Abstract
Resting and exercise right heart catheterisation is the gold standard method to diagnose and differentiate types of pulmonary hypertension (PH). As it carries technical challenges, the question arises if non-invasive exercise stress echocardiography may be used as an alternative. Exercise echocardiography can unmask exercise PH, detect the early stages of left ventricular diastolic dysfunction, and, therefore, differentiate between pre- and post-capillary PH. Regardless of the underlying aetiology, a developed PH is associated with increased mortality. Parameters of overt right ventricle (RV) dysfunction, including RV dilation, reduced RV ejection fraction, and elevated right-sided filling pressures, are detectable with resting echocardiography and are associated with worse outcome. However, these measures all fail to identify occult RV dysfunction. Echocardiographic measures of RV contractile reserve during exercise echocardiography are very promising and provide incremental prognostic information on clinical outcome. In this paper, we review pulmonary haemodynamic response to exercise, briefly describe the modalities for assessing pulmonary haemodynamics, and discuss in depth the contemporary key clinical application of exercise stress echocardiography in patients with PH.
1. Introduction
Pulmonary hypertension (PH) is a pathophysiological disorder that may involve various clinical conditions and may be associated with numerous respiratory and/or cardiovascular diseases. Regardless of the underlying pathobiological process and the site of the functional changes, PH is defined as a resting mean pulmonary arterial pressure (mPAP) of >20 mmHg measured by right heart catheterisation (RHC) [1].
However, the elevated mPAP is not sufficient to define the subgroups of PH since it could be due to increased pulmonary vascular resistance (PVR) or increased pulmonary artery wedge pressure (PAWP), or just a consequence of alteration in cardiac output (CO) or intrathoracic pressure [1,2]. Taking mPAP, PAWP, and PVR into consideration, we differentiate two main phenotypes of PH. Pre-capillary PH is the consequence of pulmonary vascular disease (e.g., pulmonary artery hypertension, lung diseases, and/or hypoxia, pulmonary artery obstructions,…) and is characterised by the presence of elevated PVR (>2 mmHg/L/min) and normal PAWP (≤15 mmHg). Post-capillary PH is due to left heart disease and is haemodynamically defined as mPAP > 20 mmHg and PAWP > 15 mmHg. The detection and risk stratification of PH patients in everyday clinical practice is often challenging due to the complexity of PH phenotypes; however, it is of paramount importance for adequate treatment.
RHC is the gold standard method to assess pulmonary haemodynamics. It enables diagnosing and classifying PH [1]. On the other hand, echocardiography takes a central role in detecting the consequences of right ventricle (RV) pressure overload, such as RV dysfunction, as well as in detecting the causes of PH, particularly in PH associated with left heart disease. Recent studies have demonstrated that exercise could reveal abnormal pulmonary haemodynamic response in patients with PH, which might provide important prognostic and functional information [3]. According to the current consensus statement, an exercise stress test during RHC is the recommended method to assess cardiopulmonary haemodynamics. However, exercise RHC carries some technical challenges, requiring experienced personnel, special equipment, and additional procedural time. This raises the question of whether non-invasive exercise stress echocardiography may be used as an alternative technique.
The purpose of this manuscript is to review the basic concept of the pulmonary haemodynamic response to exercise in health and disease and to discuss in depth the role of exercise stress echocardiography with a particular emphasis on its diagnostic and prognostic implication in patients with PH.
2. How Does Pulmonary Circulation Response to Exercise?
In healthy subjects: An increase in CO during exercise stresses the pulmonary circulation, which results in a physiological increase in pulmonary arterial pressure (PAP). The increase in PAP is strongly dependent on the level of the exercise, and at high exercise levels, it can frequently exceed the mPAP > 20 mmHg [4]. Changes in PAP during exercise are determined by the interplay between CO, pulmonary artery compliance, PVR, and PAWP and can be presented with the following simplified equitation:
mPAP = PVR × CO + PAWP.
In a normal individual, mPAP and PAWP increase significantly during exercise, while PVR slightly but not significantly decreases [3,5]. The physiological decrease in PVR during exercise is due to the recruitment and distension of the pulmonary resistance vessels with increased flow [6]. The increase in PAP is flow-dependent and needs to be reported in relation to the increase in CO (Figure 1) [7].
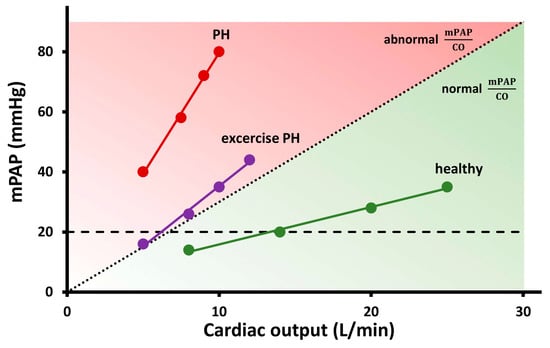
Figure 1.
Mean pulmonary arterial pressure (mPAP)–cardiac output (CO) relationship for characterisation of pulmonary haemodynamics during exercise in three representative cases: a patient with pulmonary hypertension (PH), a patient with exercise PH, and a healthy subject. The patient with PH has elevated mPAP at rest and demonstrates disproportionate increase in mPAP during exercise (mPAP/CO = 8 mmHg/L/min), while the patient with exercise, PH has normal resting pulmonary haemodynamics and abnormal response during exercise (mPAP/CO slope = 4 mmHg/L/min). Healthy subject has flow-dependent increase in mPAP, which exceeds 20 mmHg at high CO; however, mPAP/CO slope remains very low (1 mmHg/L/min).
