infiltration by MDSCs and T-cell exclusion through the increased tumor expression of CXCL1 and GM-CSF, promotes the downregulation of CCL4 expression, impeding DC recruitment, and has a pro-inflammatory role by activating the Sonic Hedgehog signaling pathway and stimulating the expression of IL-6, COX2, MMP7, and pSTAT3 [17,83,84,85,86]. Experimental data on mouse models described as the inactivation of this tumor oncogene determined the formation of pancreatic intraepithelial neoplasia (PanIN) [87,88]. Moreover, PanIN lesions were infiltrated by MDSCs, TAMs, and Tregs. On the other hand, the level of type I conventional dendritic cells (cDCs-1) decreased as PanINs progressively evolved to invasive cancers [89,90]. These data correlate with the low number of infiltrating DCs found in human PC [91,92,93]. Exome studies have discovered that activating KRAS mutations are present in more than 50% of human PC [93,94]. Therefore, an increased level of mutated KRAS genes might represent a predictive factor of PC progression and metastasis [95,96]. On these bases, a novel therapeutic strategy might be the employment of the KRAS inhibitor to sensitize PC to immunotherapy [82]. In this regard, mouse cancer models tested the pharmacologic inhibition of KRAS G12C that led to an increased level of MHC-I expression on cancer cells and promoted TME infiltration by cDCs-1 and T-cells [97]. These events favored tumor sensibilization to immune modulation. However, KRAS G12C mutations only rarely occur in PC, and no targeted therapies have been approved for the other mKRAS variants, although some strategies, such as engineered exosomes and mKRAS-specific T lymphocytes, have shown interesting results [98,99].
4. Immunotherapy: State of the Art
Despite several ICIs, including anti-CTLA4, anti-PD-1, and anti-PD-L1 antibodies, documenting high efficacy in various types of cancers, the same is not true for PC. Indeed, the results derived from the administration of immunotherapy in PC patients have been largely disappointing [6,100].
Different efforts have been performed to improve the immune infiltration of the pancreatic TME. For example, it is well known that the inhibition of CXCR4 might result in increased T-cell chemotaxis. In this regard, preclinical modes demonstrated enhanced T-cell expansion and cancer cell death through the employment of PD-1 plus CXCR4 inhibition [101].
Another strategy to enhance the anticancer activity of the TME could be represented by CD40 activation. Indeed, agonistic CD40 antibodies have been proven to enhance tumor cells killing mediated by T lymphocytes and to rescue ICI sensitivity when combined with chemotherapy [102,103,104,105]. A phase I trial evaluated sotigalimab—a CD40 agonistic monoclonal antibody—and sotigalimab plus chemotherapy in combination or not with nivolumab—a PD-1 inhibitor—as a first-line therapy for metastatic PC patients. The results reported an ORR of 58% without serious adverse events (AEs) [105].
Two phase II clinical trials tested single-agent ICIs or their combination with the aim to improve immunotherapy activity on advanced PC. However, single-agent ipilimumab and durvalumab, anti-CTLA-4 antibodies, as single-agents or in combination with tremelimumab, a PD-L1 inhibitor, did not show significant results [106,107].
At the same time, the combination of a single ICI or dual immune blockade with chemotherapy (gemcitabine plus nab-paclitaxel) proved to be poorly effective [108,109]. The addition of pembrolizumab to chemoradiation therapy in the neoadjuvant setting did not lead to an improvement compared to chemoradiation alone and an enhanced immune infiltration in the TME [110]. A phase II pilot study analyzed nivolumab plus paricalcitol and chemotherapy (gemcitabine, cisplatin, and nab-paclitaxel) in 10 advanced PC patients as a first-line treatment. Although a very low number of patients was enrolled in this study, an ORR of 80% and a disease control rate (DCR) of 100% were obtained, but no further investigations were performed [111].
