Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. In Step 1
2.3. In Step 2
2.4. In Step 3
2.5. Data Analysis
3. Results
3.1. Step 1 in the Selection of Rootstock Candidates
3.2. Step 2 in the Selection of Rootstock Candidates
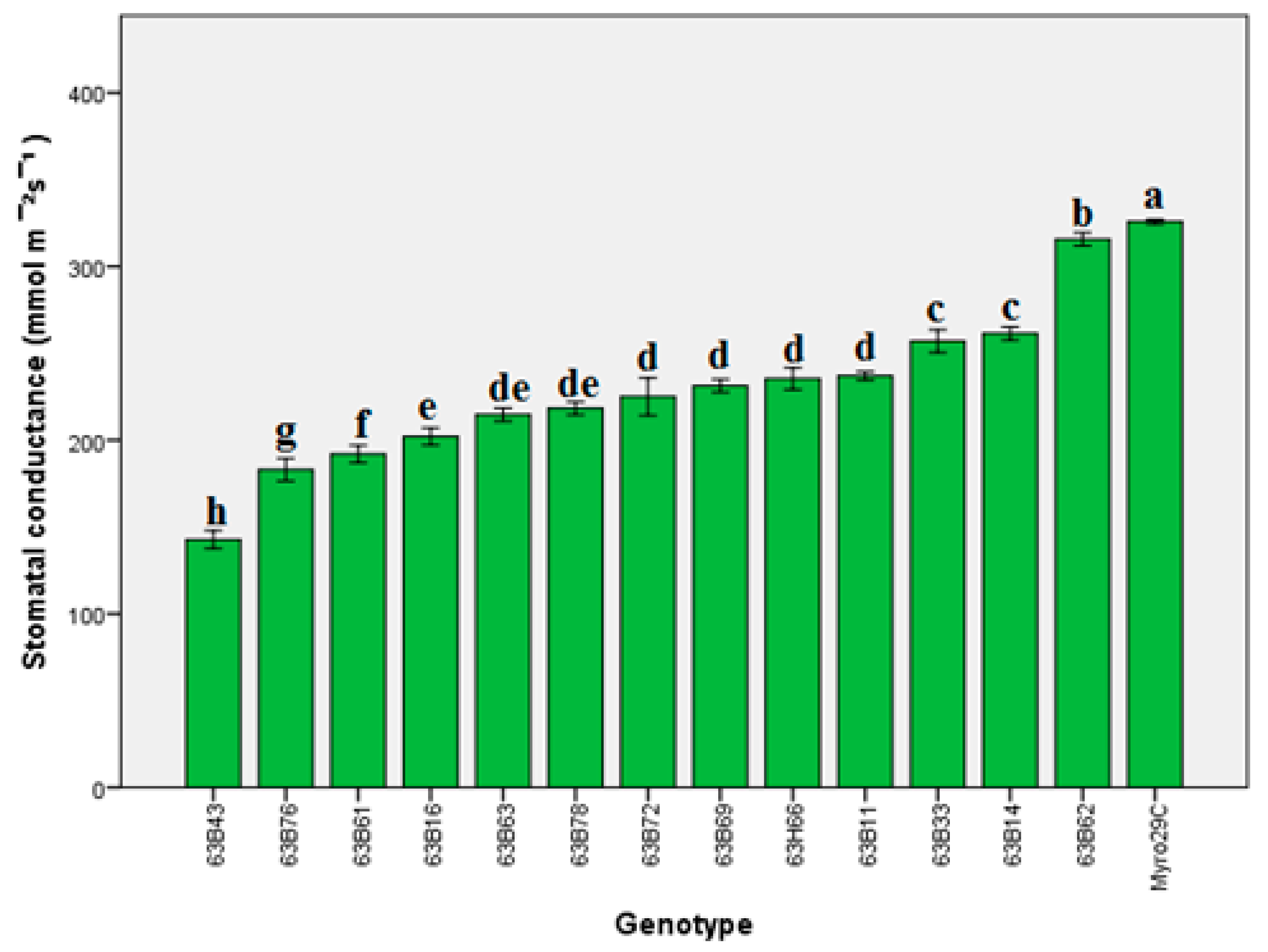
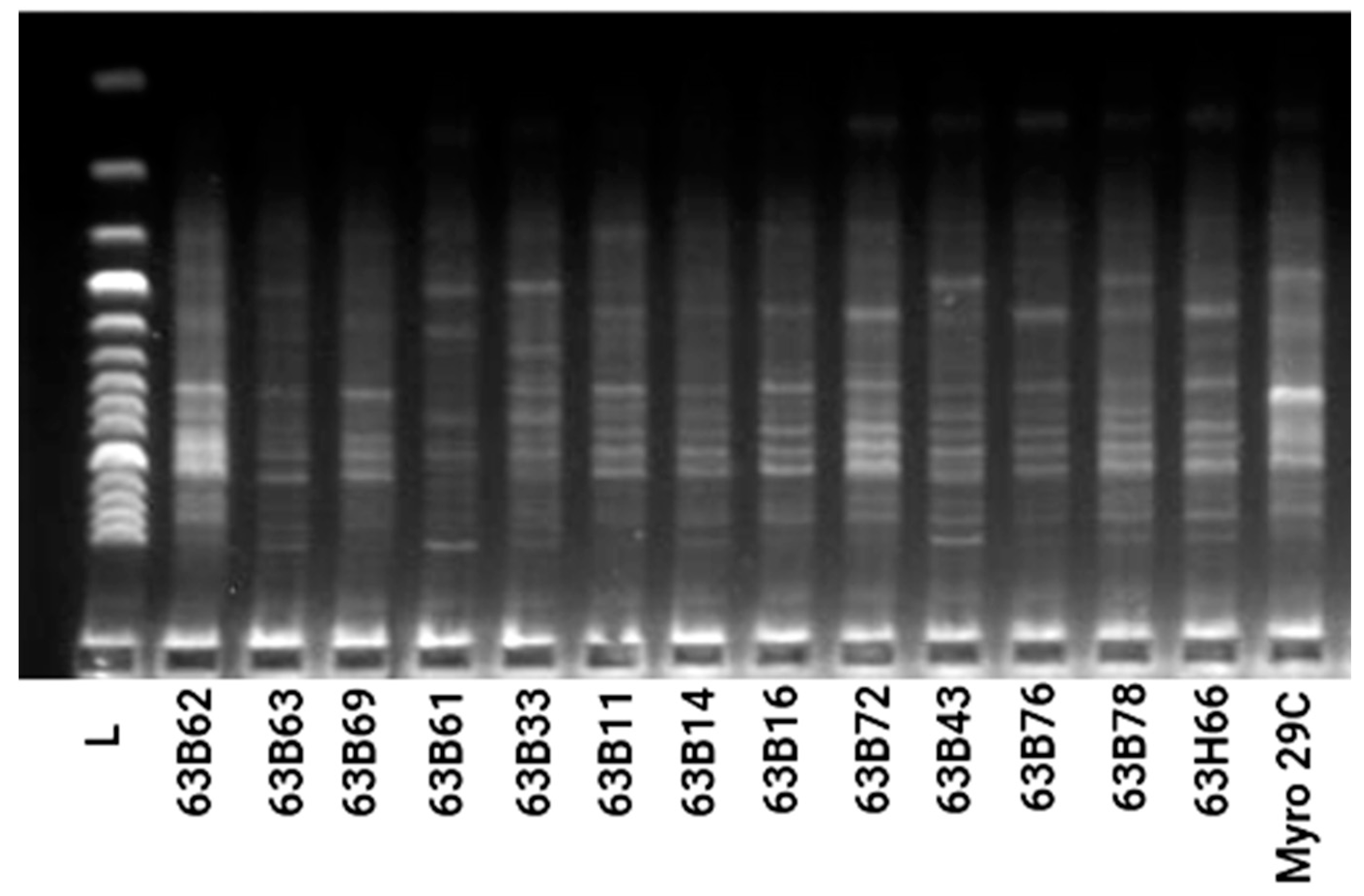
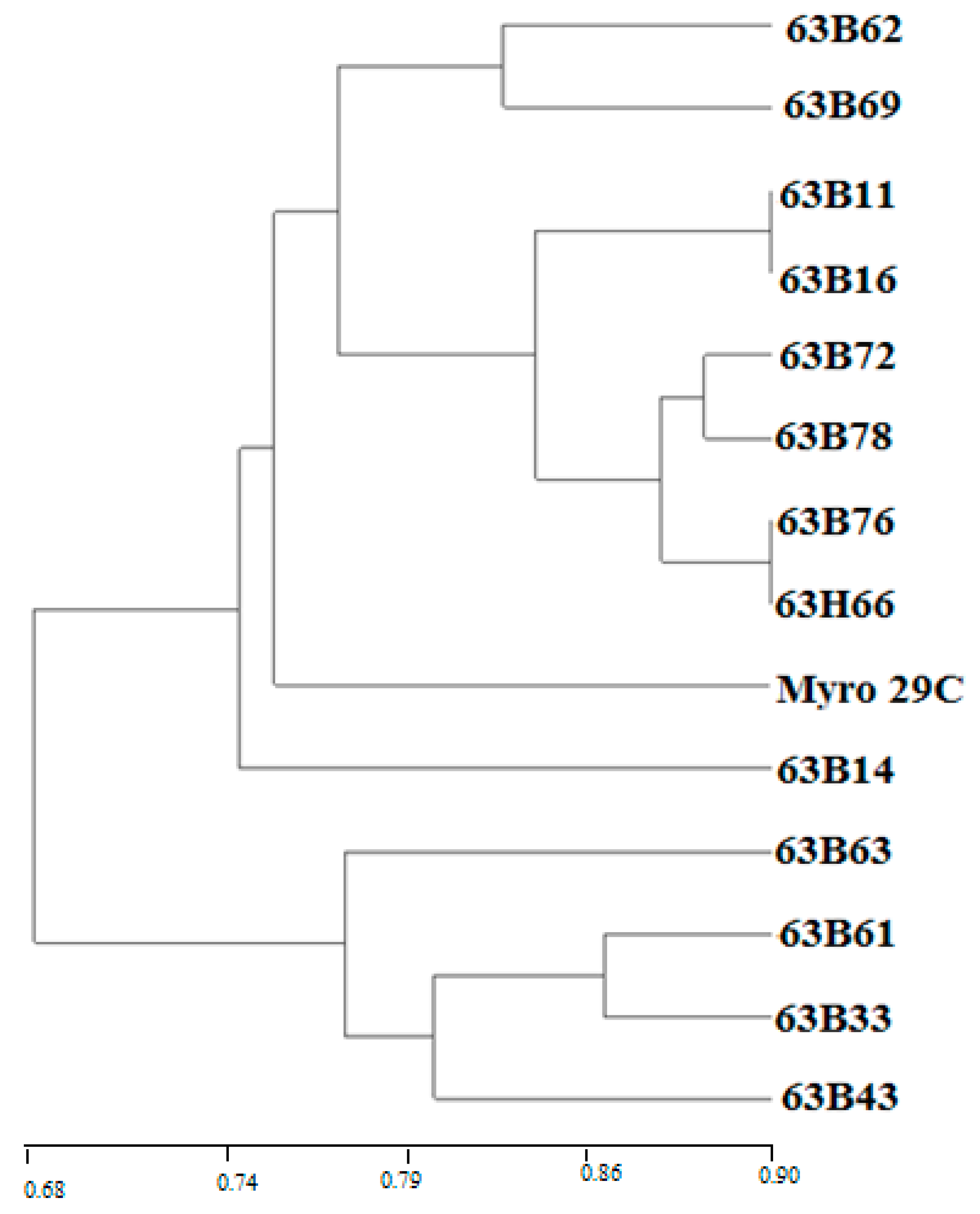
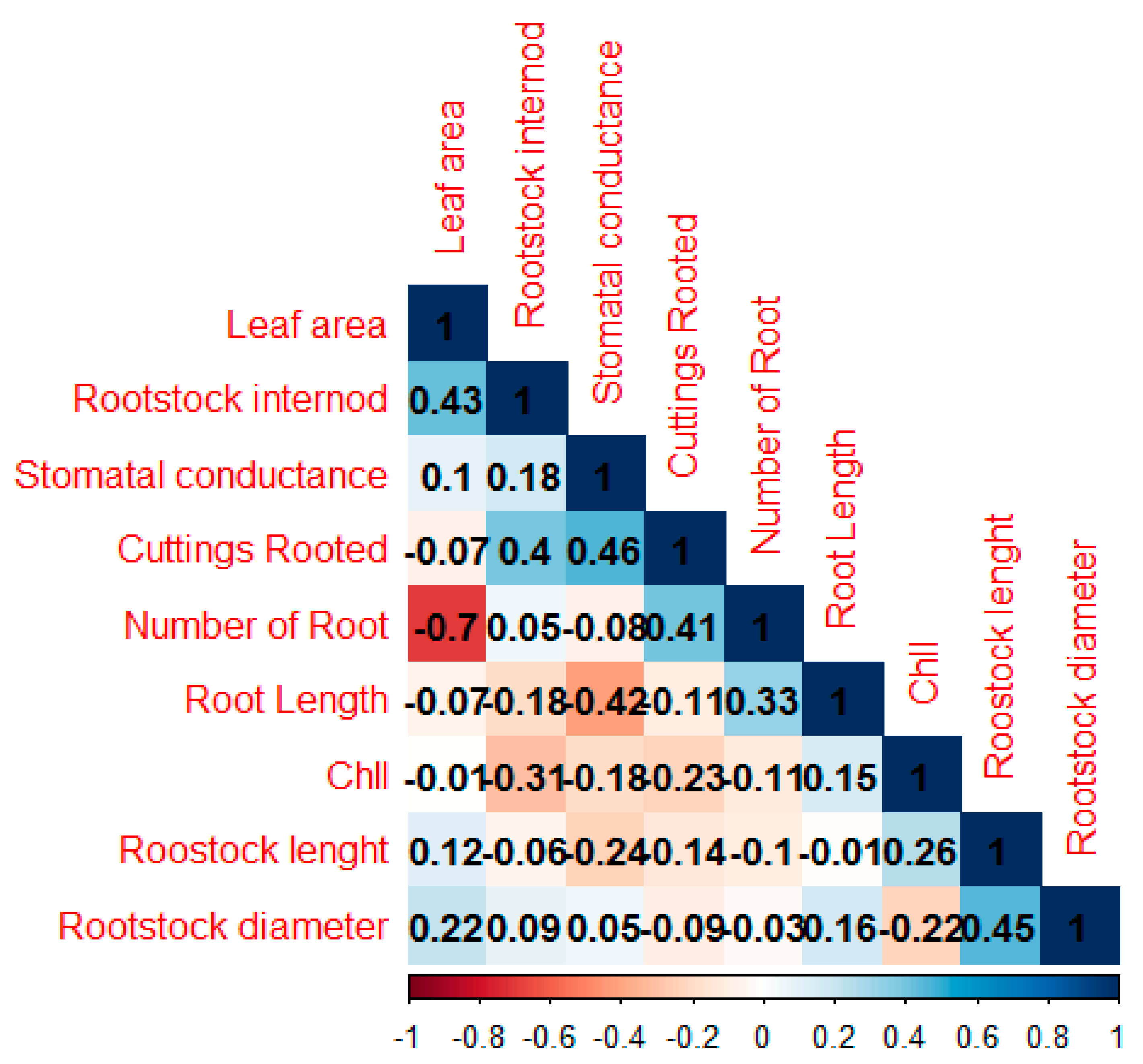
3.3. Step 3 in the Selection of Rootstock Candidates
4. Discussion
4.1. Step 1 in the Selection of Rootstock Candidates
4.2. Step 2 in the Selection of Rootstock Candidates
4.3. Step 3 in the Selection of Rootstock Candidates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, O.; Kose, C. Cell death point in flower organs of some apricot (Prunus armeniaca L.) cultivars at subzero temperatures. Sci. Hortic. 2019, 249, 299–305. [Google Scholar] [CrossRef]
- Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Kaya, O.; Kose, C. How sensitive are the flower parts of the sweet cherry in sub-zero temperatures? Use of differential thermal analysis and critical temperatures assessment. N. Z. J. Crop Hortic. Sci. 2022, 50, 17–31. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C.; Donderalp, V.; Gecim, T.; Taskın, S. Last updates on cell death point, bud death time and exothermic characteristics of flower buds for deciduous fruit species by using differential thermal analysis. Sci. Hortic. 2020, 270, 109403. [Google Scholar] [CrossRef]
- Kaya, O.; Kose, C.; Esıtken, A.; Turan, M.; Utku, O. Can organic acid and sugar compositions be used to predict cell death point limits? Receptacle and pistil organs of apricot (Prunus armeniaca L.). Rend. Lincei. Sci. Fis. Nat. 2021, 32, 493–509. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef]
- Topp, B.L.; Russell, D.M.; Neumüller, M.; Dalbó, M.A.; Liu, W. Plum. In Fruit Breeding; Springer: Berlin/Heidelberg, Germany, 2012; pp. 571–621. [Google Scholar]
- Ashton, R.W. Plums of North America; Third Millennium Publishing: Temple, AZ, USA, 2008. [Google Scholar]
- Okie, W.R. Plum rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; Wiley: New York, NY, USA, 1987; pp. 321–360. [Google Scholar]
- Kosina, J. Orchard performance of two plum cultivars on some clonal rootstocks. Hortic. Sci. 2004, 31, 93–95. [Google Scholar] [CrossRef]
- Bolat, I.; Bakır, A.G.; Korkmaz, K.; Gutiérrez-Gamboa, G.; Kaya, O. Silicon and Nitric Oxide Applications Allow Mitigation of Water Stress in Myrobalan 29C Rootstocks (Prunus cerasifera Ehrh.). Agriculture 2022, 12, 1273. [Google Scholar] [CrossRef]