In contrast, the mPAP/CO slope is largely unaffected by workload, but it is strongly age dependent. The age dependency of the mPAP/CO slope is mainly determined by the age dependency of the PAWP/CO slope [5]. Higher PAWP/CO slope with ageing is likely due to a decrease in left ventricular (LV) compliance and relaxation during exercise, which is part of the physiological ageing process [8,9]. On the other hand, the distensibility of the pulmonary vessels may remain largely unaffected by age.
In pathological conditions: Unrelated to the pathology, a disproportionate increase in PAP during low levels of exercise is observed, and this contributes to abnormally high mPAP/CO slope (>3 mmHg/L/min) (Figure 1).
In patients with heart failure (post-capillary PH), impaired early diastolic relaxation, reduced increments in suction, and poor LV contractility and/or LV compliance led to inadequate increases in stroke volume and CO with exercise and increased LV filling pressures and PAWP. The high pressure from the left heart is transmitted into the pulmonary circulation, consequently resulting in an increase in mPAP [10,11,12].
On the other hand, patients with pulmonary vascular disease have primarily an increased pulmonary vascular tone and remodelling of the small pulmonary arteries. An abnormal exercise-induced increase in PVR due to pulmonary vascular abnormalities is the most important factor contributing to exercise PAP in this patient’s population [6]. These patients often have reduced CO already at rest and/or fail to adequately augment CO through exercise [13], which results in a steeper mPAP/CO slope during exercise (Figure 1).
A small group of patients have normal pulmonary haemodynamics at rest, but during exercise, a pathological increase in PAP can be detected, evident by an mPAP/CO slope >3 mmHg/L/min between rest and exercise (Figure 1) [5]. The latest evidence demonstrated that this pathological increase in PAP during exercise is associated with a worse prognosis in patients with exercise dyspnoea [14] and several cardiovascular conditions [15,16,17,18]. This entity of exercise PH was recently reintroduced in the 2022 ESC/ERS Guidelines for the diagnosis and treatment of PH as a new phenotype of PH [1].
3. Methods for Assessing Pulmonary Haemodynamics
3.1. RHC
RHC is the gold standard method to assess pulmonary haemodynamics at rest and during exercise [1]. After confirming PH with mPAP > 20 mmHg at rest, the haemodynamic definition further distinguishes PH into two main phenotypes of PH, pre-capillary and post-capillary PH. PVR is used to differentiate between patients with post-capillary PH who have a significant pre-capillary component (PVR > 2 mmHg/L/min; combined post- and pre-capillary PH) and those who do not (PVR ≤ 2 mmHg/L/min; isolated post-capillary PH) [1]. However, discrimination between subgroups can be challenging if only resting haemodynamics is available, such as when PAWP is close to 15 mmHg or if the clinical characteristics of the patient primarily suggest heart failure with preserved ejection fraction (HFpEF) despite normal resting PAWP [3]. Therefore, the diagnostic work-up requires the use of provocative manoeuvres (e.g., exercise and fluid challenge) during RHC to elicit a dynamic response of the PAWP that may identify the occult post-capillary PH. The latest guidelines recommend using a PAWP cut-off of >25 mmHg during supine exercise for diagnosing post-capillary PH [9]. Although an increased mPAP/CO slope defines an abnormal haemodynamic response to exercise, it does not allow for differentiation between pre- and post-capillary subgroups. However, the PAWP/CO slope with a threshold > 2 mmHg/L/min may best differentiate between pre- and post-capillary causes of exercise PH [19,20].
While the use of resting RHC is widespread, the exercise RHC is a technically demanding diagnostic method as the exercise causes movement artefacts and is available only in specialised centres.
3.2. Echocardiography
Echocardiography is a non-invasive, inexpensive, and widely available imaging modality for estimating the systolic pulmonary arterial pressure (sPAP) and detecting additional signs suggestive of PH at rest. However, conventional echocardiography alone is insufficient to confirm a diagnosis of PH as the correlation of sPAP by echocardiography compared with sPAP by RHC was modest, with a correlation coefficient of 0.70 (95% CI 0.67 to 0.73) and with the trends toward underestimation of sPAP by echocardiography [21]. Furthermore, the diagnostic accuracy of echocardiography for PH was also modest, with a sensitivity and specificity of around 80% and 70%, respectively [21,22]. There is no good conventional echocardiographic method that can reliably discriminate between pre- and post-capillary PH at rest. Early studies showed only a modest correlation between the echocardiographic Doppler parameter for the estimation of LV filling pressure (E/e′) at rest and invasively measured PAWP [11]. Similar to the concept of exercise RHC, introducing an exercise test to standard Doppler echocardiography might improve the diagnostic accuracy for detecting post-capillary PH. Furthermore, exercise stress echocardiography has an added value in the management of PH patients, as it also allows us to assess complex RV mechanics (e.g., contractile reserve) and the ability of the pulmonary vasculature to accommodate the increased flow.
Ideally, a semi-supine bicycle test or an upright bicycle exercise protocol with imaging is used [11,12], but there are no universally adopted protocols (Table 1). None of these protocols have been shown to be superior to others [9]. The European Association of Cardiovascular Imaging and the American Society of Echocardiography recommend a stepped protocol on a semi-supine bicycle until the patient reaches his maximal predicted workload and/or maximal predicted heart rate (220—age in years) and/or develops limiting symptoms [12]. However, some patients cannot perform that protocol, and a ramped exercise test has also been proposed for a submaximal target heart rate of 100–110/min or until the patient develops limiting symptoms [23]. There is a paucity of data on isometric exercise, which has little or no effect on CO and may change intrathoracic and systemic arterial pressure and systemic vascular resistance. Therefore, isometric exercise might not be suitable for challenging pulmonary circulation [24].

