Serum Neurofilaments in Motor Neuron Disease and Their Utility in Differentiating ALS, PMA and PLS
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. Participants and Clinical Parameters
2.4. NFL and NFH Quantification
2.5. Statistical Methods
3. Results
3.1. Patient Characteristics
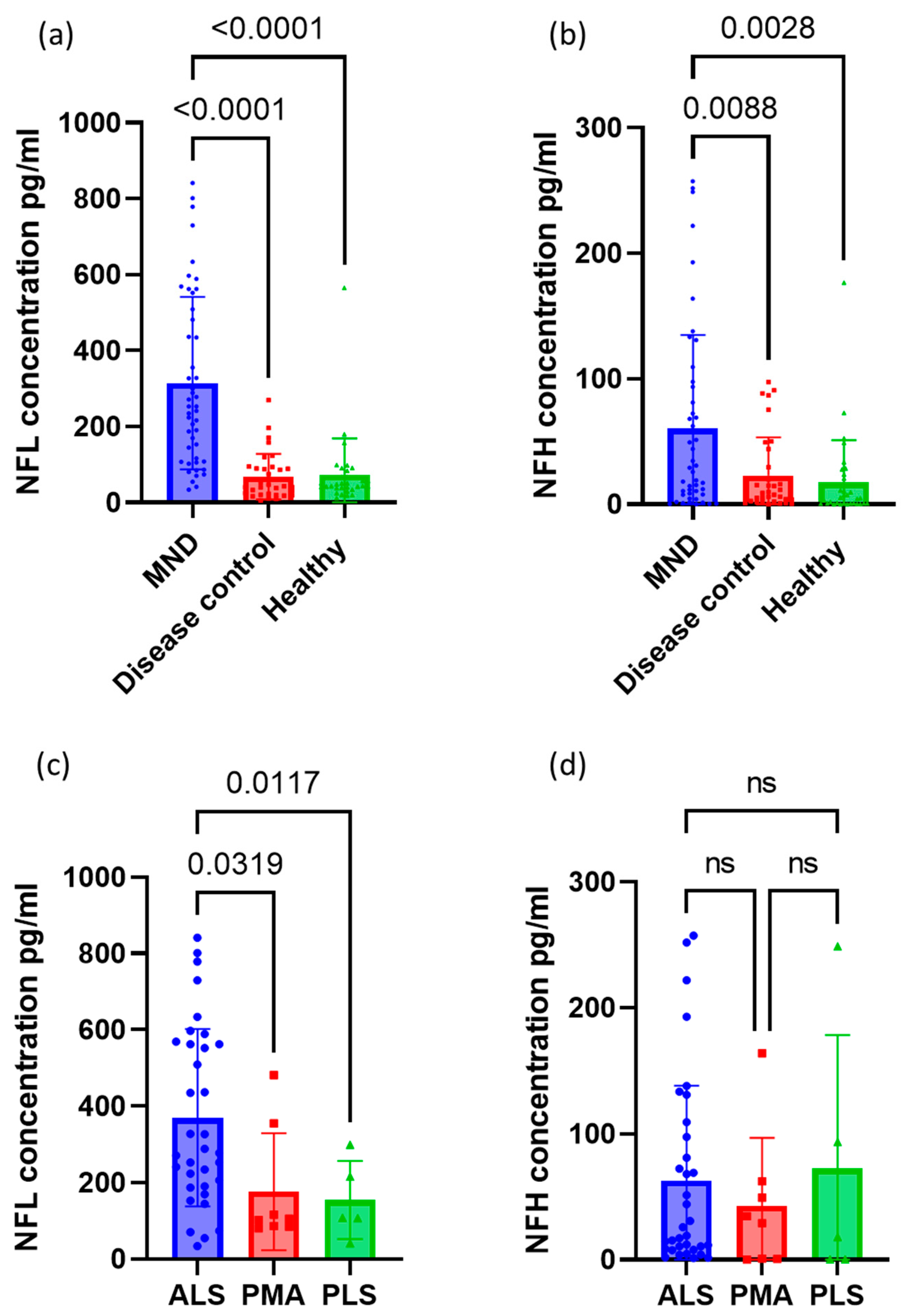
3.2. Neurofilament Concentrations between Groups
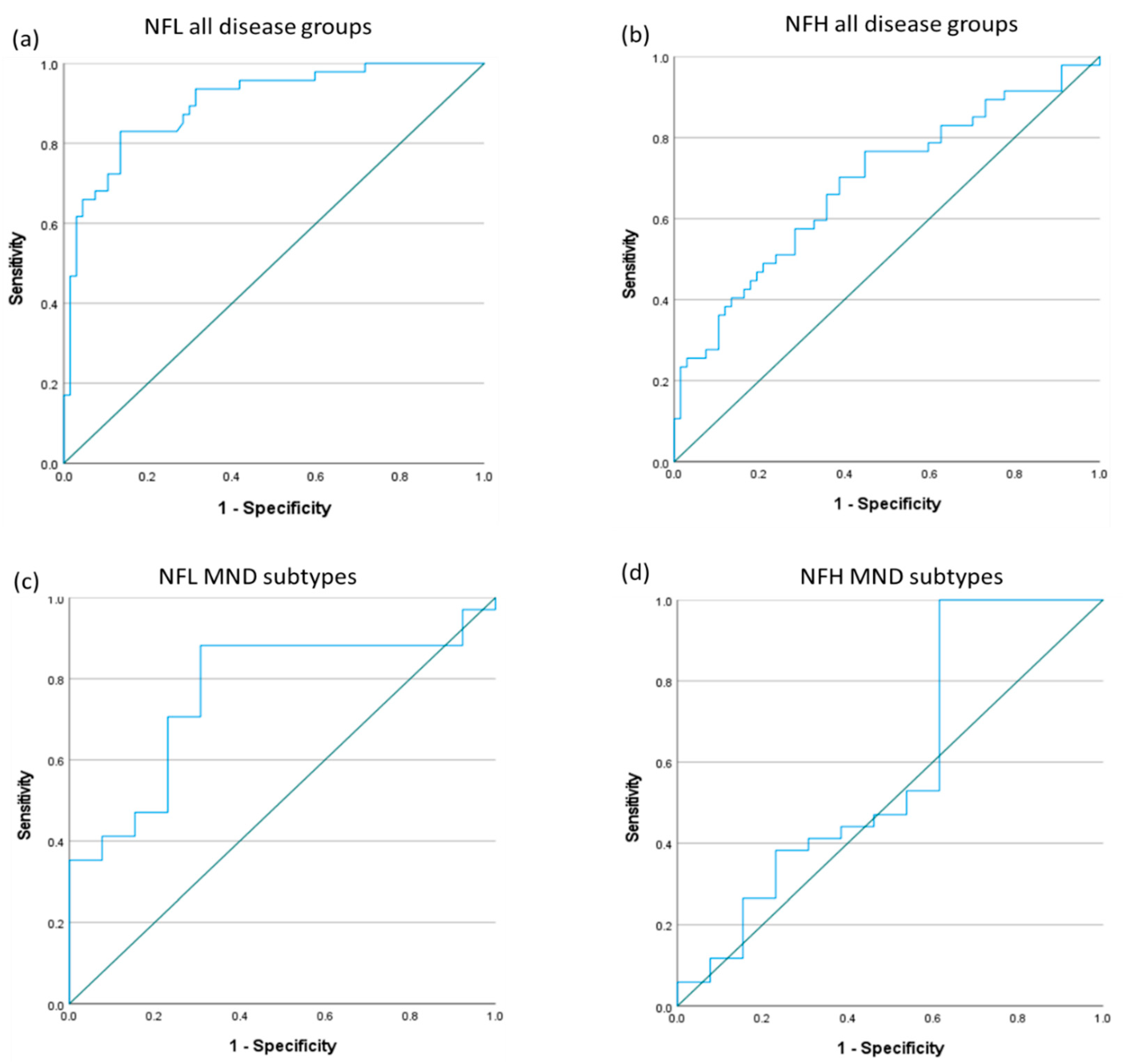
3.3. Receiver Operating Characteristics for Neurofilaments
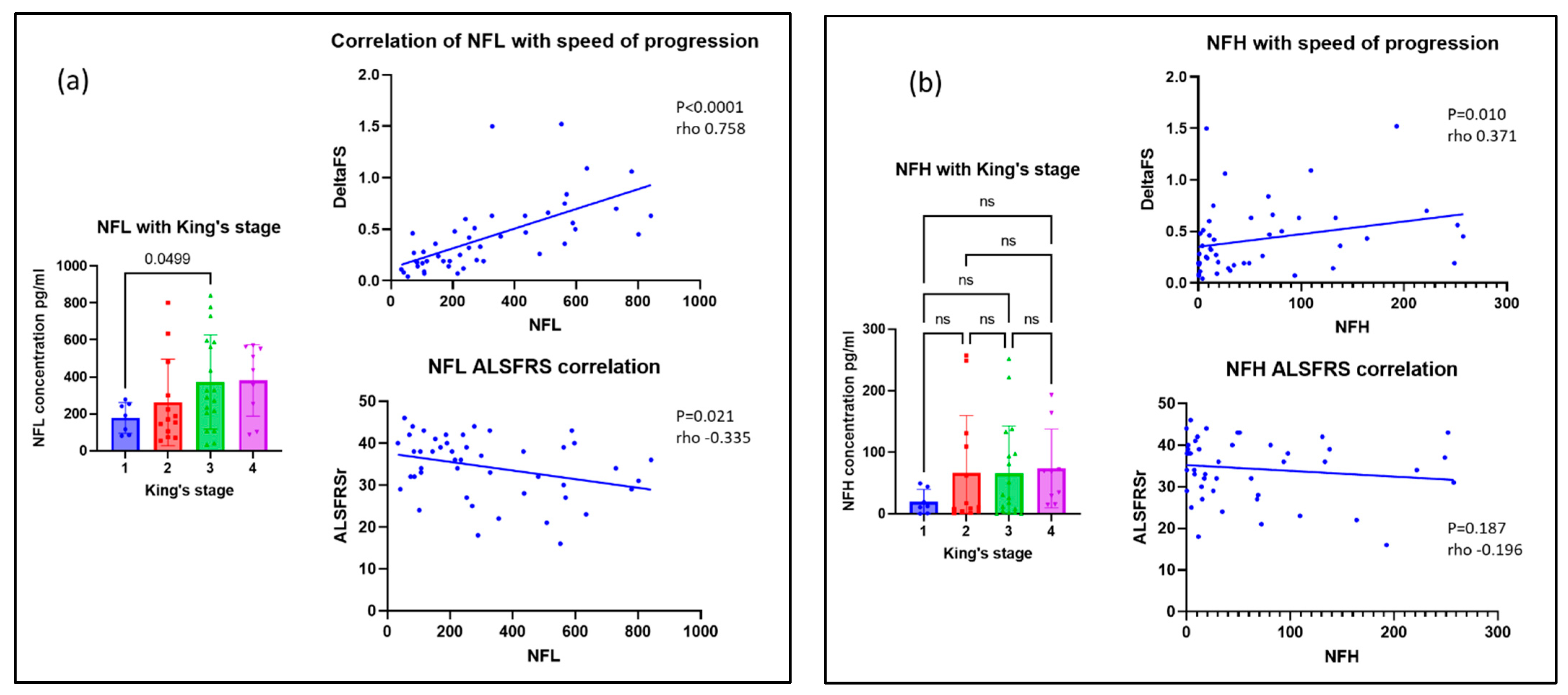
3.4. Neurofilaments for Monitoring Disease Progression in MND
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiò, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 5–6, 310–323. [Google Scholar] [CrossRef]
- Tiryaki, E.; Horak, H.A. ALS and other motor neuron diseases. Contin. Lifelong Learn. Neurol. 2014, 20, 1185–1207. [Google Scholar] [CrossRef]
- Ince, P.G.; Evans, J.; Knopp, M.; Forster, G.; Hamdalla, H.H.M.; Wharton, S.B.; Shaw, P. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003, 60, 1252–1258. [Google Scholar] [CrossRef]
- Kim, W.K.; Liu, X.; Sandner, J.; Pasmantier, M.; Andrews, J.; Rowland, L.P.; Mitsumoto, H. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology 2009, 73, 1686–1692. [Google Scholar] [CrossRef]