- Bakır, A.G.; Bolat, I.; Korkmaz, K.; Hasan, M.M.; Kaya, O. Exogenous Nitric Oxide and Silicon Applications Alleviate Water Stress in Apricots. Life 2022, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Reig, G.; Salazar, A.; Zarrouk, O.; Font I Forcada, C.F.; Val, J.; Moreno, M.Á. Long-term graft compatibility study of peach-almond hybrid and plum based rootstocks budded with European and Japanese plums. Sci. Hortic. 2019, 243, 392–400. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Romero, J.; Gogorcena, Y.; Moreno, M.Á.; Espada, J.L. Performance of peach and plum based rootstocks of different vigour on a late peach cultivar in replant and calcareous conditions. Sci. Hortic. 2011, 129, 58–63. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gómez-Aparisi, J.; Betrán, J.A.; Moreno, M.A. Influence of almond × peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigour of two peach cultivars. Sci. Hortic. 2005, 106, 502–514. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.Á. Agronomical parameters, sugar profile and antioxidant compounds of “Catherine” peach cultivar influenced by different plum rootstocks. Int. J. Mol. Sci. 2014, 15, 2237–2254. [Google Scholar] [CrossRef]
- Davies, F.T., Jr.; Geneve, R.L.; Wilson, S.B. Hartmann and Kester’s Plant Propagation Principles and Practices, 9th ed; Pearson Education: New York, NY, USA, 2018; p. 741. [Google Scholar] [CrossRef]
- Grădinariu, G.; Zlati, C.; Istrate, M.; Dascălu, M. Investigation on Anatomical and Histological Structure of Graft Union in Plum; Scientific Papers of the Research Institute for Fruit Growing Pitesti: Pitesti, Romania, 2009. [Google Scholar]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Ayanoğlu, H.; Bayazit, S.; Inan, G.; Bakır, M.; Akpınar, A.E.; Kazan, K.; Ergül, A. AFLP analysis of genetic diversity in Turkish green plum accessions (Prunus cerasifera L.) adapted to the Mediterranean region. Sci. Hortic. 2007, 114, 263–267. [Google Scholar] [CrossRef]
- Bolat, I.; Ak, B.; Acar, I.; Ikinci, A. Plum culture in Turkey. Acta Hortic. 2017, 1175, 15–18. [Google Scholar] [CrossRef]
- Demirsoy, H.; Bilgener, Ş. Çarşamba ovasından selekte edilen bazı Can erik (Prunus cerasifera Ehrh.) tiplerinin şeftali ve eriklere anaç olarak kullanılabilirliklerinin saptanması üzerinde araştırmalar. J. Fac. Agric. OMU 2002, 17, 33–43. [Google Scholar]
- Ugur, R.; Kargi, S.P. Seleksiyonla elde edilen klonal anaç adayı bazı yabani erik genotipleri üzerine aşılanan kayısı çeşitlerinin büyüme durumlarının araştırılması. Türk Tarım Doğa Bilim. Derg. 2018, 5, 56–63. [Google Scholar]
- Gür, I.; Koçal, H.; Kaçal, E.; Aydinli, M.; Yalçin, B.; Karamürsel, Ö.; Demirtaş, İ. Selekte Edilen Bazı Erik Genotiplerinin Monroe Şeftali ve Aprikoz Kayısı Çeşidi ile Aşı Uyuşma Durumlarının Belirlenmesi. Meyve Bilim. 2020, 7, 1–9. [Google Scholar]
- Ircan, M.R. Şanliurfa’s Climate Characteristics and Drought Analysis. Master’s Thesis, Çankırı Karatekin University, Çankırı, Turkey, 2020. [Google Scholar]