Another interesting strategy to improve immunotherapy efficacy is represented by the combination of ICI with vaccines. In this regard, a study tested ipilimumab plus GVAX, a granulocyte-macrophage colony-stimulating factor (GM-CSF) cell-based vaccine. The results showed an increased T-cell repertoire in the TME and better overall survival (OS) with respect to ipilimumab alone, with a statistically significant difference [112]. Another trial evaluated GVAX plus nivolumab or with nivolumab and urelumab, an anti-CD137 antibody, as a neoadjuvant or adjuvant treatment for resectable PC patients. The experimental group treated with GVAX, nivolumab, and urelumab obtained a better OS, disease-free survival (DFS), and pathologic response, but without a statistically significant difference [113]. A phase III clinical study investigated an allogenic vaccine, algenpantucel-L, made up of Gal-expressing engineered PC cell lines, combined with chemotherapy and chemoradiotherapy in the adjuvant setting. However, no significant improvements were observed [114,115].
Interestingly, oncolytic viruses have also been studied in PC, as single agents or combined with conventional treatments. They are natural or genetically modified viruses tested as treatment of different tumors. They may directly cause tumor cell killing or indirectly modify the TME indirectly favoring tumor regression [116,117,118]. Preclinical and clinical data show few promising results through the employment of reovirus, vaccinia, adenovirus, and herpes simplex 1. The poor results are probably due to the elevated density of the pancreatic TME that determines a low penetrability to viruses [119]. A phase II clinical trial tested the combination of Pelareorep, an isolate of a reovirus strain derivate, with gemcitabine. The results documented high viral replication in PC cells and an acceptable profile of safety [120]. A phase Ib trial evaluated Pelareorep plus chemotherapy and immunotherapy in pretreated PC patients reporting favorable outcomes and good tolerance [121]. Bruton tyrosine kinase (BTK) is expressed by various ICs [122]. In vivo data on PC murine models showed that the inhibition of BTK led to the differentiation of CD8 T-cells and the shift from an M2-like to M1-like macrophage phenotype [43]. In addition, when the BTK inhibition was associated with gemcitabine, an enhanced tumor shrinkage was determined [43]. In this regard, a randomized phase II clinical trial analyzed the combination of Acalabrutinib—a BTK inhibitor—combined or not with pembrolizumab, in pretreated PC patients. The combination group experienced a DCR of 29% with respect to 14.4% of the acalabrutinib-alone group [123].
Based on the favorable clinical results for those PC patients with an elevated T-lymphocytes infiltration, it is easy to assume that tumor infiltrating lymphocyte (TIL) therapy may represent a potential anti-cancer activity although most PC patients do not have pre-existing T-cell immunity, limiting this type of strategy [118,119]. The continuous research about this topic has rapidly led to very important results for patients suffering from hematological tumors through the employment of T-cell receptor T-cell (TCR-T) therapy and chimeric antigen receptor T-cell (CAR-T) therapy [120]. Unfortunately, these results were not obtained for solid tumors. However, different strategies have been tested to improve TIL therapy, for example, by means of the combination with immune modulating agents or the expression of transgenes to improve T-cell infiltration and activity [121]. The most important limitation to TCR-T and CAR-T treatments in PC corresponds to the antigen selection since they can have variable or heterogeneous expression on cancers cells, determining an elevated risk of toxicity. CEA, HER2, and mesothelin antigens have been mainly studied for CAR-T therapy in PC [122]. However, the employment of T lymphocytes directed against CEA and HER2 antigens determined serious adverse events [123,124,125]. On the other hand, mouse models [126] and early-phase clinical trials showed important activity and good safety by CAR-T engineered to target mesothelin in advanced PC patients pretreated with chemotherapy [127,128]. mKRAS has a high frequency in PC, so a neoantigen-targeted TCR therapy might represent a favorable strategy [93,129]. Currently, clinical studies are investigating this strategy [93].
Immunosuppressive ICs in the TME such as TAMs and MDSCs may represent a target of immune-based treatments with the aim of eliminating inhibitory elements that hamper T-cell responses. In this regard, CSF-1R inhibition favors antigen presentation and anti-cancer T-cell activity by TAMs that are suppressed by immune checkpoints [130]. Moreover, the combination of CSF-1R inhibition and ICI greatly promotes anti-tumor efficacy [130]. Early-phase clinical trials showed interesting results from the CSF-1R and CCR2 inhibition in advanced PC [76,131]. However, the combination of cabiralizumab, a CSF-1R blocking monoclonal antibody, with chemotherapy and nivolumab in metastatic PC patients did not improve PFS with respect to chemotherapy alone in a phase II study.