- De Vries, B.S.; Rustemeijer, L.M.M.; Bakker, L.A.; Schröder, C.D.; Veldink, J.H.; van den Berg, L.H.; Nijboer, T.C.W.; Van Es, M.A. Cognitive and behavioural changes in PLS and PMA:challenging the concept of restricted phenotypes. J. Neurol. Neurosurg. Psychiatry 2018, 90, 141–147. [Google Scholar] [CrossRef]
- Gordon, P.H.; Cheng, B.; Katz, I.B.; Pinto, M.; Hays, A.P.; Mitsumoto, H.; Rowland, L.P. The natural history of primary lateral sclerosis. Neurology 2006, 66, 647–653. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, G.; Duddy, W.; Haffey, S.; Morrison, K.; Donaghy, C.; Duguez, S. Epidemiology and survival trends of motor neurone disease in Northern Ireland from 2015 to 2019. Eur. J. Neurol. 2021, 29, 707–714. [Google Scholar] [CrossRef]
- Turner, M.R.; Barohn, R.J.; Corcia, P.; Fink, J.K.; Harms, M.B.; Kiernan, M.C.; Ravits, J.; Silani, V.; Simmons, Z.; Statland, J.; et al. Primary lateral sclerosis: Consensus diagnostic criteria. J. Neurol. Neurosurg. Psychiatry 2020, 91, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Verber, N.S.; Shepheard, S.R.; Sassani, M.; McDonough, H.E.; Moore, S.A.; Alix, J.J.; Shaw, P.J. Biomarkers in Motor Neuron Disease: A State of the Art Review. Front. Neurol. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, U.G.; Milla, V.; Cynthia Stafford, M.Y.; Bjourson, A.J.; Duddy, W.; Duguez, S.M.-R. A Systematic Review of Suggested Molecular Strata, Biomarkers and Their Tissue Sources in ALS. Front. Neurol. 