Table 1.
Commonly used exercise stress echocardiography protocols currently employed in clinical practice.
4. Clinical Application of Exercise Stress Echocardiography
4.1. Diagnostic Role
In patients with exertional dyspnoea and suspected PH due to HFpEF, there is a possibility to unmask early stages of LV diastolic dysfunction to detect increased LV filling pressures during exercise and, therefore, to differentiate between pre- and post-capillary PH.
As in resting echocardiography, the increase in the E/e′ ratio during exercise is suggestive of elevated LV filling pressures. However, studies comparing haemodynamic data acquired by echocardiography and by RHC during exercise are limited [3,25]. Even though the E/e′ ratio during exercise had only a moderate correlation with directly invasively measured PAWP (r = 0.57; p < 0.001), adding the peak exercise E/e′ ratio to the ESC proposed algorithm of diastolic dysfunction improved sensitivity (up to 90%) and can be used to rule out post-capillary PH [10]. Using low-level exercise (20 W) seems to be a good alternative, as E/e′ at 20 W could reliably predict normal PAWP during exercise (AUC: 0.77; p < 0.01) [29]. The authors proposed a cut-off of 12.4 for E/e′ at 20 W (specificity 83%, sensitivity 75%). However, these studies comprised only healthy controls and patients with HFpEF. There is only one study that tested the echocardiographic mPAP/CO ratio to identify patients with abnormal pulmonary vascular response to exercise [30]. In a study group of healthy subjects and mainly patients with chronic thromboembolic pulmonary hypertension, mPAP/CO via exercise stress echocardiography of 3.2 mmHg/L/min was identified as the most favourable threshold. Of note, this cut-off is perfectly in line with the proposed cut-off obtained by RHC.
In spite of the above-mentioned data, the diagnostic value of the stress tests during echocardiography to distinguish between PH subtypes is currently uncertain due to the lack of prospective data, especially regarding its use to identify cases of combined post- and pre-capillary PH. Based on the data from the literature, exercise echocardiography is considered abnormal if the average E/e′ ratio at peak stress increases to ≥15, with or without a peak TR velocity >3.4 m/s (Figure 2) [10,11,12]. An increase in TR velocity only should not be used to diagnose post-capillary PH because it might be a normal hyperdynamic response to exercise with increased pulmonary blood flow in the absence of LV diastolic dysfunction [31].
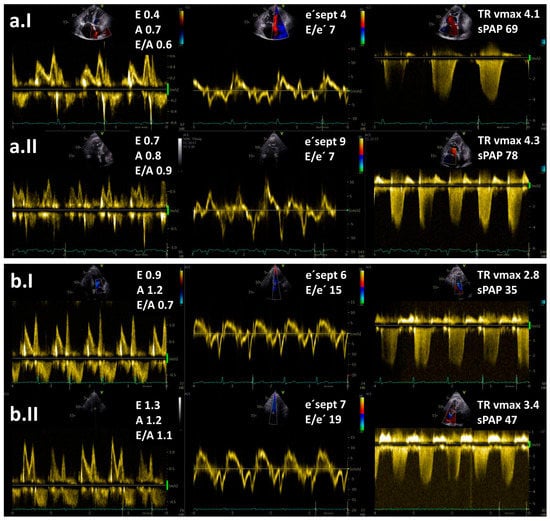
Figure 2.
Resting (I) and exercise stress echocardiography (II) in two patients with pulmonary hypertension (PH): (a) a patient with pre-capillary PH and (b) a patient with post-capillary PH. Maximal tricuspid regurgitation velocity (TR vmax) is elevated in both patients. Note an increase in E/e′ ratio at low-level exercise from 15 to 19 in a patient with post-capillary PH but no increase in a patient with pre-capillary PH. Legend: sPAP—systolic pulmonary arterial pressure.
Exercise stress echocardiography could be extremely useful as an effective gatekeeper to the RHC for patients with exertional dyspnoea of unknown aetiology and normal resting echocardiographic results and also for identifying patients with a high risk for developing PH. It has been demonstrated that exercise stress echocardiography can distinguish between noncardiac and cardiac causes of unexplained dyspnoea [10,32]. The diagnostic value of exercise stress echocardiography was also evaluated in asymptomatic relatives of patients with idiopathic and familial PAH [33]. Hypertensive response to exercise, defined by TR velocity > 3.1 m/s, was more often present in relatives of PAH patients than in control subjects. Additionally, exercise stress echocardiography is considered to be reasonable, especially in patients with connective tissue disease [34]. It has been reported that up to 50% of this patient population with normal resting mPAP had an abnormal increase in mPAP during exercise. In patients with systemic sclerosis, PH was confirmed by RHC in 81% of patients with positive exercise stress echocardiography [35]. Moreover, exercise stress echocardiography could unmask exercise PH in patients with systemic sclerosis and baseline echocardiographic PAP within the grey zone [36]. It is important to note that in both studies, authors used a definition of exercise PH, which is not in line with nowadays valid definition (an increase of 20 mmHg over the resting sPAP or sPAP > 50 mmHg was considered as a positive test result). However, the clinical value of exercise PH identified by exercise stress echocardiography remains uncertain because of the lack of validated criteria and prospective confirmatory data [1]. Therefore, data from exercise stress echocardiography are not sufficient to be a substitute for invasive haemodynamic data under all circumstances, especially if a therapeutic decision depends on the results [9].
