Organic Matter in the Asteroid Ryugu: What We Know So Far
Simple Summary
Abstract
1. Introduction
1.1. The Hayabusa2 Mission
1.2. The Origin and Evolution of Extraterestrial Organic Matter
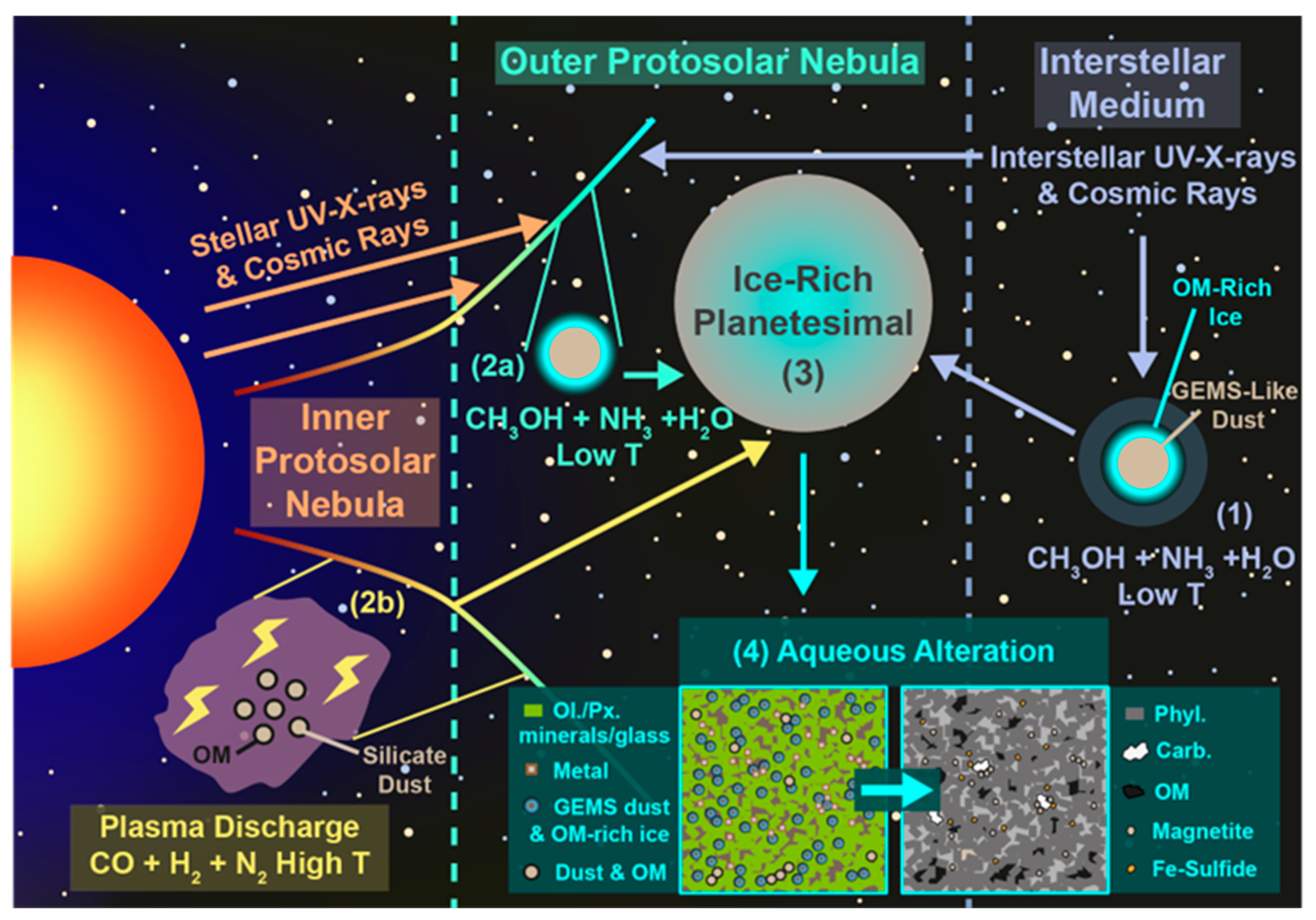
1.2.1. The Interstellar Medium
1.2.2. The Protosolar Nebula and Protostellar Disk
1.2.3. Planetesimals
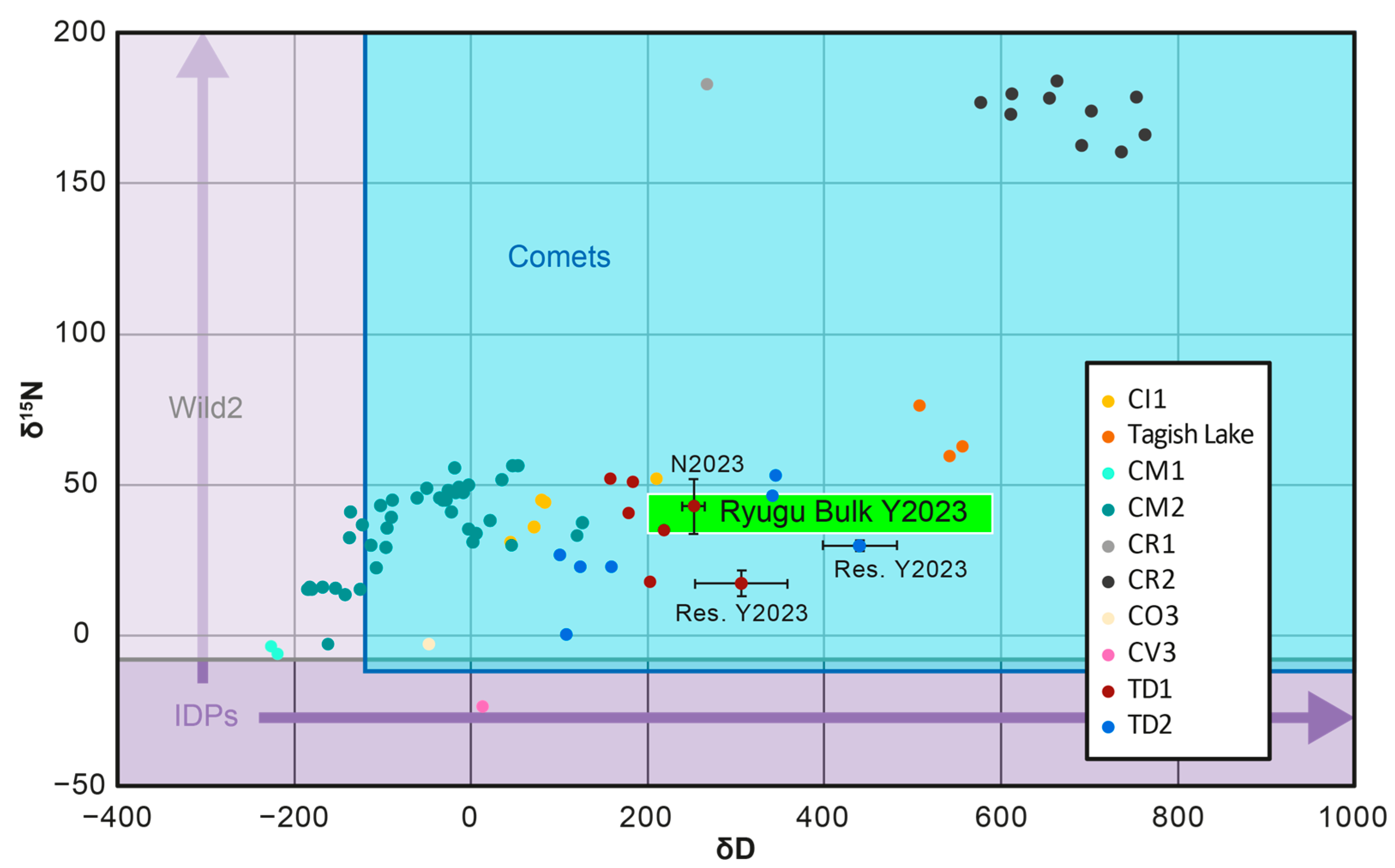
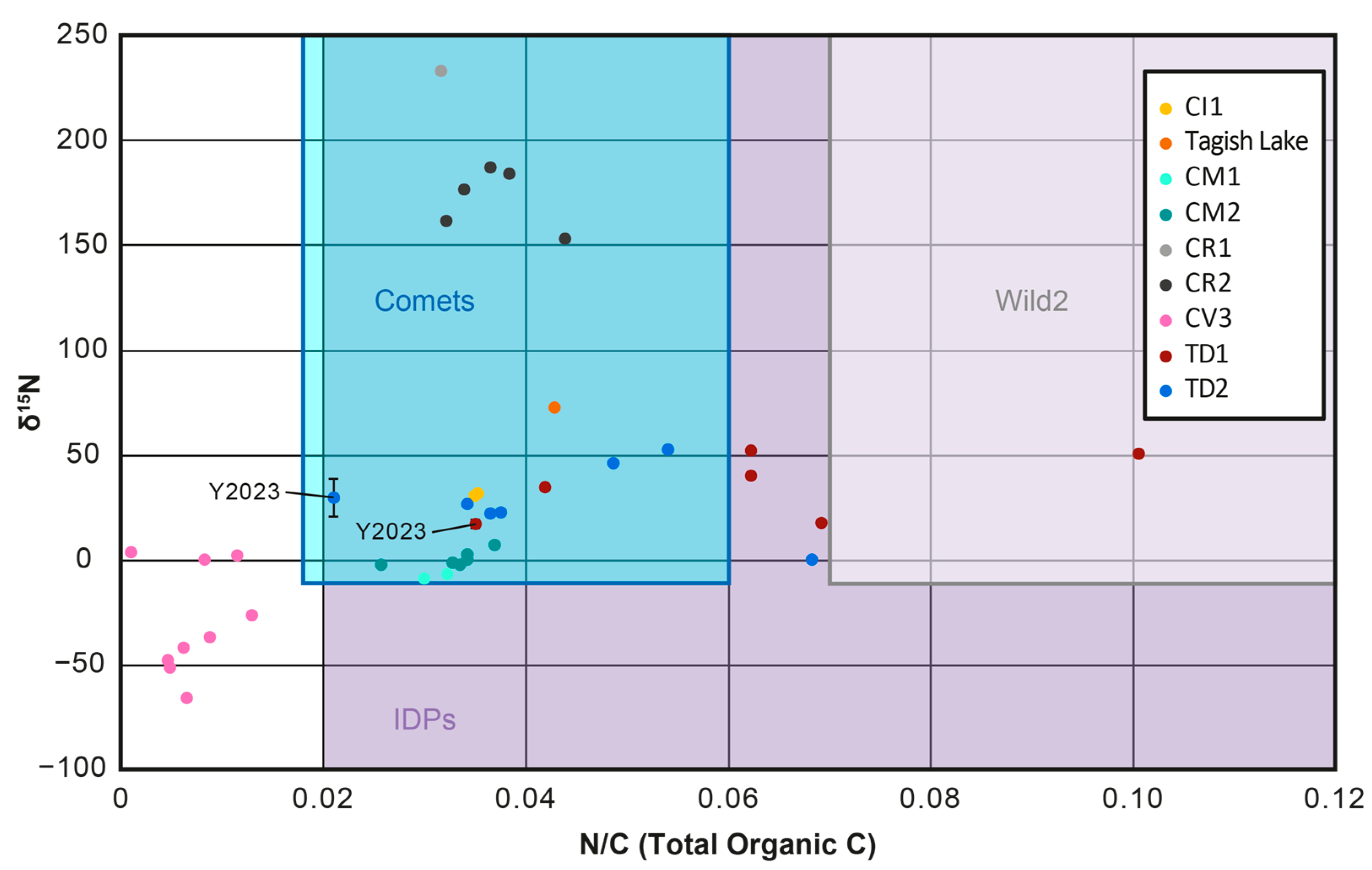
1.2.4. Isotopic Signatures
2. Ryugu (1999 JU3): Remote Sensing Observations and Predictions
3. Organic Matter in the Ryugu Return Samples
3.1. Free/Soluable Organic Matter (FOM/SOM)
3.1.1. Amino Acids
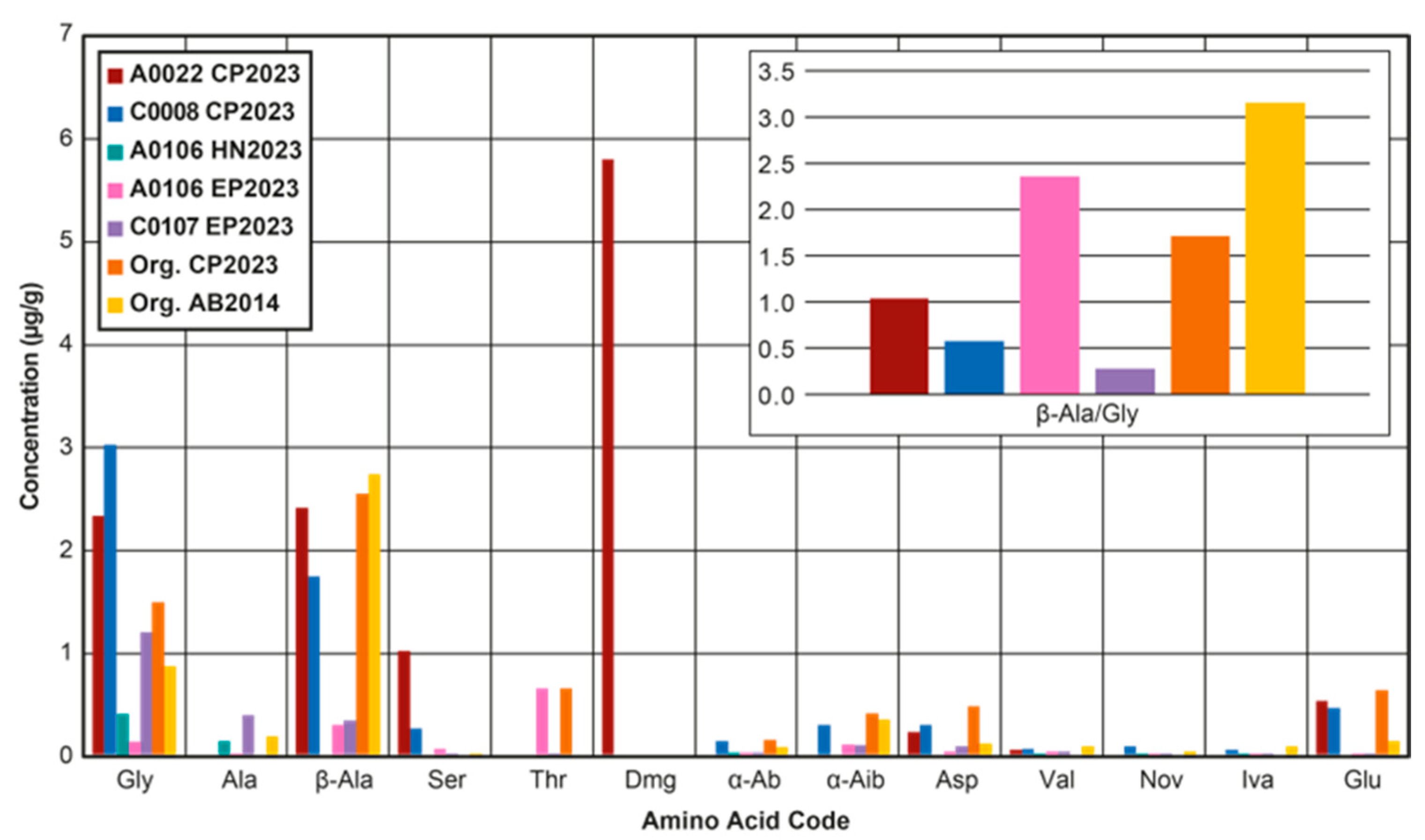
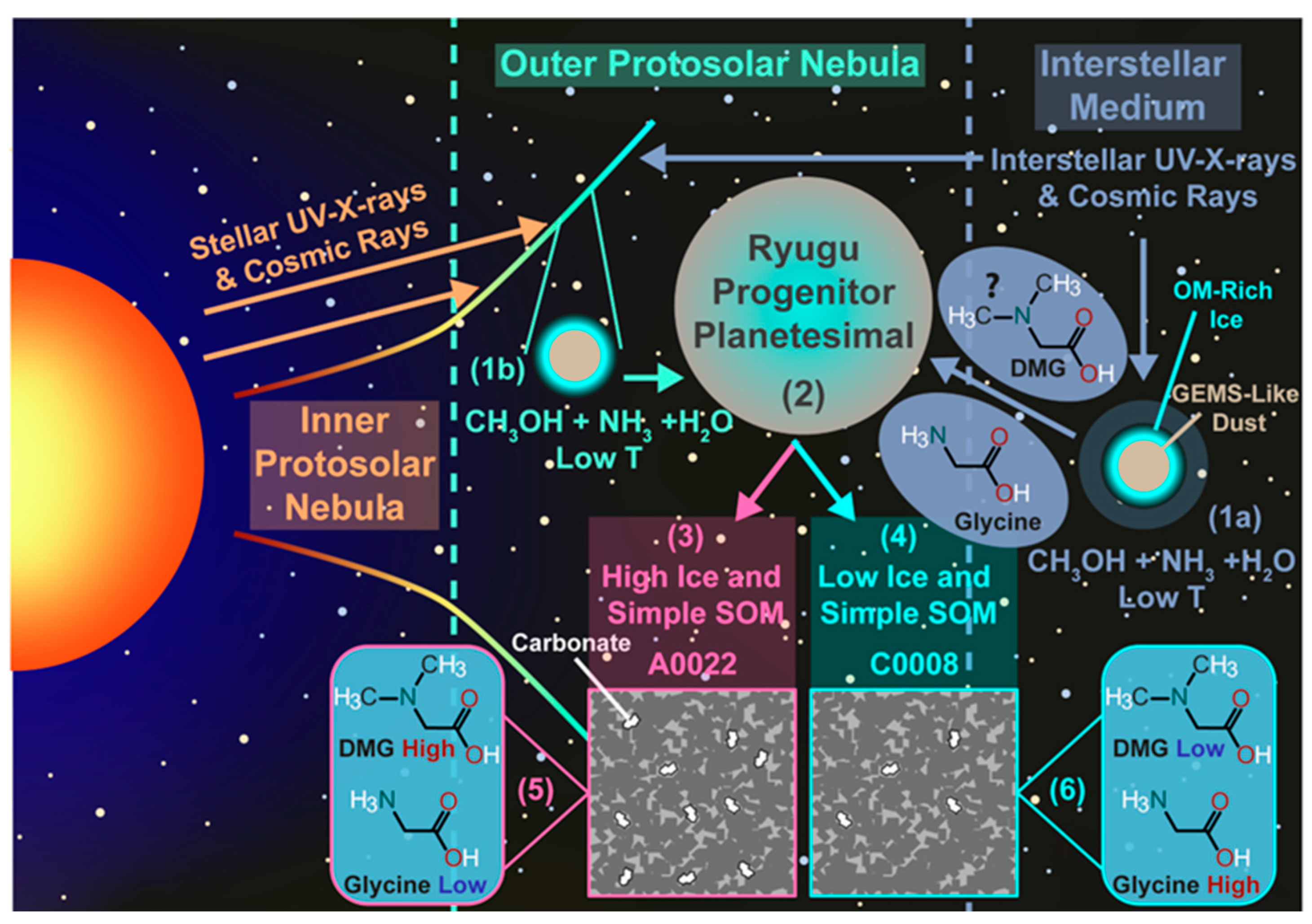
3.1.2. Amines
3.1.3. Nitrogen Heterocycles
3.1.4. Other Compounds
3.2. Macromolecular/Insoluable Organic Matter (MOM/IOM)
3.2.1. Micrometer Scale Observations
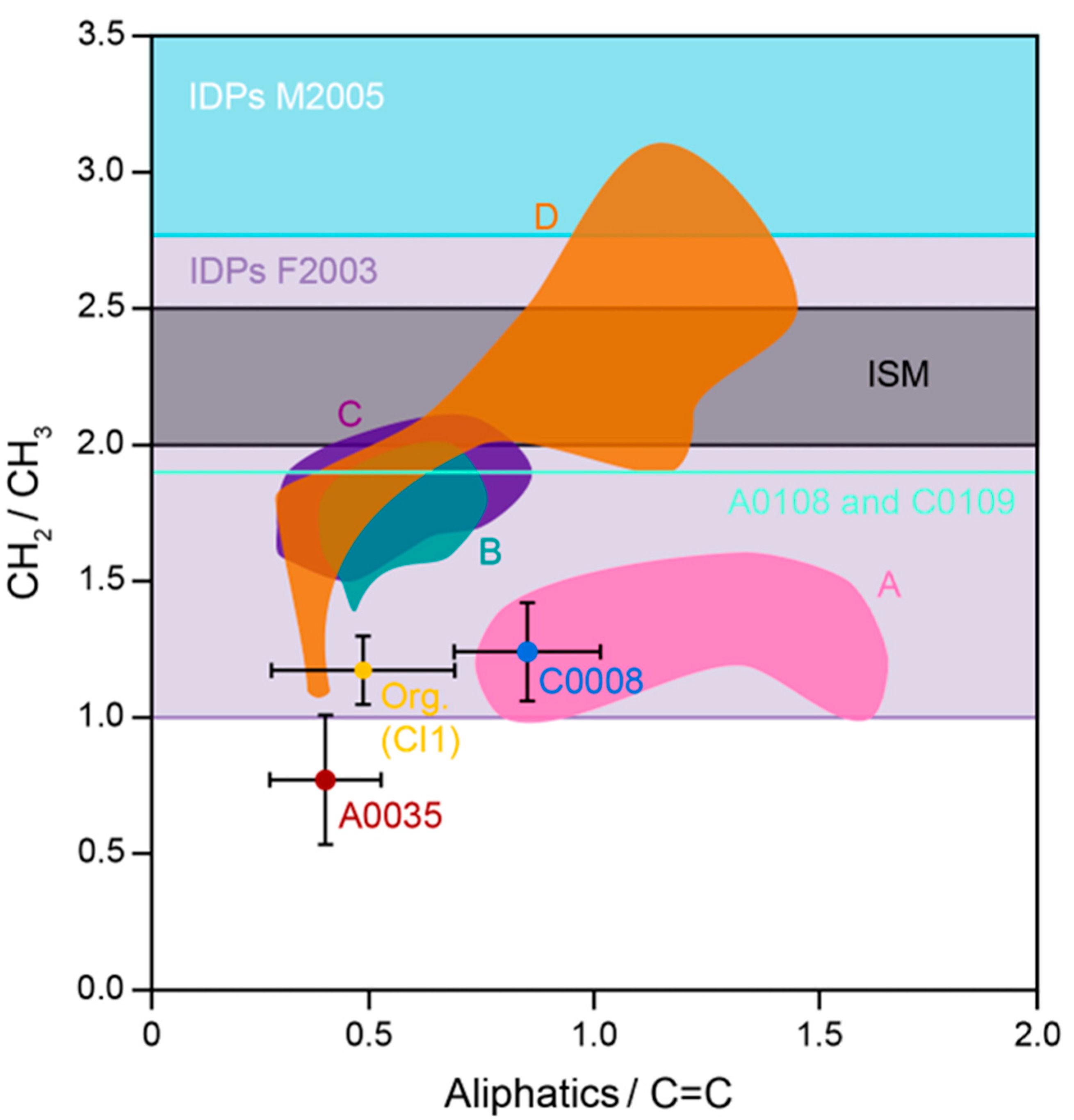
3.2.2. Nanometer Scale Observations
3.2.3. Elemental and Isotopic Characteristics
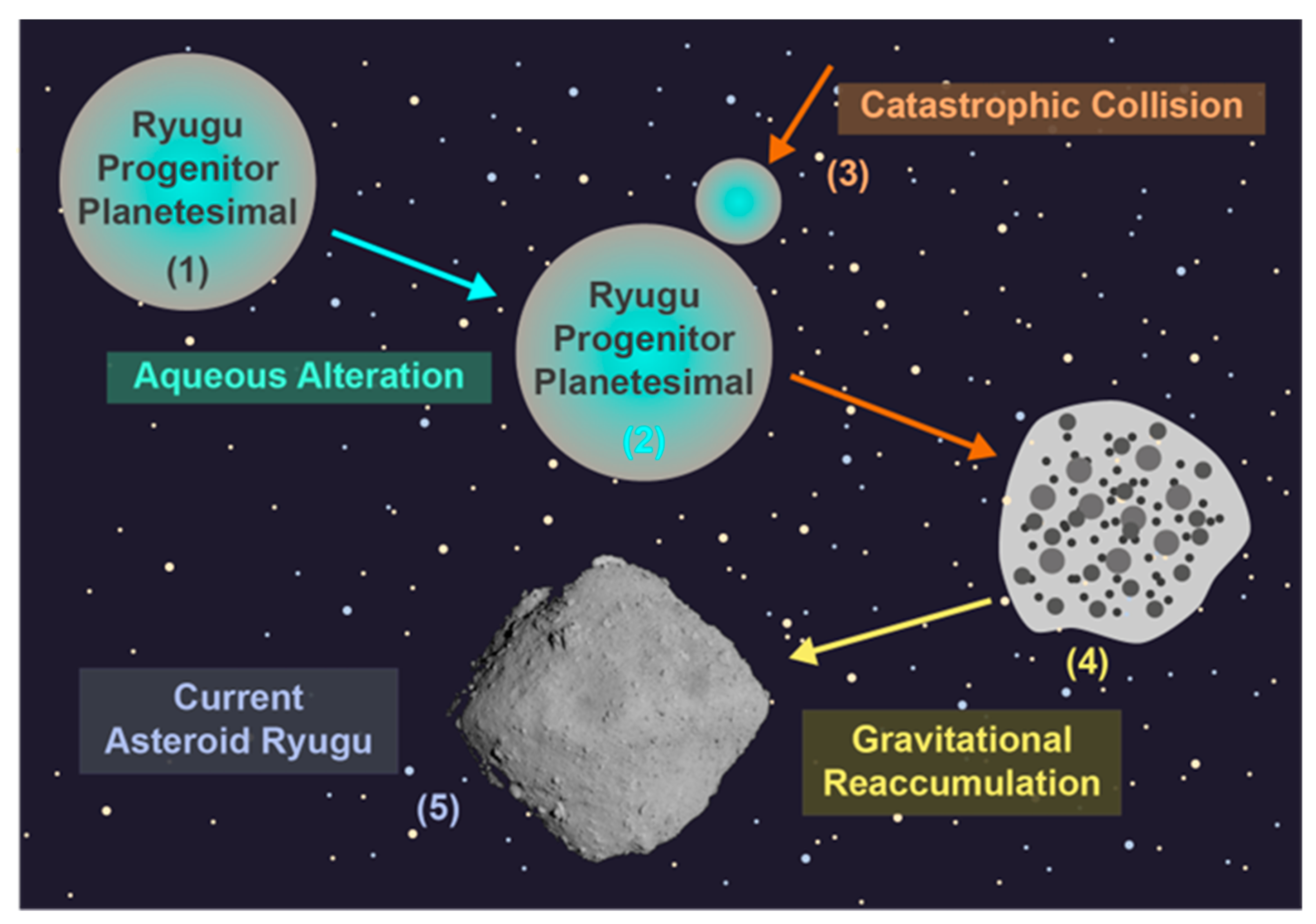
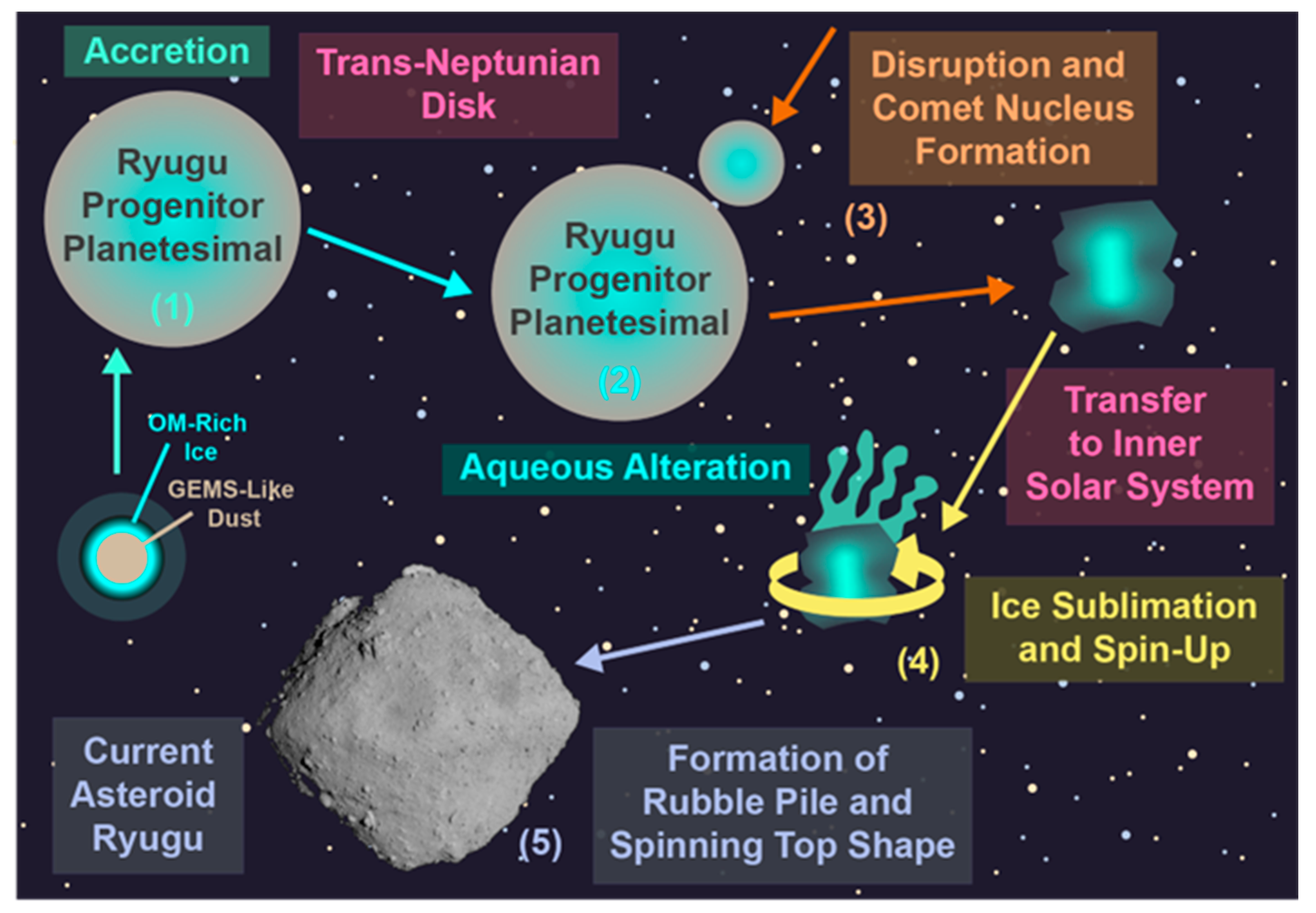
4. The Formation and Evolution of Ryugu
4.1. Catastrophic Collsion Model
4.2. Cometary Nucleus Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawaguchi, J.; Uesugi, K.; Fujiwara, A. The MUSES-C Mission for the Sample and Return—Its Technology Development Status and Readiness. Acta Astronaut. 2003, 52, 117–123. [Google Scholar] [CrossRef]
- Nakamura, E.; Makishima, A.; Moriguti, T.; Kobayashi, K.; Tanaka, R.; Kunihiro, T.; Tsujimori, T.; Sakaguchi, C.; Kitagawa, H.; Ota, T.; et al. Space Environment of an Asteroid Preserved on Micrograins Returned by the Hayabusa Spacecraft. Proc. Natl. Acad. Sci. USA 2012, 109, E624–E629. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsuda, Y.; Yoshikawa, M.; Tanaka, S.; Saiki, T.; Nakazawa, S. Hayabusa2 Mission Overview. Space Sci. Rev. 2017, 208, 3–16. [Google Scholar] [CrossRef]
- Watanabe, S.; Hirabayashi, M.; Hirata, N.; Hirata, N.; Noguchi, R.; Shimaki, Y.; Ikeda, H.; Tatsumi, E.; Yoshikawa, M.; Kikuchi, S.; et al. Hayabusa2 Arrives at the Carbonaceous Asteroid 162173 Ryugu—A Spinning Top-Shaped Rubble Pile. Science 2019, 364, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Honda, R.; Morota, T.; Kameda, S.; Sawada, H.; Tatsumi, E.; Yamada, M.; Honda, C.; Yokota, Y.; Kouyama, T.; et al. The Geomorphology, Color, and Thermal Properties of Ryugu: Implications for Parent-Body Processes. Science 2019, 364, eaaw0422. [Google Scholar] [CrossRef] [PubMed]
- Kitazato, K.; Milliken, R.E.; Iwata, T.; Abe, M.; Ohtake, M.; Matsuura, S.; Arai, T.; Nakauchi, Y.; Nakamura, T.; Matsuoka, M.; et al. The Surface Composition of Asteroid 162173 Ryugu from Hayabusa2 Near-Infrared Spectroscopy. Science 2019, 275, 272–275. [Google Scholar] [CrossRef]
- Jaumann, R.; Schmitz, N.; Otto, K.A.; Stephan, K.; Elgner, S.; Krohn, K.; Preusker, F.; Scholten, F.; Biele, J.; Ulamec, S.; et al. Images from the Surface of Asteroid Ryugu Show Rocks Similar to Carbonaceous Chondrite Meteorites. Science 2019, 820, 817–820. [Google Scholar] [CrossRef]
- Morota, T.; Sugita, S.; Cho, Y.; Kanamaru, M.; Tatsumi, E.; Sakatani, N.; Honda, R.; Hirata, N.; Kikuchi, H.; Yamada, M.; et al. Sample Collection from Asteroid (162173) Ryugu by Hayabusa2: Implications for Surface Evolution. Science 2020, 368, 654–659. [Google Scholar] [CrossRef]
- Kikuchi, S.; Saiki, T.; Takei, Y.; Terui, F.; Ogawa, N.; Mimasu, Y.; Ono, G.; Yoshikawa, K.; Sawada, H.; Takeuchi, H.; et al. Hayabusa2 Pinpoint Touchdown near the Artificial Crater on Ryugu: Trajectory Design and Guidance Performance. Adv. Space Res. 2021, 68, 3093–3140. [Google Scholar] [CrossRef]
- Potiszil, C.; Tanaka, R.; Kobayashi, K.; Kunihiro, T.; Nakamura, E. The Albedo of Ryugu: Evidence for a High Organic Abundance, as Inferred from the Hayabusa2 Touchdown Maneuver. Astrobiology 2020, 20, 916–921. [Google Scholar] [CrossRef]
- Tatsumi, E.; Domingue, D.; Schröder, S.; Yokota, Y.; Kuroda, D.; Ishiguro, M.; Hasegawa, S.; Hiroi, T.; Honda, R.; Hemmi, R.; et al. Global Photometric Properties of (162173) Ryugu. Astron. Astrophys. 2020, 639, A83. [Google Scholar] [CrossRef]
- Saiki, T.; Takei, Y.; Mimasu, Y.; Sawada, H.; Ogawa, N.; Ono, G.; Yoshikawa, K.; Terui, F.; Arakawa, M.; Sugita, S.; et al. Hayabusa2’s Kinetic Impact Experiment: Operational Planning and Results. Acta Astronaut. 2020, 175, 362–374. [Google Scholar] [CrossRef]
- Arakawa, M.; Saiki, T.; Wada, K.; Ogawa, K.; Kadono, T.; Shirai, K.; Sawada, H.; Ishibashi, K.; Honda, R.; Sakatani, N.; et al. An Artificial Impact on the Asteroid (162173) Ryugu Formed a Crater in the Gravity-Dominated Regime. Science 2020, 368, 67–71. [Google Scholar] [CrossRef]
- Yada, T.; Abe, M.; Okada, T.; Nakato, A.; Yogata, K.; Miyazaki, A.; Hatakeda, K.; Kumagai, K.; Nishimura, M.; Hitomi, Y.; et al. Preliminary Analysis of the Hayabusa2 Samples Returned from C-Type Asteroid Ryugu. Nat. Astron. 2021, 6, 214–220. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic Geochemistry of Meteorites. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2013; Volume 12, pp. 1–31. ISBN 9780080983004. [Google Scholar]