Another strategy is to target stromal elements, such as hyaluronan, the vitamin D receptor (VDR), focal adhesion kinase (FAK), and fibroblast activation protein (FAP), that promote the desmoplastic reaction and the consequent chemo-resistance of PC [132]. PEGPH20 is a hyaluronidase that has been evaluated in combination with chemotherapy compared to chemotherapy alone for untreated metastatic PC patients with high hyaluronan levels in a phase III trial [133]. However, the results did not demonstrate an improvement of OS. PEGPH20 has also been examined in association with mFOLFIRINOX in a phase II trial [134]. However, the study was prematurely closed because of poor results and elevated toxicity. These unsatisfactory outcomes suggest that targeting stromal elements alone is insufficient to overcome PC immune resistance. Currently, treatment strategies that combine ICIs plus targeting desmoplasia are ongoing. Early-phase clinical studies are currently under investigation with the aim to test FAK inhibitors plus αPD1 antibodies [135]. Moreover, FAP-directed CAR-T therapy showed an anti-cancer response in tumor mouse models [136,137].
As mentioned before, MSI-H is present in 1–2% of PC, and high TMB is very rare in this tumor [78,138,139]. The KEYNOTE-158 trial investigated pembrolizumab in patients affected by advanced non-colorectal cancers with MSI-H/MMR-d including 22 PC patients. This group of patients experienced an ORR of 18.2%, a median duration of response of 13.4 months, and a PFS and OS of 2.1 and 4.0 months, respectively. However, these results were considerably lower than patients who suffered from other tumors [64]. Table 1 summarizes all ongoing clinical studies evaluating immunotherapy in PC patients.

Table 1.
Ongoing clinical studies evaluating immunotherapy in PC patients.
5. Conclusions
To date, no clinical trial testing immunotherapy or targeted therapy for PC patients has led to practice-changing results. This is probably due to the peculiar pancreatic TME and its genomic landscape. Further studies are needed to better understand TME features that make PC resistant to immunotherapy with the aim to design modification mechanisms so that PC becomes more immunosensitive. A better selection of patients that might benefit more from immunotherapy is also required. An useful approach could derive from the identification of biomarkers able to predict clinical outcomes following the administration of immunotherapy.
Author Contributions
Conceptualization, C.L. and P.F.; methodology, F.M.M.; software, M.L.I.; validation, A.N.S., M.P. and P.D.S.; formal analysis, R.M. and C.G.; investigation, C.G.; resources, C.L.; data curation, R.M., F.A. and P.F.; writing—original draft preparation, C.L.; writing—review and editing, P.F.; visualization, F.M.M. and M.L.; supervision, P.F.; project administration, C.L.; funding acquisition, A.N.S. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laface, C.; Laforgia, M.; Molinari, P.; Foti, C.; Ambrogio, F.; Gadaleta, C.D.; Ranieri, G. Intra-Arterial Infusion Chemotherapy in Advanced Pancreatic Cancer: A Comprehensive Review. Cancers 2022, 14, 450. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Currò, G.; Laface, C.; Zuccalà, V.; Memeo, R.; Luposella, F.; Laforgia, M.; Zizzo, N.; Zito, A.; Loisi, D.; et al. Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells 2021, 10, 444. [Google Scholar] [CrossRef]
- Laface, C.; Laforgia, M.; Zito, A.F.; Loisi, D.; Zizzo, N.; Tamma, R.; Gadaleta, C.D.; Porcelli, M.; Currò, G.; Ammendola, M.; et al. Chymase-positive Mast cells correlate with tumor angiogenesis: First report in pancreatic cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6862–6873. [Google Scholar] [CrossRef]