- Khan, A.N.; Qureshi, R.H.; Ahmad, N. Salt Tolerance of Cotton Cultivars in Relation to Relative Growth Rate in Saline Environments. Int. J. Agric. Biol. 2004, 6, 786–787. [Google Scholar]
- Dodd, I.C. Leaf area development of ABA-deficient and wild-type peas at two levels of nitrogen supply. Funct. Plant Biol. 2003, 30, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.H. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Uzun, A.; Gulsen, O.; Kafa, G.; Seday, U.; Tuzcu, O.; Yesiloglu, T. Characterization for yield, fruit quality, and molecular profiles of lemon genotypes tolerant to ‘mal secco’ disease. Sci. Hortic. 2009, 122, 556–561. [Google Scholar] [CrossRef]
- Kovach, W.L. MVSP-A Multivariate Statistical Package for Windows; Version 3.1; Kovach Computing Services: Wales, UK, 1999; p. 133. [Google Scholar]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Moreno, M.A. Breeding and selection of Prunus rootstocks at the Aula Dei experimental station, Zaragoza, Spain. In I International Symposium on Rootstocks for Deciduous Fruit Tree Species; ISHS: Korbeek-Lo, Belgium, 2002; pp. 519–528. [Google Scholar]
- Suranyi, D. Long term investigations of flowers and leaves on mainly non-domestica plums. Int. J. Hortic. Sci. 2005, 11, 73–79. [Google Scholar] [CrossRef]
- Howard, B.H.; Nahlawif, N. Factors affecting the rooting of plum hardwood cuttings. J. Hortic. Sci. 1969, 44, 303–310. [Google Scholar] [CrossRef]
- Stanica, F. Propagation of Prunus rootstocks by hardwood cuttıngs on composed rootıng substrates. Acta Hortic. 2007, 734, 309–311. [Google Scholar] [CrossRef]
- Sharma, S.D.; Aier, N.B. Seasonal rooting behaviour of cuttings of plum cultivars as influenced by IBA treatments. Sci. Hortic. 1989, 40, 297–303. [Google Scholar] [CrossRef]
- Moreno, M.A.; Montañés, L.; Tabuenca, M.C.; Cambra, R. The performance of Adara as a cherry rootstock. Sci. Hortic. 1996, 65, 85–91. [Google Scholar] [CrossRef]
- Pinochet, J.; Calvet, C.; Hernández-Dorrego, A.; Bonet, A.; Felipe, A.; Moreno, M. Resistance of peach and plum rootstocks from Spain, France, and Italy to root-knot nematode Meloidogyne javanica. Sci. Hortic. 1999, 34, 1259–1262. [Google Scholar] [CrossRef]
- Webster, A.D. Rootstock and İnterstock Effects on Deciduous Fruit Tree Vigour, Precocity, and Yield Productivity. N. Z. J. Crop Hortic. Sci. 1995, 23, 273–382. [Google Scholar] [CrossRef]
- Webster, A.D. Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. In I International Symposium on Rootstocks for Deciduous Fruit Tree Species; ISHS: Korbeek-Lo, Belgium, 2002; Volume 658, pp. 29–41. [Google Scholar] [CrossRef]