- Glavin, D.P.; Alexander, C.M.O.; Aponte, J.C.; Dworkin, J.P.; Elsila, J.E.; Yabuta, H. The Origin and Evolution of Organic Matter in Carbonaceous Chondrites and Links to Their Parent Bodies. In Primitive Meteorites and Asteroids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–271. [Google Scholar]
- Nakamura, E.; Kobayashi, K.; Tanaka, R.; Kunihiro, T.; Kitagawa, H.; Potiszil, C.; Ota, T.; Sakaguchi, C.; Yamanaka, M.; Ratnayake, D.M.; et al. On the Origin and Evolution of the Asteroid Ryugu: A Comprehensive Geochemical Perspective. Proc. Jpn. Acad. Ser. B 2022, 98, 227–282. [Google Scholar] [CrossRef]
- Potiszil, C.; Ota, T.; Yamanaka, M.; Sakaguchi, C.; Kobayashi, K.; Tanaka, R.; Kunihiro, T.; Kitagawa, H.; Abe, M.; Miyazaki, A.; et al. Insights into the Formation and Evolution of Extraterrestrial Amino Acids from the Asteroid Ryugu. Nat. Commun. 2023, 14, 1482. [Google Scholar] [CrossRef]
- Martins, Z. Organic Molecules in Meteorites and Their Astrobiological Significance. In Handbook of Astrobiology; Kolb, V.M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 177–194. [Google Scholar]
- McSween, H., Jr.; Huss, G. Cosmochemistry; Cambridge University Press: Cambridge, UK, 2022; ISBN 9781108885263. [Google Scholar]
- Cami, J.; Bernard-Salas, J.; Peeters, E.; Malek, S.E. Detection of C60 and C70 in a Young Planetary Nebula. Science 2010, 329, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Bernard-Salas, J.; Lloyd Evans, T.; Volk, K.M.; Hrivnak, B.J.; Sloan, G.C.; Chu, Y.-H.; Gruendl, R.; Kraemer, K.E.; Peeters, E.; et al. Spitzer Space Telescope Spectra of Post-AGB Stars in the Large Magellanic Cloud—Polycyclic Aromatic Hydrocarbons at Low Metallicities. Mon. Not. R. Astron. Soc. 2014, 439, 1472–1493. [Google Scholar] [CrossRef]
- Sandford, S.A.; Nuevo, M.; Bera, P.P.; Lee, T.J. Prebiotic Astrochemistry and the Formation of Molecules of Astrobiological Interest in Interstellar Clouds and Protostellar Disks. Chem. Rev. 2020, 120, 4616–4659. [Google Scholar] [CrossRef]
- Tielens, A.G.G.M. Molecular Astrophysics; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781316718490. [Google Scholar]
- Potapov, A.; Canosa, A.; Jiménez, E.; Rowe, B. Uniform Supersonic Chemical Reactors: 30 Years of Astrochemical History and Future Challenges. Angew. Chem. Int. Ed. 2017, 56, 8618–8640. [Google Scholar] [CrossRef]
- Guélin, M.; Cernicharo, J. Organic Molecules in Interstellar Space: Latest Advances. Front. Astron. Space Sci. 2022, 9, 787567. [Google Scholar] [CrossRef]
- Pauly, T.; Garrod, R.T. The effects of grain size and temperature distributions on the formation of interstellar ice mantles. Astrophys. J. 2016, 817, 146. [Google Scholar] [CrossRef]
- Cuppen, H.M.; van Dishoeck, E.F.; Herbst, E.; Tielens, A.G.G.M. Microscopic Simulation of Methanol and Formaldehyde Ice Formation in Cold Dense Cores. Astron. Astrophys. 2009, 508, 275–287. [Google Scholar] [CrossRef]
- Boogert, A.C.A.; Pontoppidan, K.M.; Knez, C.; Lahuis, F.; Kessler-Silacci, J.; van Dishoeck, E.F.; Blake, G.A.; Augereau, J.-C.; Bisschop, S.E.; Bottinelli, S.; et al. The C2d Spitzer Spectroscopic Survey of Ices around Low-Mass Young Stellar Objects. I. H 2 O and the 5–8 Μm Bands1,2. Astrophys. J. 2008, 678, 985–1004. [Google Scholar] [CrossRef]
- Boogert, A.C.A.; Gerakines, P.A.; Whittet, D.C.B. Observations of the Icy Universe. Annu. Rev. Astron. Astrophys. 2015, 53, 541–581. [Google Scholar] [CrossRef]
- Prasad, S.S.; Tarafdar, S.P. UV Radiation Field inside Dense Clouds—Its Possible Existence and Chemical Implications. Astrophys. J. 1983, 267, 603. [Google Scholar] [CrossRef]
- Dworkin, J.P.; Seb Gillette, J.; Bernstein, M.P.; Sandford, S.A.; Allamandola, L.J.; Elsila, J.E.; Ryan McGlothlin, D.; Zare, R.N. An Evolutionary Connection between Interstellar Ices and IDPs? Clues from Mass Spectroscopy Measurements of Laboratory Simulations. Adv. Space Res. 2004, 33, 67–71. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones Segovia, A.; Rosenbauer, H.; Thiemann, W.H.-P.; Brack, A.; Greenberg, J.M. Amino Acids from Ultraviolet Irradiation of Interstellar Ice Analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef]
- Meinert, C.; Filippi, J.-J.; de Marcellus, P.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. N-(2-Aminoethyl)Glycine and Amino Acids from Interstellar Ice Analogues. Chempluschem 2012, 77, 186–191. [Google Scholar] [CrossRef]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; d’Hendecourt, L.L.S.; Meierhenrich, U.J. Ribose and Related Sugars from Ultraviolet Irradiation of Interstellar Ice Analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef]
- Nuevo, M.; Cooper, G.; Sandford, S.A. Deoxyribose and Deoxysugar Derivatives from Photoprocessed Astrophysical Ice Analogues and Comparison to Meteorites. Nat. Commun. 2018, 9, 5276. [Google Scholar] [CrossRef] [PubMed]