- Ranieri, G.; Laface, C. Loco-Regional and Systemic Chemotherapies for Hepato-Pancreatic Tumors: Integrated Treatments. Cancers 2020, 12, 2737. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Sablone, S.; Fazio, V.; De Ceglia, D.; Porcelli, M.; Molinari, P.; Fucci, L.; Laface, C.; Gadaleta, C.D. A Patient With Stage III Locally Advanced Pancreatic Adenocarcinoma Treated With Intra-Arterial Infusion FOLFIRINOX: Impressive Tumoral Response and Death due to Legionella pneumophila Infection: A Unique Case Report. Front. Oncol. 2022, 12, 877334. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.; Dyhl-Polk, A.; Chen, I.; Nielsen, D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat. Rev. 2019, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer-clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Farrow, B.; Albo, D.; Berger, D.H. The Role of the Tumor Microenvironment in the Progression of Pancreatic Cancer. J. Surg. Res. 2008, 149, 319–328. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef]
- Ligorio, M.; Sil, S.; Malagon-Lopez, J.; Nieman, L.T.; Misale, S.; Di Pilato, M.; Ebright, R.Y.; Karabacak, M.N.; Kulkarni, A.S.; Liu, A.; et al. Stromal Microenvironment Shapes the Intratumoral Architecture of Pancreatic Cancer. Cell 2019, 178, 160–175.e27. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe Di, C.; Nina, C.; Giovanni Francesco, C.; Fabio, G.; Francesca, G.; Cristina, R.; Giovanni, C.; Rossana, M.; Jelena, T.; Alessandro, Z.; et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut 2016, 65, 1710. [Google Scholar] [CrossRef]
- Heidi, G.; Christof, D.; Nada, M.; Bence, S.; Jonas, R.; Thomas, M.G.; Patrick, M. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut 2017, 66, 1278. [Google Scholar] [CrossRef]
- Filippini, D.; Agosto, S.D.; Delfino, P.; Simbolo, M.; Piro, G.; Rusev, B.; Veghini, L.; Cantù, C.; Lupo, F.; Ugel, S.; et al. Immunoevolution of mouse pancreatic organoid isografts from preinvasive to metastatic disease. Sci. Rep. 2019, 9, 12286. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Bayne, L.J.; Beatty, G.L.; Jhala, N.; Clark, C.E.; Rhim, A.D.; Stanger, B.Z.; Vonderheide, R.H. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012, 21, 822–835. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Lee, K.E.; Hajdu, C.H.; Miller, G.; Bar-Sagi, D. Oncogenic Kras-Induced GM-CSF Production Promotes the Development of Pancreatic Neoplasia. Cancer Cell 2012, 21, 836–847. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012, 22, 275–281. [Google Scholar] [CrossRef]
- Pinton, L.; Solito, S.; Damuzzo, V.; Francescato, S.; Pozzuoli, A.; Berizzi, A.; Mocellin, S.; Rossi, C.R.; Bronte, V.; Mandruzzato, S. Activated T cells sustain myeloid-derived suppressor cell-mediated immune suppression. Oncotarget 2016, 7, 1168. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-Talk between Myeloid-Derived Suppressor Cells and Macrophages Subverts Tumor Immunity toward a Type 2 Response1. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, P.-Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.-H. Gr-1+CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dhar, P.; Wu, J.D. NK Cell Plasticity in Cancer. J. Clin. Med. 2019, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef]
- Funa, K.; Nilsson, B.; Jacobsson, G.; Alm, G.V. Decreased natural killer cell activity and interferon production by leucocytes in patients with adenocarcinoma of the pancreas. Br. J. Cancer 1984, 50, 231–233. [Google Scholar] [CrossRef]
- Lim, S.A.; Kim, J.; Jeon, S.; Shin, M.H.; Kwon, J.; Kim, T.-J.; Im, K.; Han, Y.; Kwon, W.; Kim, S.-W.; et al. Defective Localization With Impaired Tumor Cytotoxicity Contributes to the Immune Escape of NK Cells in Pancreatic Cancer Patients. Front. Immunol. 2019, 10, 00496. [Google Scholar] [CrossRef]
- Wahl, S.M.; Wen, J.; Moutsopoulos, N.M. The kiss of death: Interrupted by NK-cell close encounters of another kind. Trends Immunol. 2006, 27, 161–164. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef]