- Foster, T.M.; Celton, J.M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, Dw1 and Dw2, are primarily responsible for rootstock-induced dwarfing in apple. Hortic. Res. 2015, 2, 15001. [Google Scholar] [CrossRef]
- Sakamoto, T.; Miura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Fleet, C.M.; Sun, T.P. A DELLAcate balance: The role of gibberellin in plant morphogenesis. Curr. Opin. Plant Biol. 2005, 8, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Hou, L.; Ma, C.; Li, G.; Lin, R.; Zhao, Y.; Wang, X. Comparative transcriptome analysis of the peanut semi-dwarf mutant 1 reveals regulatory mechanism involved in plant height. Gene 2021, 791, 145722. [Google Scholar] [CrossRef]
- Xing, M.; Su, H.; Liu, X.; Yang, L.; Zhang, Y.; Wang, Y.; Fang, Z.; Lv, H. Morphological, transcriptomics and phytohormone analysis shed light on the development of a novel dwarf mutant of cabbage (Brassica oleracea). Plant Sci. 2020, 290, 110283. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Molecular basis of plant architecture. Annu. Rev. Plant Biol. 2008, 59, 253–279. [Google Scholar] [CrossRef]
- Everard, J.D.; Cucci, R.; Kann, S.C.; Flore, J.A.; Loescher, W.H. Gas Exchange and Carbon Partitioning in the leaves of Celery (Apium graveolens L.) at Various levels of Root Zone Salinity. Plant Physiol. 1994, 106, 281–292. [Google Scholar] [CrossRef]
- Cristofori, V.; Rouphael, Y.; Gyves, E.M.; Bignami, C.A. Simple model for estimating leaf area of hazelnut from linear measurements. Sci. Hortic. 2007, 113, 221–225. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Ruiz-Sánchez, M.C.; Domingoa, R. Effects of timing and intensity of deficit irrigation on vegetative and fruit growth of apricot trees. Agric. Water Manag. 2014, 134, 110–118. [Google Scholar] [CrossRef]
- Das Neves, T.R.; Mayer, N.A.; Ueno, B. Graft incompatibility in Prunus spp. preceded by SPAD index reduction. Ciências Agrárias Londrina 2017, 38, 635–648. [Google Scholar] [CrossRef]
- Madakadze, I.C.; Stewart, K.A.; Madakadze, R.M.; Peterson, P.R.; Coulman, B.E.; Smith, D.L. Field Evaluation of the Chlorophyll Meter to Predict Yield and Nitrogen Concentration of Switchgrass. J. Plant Nutr. 1999, 22, 1001–1010. [Google Scholar] [CrossRef]
- Peterson, R.B.; Oja, V.; Laisk, A. Chlorophyll fluorescence at 680 and 730 nm and leaf photosynthesis. Photosynth. Res. 2001, 70, 185–196. [Google Scholar] [CrossRef]
- Percival, G.C.; Keary, I.P.; Noviss, K. The Potential of a Chlorophyll Content SPAD Meter to Quantify Nutrient Stress in Foliar Tissue of Sycamore (Acer pseudoplatanus), English Oak (Quercus robur), and European Beech (Fagus sylvatica). Arboric. Urban For. 2008, 34, 89–100. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Pence, H.E.; Hasegawa, P.M.; Mickelbart, M.V. Regulation of transpiration to improve crop water use. Crit. Rev. Plant Sci. 2009, 28, 410–431. [Google Scholar] [CrossRef]