- Materese, C.K.; Nuevo, M.; McDowell, B.L.; Buffo, C.E.; Sandford, S.A. The Photochemistry of Purine in Ice Analogs Relevant to Dense Interstellar Clouds. Astrophys. J. 2018, 864, 44. [Google Scholar] [CrossRef]
- Nuevo, M.; Materese, C.K.; Sandford, S.A. The photochemistry of pyrimidine in realistic astrophysical ices and the production of nucleobases. Astrophys. J. 2014, 793, 125. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Naraoka, H.; Watanabe, N.; Kouchi, A. Nucleobase Synthesis in Interstellar Ices. Nat. Commun. 2019, 10, 4413. [Google Scholar] [CrossRef]
- Materese, C.K.; Nuevo, M.; Sandford, S.A. N- and O-heterocycles produced from the irradiation of benzene and naphthalene in H2O/NH3-containing ices. Astrophys. J. 2015, 800, 116. [Google Scholar] [CrossRef]
- Henning, T.; Semenov, D. Chemistry in Protoplanetary Disks. Chem. Rev. 2013, 113, 9016–9042. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Millar, T.J.; Nomura, H.; Herbst, E.; Weaver, S.W.; Aikawa, Y.; Laas, J.C.; Vasyunin, A.I. Complex Organic Molecules in Protoplanetary Disks. Astron. Astrophys. 2014, 563, A33. [Google Scholar] [CrossRef]
- Ciesla, F.J.; Sandford, S.A. Organic Synthesis via Irradiation and Warming of Ice Grains in the Solar Nebula. Science 2012, 336, 452–454. [Google Scholar] [CrossRef]
- Nuevo, M.; Milam, S.N.; Sandford, S.A.; de Gregorio, B.T.; Cody, G.D.; Kilcoyne, A.L.D. XANES Analysis of Organic Residues Produced from the UV Irradiation of Astrophysical Ice Analogs. Adv. Space Res. 2011, 48, 1126–1135. [Google Scholar] [CrossRef]
- Biron, K.; Derenne, S.; Robert, F.; Rouzaud, J.N. Toward an Experimental Synthesis of the Chondritic Insoluble Organic Matter. Meteorit. Planet. Sci. 2015, 50, 1408–1422. [Google Scholar] [CrossRef]
- Robert, F.; Derenne, S.; Lombardi, G.; Hassouni, K.; Michau, A.; Reinhardt, P.; Duhamel, R.; Gonzalez, A.; Biron, K. Hydrogen Isotope Fractionation in Methane Plasma. Proc. Natl. Acad. Sci. USA 2017, 114, 870–874. [Google Scholar] [CrossRef]
- Kuga, M.; Marty, B.; Marrocchi, Y.; Tissandier, L. Synthesis of Refractory Organic Matter in the Ionized Gas Phase of the Solar Nebula. Proc. Natl. Acad. Sci. USA 2015, 112, 7129–7134. [Google Scholar] [CrossRef] [PubMed]
- Kuga, M.; Cernogora, G.; Marrocchi, Y.; Tissandier, L.; Marty, B. Processes of Noble Gas Elemental and Isotopic Fractionations in Plasma-Produced Organic Solids: Cosmochemical Implications. Geochim. Cosmochim. Acta 2017, 217, 219–230. [Google Scholar] [CrossRef]
- Bekaert, D.V.; Derenne, S.; Tissandier, L.; Marrocchi, Y.; Anquetil, C.; Marty, B.; Charnoz, S. High-Temperature Ionization-Induced Synthesis of Biologically Relevant Molecules in the Protosolar Nebula. Astrophys. J. 2018, 859, 142. [Google Scholar] [CrossRef]
- Youdin, A.N.; Goodman, J. Streaming Instabilities in Protoplanetary Disks. Astrophys. J. 2005, 620, 459–469. [Google Scholar] [CrossRef]
- Cuzzi, J.N.; Hogan, R.C.; Shariff, K. Toward Planetesimals: Dense Chondrule Clumps in the Protoplanetary Nebula. Astrophys. J. 2008, 687, 1432–1447. [Google Scholar] [CrossRef]
- Raettig, N.; Klahr, H.; Lyra, W. Particle trapping and streaming instability in vortices in protoplanetary disks. Astrophys. J. 2015, 804, 35. [Google Scholar] [CrossRef]
- Wahlberg Jansson, K.; Johansen, A. Formation of Pebble-Pile Planetesimals. Astron. Astrophys. 2014, 570, A47. [Google Scholar] [CrossRef]
- Windmark, F.; Birnstiel, T.; Güttler, C.; Blum, J.; Dullemond, C.P.; Henning, T. Planetesimal Formation by Sweep-up: How the Bouncing Barrier Can Be Beneficial to Growth. Astron. Astrophys. 2012, 540, A73. [Google Scholar] [CrossRef]
- Davidsson, B.J.R.; Sierks, H.; Güttler, C.; Marzari, F.; Pajola, M.; Rickman, H.; A’Hearn, M.F.; Auger, A.-T.; El-Maarry, M.R.; Fornasier, S.; et al. The Primordial Nucleus of Comet 67P/Churyumov-Gerasimenko. Astron. Astrophys. 2016, 592, A63. [Google Scholar] [CrossRef]
- Brearley, A.J. The Action of Water. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 587–624. ISBN 9781137002778. [Google Scholar]
- le Guillou, C.; Brearley, A. Relationships between Organics, Water and Early Stages of Aqueous Alteration in the Pristine CR3.0 Chondrite MET 00426. Geochim. Cosmochim. Acta 2014, 131, 344–367. [Google Scholar] [CrossRef]
- le Guillou, C.; Bernard, S.; Brearley, A.J.; Remusat, L. Evolution of Organic Matter in Orgueil, Murchison and Renazzo during Parent Body Aqueous Alteration: In Situ Investigations. Geochim. Cosmochim. Acta 2014, 131, 368–392. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Chan, Q.H.S.; Tachibana, S.; Kobayashi, K.; Zolensky, M.E. One-Pot Synthesis of Amino Acid Precursors with Insoluble Organic Matter in Planetesimals with Aqueous Activity. Sci. Adv. 2017, 3, e1602093. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Iwasa, Y.; Chikaraishi, Y. Synthesis of 13 C-Enriched Amino Acids with 13 C-Depleted Insoluble Organic Matter in a Formose-Type Reaction in the Early Solar System. Sci. Adv. 2021, 7, 3575–3603. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, E.T.; Bada, J.L.; Schlesinger, G.; Miller, S.L. The Chemical Conditions on the Parent Body of the Murchison Meteorite: Some Conclusions Based on Amino, Hydroxy and Dicarboxylic Acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dworkin, J.P. The Search for Chiral Asymmetry as a Potential Biosignature in Our Solar System. Chem. Rev. 2020, 120, 4660–4689. [Google Scholar] [CrossRef]