4.2. Prognostic Role
Regardless of the underlying aetiology, the developed PH is associated with worsening symptoms and substantially increased mortality [37]. Even though the detection of exercise PH via exercise stress echocardiography is considered an early and mild phase of PAH [38], patients with exercise PH already had worse outcomes than subjects without exercise PH [39].
The survival of PH patients depends on the capability of the RV to adapt to chronically elevated PAP [40]. Over time, adaptive concentric RV hypertrophy with preserved RV function can evolve into RV dilatation and systolic dysfunction [41,42]. RV function is a major determinant of functional capacity and prognosis when RV afterload is elevated [43,44,45]. Echocardiographic measures of RV function that are independent predictors of mortality in PH include the tricuspid annular plane systolic excursion (TAPSE < 18 mm [46,47,48]), RV fractional area change (FAC < 35% [49,50]), peak systolic tricuspid lateral annular velocity (S’ < 9.7 cm/s [51]) and Tei index (>0.40 by pulse Doppler or >0.55 by tissue Doppler [52]). Conventional 2-dimensional echocardiographic evaluation of the RV is difficult due to the complex 3-dimensional (3D) anatomical shape of the RV. This limitation can be overcome with 3D echocardiography and/or cardiac magnetic resonance [53,54] and recently, an increased 3D RVESVi has been shown to correlate with increased mortality [55].
However, these parameters all fail to identify occult RV dysfunction in patients with PH [56] as they reflect already established RV dysfunction. Subtle RV dysfunction could possibly be recognised by the use of advanced echocardiographic techniques, such as strain/myocardial deformation and myocardial work [57,58,59]. Previous studies demonstrated that RV longitudinal strain was a powerful predictor of survival in patients with PH and provided incremental prognostic value over conventional clinical and echocardiographic variables [60,61].
Additionally, the assessment of RV contractile reserve via RV–pulmonary arterial (PA) coupling shows promising results in detecting subclinical RV systolic dysfunction [56,62,63,64,65,66]. Gold standard measurement of RV–PA coupling involves conductance catheter measurement of “multi-beat” RV end-systolic elastance (Ees), a method that remains costly, impractical and clinically challenging [62]. However, new echocardiographic indices, such as the TAPSE/sPAP ratio [45,67,68,69] and RV free wall longitudinal strain/sPAP [59], are tightly linked to RV–PA coupling and are associated with outcomes in patients with PH.
A possible non-invasive measure of the RV contractile reserve using exercise stress echocardiography was first proposed by Grünig et al. They demonstrated that an exercise-induced increase in sPAP was a measure of the RV contractile reserve and was an independent prognostic factor in patients with pre-capillary PH (Table 1) [70]. A lower sPAP increase may reveal an impaired ability of the RV to adapt to pulmonary load and exercise and to further increase pressure and pulmonary blood flow. Similarly, an initial steep increment in PAP during exercise followed by a plateau with a linear pattern was associated with decreased exercise capacity and survival in patients with heart failure [17]. Echocardiographic studies focused only on the peak exercise sPAP or the peak change in sPAP [17,70]; however, it would be preferable to interpret exercise PAP pattern relative to the increase in blood flow (PAP/CO ratio). Invasively obtained haemodynamic data clearly showed that high mPAP/CO during exercise was associated with impaired survival in a heterogeneous group of different PH phenotypes [5,14]. Echocardiographic studies analysing mPAP/CO are limited, but initial results are very promising. A disproportionate increase in mPAP/CO slope during exercise was independently associated with adverse clinical outcomes in patients with HFpEF (Table 2) [71], and this parameter had an incremental value even in patients with preserved RV-PA coupling at rest.
Other authors assessed RV contractile reserve based on echocardiographic parameters of RV systolic function (e.g., change in TAPSE, change in RV FAC and change in S’) (Table 2) [72,73]. The magnitude of the increase in all three parameters was significantly lower in patients with pre-capillary PH than in healthy controls [72,73]; however, no prognostic data have been available for these parameters. Ireland et al. prospectively studied RV contractile reserve in PH patients who underwent cardiac magnetic resonance, echocardiography, and supine invasive cardiopulmonary exercise testing with concomitant RV pressure-volume catheterisation. RV contractile reserve during exercise, measured by Ees during exertion, was associated with an elevation in PAP but the preservation of RV volumes. The lack of RV reserve, on the other hand, was tightly coupled with acute RV dilation during exercise [62]. RV ejection fraction during exercise was shown to be a robust surrogate for RV contractile reserve (Table 2), and it best predicted occult RV dysfunction among a variety of resting and exercise RV measures and was also associated with clinical worsening [62]. Therefore, echocardiographic parameters of RV contractile reserve and exercise stress echocardiography could be useful for follow-up assessment, especially to identify PH patients at high risk [70].

Table 2.
Non-invasive measures of the right ventricle (RV) contractile reserve during exercise stress echocardiography.
Table 2.