- Gülen, H.; Köksal, N.; Atilla, E. Farklı anaçlar üzerine aşılı bazı kiraz ve elma çeşitlerinde stoma yoğunluğu ve stoma boyutları. Bahçe 2004, 33, 1–5. [Google Scholar]
- Wünsche, J.N.; Palmer, J.W.; Greer, D.H. Effects of Crop Load on Fruiting and Gas-exchange Characteristics of Braeburn’/M. 26 Apple Trees at Full Canopy. J. Am. Soc. Hortic. Sci. 2000, 125, 93–99. [Google Scholar] [CrossRef]
- Marshall, E.; Costa, L.M.; Gutierrez-Marcos, J. Cysteine-rich peptides (CRPs) mediate diverse aspects of cell–cell communication in plant reproduction and development. J. Exp. Bot. 2011, 62, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, D.; Zhang, A.; Feng, C.; Yang, J.; Yoon, J.; Li, S. Genetic diversity and phylogenetic relationships among plum germplasm resources in China assessed with Inter-simple Sequence Repeat markers. J. Am. Soc. Hortic. Sci. 2007, 132, 619–628. [Google Scholar] [CrossRef]
- Carrasco, B.; Diaz, C.; Moya, M.; Gebauer, M.; Garcia-Gonzalez, R. Genetic characterization of Japanese plum cultivars (Prunus salicina) using SSR and ISSR molecular markers. Cienc. Investig. Agrar. 2012, 39, 533–543. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Q.; Xie, J.; Pan, Y.B.; Zhou, F.; Guo, Y.; Qiu, Y. SSR marker-assisted management of parental germplasm in sugarcane (Saccharum spp. hybrids) breeding programs. Agronomy 2019, 9, 449. [Google Scholar] [CrossRef]
- Athanasiadis, I.; Nikoloudakis, N.; Hagidimitriou, M. Genetic relatedness among cultivars of the Greek plum germplasm. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 491–498. [Google Scholar] [CrossRef]
- Casas, A.M.; Igartua, E.; Balaguer, G.; Moreno, M.A. Genetic diversity of Prunus rootstocks analyzed by RAPD markers. Euphytica 1999, 110, 139–149. [Google Scholar] [CrossRef]
- Shimada, T.; Hayama, H.; Haji, T.; Yamaguchi, M.; Yosida, M. Genetic diversity of plums characterized by random amplified polymorphic DNA analysis. Euphytica 1999, 109, 143–147. [Google Scholar] [CrossRef]
- Goulao, L.; Monte-Corvo, L.; Oliveira, C.M. Phenetic characterization of plum cultivars by high multiplex ratio markers: Amplified fragment length polymorphisms and intersimple sequence repeats. J. Am. Soc. Hortic. Sci. 2001, 126, 72–77. [Google Scholar] [CrossRef]
- Bayazit, S.; Kazan, K.; Gülbitti, S.; Cevik, V.; Ayanoğlu, H.; Ergül, A. AFLP analysis of genetic diversity in low chill requiring walnut (Juglans regia L.) genotypes from Hatay, Turkey. Sci. Hortic. 2007, 111, 394–398. [Google Scholar] [CrossRef]
- Sehic, J.; Nybom, H.; Hjeltnes, S.H.; Gasi, F. Genetic diversity and structure of Nordic plum germplasm preserved ex situ and on-farm. Sci. Hortic. 2015, 190, 195–202. [Google Scholar] [CrossRef]