- Miller, S.L. The Mechanism of Synthesis of Amino Acids by Electric Discharges. Biochim. Biophys. Acta 1957, 23, 480–489. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Bernard, S.; le Guillou, C.; Remusat, L. Evolution of Interstellar Organic Compounds under Asteroidal Hydrothermal Conditions. Icarus 2018, 305, 358–370. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial Nucleobases in the Murchison Meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Levy, M.; Miller, S.L.; Oró, J. Production of Guanine from NH4CN Polymerizations. J. Mol. Evol. 1999, 49, 165–168. [Google Scholar] [CrossRef]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The Cold Origin of Life: B. Implications Based on Pyrimidines and Purines Produced From Frozen Ammonium Cyanide Solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Sanchez, R.A.; Orgel, L.E. Studies in Prebiotic Synthesis. J. Mol. Biol. 1968, 33, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Rios, A.C.; Nuevo, M. Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses. Life 2018, 8, 36. [Google Scholar] [CrossRef]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial Ribose and Other Sugars in Primitive Meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef] [PubMed]
- Kebukawa, Y.; David Kilcoyne, A.L.; Cody, G.D. Exploring the Potential Formation of Organic Solids in Chondrites and Comets through Polymerization of Interstellar Formaldehyde. Astrophys. J. 2013, 771, 19. [Google Scholar] [CrossRef]
- Füri, E.; Marty, B. Nitrogen Isotope Variations in the Solar System. Nat. Geosci. 2015, 8, 515–522. [Google Scholar] [CrossRef]
- Owen, T.; Mahaffy, P.R.; Niemann, H.B.; Atreya, S.; Wong, M. Protosolar Nitrogen. Astrophys. J. 2001, 553, L77–L79. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Donahue, T.M.; Atreya, S.K.; Owen, T.C.; Niemann, H.B. Galileo Probe Measurements of D/H and 3He/4He in Jupiter’s Atmosphere. Space Sci. Rev. 1998, 84, 251–263. [Google Scholar] [CrossRef]
- Robert, F.; Gautier, D.; Dubrulle, B. The Solar System D/H Ratio: Observations and Theories. Space Sci. Rev. 2000, 92, 201–224. [Google Scholar] [CrossRef]
- Marty, B.; Chaussidon, M.; Wiens, R.C.; Jurewicz, A.J.G.; Burnett, D.S. A 15N-Poor Isotopic Composition for the Solar System As Shown by Genesis Solar Wind Samples. Science 2011, 332, 1533–1536. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Bowden, R.; Fogel, M.L.; Howard, K.T.; Herd, C.D.K.; Nittler, L.R. The Provenances of Asteroids, and Their Contributions to the Volatile Inventories of the Terrestrial Planets. Science 2012, 337, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.M.O.; Howard, K.T.; Bowden, R.; Fogel, M.L. The Classification of CM and CR Chondrites Using Bulk H, C and N Abundances and Isotopic Compositions. Geochim. Cosmochim. Acta 2013, 123, 244–260. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Calmonte, U.; Charnley, S.; Duprat, J.; Engrand, C.; Gicquel, A.; Hässig, M.; Jehin, E.; Kawakita, H.; Marty, B.; et al. Cometary Isotopic Measurements. Space Sci. Rev. 2015, 197, 47–83. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Fuselier, S.A. Cometary Chemistry and the Origin of Icy Solar System: The View after Rosetta. Annu. Rev. Astron. Astrophys. 2019, 57, 113–155. [Google Scholar] [CrossRef]
- Stadermann, F.J.; Hoppe, P.; Floss, C.; Heck, P.R.; Hörz, F.; Huth, J.; Kearsley, A.T.; Leitner, J.; Marhas, K.K.; Mckeegan, K.D.; et al. Stardust in Stardust-The C, N, and O Isotopic Compositions of Wild 2 Cometary Matter in Al Foil Impacts. Meteorit. Planet. Sci. 2008, 43, 299–313. [Google Scholar] [CrossRef]
- McKeegan, K.D.; Aléon, J.; Bradley, J.; Brownlee, D.; Busemann, H.; Butterworth, A.; Chaussidon, M.; Fallon, S.; Floss, C.; Gilmour, J.; et al. Isotopic Compositions of Cometary Matter Returned by Stardust. Science 2006, 314, 1724–1728. [Google Scholar] [CrossRef]
- Sandford, S.A. Interstellar Processes Leading to Molecular Deuterium Enrichment and Their Detection. Planet. Space Sci. 2002, 50, 1145–1154. [Google Scholar] [CrossRef][Green Version]
- Lee, J.-E.; Bergin, E.A. The D/H ratio of water ice at low temperatures. Astrophys. J. 2015, 799, 104. [Google Scholar] [CrossRef]
- Allamandola, L.J.; Tielens, G.G.M.; Barker, J.R. Interstellar Polycyclic Aromatic Hydrocarbons—The Infrared Emission Bands, the Excitation/Emission Mechanism, and the Astrophysical Implications. Astrophys. J. Suppl. Ser. 1989, 71, 733. [Google Scholar] [CrossRef]
- Roueff, E.; Loison, J.C.; Hickson, K.M. Isotopic Fractionation of Carbon, Deuterium, and Nitrogen: A Full Chemical Study. Astron. Astrophys. 2015, 576, A99. [Google Scholar] [CrossRef]