Non-invasive measures of the right ventricle (RV) contractile reserve during exercise stress echocardiography.
| Author | Subjects (n) | Echocardiographic Parameters | Most Relevant Findings |
|---|---|---|---|
| Grünig, 2013 [70] | 124 PH patients (PAH, CTEPH) and impaired RV systolic function | ∆sPAP | Exercise-induced sPAP increase ≤ 30 mmHg was related to the worst outcome (HR 2.84, 95% CI 1.92–6.78; p = 0.018). |
| Almeida, 2014 [73] | 14 subjects (7 controls, 7 patients with PH) | ∆S’, ∆TAPSE and ∆FAC | The magnitude of increase in ∆S’, ∆TAPSE and ∆FAC in healthy controls was higher than in patients (all p < 0.05. |
| Guo, 2019 [72] | 46 subjects (31 patients with pre-capillary PH, 15 controls) | ∆S’, ∆TAPSE and ∆FAC | Significant increase in ∆S’ (p = 0.002), ∆TAPSE (p < 0.001) and ∆FAC (p < 0.001) was noted only in healthy controls. |
| Ireland, 2021 [62] | 35 subjects with known or suspected PH | Exercise RVEF | Exercise RVEF can detect occult RV dysfunction (AUC = 0.81, cut off of exercise RVEF = 38%). Patients with exercise RVEF < 38% had an increased propensity for clinical worsening over 4 years compared to patients with RVEF > 38% (p = 0.014). |
| Saito, 2023 [71] | 345 patients (1666 HFpEF, 179 controls) | mPAP/CO slope | Patients with mPAP/CO slope > 5.2 mmHg/L/min had a higher rate of adverse events (all-cause mortality, HF events) compared to those with mPAP/CO slope < 5.2 mmHg/L/min (p = 0.0002). |
Legend: CO—cardiac output, CTEPH—chronic thromboembolic pulmonary hypertension, FAC—fractional area change, HFpEF—heart failure with preserved ejection fraction, mPAP—mean pulmonary arterial pressure, PAH—pulmonary arterial hypertension, PH—pulmonary hypertension, RVEF—right ventricular ejection fraction, sPAP—systolic pulmonary arterial pressure, S’ -Doppler-derived tricuspid lateral annular peak systolic velocity, and TAPSE—tricuspid annular plane systolic excursion.
4.3. Practical Approach to Exercise Stress Echocardiography
Detailed practical guidelines on stress echocardiography have already been published [74]. For the purpose of this review, only some important factors are emphasised:
- In the case of a step protocol, haemodynamic measurements are performed towards the end of each exercise level when a steady state in oxygen consumption on a given exercise level is achieved (usually in 3–5 min). For practical reasons, shorter time intervals can be chosen (e.g., 2 min steps aiming for a duration of the exercise time of∼10 min), which appear to be a good compromise [3].
- The feasibility of obtaining diagnostic-quality measurements of TR velocity decreases with increasing exercise load, with 54% at low exercise (20 W) and 49% at peak exercise [10]. The administration of agitated colloids enhances a continuous Doppler tricuspid regurgitation signal and allows reliable estimation of sPAP during exercise [30].
- At higher heart rates, the fusion of the mitral E and A waves prevents the estimation of the LV filling pressures. It was reported that E/e′ could not be measured in about 10% of subjects during submaximal exercise (20 W) and in about 25% of patients during peak exercise [29]. Therefore, the acquisition of images during the submaximal phase before the fusion of E and A waves is advised (heart rate 100–110 bpm) [75].
- Acquisition during early recovery is not optimal, as haemodynamics change very rapidly after cessation of exercise, and previous invasive studies demonstrated that PAWP returned to the baseline levels already 1 min post-exercise [76].
5. Future Perspectives
Many different protocols of exercise stress echocardiography are used in everyday clinical practice, and further research is needed to compare these protocols and possibly universally adopt one of them. The diagnosis of exercise PH, which was reintroduced in the newest ESC Guidelines for the diagnosis and treatment of PH, is challenging [1]. It requires exercise RHC, which is not readily available. Further research is needed to determine non-invasive cut-offs that would reveal possibly abnormal exercise pulmonary haemodynamics. Moreover, there is no reliable non-invasive method for discrimination between pre-capillary and post-capillary PH in dubious cases. There are some non-invasive stress tests that could be used in such cases to avoid invasive procedures, but the data about their usability are limited. RV dysfunction is the most important determinant of survival in patients with PH [43,44]. However, the manifestations of RV dysfunction not only include changes in global RV systolic function but also abnormalities in the pattern of contraction and synchrony that can be analysed using RV strain and RV myocardial work. The use of these novel parameters for prognostic assessment of PH patients is relatively new and mainly used for research purposes [57,58,59]. Further data are needed to implement them into exercise stress echocardiography in PH patients.
6. Conclusions
Exercise stress echocardiography is a promising non-invasive tool for the assessment of pulmonary haemodynamics, as it provides clinically relevant diagnostic and prognostic information in patients with PH. However, studies comparing echocardiography and RHC data during exercise are limited, and there is no single echocardiographic parameter that reliably identifies the underlying aetiology of PH. Therefore, RHC remains the gold standard method for the diagnosis and classification of different types of PH. In contrast to RHC, echocardiography has the ability to identify RV remodelling and systolic dysfunction, which are important determinants of survival in patients with PH. However, more research is needed since there are still many different protocols of exercise stress echocardiography used in everyday clinical practice, and many new promising echocardiographic parameters for the prognostic assessment of PH patients are emerging.