2019, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Berg, L.H.v.D.; Sorenson, E.; Gronseth, G.; Macklin, E.A.; Andrews, J.; Baloh, R.H.; Benatar, M.; Berry, J.D.; Chio, A.; Corcia, P.; et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology 2019, 92, e1610–e1623. [Google Scholar] [CrossRef]
- Zucchi, E.; Bonetto, V.; Sorarù, G.; Martinelli, I.; Parchi, P.; Liguori, R.; Mandrioli, J. Neurofilaments in motor neuron disorders: Towards promising diagnostic and prognostic biomarkers. Mol. Neurodegener. 2020, 15, 58–63. [Google Scholar] [CrossRef]
- Gordon, B.A. Neurofilaments in disease: What do we know? Curr. Opin. Neurobiol. 2020, 61, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Alagaratnam, J.; von Widekind, S.; De Francesco, D.; Underwood, J.; Edison, P.; Winston, A.; Zetterberg, H.; Fidler, S. Correlation between CSF and blood neurofilament light chain protein: A systematic review and meta-analysis. BMJ Neurol. Open 2021, 3, e000143. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef]
- Kouchakim, E.; Dashti, F.; Mirazimi, S.M.A.; Alirezaei, Z.; Jafari, S.H.; Hamblin, M.R.; Mirzaei, H. Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J. 2021, 20, 1308–1325. [Google Scholar]
- Zhao, Y.; Xin, Y.; Meng, S.; He, Z.; Hu, W. Neurofilament light chain protein in neurodegenerative dementia: A systematic review and network meta-analysis. Neurosci. Biobehav. Rev. 2019, 102, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef] [PubMed]