- Brandau, S.; Dumitru, C.A.; Lang, S. Protumor and antitumor functions of neutrophil granulocytes. Semin. Immunopathol. 2013, 35, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.-j.; Hu, T.; Wang, Y.; Wang, H.; Xu, L.; Cui, N. Neutrophil–lymphocyte ratio (NLR) was associated with prognosis and immunomodulatory in patients with pancreatic ductal adenocarcinoma (PDAC). Biosci. Rep. 2020, 40, BSR20201190. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhang, L.; Peng, Y.; Tao, M.; Li, L.; Xiu, D.; Yuan, C.; Ma, Z.; Jiang, B. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma: A new hypothesis and a new predictor for distant metastasis. Medicine 2016, 95, e4932. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Nasca, V.; Chiaravalli, M.; Piro, G.; Esposito, A.; Salvatore, L.; Tortora, G.; Corbo, V.; Carbone, C. Intraductal Pancreatic Mucinous Neoplasms: A Tumor-Biology Based Approach for Risk Stratification. Int. J. Mol. Sci. 2020, 21, 6386. [Google Scholar] [CrossRef] [PubMed]
- Vizio, B.; Novarino, A.; Giacobino, A.; Cristiano, C.; Prati, A.; Ciuffreda, L.; Montrucchio, G.; Bellone, G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp. Ther. Med. 2012, 4, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int. J. Mol. Sci. 2011, 12, 7424–7437. [Google Scholar] [CrossRef]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef]
- Tassi, E.; Gavazzi, F.; Albarello, L.; Senyukov, V.; Longhi, R.; Dellabona, P.; Doglioni, C.; Braga, M.; Di Carlo, V.; Protti, M.P. Carcinoembryonic Antigen-Specific but Not Antiviral CD4+ T Cell Immunity Is Impaired in Pancreatic Carcinoma Patients1. J. Immunol. 2008, 181, 6595–6603. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Liu, Y.; Henne-Bruns, D.; Kornmann, M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: Evidence for autocrine and paracrine actions. Br. J. Cancer 2005, 92, 921–928. [Google Scholar] [CrossRef] [PubMed]
- De Monte, L.; Wörmann, S.; Brunetto, E.; Heltai, S.; Magliacane, G.; Reni, M.; Paganoni, A.M.; Recalde, H.; Mondino, A.; Falconi, M.; et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016, 76, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Piro, G.; Simionato, F.; Carbone, C.; Frizziero, M.; Malleo, G.; Zanini, S.; Casolino, R.; Santoro, R.; Mina, M.M.; Zecchetto, C.; et al. A circulating TH2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. OncoImmunology 2017, 6, e1322242. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Liu, X.; Liang, C.; Hua, J.; Xu, J.; Wang, W.; Meng, Q.; Liu, J.; Zhang, B.; Yu, X.; et al. Deciphering the Prognostic Implications of the Components and Signatures in the Immune Microenvironment of Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2021, 12, 648917. [Google Scholar] [CrossRef]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2019, 68, 159–171. [Google Scholar] [CrossRef]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Belle, J.I.; DeNardo, D.G. A Single-Cell Window into Pancreas Cancer Fibroblast Heterogeneity. Cancer Discov. 2019, 9, 1001–1002. [Google Scholar] [CrossRef]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell–Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel, C.H., 3rd; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H.; et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Oettle, H.; Kozloff, M.; Cleverly, A.; et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 2018, 119, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; O’Reilly, E.M.; Wolpin, B.M.; Ryan, D.P.; Bullock, A.J.; Britten, C.D.; Linehan, D.C.; Belt, B.A.; Gamelin, E.C.; Ganguly, B.; et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Investig. New Drugs 2020, 38, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Shon, Y.; Kim, J.; Oh, Y.-K. Selective Activation of Anticancer Chemotherapy by Cancer-Associated Fibroblasts in the Tumor Microenvironment. JNCI J. Natl. Cancer Inst. 2016, 109, 186. [Google Scholar] [CrossRef]