Author Contributions
Conception and design of the manuscript, or both (M.Š. and M.C.); Manuscript drafting or critical revision for important intellectual content (M.Š., J.A., J.T. and M.C.); Final approval of the manuscript submitted (M.Š., J.A., J.T. and M.C.). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef]
- Kovacs, G.; Herve, P.; Barbera, J.A.; Chaouat, A.; Chemla, D.; Condliffe, R.; Garcia, G.; Grünig, E.; Howard, L.; Humbert, M.; et al. An official European Respiratory Society statement: Pulmonary haemodynamics during exercise. Eur. Respir. J. 2017, 50, 1700578. [Google Scholar] [CrossRef]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef]
- Zeder, K.; Banfi, C.; Steinrisser-Allex, G.; Maron, B.A.; Humbert, M.; Lewis, G.D.; Berghold, A.; Olschewski, H.; Kovacs, G. Diagnostic, prognostic and differential-diagnostic relevance of pulmonary haemodynamic parameters during exercise: A systematic review. Eur. Respir. J. 2022, 60, 2103181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Bossone, E.; Naeije, R.; Grünig, E.; Saggar, R.; Lancellotti, P.; Ghio, S.; Varga, J.; Rajagopalan, S.; Oudiz, R.J.; et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013, 128, 1470–1479. [Google Scholar] [CrossRef]
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.-L.; Lewis, G.D. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am. J. Respir. Crit. Care Med. 2013, 178, 576–583. [Google Scholar] [CrossRef]
- Wolsk, E.; Bakkestrøm, R.; Thomsen, J.H.; Balling, L.; Andersen, M.J.; Dahl, J.S.; Hassager, C.; Møller, J.E.; Gustafsson, F. The Influence of Age on Hemodynamic Parameters During Rest and Exercise in Healthy Individuals. JACC Heart Fail. 2017, 5, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, A.R.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.; Claessen, G.; La Gerche, A.; Petit, T.; Belge, C.; Meyns, B.; Bogaert, J.; Willems, R.; Claus, P.; Delcroix, M. Impaired Cardiac Reserve and Abnormal Vascular Load Limit Exercise Capacity in Chronic Thromboembolic Disease. JACC Cardiovasc. Imaging 2019, 12, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Zern, E.K.; Lau, E.S.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Farrell, R.; Sbarbaro, J.A.; et al. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients With Dyspnea on Effort. J. Am. Coll. Cardiol. 2020, 75, 17–26. [Google Scholar] [CrossRef]
- Stamm, A.; Saxer, S.; Lichtblau, M.; Hasler, E.D.; Jordan, S.; Huber, L.C.; Bloch, K.E.; Distler, O.; Ulrich, S. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur. Respir. J. 2016, 48, 1658–1667. [Google Scholar] [CrossRef]
- Zeder, K.; Avian, A.; Bachmaier, G.; Douschan, P.; Foris, V.; Sassmann, T.; Moazedi-Fuerst, F.C.; Graninger, W.B.; Hafner, F.; Brodmann, M.; et al. Exercise Pulmonary Resistances Predict Long-Term Survival in Systemic Sclerosis. Chest 2021, 159, 781–790. [Google Scholar] [CrossRef]
- Lewis, G.D.; Murphy, R.M.; Shah, R.V.; Pappagianopoulos, P.P.; Malhotra, R.; Bloch, K.D.; Systrom, D.M.; Semigran, M.J. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ. Heart Fail. 2011, 4, 276–285. [Google Scholar] [CrossRef]
- Hasler, E.D.; Müller-Mottet, S.; Furian, M.; Saxer, S.; Huber, L.C.; Maggiorini, M.; Speich, R.; Bloch, K.E.; Ulrich, S. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest 2016, 150, 57–67. [Google Scholar] [CrossRef]
- Bentley, R.F.; Barker, M.; Esfandiari, S.; Wright, S.P.; Valle, F.H.; Granton, J.T.; Mak, S. Normal and abnormal relationships of pulmonary artery to wedge pressure during exercise. J. Am. Heart Assoc. 2020, 9, e016339. [Google Scholar] [CrossRef]
- Eisman, A.S.; Shah, R.V.; Dhakal, B.P.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.; Cunningham, T.F.; Hardin, K.M.; Baggish, A.L.; Ho, J.E.; et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ. Heart Fail. 2018, 11, e004750. [Google Scholar] [CrossRef]
- Janda, S.; Shahidi, N.; Gin, K.; Swiston, J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart 2011, 97, 612–622. [Google Scholar] [CrossRef]
- Ni, J.-R.; Yan, P.-J.; Liu, S.-D.; Hu, Y.; Yang, K.-H.; Song, B.; Lei, J.-Q. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: A systematic review and meta-analysis. BMJ Open 2019, 9, e033084. [Google Scholar] [CrossRef]
- Erdei, T.; Smiseth, O.A.; Marino, P.; Fraser, A.G. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU-FP7 MEDIA study group. Eur. J. Heart Fail. 2014, 16, 1345–1361. [Google Scholar] [CrossRef]
- Motoji, Y.; Forton, K.; Pezzuto, B.; Faoro, V.; Naeije, R. Resistive or dynamic exercise stress testing of the pulmonary circulation and the right heart. Eur. Respir. J. 2017, 50, 1700151. [Google Scholar] [CrossRef]
- Ha, J.W.; Andersen, O.S.; Smiseth, O.A. Diastolic Stress Test: Invasive and Noninvasive Testing. JACC Cardiovasc. Imaging 2020, 13, 272–282. [Google Scholar] [CrossRef]
- Mottram, P.M.; Haluska, B.A.; Marwick, T.H. Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: Correlation with cardiac function, hemodynamics, and workload. Am. Heart J. 2004, 148, 365–370. [Google Scholar] [CrossRef]