- Colzi, L.; Fontani, F.; Caselli, P.; Leurini, S.; Bizzocchi, L.; Quaia, G. First Interferometric Study of Enhanced N-Fractionation in N2H+: The High-Mass Star-Forming Region IRAS 05358+3543. Mon. Not. R. Astron. Soc. 2019, 485, 5543–5558. [Google Scholar] [CrossRef]
- Fontani, F.; Barnes, A.T.; Caselli, P.; Henshaw, J.D.; Cosentino, G.; Jiménez-Serra, I.; Tan, J.C.; Pineda, J.E.; Law, C.Y. ALMA-IRDC—II. First High-Angular Resolution Measurements of the 14N/15N Ratio in a Large Sample of Infrared-Dark Cloud Cores. Mon. Not. R. Astron. Soc. 2021, 503, 4320–4335. [Google Scholar] [CrossRef]
- Clayton, R.N. Self-Shielding in the Solar Nebula. Nature 2002, 415, 860–861. [Google Scholar] [CrossRef]
- Chakraborty, S.; Muskatel, B.H.; Jackson, T.L.; Ahmed, M.; Levine, R.D.; Thiemens, M.H. Massive Isotopic Effect in Vacuum UV Photodissociation of N2 and Implications for Meteorite Data. Proc. Natl. Acad. Sci. USA 2014, 111, 14704–14709. [Google Scholar] [CrossRef]
- Binzel, R. Spectral Properties of Near-Earth Objects: Palomar and IRTF Results for 48 Objects Including Spacecraft Targets (9969) Braille and (10302) 1989 ML. Icarus 2001, 151, 139–149. [Google Scholar] [CrossRef]
- Vilas, F.; Gaffey, M.J. Phyllosilicate Absorption Features in Main-Belt and Outer-Belt Asteroid Reflectance Spectra. Science 1989, 246, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Vilas, F.; Jarvis, K.S.; Gaffey, M.J. Iron Alteration Minerals in the Visible and Near-Infrared Spectra of Low-Albedo Asteroids. Icarus 1994, 109, 274–283. [Google Scholar] [CrossRef]
- BINZEL, R.P.; PEROZZI, E.; RIVKIN, A.S.; ROSSI, A.; HARRIS, A.W.; BUS, S.J.; VALSECCHI, G.B.; SLIVAN, S.M. Dynamical and Compositional Assessment of Near-Earth Object Mission Targets. Meteorit. Planet. Sci. 2004, 39, 351–366. [Google Scholar] [CrossRef]
- Tsuda, Y.; Yoshikawa, M.; Abe, M.; Minamino, H.; Nakazawa, S. System Design of the Hayabusa 2—Asteroid Sample Return Mission to 1999 JU3. Acta Astronaut. 2013, 91, 356–362. [Google Scholar] [CrossRef]
- Perna, D.; Barucci, M.A.; Ishiguro, M.; Alvarez-Candal, A.; Kuroda, D.; Yoshikawa, M.; Kim, M.-J.; Fornasier, S.; Hasegawa, S.; Roh, D.-G.; et al. Spectral and Rotational Properties of Near-Earth Asteroid (162173) Ryugu, Target of the Hayabusa2 Sample Return Mission. Astron. Astrophys. 2017, 599, L1. [Google Scholar] [CrossRef]
- Le Corre, L.; Sanchez, J.A.; Reddy, V.; Takir, D.; Cloutis, E.A.; Thirouin, A.; Becker, K.J.; Li, J.-Y.; Sugita, S.; Tatsumi, E. Ground-Based Characterization of Hayabusa2 Mission Target Asteroid 162173 Ryugu: Constraining Mineralogical Composition in Preparation for Spacecraft Operations. Mon. Not. R. Astron. Soc. 2018, 475, 614–623. [Google Scholar] [CrossRef]
- Flynn, G.J.; Keller, L.P.; Jacobsen, C.; Wirick, S. An Assessment of the Amount and Types of Organic Matter Contributed to the Earth by Interplanetary Dust. Adv. Space Res. 2004, 33, 57–66. [Google Scholar] [CrossRef]
- Vernazza, P.; Marsset, M.; Beck, P.; Binzel, R.P.; Birlan, M.; Brunetto, R.; Demeo, F.E.; Djouadi, Z.; Dumas, C.; Merouane, S.; et al. Interplanetary dust particles as samples of icy asteroids. Astrophys. J. 2015, 806, 204. [Google Scholar] [CrossRef]
- Wirick, S.; Flynn, G.J.; Keller, L.P.; Nakamura-Messenger, K.; Peltzer, C.; Jacobsen, C.; Sandford, S.; Zolensky, M. Organic Matter from Comet 81P/Wild 2, IDPs, and Carbonaceous Meteorites; Similarities and Differences. Meteorit. Planet. Sci. 2009, 44, 1611–1626. [Google Scholar] [CrossRef]
- Busemann, H.; Nguyen, A.N.; Cody, G.D.; Hoppe, P.; Kilcoyne, A.L.D.; Stroud, R.M.; Zega, T.J.; Nittler, L.R. Ultra-Primitive Interplanetary Dust Particles from the Comet 26P/Grigg–Skjellerup Dust Stream Collection. Earth Planet. Sci. Lett. 2009, 288, 44–57. [Google Scholar] [CrossRef]
- Gounelle, M.; Zolensky, M.E. The Orgueil Meteorite: 150 Years of History. Meteorit. Planet. Sci. 2014, 49, 1769–1794. [Google Scholar] [CrossRef]
- Naraoka, H.; Takano, Y.; Dworkin, J.P.; Oba, Y.; Hamase, K.; Furusho, A.; Ogawa, N.O.; Hashiguchi, M.; Fukushima, K.; Aoki, D.; et al. Soluble Organic Molecules in Samples of the Carbonaceous Asteroid (162173) Ryugu. Science 2023, 379, eabn9033. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Grunsfeld, S.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. The Effects of Parent-Body Hydrothermal Heating on Amino Acid Abundances in CI-like Chondrites. Polar Sci. 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Furusho, A.; Akita, T.; Mita, M.; Naraoka, H.; Hamase, K. Three-Dimensional High-Performance Liquid Chromatographic Analysis of Chiral Amino Acids in Carbonaceous Chondrites. J. Chromatogr. A 2020, 1625, 461255. [Google Scholar] [CrossRef]
- Parker, E.T.; McLain, H.L.; Glavin, D.P.; Dworkin, J.P.; Elsila, J.E.; Aponte, J.C.; Naraoka, H.; Takano, Y.; Tachibana, S.; Yabuta, H.; et al. Extraterrestrial Amino Acids and Amines Identified in Asteroid Ryugu Samples Returned by the Hayabusa2 Mission. Geochim. Cosmochim. Acta 2023, 347, 42–57. [Google Scholar] [CrossRef]