- Maroof, M.S.; Allard, R.W.; Zhang, Q.F. Genetic diversity and ecogeographical differentiation among ribosomal DNA alleles in wild and cultivated barley. Proc. Natl. Acad. Sci. USA 1990, 87, 8486–8490. [Google Scholar] [CrossRef] [PubMed]
- Dawson, I.K.; Chalmers, K.J.; Waugh, R.; Powell, W. Detection and analysis of genetic variation in Hordeum spontaneum populations from Israel using RAPD markers. Mol. Ecol. 1993, 2, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.E.; Rodrigo, J. Japanese plum pollination: A review. Sci. Hortic. 2015, 197, 674–686. [Google Scholar] [CrossRef]





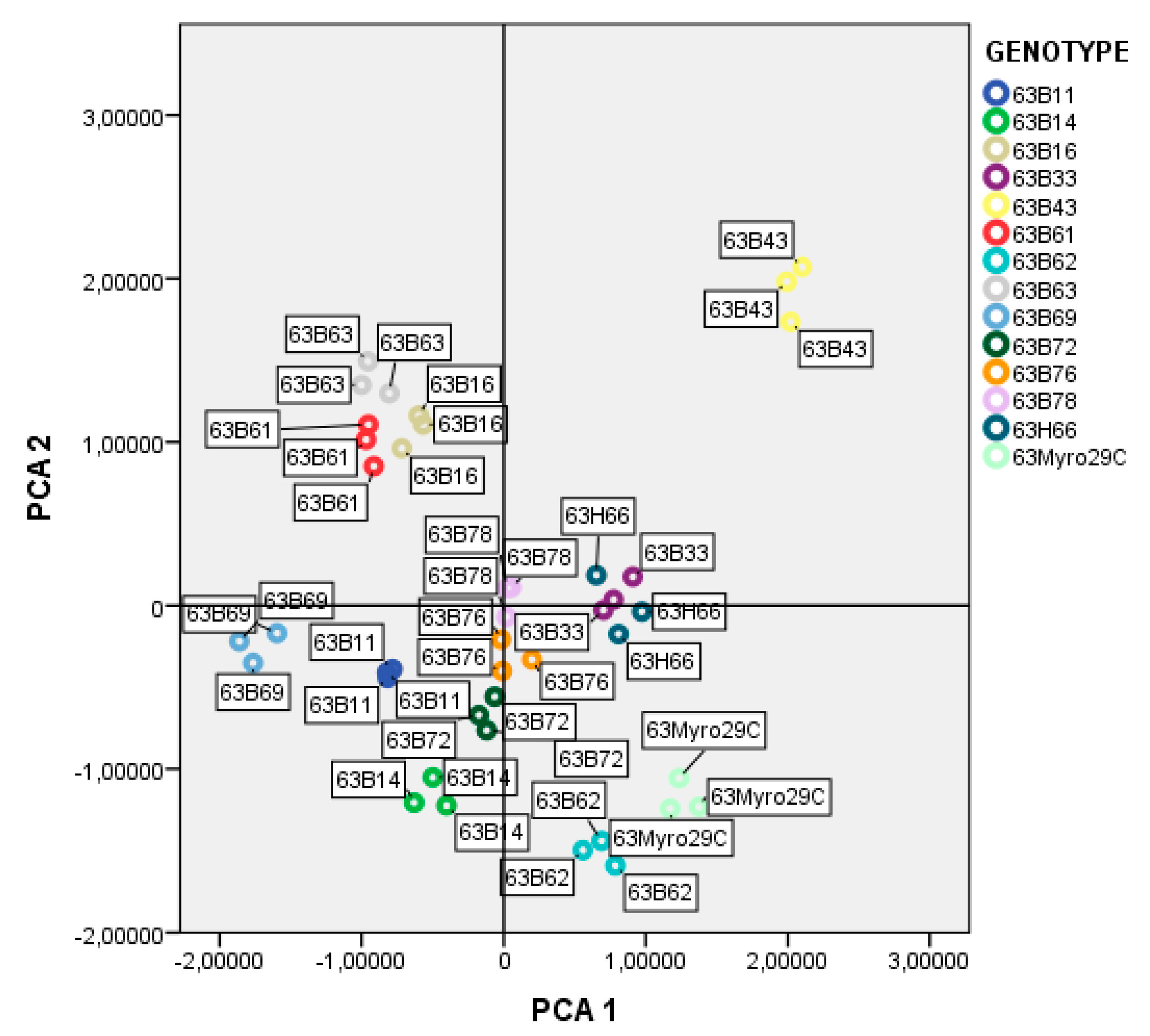
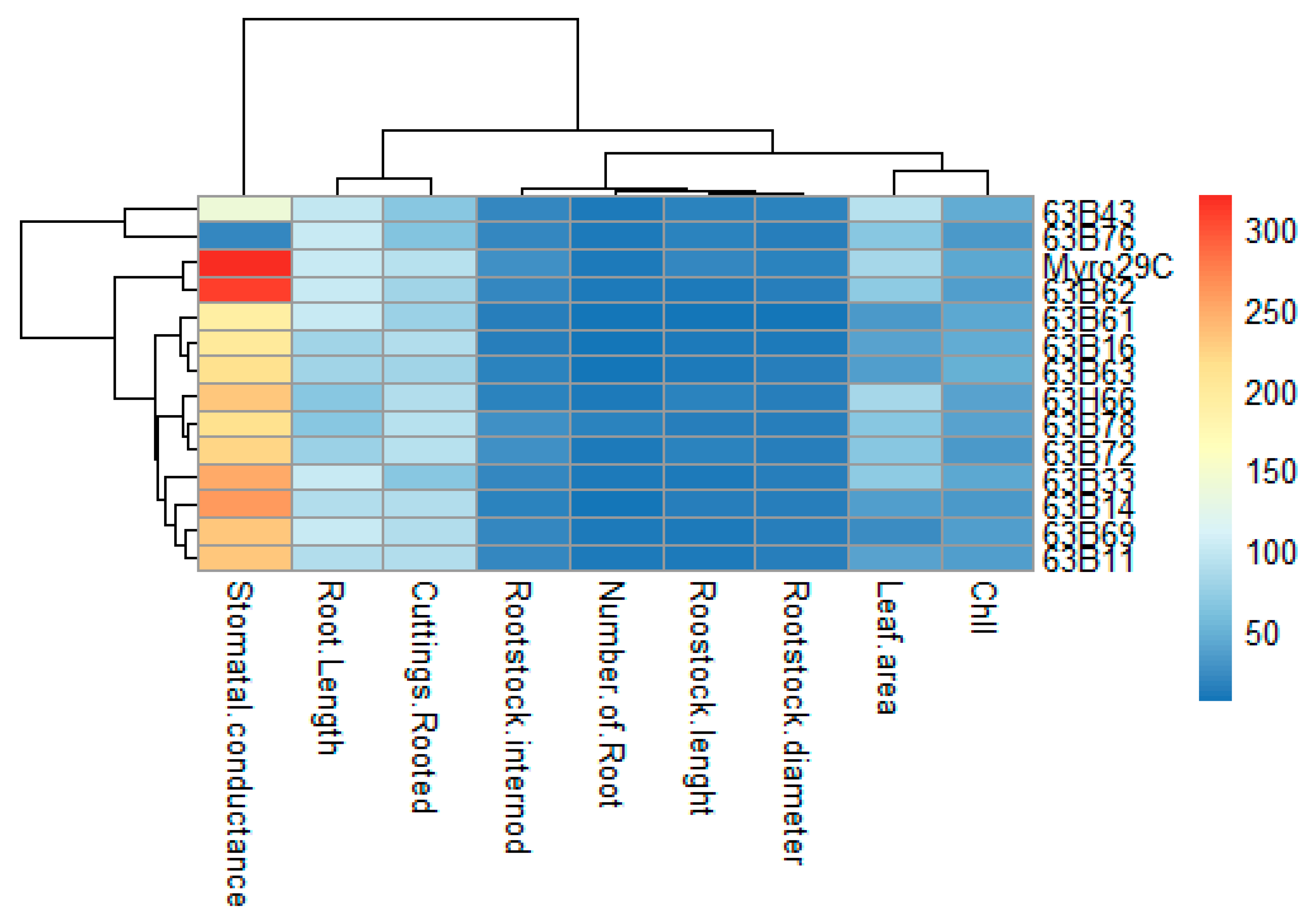
| Number of Rootstock Candidates | Rootstock Candidates | Root Length (mm) | Number of Roots | Cuttings Rooted (%) |
|---|---|---|---|---|
| 1 | 63B78 | 67.50 d | 16.32 a | 93.86 a |
| 2 | 63B72 | 77.50 d | 9.40 cd | 92.40 a |
| 3 | Myrobalan 29C | 100 a | 11.60 b | 92.40 a |
| 4 | 63B69 | 100 a | 9.00 e | 90.33 a |
| 5 | 63H66 | 67.50 d | 10.00 cd | 89.46 a |
| 6 | 63B11 | 90.00 b | 9.23 d | 88.06 a |
| 7 | 63B14 | 87.5 c | 7.66 e | 88.06 a |
| 8 | 63B16 | 80.00 c | 7.00 e | 85.30 ab |
| 9 | 63B62 | 100 a | 10.00 cd | 78.66 bc |
| 10 | 63B63 | 80.00 c | 7.80 e | 78.60 bc |
| 11 | 63B61 | 100 a | 5.40 f | 75.63 cd |
| 12 | 63B33 | 100 a | 11.80 b | 68.90 de |
| 13 | 63B43 | 99.66 b | 10.26 c | 68.16 d–f |
| 14 | 63B76 | 100 a | 10.00 cd | 64.70 ef |
| 15 | 63H65 | 57.50 e | 4.66 fg | 59.30 fg |
| 16 | 63B68 | 57.50 e | 4.66 fg | 53.60 gh |
| 17 | 63B60 | 57.50 e | 4.33 gh | 51.83 hi |
| 18 | 63B66 | 57.50 e | 4.33 gh | 48.16 h–j |
| 19 | 63B13 | 52.50 ef | 4.33 gh | 46.83 i–k |
| 20 | 63B34 | 52.50 ef | 4.33 gh | 46.16 i–l |
| 21 | 63B77 | 45.00 gh | 4.00 c–h | 41.66 i–l |
| 22 | 63B70 | 47.50 fg | 2.00 e–h | 40.03 j–m |
| 23 | 63H30 | 45.00 g–i | 3.66 g–i | 39.80 j–n |
| 24 | 63H39 | 45.00 g–i | 3.66 g–i | 39.13 j–n |
| 25 | 63B45 | 45.00 g–i | 2.00 e–h | 38.86 k–o |
| 26 | 63H46 | 42.66 g–i | 4.33 gh | 38.83 k–p |
| 27 | 63B19 | 42.50 g–i | 3.66 g–i | 36.56 k–q |
| 28 | 63B12 | 41.33 h–k | 3.66 g–i | 35.77 k–r |
| 29 | 63B56 | 40.00 i–l | 3.66 g–i | 34.80 l–s |
| 30 | 63B42 | 39.00 i–m | 3.66 g–i | 33.40 l–s |
| 31 | 63B53 | 38.33 j–n | 3.66 g–i | 31.93 l–t |
| 32 | 63B47 | 36.00 j–n | 2.40 e–h | 31.80 l–u |
| 33 | 63B54 | 35.00 k–o | 2.40 e–h | 31.60 l–u |
| 34 | 63H27 | 35.00 k–o | 3.00 d–h | 31.46 m–v |
| 35 | 63B46 | 34.00 l–p | 2.30 e–h | 31.20 m–w |
| 36 | 63B51 | 34.00 l–p | 2.15 e–h | 30.53 m–x |
| 37 | 63B73 | 34.00 l–p | 2.00 e–h | 30.40 n–x |
| 38 | 63H35 | 34.00 l–p | 2.00 e–h | 30.20 n–x |
| 39 | 63B15 | 33.66 l–q | 2.00 e–h | 30.06 n–x |