- Albrengues, J.; Bourget, I.; Pons, C.; Butet, V.; Hofman, P.; Tartare-Deckert, S.; Feral, C.C.; Meneguzzi, G.; Gaggioli, C. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014, 7, 1664–1678. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Lee, B.; Gibbs, P. Inflammation, Biomarkers and Immuno-Oncology Pathways in Pancreatic Cancer. J. Pers. Med. 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, R.T.; Mattiolo, P.; Mafficini, A.; Hong, S.-M.; Piredda, M.L.; Taormina, S.V.; Malleo, G.; Marchegiani, G.; Pea, A.; Salvia, R.; et al. Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Pancreatic Cancer: Systematic Review and Still-Open Questions. Cancers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Nomi, T.; Sho, M.; Akahori, T.; Hamada, K.; Kubo, A.; Kanehiro, H.; Nakamura, S.; Enomoto, K.; Yagita, H.; Azuma, M.; et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1 Ligand/Programmed Death-1 Pathway in Human Pancreatic Cancer. Clin. Cancer Res. 2007, 13, 2151–2157. [Google Scholar] [CrossRef]
- Blando, J.; Sharma, A.; Higa, M.G.; Zhao, H.; Vence, L.; Yadav, S.S.; Kim, J.; Sepulveda, A.M.; Sharp, M.; Maitra, A.; et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 1692–1697. [Google Scholar] [CrossRef]
- Karamitopoulou, E.; Andreou, A.; Pahud de Mortanges, A.; Tinguely, M.; Gloor, B.; Perren, A. PD-1/PD-L1-Associated Immunoarchitectural Patterns Stratify Pancreatic Cancer Patients into Prognostic/Predictive Subgroups. Cancer Immunol. Res. 2021, 9, 1439–1450. [Google Scholar] [CrossRef]
- McGuigan, A.J.; Coleman, H.G.; McCain, R.S.; Kelly, P.J.; Johnston, D.I.; Taylor, M.A.; Turkington, R.C. Immune cell infiltrates as prognostic biomarkers in pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. J. Pathol. Clin. Res. 2021, 7, 99–112. [Google Scholar] [CrossRef]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099.e6. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef]
- Lu, C.; Paschall, A.V.; Shi, H.; Savage, N.; Waller, J.L.; Sabbatini, M.E.; Oberlies, N.H.; Pearce, C.; Liu, K. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. JNCI J. Natl. Cancer Inst. 2017, 109, djw283. [Google Scholar] [CrossRef]
- Lemery, S.; Keegan, P.; Pazdur, R. First FDA Approval Agnostic of Cancer Site-When a Biomarker Defines the Indication. N. Engl. J. Med. 2017, 377, 1409–1412. [Google Scholar] [CrossRef]
- Macherla, S.; Laks, S.; Naqash, A.R.; Bulumulle, A.; Zervos, E.; Muzaffar, M. Emerging Role of Immune Checkpoint Blockade in Pancreatic Cancer. Int. J. Mol. Sci. 2018, 19, 3505. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: Histology, molecular pathology and clinical implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018, 10, 3342. [Google Scholar] [CrossRef]
- Dias Carvalho, P.; Guimarães, C.F.; Cardoso, A.P.; Mendonça, S.; Costa, Â.M.; Oliveira, M.J.; Velho, S. KRAS Oncogenic Signaling Extends beyond Cancer Cells to Orchestrate the Microenvironment. Cancer Res. 2018, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; El-Sherbiny, Y.M.; Scott, G.B.; Morgan, R.S.M.; Prestwich, R.; Bowles, P.A.; Blair, G.E.; Tanaka, T.; Rabbitts, T.H.; Meade, J.L.; et al. Blocking oncogenic RAS enhances tumour cell surface MHC class I expression but does not alter susceptibility to cytotoxic lymphocytes. Mol. Immunol. 2014, 58, 160–168. [Google Scholar] [CrossRef]
- Bear, A.S.; Vonderheide, R.H.; O’Hara, M.H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer cell 2020, 38, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Patruno, R.; Passantino, G.; Laface, C.; Tinelli, A.; Zito, A.; Ruggieri, R.; Luposella, F.; Gadaleta, P.; Laforgia, M.; Lacitignola, L.; et al. Microvascular Density, Endothelial Area, and Ki-67 Proliferative Index Correlate Each Other in Cat Post-Injection Fibrosarcoma. Cells 2020, 10, 31. [Google Scholar] [CrossRef]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018, 49, 178–193.e7. [Google Scholar] [CrossRef]