- Simkus, D.N.; Aponte, J.C.; Elsila, J.E.; Parker, E.T.; Glavin, D.P.; Dworkin, J.P. Methodologies for Analyzing Soluble Organic Compounds in Extraterrestrial Samples: Amino Acids, Amines, Monocarboxylic Acids, Aldehydes, and Ketones. Life 2019, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Elsila, J.E.; Callahan, M.P.; Martin, M.G.; Glavin, D.P.; Johnson, N.M.; Dworkin, J.P. A Propensity for N-ω-Amino Acids in Thermally Altered Antarctic Meteorites. Meteorit. Planet. Sci. 2012, 47, 374–386. [Google Scholar] [CrossRef]
- Burton, A.; Berger, E. Insights into Abiotically-Generated Amino Acid Enantiomeric Excesses Found in Meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Koga, T.; Takano, Y.; Ogawa, N.O.; Ohkouchi, N.; Sasaki, K.; Sato, H.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H.; et al. Uracil in the Carbonaceous Asteroid (162173) Ryugu. Nat. Commun. 2023, 14, 1292. [Google Scholar] [CrossRef]
- Clarke, H.T.; Gillespie, H.B.; Weisshaus, S.Z. The Action of Formaldehyde on Amines and Amino Acids. J. Am. Chem. Soc. 1933, 55, 4571–4587. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Calmonte, U.; Fuselier, S.A.; Goesmann, F.; Gasc, S.; Gombosi, T.I.; Le Roy, L.; et al. Organics in Comet 67P—A First Comparative Analysis of Mass Spectra from ROSINA–DFMS, COSAC and Ptolemy. Mon. Not. R. Astron. Soc. 2017, 469, S130–S141. [Google Scholar] [CrossRef]
- Aponte, J.C.; Abreu, N.M.; Glavin, D.P.; Dworkin, J.P.; Elsila, J.E. Distribution of Aliphatic Amines in CO, CV, and CK Carbonaceous Chondrites and Relation to Mineralogy and Processing History. Meteorit. Planet. Sci. 2017, 52, 2632–2646. [Google Scholar] [CrossRef]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Indigenous Aliphatic Amines in the Aqueously Altered Orgueil Meteorite. Meteorit. Planet. Sci. 2015, 50, 1733–1749. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Naraoka, H. High-Mass Resolution Molecular Imaging of Organic Compounds on the Surface of Murchison Meteorite. Meteorit. Planet. Sci. 2018, 54, 452–468. [Google Scholar] [CrossRef]
- Naraoka, H.; Hashiguchi, M. In Situ Organic Compound Analysis on a Meteorite Surface by Desorption Electrospray Ionization Coupled with an Orbitrap Mass Spectrometer. Rapid Commun. Mass. Spectrom. 2018, 32, 959–964. [Google Scholar] [CrossRef]
- Naraoka, H.; Yamashita, Y.; Yamaguchi, M.; Orthous-Daunay, F.R. Molecular Evolution of N-Containing Cyclic Compounds in the Parent Body of the Murchison Meteorite. ACS Earth Space Chem. 2017, 1, 540–550. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the Wide Diversity of Extraterrestrial Purine and Pyrimidine Nucleobases in Carbonaceous Meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Potiszil, C.; Tanaka, R.; Ota, T.; Kunihiro, T.; Kobayashi, K.; Nakamura, E. Concentration of Meteoritic Free Organic Matter by Fluid Transport and Adsorption. Geochem. Perspect. Lett. 2020, 13, 30–35. [Google Scholar] [CrossRef]
- Naraoka, H.; Hashiguchi, M. Distinct Distribution of Soluble N-Heterocyclic Compounds between CM and CR Chondrites. Geochem. J. 2019, 53, 33–40. [Google Scholar] [CrossRef]
- Cech, N.B.; Enke, C.G. Practical Implications of Some Recent Studies in Electrospray Ionization Fundamentals. Mass. Spectrom. Rev. 2001, 20, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.; Blair, N.; Des Marais, D.J.; Chang, S. Carbon Isotope Composition of Low Molecular Weight Hydrocarbons and Monocarboxylic Acids from Murchison Meteorite. Nature 1984, 307, 252–254. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and Compound-Specific Isotopic Characterization of Monocarboxylic Acids in Carbonaceous Meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Aponte, J.C.; Woodward, H.K.; Abreu, N.M.; Elsila, J.E.; Dworkin, J.P. Molecular Distribution, 13C-isotope, and Enantiomeric Compositions of Carbonaceous Chondrite Monocarboxylic Acids. Meteorit. Planet. Sci. 2019, 54, 415–430. [Google Scholar] [CrossRef]
- Basile, B.P.; Middleditch, B.S.; Oró, J. Polycyclic Aromatic Hydrocarbons in the Murchison Meteorite. Org. Geochem. 1984, 5, 211–216. [Google Scholar] [CrossRef]
- Wing, M.R.; Bada, J.L. Geochromatography on the Parent Body of the Carbonaceous Chondrite Ivuna. Geochim. Cosmochim. Acta 1991, 55, 2937–2942. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Potiszil, C.; Montgomery, W.; Sephton, M.A. Heterogeneity within Refractory Organic Matter from CM2 Carbonaceous Chondrites: Evidence from Raman Spectroscopy. Earth Planet. Sci. Lett. 2021, 574, 117149. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Matrajt, G.; Dartois, E.; Nuevo, M.; D’Hendecourt, L.; Deboffle, D.; Montagnac, G.; Chauvin, N.; Boukari, C.; Le Du, D. Nature and Evolution of the Dominant Carbonaceous Matter in Interplanetary Dust Particles: Effects of Irradiation and Identification with a Type of Amorphous Carbon. Astron. Astrophys. 2006, 459, 147–159. [Google Scholar] [CrossRef]
- Yabuta, H.; Cody, G.D.; Engrand, C.; Kebukawa, Y.; De Gregorio, B.; Bonal, L.; Remusat, L.; Stroud, R.; Quirico, E.; Nittler, L.; et al. Macromolecular Organic Matter in Samples of the Asteroid (162173) Ryugu. Science 2023, 379, 25. [Google Scholar] [CrossRef] [PubMed]