| 40 | 63B18 | 33.66 l–q | 3.00 d–h | 30.00 o–x |
| 41 | 63B55 | 33.00 m–r | 2.66 j–o | 29.90 o–x |
| 42 | 63H40 | 32.50 n–s | 3.33 h–k | 29.80 o–y |
| 43 | 63B49 | 32.00 n–t | 3.00 d–h | 29.53 o–y |
| 44 | 63H26 | 30.00 o–u | 3.33 h–k | 28.80 o–y |
| 45 | 63H60 | 42.66 g–i | 3.10 d–h | 28.40 o–z |
| 46 | 63H36 | 29.00 p–v | 2.00 e–h | 28.36 p–a1 * |
| 47 | 63B77 | 29.00 p–v | 3.33 h–k | 27.93 q–a1 |
| 48 | 63B75 | 28.33 q–w | 2.66 j–o | 26.93 r–a1 |
| 49 | 63H28 | 28.00 r–w | 2.66 j–o | 26.26 r–a1 |
| 50 | 63H55 | 28.00 r–w | 2.66 j–o | 25.73 r–a1 |
| 51 | 63H37 | 28.00 r–w | 2.66 j–o | 25.60 s–b1 |
| 52 | 63B70 | 28.00 r–w | 2.66 j–o | 24.66 s–b1 |
| 53 | 63B50 | 27.50 s–x | 2.66 j–o | 23.66 s–b1 |
| 54 | 63B65 | 27.00 t–x | 2.66 j–o | 23.06 t–b1 |
| 55 | 63B36 | 27.00 t–x | 2.66 j–o | 23.00 u–b1 |
| 56 | 63B74 | 26.33 u–y | 2.63 k–q | 22.83 v–c1 |
| 57 | 63H45 | 25.00 u–z | 2.46 k–r | 22.63 w–d1 |
| 58 | 63B71 | 25.00 u–z | 2.46 k–r | 22.13 x–d1 |
| 59 | 63B66 | 24.00 v–a1* | 2.33 k–s | 21.46 x–d1 |
| 60 | 63H34 | 23.33 w–a1 | 2.33 k–s | 21.40 y–d1 |
| 61 | 63B64 | 22.50 x–b1 | 2.33 k–s | 21.33 y–d1 |
| 62 | 63H54 | 22.50 x–b1 | 2.33 k–s | 20.13 z–d1 |
| 63 | 63B32 | 22.50 x–b1 | 2.33 k–s | 20.00 z–d1 |
| 64 | 63H22 | 22.50 x–b1 | 2.33 k–s | 19.36 a1–d1 |
| 65 | 63B79 | 21.33 y–c1 | 2.30 l–s | 19.33 b1–e1 |
| 66 | 63B68 | 20.00 z–d1 | 2.00 l–t | 18.66 b1–e1 |
| 67 | 63B31 | 19.00 a1–d1 | 1.93 m–u | 15.53 c1–e1 |
| 68 | 63H44 | 17.50 b1–d1 | 1.93 m–u | 14.96 d1 e1 |
| 69 | 63B48 | 16.33 c1–e1 | 1.83 n–v | 13.40 e1 f1 |
| 70 | 63B67 | 14.66 d1–f1 | 1.66 o–w | 12.53 e1 f1 |
| 71 | 63H41 | 11.66 e1–f1 | 1.60 p–x | 7.86 e1 f1 |
| 72 | 63B48 | 10.33 f1–g1 | 1.60 p–x | 7.80 e1 f1 |
| 73 | 63B67 | 5.00 g1–h1 | 1.60 p–x | 6.80 e1 f1 |
| 74 | 63H41 | 0 h1 | 0 a1 * | 0 f1 |
| 75 | 63B30 | 0 h1 | 0 a1 | 0 f1 |
| 76 | 63B44 | 0 h1 | 0 a1 | 0 f1 |
| 77 | 63B20 | 0 h1 | 0 a1 | 0 f1 |
| 78 | 63B21 | 0 h1 | 0 a1 | 0 f1 |
| 79 | 63B22 | 0 h1 | 0 a1 | 0 f1 |
| 80 | 63B23 | 0 h1 | 0 a1 | 0 f1 |
| Number of Rootstock Candidates | Rootstock Candidates | Internode Height of the Leader (mm) | Height of the Leader (cm) | Trunk Diameter of the Leader (mm) |
|---|---|---|---|---|
| 1 | 63B78 | 10.46 r | 69 o | 5.46 i |
| 2 | 63B72 | 9.31 st | 82 g | 5.23 j |
| 3 | Myrobalan 29C | 11.21 q | 87 cd | 6.01 e |
| 4 | 63B69 | 7.63 v | 43 t | 5.06 l |
| 5 | 63H66 | 5.35 z | 61 r | 5.21 j |
| 6 | 63B11 | 8.84 u | 58 s | 6.11 d |
| 7 | 63B14 | 7.61 v | 66 pq | 5.88 f |
| 8 | 63B16 | 5.41 z | 41 u | 4.74 n |
| 9 | 63B62 | 6.66 w | 37 v | 5.78 g |
| 10 | 63B63 | 6.22 x | 35 w | 7.20 a |
| 11 | 63B61 | 5.86 y | 31 x | 3.74 u |
| 12 | 63B33 | 9.16 t | 57 s | 6.35 c |
| 13 | 63B43 | 9.46 s | 82 g | 6.49 b |
| 14 | 63B76 | 9.27 t | 84 f | 5.64 h |