- Collins, M.A.; Bednar, F.; Zhang, Y.; Brisset, J.C.; Galbán, S.; Galbán, C.J.; Rakshit, S.; Flannagan, K.S.; Adsay, N.V.; Pasca di Magliano, M. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Investig. 2012, 122, 639–653. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Lee, J.W.; Komar, C.A.; Bengsch, F.; Graham, K.; Beatty, G.L. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+); LSL-Trp53(R172H/+); Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr. Protoc. Pharmacol. 2016, 73, 14.39.1–14.39.20. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Huffman, A.P.; Wattenberg, M.M.; Walter, D.M.; Carpenter, E.L.; Feldser, D.M.; Beatty, G.L.; Furth, E.E.; Vonderheide, R.H. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 2020, 217, 673. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Krisnawan, V.E.; Herzog, B.H.; Zuo, C.; Breden, M.A.; Knolhoff, B.L.; Hogg, G.D.; Tang, J.P.; Baer, J.M.; Mpoy, C.; et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell 2020, 37, 289–307.e9. [Google Scholar] [CrossRef] [PubMed]
- Dallal, R.M.; Christakos, P.; Lee, K.; Egawa, S.; Son, Y.I.; Lotze, M.T. Paucity of dendritic cells in pancreatic cancer. Surgery 2002, 131, 135–138. [Google Scholar] [CrossRef]
- Hiraoka, N.; Yamazaki-Itoh, R.; Ino, Y.; Mizuguchi, Y.; Yamada, T.; Hirohashi, S.; Kanai, Y. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology 2011, 140, 310–321. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Figueroa, E.F.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef]
- Mueller, S.; Engleitner, T.; Maresch, R.; Zukowska, M.; Lange, S.; Kaltenbacher, T.; Konukiewitz, B.; Öllinger, R.; Zwiebel, M.; Strong, A.; et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 2018, 554, 62–68. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Lu, Y.C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Hester, R.; Mazur, P.K.; McAllister, F. Immunotherapy in Pancreatic Adenocarcinoma: Beyond “Copy/Paste”. Clin. Cancer Res. 2021, 27, 6287–6297. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.D.; Jiang, X.; Sullivan, K.M.; Jalikis, F.G.; Smythe, K.S.; Abbasi, A.; Vignali, M.; Park, J.O.; Daniel, S.K.; Pollack, S.M.; et al. Mobilization of CD8+ T Cells via CXCR4 Blockade Facilitates PD-1 Checkpoint Therapy in Human Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 3934–3945. [Google Scholar] [CrossRef]
- Vonderheide, R.H. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell 2018, 33, 563–569. [Google Scholar] [CrossRef]
- Byrne, K.T.; Vonderheide, R.H. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep. 2016, 15, 2719–2732. [Google Scholar] [CrossRef]
- Winograd, R.; Byrne, K.T.; Evans, R.A.; Odorizzi, P.M.; Meyer, A.R.L.; Bajor, D.L.; Clendenin, C.; Stanger, B.Z.; Furth, E.E.; Wherry, E.J.; et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res. 2015, 3, 399–411. [Google Scholar] [CrossRef]
- O’Hara, M.H.; O’Reilly, E.M.; Varadhachary, G.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: An open-label, multicentre, phase 1b study. Lancet Oncol. 2021, 22, 118–131. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-C.; Vlahovic, G.; et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs 2018, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar] [CrossRef]
- Rahma, O.E.; Katz, M.H.G.; Wolpin, B.M.; Dias-Costa, A.; Nowak, J.; Rodig, S.J.; Dougan, S.; Bekaii-Saab, T.S.; Stucky, C.-C.H.; Elias, R.; et al. Randomized multicenter phase Ib/II study of neoadjuvant chemoradiation therapy (CRT) alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic cancer. J. Clin. Oncol. 2021, 39, 4128. [Google Scholar] [CrossRef]
- Borazanci, E.H.; Jameson, G.S.; Borad, M.J.; Ramanathan, R.K.; Korn, R.L.; Caldwell, L.; Ansaldo, K.; Hendrickson, K.; Marceau, K.; Von Hoff, D.D. A phase II pilot trial of nivolumab (N) + albumin bound paclitaxel (AP) + paricalcitol (P) + cisplatin (C) + gemcitabine (G) (NAPPCG) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC). J. Clin. Oncol. 2018, 36, 358. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.J.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef]
- Heumann, T.R.; Judkins, C.; Lim, S.J.; Wang, H.; Parkinson, R.; Gai, J.; Celiker, B.; Durham, J.N.; Laheru, D.A.; De Jesus-Acosta, A.; et al. Neoadjuvant and adjuvant antitumor vaccination alone or combination with PD1 blockade and CD137 agonism in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2022, 40, 558. [Google Scholar] [CrossRef]