- Kebukawa, Y.; Alexander, C.M.O.; Cody, G.D. Compositional Diversity in Insoluble Organic Matter in Type 1, 2 and 3 Chondrites as Detected by Infrared Spectroscopy. Geochim. Cosmochim. Acta 2011, 75, 3530–3541. [Google Scholar] [CrossRef]
- Sandford, S.A.; Allamandola, L.J.; Tielens, A.G.G.M.; Sellgren, K.; Tapia, M.; Pendleton, Y. The Interstellar C-H Stretching Band near 3.4 Microns—Constraints on the Composition of Organic Material in the Diffuse Interstellar Medium. Astrophys. J. 1991, 371, 607. [Google Scholar] [CrossRef]
- Pendleton, Y.J.; Sandford, S.A.; Allamandola, L.J.; Tielens, A.G.G.M.; Sellgren, K. Near-Infrared Absorption Spectroscopy of Interstellar Hydrocarbon Grains. Astrophys. J. 1994, 437, 683–696. [Google Scholar] [CrossRef]
- Flynn, G.J.; Keller, L.P.; Feser, M.; Wirick, S.; Jacobsen, C. The Origin of Organic Matter in the Solar System: Evidence from the Interplanetary Dust Particles. Geochim. Cosmochim. Acta 2003, 67, 4791–4806. [Google Scholar] [CrossRef]
- Matrajt, G.; Muñoz Caro, G.M.; Dartois, E.; D’Hendecourt, L.; Deboffle, D.; Borg, J. FTIR Analysis of the Organics in IDPs: Comparison with the IR Spectra of the Diffuse Interstellar Medium. Astron. Astrophys. 2005, 433, 979–995. [Google Scholar] [CrossRef]
- De Gregorio, B.T.; Stroud, R.M.; Nittler, L.R.; Alexander, C.M.O.; Bassim, N.D.; Cody, G.D.; Kilcoyne, A.L.D.; Sandford, S.A.; Milam, S.N.; Nuevo, M.; et al. Isotopic and Chemical Variation of Organic Nanoglobules in Primitive Meteorites. Meteorit. Planet. Sci. 2013, 48, 904–928. [Google Scholar] [CrossRef]
- De Gregorio, B.T.; Stroud, R.M.; Nittler, L.R.; Alexander, C.M.O.; Kilcoyne, A.L.D.; Zega, T.J. Isotopic Anomalies in Organic Nanoglobules from Comet 81P/Wild 2: Comparison to Murchison Nanoglobules and Isotopic Anomalies Induced in Terrestrial Organics by Electron Irradiation. Geochim. Cosmochim. Acta 2010, 74, 4454–4470. [Google Scholar] [CrossRef]
- Floss, C.; Stadermann, F.J.; Bradley, J.P.; Dai, Z.R.; Bajt, S.; Graham, G.; Lea, A.S. Identification of Isotopically Primitive Interplanetary Dust Particles: A NanoSIMS Isotopic Imaging Study. Geochim. Cosmochim. Acta 2006, 70, 2371–2399. [Google Scholar] [CrossRef]
- Fray, N.; Bardyn, A.; Cottin, H.; Baklouti, D.; Briois, C.; Engrand, C.; Fischer, H.; Hornung, K.; Isnard, R.; Langevin, Y.; et al. Nitrogen-to-Carbon Atomic Ratio Measured by COSIMA in the Particles of Comet 67P/Churyumov–Gerasimenko. Mon. Not. R. Astron. Soc. 2017, 469, S506–S516. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Fogel, M.; Yabuta, H.; Cody, G.D. The Origin and Evolution of Chondrites Recorded in the Elemental and Isotopic Compositions of Their Macromolecular Organic Matter. Geochim. Cosmochim. Acta 2007, 71, 4380–4403. [Google Scholar] [CrossRef]
- Ishii, H.A.; Bradley, J.P.; Bechtel, H.A.; Brownlee, D.E.; Bustillo, K.C.; Ciston, J.; Cuzzi, J.N.; Floss, C.; Joswiak, D.J. Multiple Generations of Grain Aggregation in Different Environments Preceded Solar System Body Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 6608–6613. [Google Scholar] [CrossRef] [PubMed]
- Cody, G.D.; Ade, H.; Alexander, M.O.; Araki, T.; Butterworth, A.; Fleckenstein, H.; Flynn, G.; Gilles, M.K.; Jacobsen, C.; Kilcoyne, A.L.D.; et al. Quantitative Organic and Light-Element Analysis of Comet 81P/Wild 2 Particles Using C-, N-, and O-μ-XANES. Meteorit. Planet. Sci. 2008, 43, 353–365. [Google Scholar] [CrossRef]
- Altwegg, K.; Balsiger, H.; Hänni, N.; Rubin, M.; Schuhmann, M.; Schroeder, I.; Sémon, T.; Wampfler, S.; Berthelier, J.-J.; Briois, C.; et al. Evidence of Ammonium Salts in Comet 67P as Explanation for the Nitrogen Depletion in Cometary Comae. Nat. Astron. 2020, 4, 533–540. [Google Scholar] [CrossRef]
- Poch, O.; Istiqomah, I.; Quirico, E.; Beck, P.; Schmitt, B.; Theulé, P.; Faure, A.; Hily-Blant, P.; Bonal, L.; Raponi, A.; et al. Ammonium Salts Are a Reservoir of Nitrogen on a Cometary Nucleus and Possibly on Some Asteroids. Science 2020, 367, eaaw7462. [Google Scholar] [CrossRef]
- Michel, P.; Ballouz, R.L.; Barnouin, O.S.; Jutzi, M.; Walsh, K.J.; May, B.H.; Manzoni, C.; Richardson, D.C.; Schwartz, S.R.; Sugita, S.; et al. Collisional Formation of Top-Shaped Asteroids and Implications for the Origins of Ryugu and Bennu. Nat. Commun. 2020, 11, 2655. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumoto, M.; Amano, K.; Enokido, Y.; Zolensky, M.E.; Mikouchi, T.; Genda, H.; Tanaka, S.; Zolotov, M.Y.; Kurosawa, K.; et al. Formation and Evolution of Carbonaceous Asteroid Ryugu: Direct Evidence from Returned Samples. Science 2022, 379, eabn8671. [Google Scholar] [CrossRef]
- Young, E.D.; Zhang, K.K.; Schubert, G. Conditions for Pore Water Convection within Carbonaceous Chondrite Parent Bodies—Implications for Planetesimal Size and Heat Production. Earth Planet. Sci. Lett. 2003, 213, 249–259. [Google Scholar] [CrossRef]
- TRAVIS, B.; SCHUBERT, G. Hydrothermal Convection in Carbonaceous Chondrite Parent Bodies. Earth Planet. Sci. Lett. 2005, 240, 234–250. [Google Scholar] [CrossRef]