| 15 | 63H65 | 11.87 p | 93 b | 4.77 n |
| 16 | 63B68 | 14.07 h | 97 a | 5.10 k |
| 17 | 63B60 | 12.14 o | 85 ef | 3.91 t |
| 18 | 63B66 | 12.79 l | 86 de | 4.54 o |
| 19 | 63B13 | 14.33 fg | 82 g | 4.75 n |
| 20 | 63B34 | 14.24 g | 81 gh | 5.24 j |
| 21 | 63B77 | 15.36 e | 88 c | 5.12 k |
| 22 | 63B70 | 15.80 d | 84 f | 4.91 m |
| 23 | 63H30 | 15.48 e | 80 hi | 3.75 u |
| 24 | 63H39 | 15.40 e | 77 kl | 3.50 w |
| 25 | 63B45 | 17.11 a | 79 ij | 4.55 o |
| 26 | 63H46 | 16.40 c | 82 g | 4.24 q |
| 27 | 63B19 | 14.41 f | 81 gh | 5.11 k |
| 28 | 63B12 | 13.63 i | 78 jk | 5.24 j |
| 29 | 63B56 | 16.83 b | 79 ij | 4.35 p |
| 30 | 63B42 | 13.36 j | 76 lm | 3.97 t |
| 31 | 63B53 | 13.45 j | 79 ij | 4.12 r |
| 32 | 63B47 | 13.18 k | 75 mn | 3.24 x |
| 33 | 63B54 | 13.30 jk | 74 n | 3.52 vw |
| 34 | 63H27 | 13.64 i | 69 o | 3.78 u |
| 35 | 63B46 | 12.06 o | 66 pq | 4.24 q |
| 36 | 63B51 | 12.57 m | 67 p | 4.51 o |
| 37 | 63B73 | 12.83 l | 70 o | 3.94 t |
| 38 | 63H35 | 12.35 n | 66 pq | 3.72 u |
| 39 | 63B15 | 12.33 n | 65 q | 3.57 v |
| 40 | 63B18 | 13.74 i | 70 o | 4.04 s |
| Primers | 5′ -> 3′ Base Sequences of Primers | TBN | PBN | PR (%) |
|---|---|---|---|---|
| DBDACA7 | DBDACACACACACACACA | 12 | 11 | 91.7 |
| VHVGTG7 | VHVGTGTGTGTGTGTGTG | 15 | 15 | 100.0 |
| GACA4 | GACAGACAGACAGACA | 7 | 6 | 85.7 |
| CAC3GC | CACCACCACGC | 8 | 2 | 25.0 |
| BDBCA7C | BDBCACACACACACACAC | 5 | 4 | 80.0 |
| CT8TG | CTCTCTCTCTCTCTCTTG | 7 | 7 | 100.0 |
| GT6GG | GTGTGTGTGTGTGG | 5 | 5 | 100.0 |
| AG8T | AGAGAGAGAGAGAGAGT | 7 | 7 | 100.0 |
| AGC6G | AGCAGCAGCAGCAGCAGCG | 5 | 5 | 100.0 |
| CA6AC | CACACACACACAAC | 3 | 3 | 100.0 |
| CAB12 | CABCABCABCABCABCABCABCABCABCABCAB | 5 | 5 | 100.0 |
| TCC5RY | TCCTCCTCCTCCTCCRY | 2 | 2 | 100.0 |
| GAT7C | GAT GATGATGATGATGATGATC | - | - | - |
| GT8TG | GTGTGTGTGTGTGTGTTG | - | - | - |
| TAA8 | TAA TAATAATAATAATAATAATAA | - | - | - |
| GAA6 | GAAGAAGAAGAAGAAGAA | - | - | - |
| Total | 81 | 72 | 88.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, K.; Bolat, I.; Uzun, A.; Sahin, M.; Kaya, O. Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees. Life 2023, 13, 1476. https://doi.org/10.3390/life13071476
Korkmaz K, Bolat I, Uzun A, Sahin M, Kaya O. Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees. Life. 2023; 13(7):1476. https://doi.org/10.3390/life13071476
Chicago/Turabian StyleKorkmaz, Kubra, Ibrahim Bolat, Aydın Uzun, Muge Sahin, and Ozkan Kaya. 2023. "Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees" Life 13, no. 7: 1476. https://doi.org/10.3390/life13071476
APA StyleKorkmaz, K., Bolat, I., Uzun, A., Sahin, M., & Kaya, O. (2023). Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees. Life, 13(7), 1476. https://doi.org/10.3390/life13071476