- Hardacre, J.M.; Mulcahy, M.; Small, W.; Talamonti, M.; Obel, J.; Krishnamurthi, S.; Rocha-Lima, C.S.; Safran, H.; Lenz, H.-J.; Chiorean, E.G. Addition of Algenpantucel-L Immunotherapy to Standard Adjuvant Therapy for Pancreatic Cancer: A Phase 2 Study. J. Gastrointest. Surg. 2013, 17, 94–101. [Google Scholar] [CrossRef]
- Hewitt, D.B.; Nissen, N.; Hatoum, H.; Musher, B.; Seng, J.; Coveler, A.L.; Al-Rajabi, R.; Yeo, C.J.; Leiby, B.; Banks, J.; et al. A Phase 3 Randomized Clinical Trial of Chemotherapy With or Without Algenpantucel-L (HyperAcute-Pancreas) Immunotherapy in Subjects With Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer. Ann. Surg. 2022, 275, 45–53. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.-W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Vähä-Koskela, M.J.V.; Heikkilä, J.E.; Hinkkanen, A.E. Oncolytic viruses in cancer therapy. Cancer Lett. 2007, 254, 178–216. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Musher, B. Oncolytic viral therapy for pancreatic cancer. J. Surg. Oncol. 2017, 116, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.D.; Monaco, M.L.; Essani, K. The Present Status of Immuno-Oncolytic Viruses in the Treatment of Pancreatic Cancer. Viruses 2020, 12, 1318. [Google Scholar] [CrossRef]
- Ahn, D.H.; Bekaii-Saab, T. The Continued Promise and Many Disappointments of Oncolytic Virotherapy in Gastrointestinal Malignancies. Biomedicines 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Soucek, L.; Buggy, J.J.; Kortlever, R.; Adimoolam, S.; Monclús, H.A.; Allende, M.T.S.; Swigart, L.B.; Evan, G.I. Modeling Pharmacological Inhibition of Mast Cell Degranulation as a Therapy for Insulinoma. Neoplasia 2011, 13, 1093–1100. [Google Scholar] [CrossRef]
- Michael, O.; Milind, J.; Richard, E.D.; Pankaj, V.; Chandan, K.-S.; Lianchun, X.; Niharika, B.M.; Edwin, R.P.; Al, B.B.; Charles, D.L.; et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J. ImmunoTherapy Cancer 2020, 8, e000587. [Google Scholar] [CrossRef]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Fukunaga, A.; Miyamoto, M.; Cho, Y.; Murakami, S.; Kawarada, Y.; Oshikiri, T.; Kato, K.; Kurokawa, T.; Suzuoki, M.; Nakakubo, Y.; et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004, 28, e26–e31. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.I.; Oliver, A.J.; Samiei, T.; Chan, J.D.; Kershaw, M.H.; Slaney, C.Y. Genetic Redirection of T Cells for the Treatment of Pancreatic Cancer. Front. Oncol. 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. CII 2017, 66, 1425–1436. [Google Scholar] [CrossRef]
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA 2009, 106, 3360–3365. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef]
- Rech, A.J.; Balli, D.; Mantero, A.; Ishwaran, H.; Nathanson, K.L.; Stanger, B.Z.; Vonderheide, R.H. Tumor Immunity and Survival as a Function of Alternative Neopeptides in Human Cancer. Cancer Immunol. Res. 2018, 6, 276–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.G.; Shields, A.F.; Behl, D.; et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; Knolhoff, B.L.; Zhu, Y.; Herndon, J.M.; Meyer, M.A.; Nywening, T.M.; Hawkins, W.G.; Shapiro, I.M.; Weaver, D.T.; et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016, 22, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Di Federico, A.; Tateo, V.; Parisi, C.; Formica, F.; Carloni, R.; Frega, G.; Rizzo, A.; Ricci, D.; Di Marco, M.; Palloni, A.; et al. Hacking Pancreatic Cancer: Present and Future of Personalized Medicine. Pharmaceuticals 2021, 14, 677. [Google Scholar] [CrossRef]
- Singhi, A.D.; George, B.; Greenbowe, J.R.; Chung, J.; Suh, J.; Maitra, A.; Klempner, S.J.; Hendifar, A.; Milind, J.M.; Golan, T.; et al. Real-Time Targeted Genome Profile Analysis of Pancreatic Ductal Adenocarcinomas Identifies Genetic Alterations That Might Be Targeted With Existing Drugs or Used as Biomarkers. Gastroenterology 2019, 156, 2242–2253.e4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).