- Samuel, T.J.; Beaudry, R.; Haykowsky, M.J.; Sarma, S.; Park, S.; Dombrowsky, T.; Bhella, P.S.; Nelson, M.D. Isometric handgrip echocardiography: A noninvasive stress test to assess left ventricular diastolic function. Clin. Cardiol. 2017, 40, 1247–1255. [Google Scholar] [CrossRef]
- Pongpaopattanakul, P.; Imerbtham, T.; Kitimala, J.; Phatthanawayu, S.; Kaewkong, P. Effects of a dynamic handgrip exercise on left ventricular diastolic functions in diabetes mellitus patients: A preliminary clinical data. Chula. Med. J. 2022, 66, 283–291. [Google Scholar] [CrossRef]
- Effects of a dynamic handgrip exercise on left ventricular diastolic functions in diabetes mellitus patients: A preliminary clinical data Utility of E/e′ Ratio During Low-Level Exercise to Diagnose Heart Failure With Preserved. JACC Cardiovasc. Imaging 2023, 16, 145–155. [CrossRef] [PubMed]
- Claessen, G.; La Gerche, A.; Voigt, J.-U.; Dymarkowski, S.; Schnell, F.; Petit, T.; Willems, R.; Claus, P.; Delcroix, M.; Heidbuchel, H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc. Imaging 2016, 9, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Chang, S.M.; Nabi, F.; Shah, D.J.; Estep, J.D. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc. Imaging 2017, 10, e006547. [Google Scholar] [CrossRef]
- Talreja, D.R.; Nishimura, R.A.; Oh, J.K. Estimation of Left Ventricular Filling Pressure with Exercise by Doppler Echocardiography in Patients with Normal Systolic Function: A Simultaneous Echocardiographic-Cardiac Catheterization Study. J. Am. Soc. Echocardiogr. 2007, 20, 477–479. [Google Scholar] [CrossRef]
- Grünig, E.; Weissmann, S.; Ehlken, N.; Fijalkowska, A.; Fischer, C.; Fourme, T.; Galié, N.; Ghofrani, A.; Harrison, R.E.; Huez, S.; et al. Stress doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension results of a multicenter european analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009, 119, 1747–1757. [Google Scholar] [CrossRef]
- Shaikh, F.; Anklesaria, Z.; Shagroni, T.; Saggar, R.; Gargani, L.; Bossone, E.; Ryan, M.; Channick, R.; Saggar, R. A review of exercise pulmonary hypertension in systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 225–237. [Google Scholar] [CrossRef]
- Steen, V.; Chou, M.; Shanmugam, V.; Mathias, M.; Kuru, T.; Morrissey, R. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008, 134, 146–151. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Papangelopoulou, K.; Anthi, A.; Tsangaris, I.; Varounis, C.; Makavos, G.; Konstantonis, D.; Vlachoyiannopoulos, P.; Orfanos, S.E.; Iliodromitis, E.K. The role of exercise doppler echocardiography to unmask pulmonary arterial hypertension in selected patients with systemic sclerosis and equivocal baseline echocardiographic values for pulmonary hypertension. Diagnostics 2021, 11, 1200. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Tolle, J.J.; Waxman, A.B.; Van Horn, T.L.; Pappagianopoulos, P.P.; Systrom, D.M. Exercise-induced pulmonary arterial hypertension. Circulation 2008, 118, 2183–2189. [Google Scholar] [CrossRef]
- Shim, C.Y.; Kim, S.-A.; Choi, D.; Yang, W.-I.; Kim, J.-M.; Moon, S.-H.; Lee, H.-J.; Park, S.; Choi, E.-Y.; Chung, N.; et al. Clinical outcomes of exercise-induced pulmonary hypertension in subjects with preserved left ventricular ejection fraction: Implication of an increase in left ventricular filling pressure during exercise. Heart 2011, 97, 1417–1424. [Google Scholar] [CrossRef]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right Heart Adaptation to Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; et al. A report of the american college of cardiology foundation task force on expert consensus documents and the american heart association. Circulation 2009, 119, 2250–2294. [Google Scholar] [CrossRef]
- D’Alonzo, G.E. Survival in Patients with Primary Pulmonary Hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef]
- Burke, M.A.; Katz, D.H.; Beussink, L.; Selvaraj, S.; Gupta, D.K.; Fox, J.; Chakrabarti, S.; Sauer, A.J.; Rich, J.D.; Freed, B.H.; et al. Prognostic Importance of Pathophysiologic Markers in Patients With Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 288–299. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, I.; AbouEzzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right Ventricular Function in Heart Failure With Preserved Ejection Fraction. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef]
- Mauritz, G.-J.; Kind, T.; Marcus, J.T.; Bogaard, H.-J.; van de Veerdonk, M.; Postmus, P.E.; Boonstra, A.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive Changes in Right Ventricular Geometric Shortening and Long-term Survival in Pulmonary Arterial Hypertension. Chest 2012, 141, 935–943. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef]
- Champion, H.C.; Michelakis, E.D.; Hassoun, P.M. Comprehensive Invasive and Noninvasive Approach to the Right Ventricle–Pulmonary Circulation Unit. Circulation 2009, 120, 992–1007. [Google Scholar] [CrossRef]