- Alexander, C.M.O. Quantitative Models for the Elemental and Isotopic Fractionations in Chondrites: The Carbonaceous Chondrites. Geochim. Cosmochim. Acta 2019, 254, 277–309. [Google Scholar] [CrossRef]
- Prialnik, D.; Podolak, M. Radioactive Heating of Porous Comet Nuclei. Icarus 1995, 117, 420–430. [Google Scholar] [CrossRef]
- Miura, H.; Nakamura, E.; Kunihiro, T. The Asteroid 162173 Ryugu: A Cometary Origin. Astrophys. J. Lett. 2022, 925, L15. [Google Scholar] [CrossRef]
- Ota, T.; Potiszil, C.; Kobayashi, K.; Tanaka, R.; Kitagawa, H.; Kunihiro, T.; Sakaguchi, C.; Yamanaka, M.; Nakamura, E. The Formation of a Rubble Pile Asteroid: Insights from the Asteroid Ryugu. Universe 2023, 9, 293. [Google Scholar] [CrossRef]
- Gounelle, M.; Morbidelli, A.; Bland, P.A.; Young, E.D.; Sephton, M. Meteorites from the Outer Solar System? In The Solar System beyond Neptune; Barucci, M.A., Boehnhardt, H., Cruikshank, D.P., Morbidelli, A., Eds.; University of Arizona Press: Tucson, AZ, USA, 2008; pp. 525–541. ISBN 9780816527557. [Google Scholar]
- Dones, L.; Brasser, R.; Kaib, N.; Rickman, H. Origin and Evolution of the Cometary Reservoirs. Space Sci. Rev. 2015, 197, 191–269. [Google Scholar] [CrossRef]
- Morbidelli, A.; Rickman, H. Comets as Collisional Fragments of a Primordial Planetesimal Disk. Astron. Astrophys. 2015, 583, A43. [Google Scholar] [CrossRef]
- Schwartz, S.R.; Michel, P.; Jutzi, M.; Marchi, S.; Zhang, Y.; Richardson, D.C. Catastrophic Disruptions as the Origin of Bilobate Comets. Nat. Astron. 2018, 2, 379–382. [Google Scholar] [CrossRef]
- Jutzi, M.; Benz, W. Formation of Bi-Lobed Shapes by Sub-Catastrophic Collisions. Astron. Astrophys. 2017, 597, A62. [Google Scholar] [CrossRef]
- Morbidelli, A.; Nesvorný, D. Kuiper Belt: Formation and Evolution. In The Trans-Neptunian Solar System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–59. [Google Scholar]
- Gomes, R.; Levison, H.F.; Tsiganis, K.; Morbidelli, A. Origin of the Cataclysmic Late Heavy Bombardment Period of the Terrestrial Planets. Nature 2005, 435, 466–469. [Google Scholar] [CrossRef]
- Tsiganis, K.; Gomes, R.; Morbidelli, A.; Levison, H.F. Origin of the Orbital Architecture of the Giant Planets of the Solar System. Nature 2005, 435, 459–461. [Google Scholar] [CrossRef]
- Nesvorný, D.; Morbidelli, A. Statistical study of the early solar system’s instability with four, five, and six giant planets. Astron. J. 2012, 144, 117. [Google Scholar] [CrossRef]
- Hasegawa, S.; Marsset, M.; DeMeo, F.E.; Bus, S.J.; Geem, J.; Ishiguro, M.; Im, M.; Kuroda, D.; Vernazza, P. Discovery of Two TNO-like Bodies in the Asteroid Belt. Astrophys. J. Lett. 2021, 916, L6. [Google Scholar] [CrossRef]
- Raymond, S.N.; Izidoro, A. The Empty Primordial Asteroid Belt. Sci. Adv. 2017, 3, e1701138. [Google Scholar] [CrossRef] [PubMed]
- Nuth, J.A.; Abreu, N.; Ferguson, F.T.; Glavin, D.P.; Hergenrother, C.; Hill, H.G.M.; Johnson, N.M.; Pajola, M.; Walsh, K. Volatile-Rich Asteroids in the Inner Solar System. Planet. Sci. J. 2020, 1, 82. [Google Scholar] [CrossRef]
- Tripathi, H.; Potiszil, C.; Tanaka, R.; Nakamura, E. The Ice-Organic-Silicate Contents of Small Solar System Bodies: Indicators for a Comet to Asteroid Evolutionary Pathway. Mon. Not. R. Astron. Soc. 2022, 513, 3734–3741. [Google Scholar] [CrossRef]
- Fornasier, S.; Hoang, V.H.; Hasselmann, P.H.; Feller, C.; Barucci, M.A.; Deshapriya, J.D.P.; Sierks, H.; Naletto, G.; Lamy, P.L.; Rodrigo, R.; et al. Linking Surface Morphology, Composition, and Activity on the Nucleus of 67P/Churyumov-Gerasimenko. Astron. Astrophys. 2019, 630, A7. [Google Scholar] [CrossRef]
- Bruck Syal, M.; Schultz, P.H.; Sunshine, J.M.; A’Hearn, M.F.; Farnham, T.L.; Dearborn, D.S.P. Geologic Control of Jet Formation on Comet 103P/Hartley 2. Icarus 2013, 222, 610–624. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial Amino Acids in Orgueil and Ivuna: Tracing the Parent Body of CI Type Carbonaceous Chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potiszil, C.; Yamanaka, M.; Sakaguchi, C.; Ota, T.; Kitagawa, H.; Kunihiro, T.; Tanaka, R.; Kobayashi, K.; Nakamura, E. Organic Matter in the Asteroid Ryugu: What We Know So Far. Life 2023, 13, 1448. https://doi.org/10.3390/life13071448
Potiszil C, Yamanaka M, Sakaguchi C, Ota T, Kitagawa H, Kunihiro T, Tanaka R, Kobayashi K, Nakamura E. Organic Matter in the Asteroid Ryugu: What We Know So Far. Life. 2023; 13(7):1448. https://doi.org/10.3390/life13071448
Chicago/Turabian StylePotiszil, Christian, Masahiro Yamanaka, Chie Sakaguchi, Tsutomu Ota, Hiroshi Kitagawa, Tak Kunihiro, Ryoji Tanaka, Katsura Kobayashi, and Eizo Nakamura. 2023. "Organic Matter in the Asteroid Ryugu: What We Know So Far" Life 13, no. 7: 1448. https://doi.org/10.3390/life13071448
APA StylePotiszil, C., Yamanaka, M., Sakaguchi, C., Ota, T., Kitagawa, H., Kunihiro, T., Tanaka, R., Kobayashi, K., & Nakamura, E. (2023). Organic Matter in the Asteroid Ryugu: What We Know So Far. Life, 13(7), 1448. https://doi.org/10.3390/life13071448