- Yeo, T.C.; Dujardin, K.S.; Tei, C.; Mahoney, D.W.; McGoon, M.D.; Seward, J.B. Value of a Doppler-Derived Index Combining Systolic and Diastolic Time Intervals in Predicting Outcome in Primary Pulmonary Hypertension. Am. J. Cardiol. 1998, 81, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Conroy, J.; Narula, J. Imaging of the Right Ventricle. Cardiol Clin. 2012, 30, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Bhave, N.M.; Tabit, C.E.; Gomberg-Maitland, M.; Freed, B.H.; Dill, K.E.; Lang, R.M.; Mor-Avi, V.; Patel, A.R. Sample Size and Cost Analysis for Pulmonary Arterial Hypertension Drug Trials Using Various Imaging Modalities to Assess Right Ventricular Size and Function End Points. Circ. Cardiovasc. Imaging 2014, 7, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ryo, K.; Goda, A.; Onishi, T.; Delgado-Montero, A.; Tayal, B.; Champion, H.C.; Simon, M.A.; Mathier, M.A.; Gladwin, M.T.; Gorcsan, J., 3rd. Characterization of Right Ventricular Remodeling in Pulmonary Hypertension Associated With Patient Outcomes by 3-Dimensional Wall Motion Tracking Echocardiography. Circ. Cardiovasc. Imaging 2015, 8, e003176. [Google Scholar] [CrossRef]
- Richter, M.J.; Peters, D.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Sommer, N.; Gall, H.; Grimminger, F.; Seeger, W.; Tello, K. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 116–119. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’antonio, A.; Ambrosio, G. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: Superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; Van Der Bijl, P.; Van Der Velde, E.T.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef]
- Ünlü, S.; Bézy, S.; Cvijic, M.; Duchenne, J.; Delcroix, M.; Voigt, J.-U. Right ventricular strain related to pulmonary artery pressure predicts clinical outcome in patients with pulmonary arterial hypertension. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 635–642. [Google Scholar] [CrossRef]
- Haeck, M.L.; Scherptong, R.W.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Vliegen, H.W.; Delgado, V. Prognostic Value of Right Ventricular Longitudinal Peak Systolic Strain in Patients With Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2012, 5, 628–636. [Google Scholar] [CrossRef]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome Prediction by Quantitative Right Ventricular Function Assessment in 575 Subjects Evaluated for Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ireland, C.G.; Damico, R.L.; Kolb, T.M.; Mathai, S.C.; Mukherjee, M.; Zimmerman, S.L.; Shah, A.A.; Wigley, F.M.; Houston, B.A.; Hassoun, P.M.; et al. Exercise right ventricular ejection fraction predicts right ventricular contractile reserve. J. Heart Lung. Transplant. 2021, 40, 504–512. [Google Scholar] [CrossRef]
- Hsu, S.; Houston, B.A.; Tampakakis, E.; Bacher, A.C.; Rhodes, P.S.; Mathai, S.C.; Damico, R.L.; Kolb, T.M.; Hummers, L.K.; Shah, A.A.; et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016, 133, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Kokkonen-Simon, K.M.; Kirk, J.A.; Kolb, T.M.; Damico, R.L.; Mathai, S.C.; Mukherjee, M.; Shah, A.A.; Wigley, F.M.; Margulies, K.B.; et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation 2018, 137, 2360–2370. [Google Scholar] [CrossRef]
- Tedford, R.J.; Mudd, J.O.; Girgis, R.E.; Mathai, S.C.; Zaiman, A.L.; Housten-Harris, T.; Boyce, D.; Kelemen, B.W.; Bacher, A.C.; Shah, A.A.; et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 953–963. [Google Scholar] [CrossRef]
- Rommel, K.-P.; von Roeder, M.; Oberueck, C.; Latuscynski, K.; Besler, C.; Blazek, S.; Stiermaier, T.; Fengler, K.; Adams, V.; Sandri, M.; et al. Load-independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure–volume loops. Circ. Heart Fail. 2018, 11, e004121. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Huston, J.H.; Maron, B.A.; French, J.; Huang, S.; Thayer, T.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; Brittain, E.L. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol. 2019, 4, 1112–1121. [Google Scholar] [CrossRef]
- Grünig, E.; Tiede, H.; Enyimayew, E.O.; Ehlken, N.; Seyfarth, H.-J.; Bossone, E.; D’andrea, A.; Naeije, R.; Olschewski, H.; Ulrich, S.; et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013, 128, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Obokata, M.; Harada, T.; Kagami, K.; Sorimachi, H.; Yuasa, N.; Kato, T.; Wada, N.; Okumura, Y.; Ishii, H. Disproportionate exercise-induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: A non-invasive echocardiographic study. Eur. J. Heart Fail. 2023, Online ahead of print. [CrossRef] [PubMed]
- Guo, D.-C.; Li, Y.-D.; Yang, Y.-H.; Zhu, W.-W.; Sun, L.-L.; Jiang, W.; Ye, X.; Cai, Q.-Z.; Lu, X.-Z.; Ye, X.-G. Influence of impaired right ventricular contractile reserve on exercise capacity in patients with precapillary pulmonary hypertension: A study with exercise stress echocardiography. Echocardiography 2019, 36, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Loureiro, M.J.; Lopes, L.; Cotrim, C.; Lopes, L.; Repolho, D.; Pereira, H. Echocardiographic assessment of right ventricular contractile reserve in patients with pulmonary hypertension. Rev. Port. Cardiol. 2014, 33, 155–163. [Google Scholar] [CrossRef]
- Suzuki, K.; Hirano, Y.; Yamada, H.; Murata, M.; Daimon, M.; Takeuchi, M.; Seo, Y.; Izumi, C.; Akaishi, M. Practical guidance for the implementation of stress echocardiography. J. Echocardiogr. 2018, 16, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.; Redfield, M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).