- Poesen, K.; Van Damme, P. Diagnostic and Prognostic Performance of Neurofilaments in ALS. Front. Neurol. 2019, 9, 1167. [Google Scholar] [CrossRef]
- Gagliardi, D.; Meneri, M.; Saccomanno, D.; Bresolin, N.; Comi, G.P.; Corti, S. Diagnostic and Prognostic Role of Blood and Cerebrospinal Fluid and Blood Neurofilaments in Amyotrophic Lateral Sclerosis: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 4152. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate–Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Zhang, L.; Wang, L.; Granit, V.; Statland, J.; Barohn, R.; Swenson, A.; Ravits, J.; Jackson, C.; Burns, T.M.; et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 2020, 95, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Feneberg, E.; Weishaupt, J.; Brettschneider, J.; Tumani, H.; Andersen, P.M.; Von Arnim, C.A.F.; Böhm, S.; Kassubek, J.; Kubisch, C.; et al. Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 2015, 87, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, A.; Martinelli, I.; Bello, L.; Querin, G.; Puthenparampil, M.; Ruggero, S.; Sorarù, G. Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis: Neurofilament Light Chain Levels in Definite Subtypes of Disease. JAMA Neurol. 2017, 74, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Gille, B.; De Schaepdryver, M.; Goossens, J.; Dedeene, L.; De Vocht, J.; Oldoni, E.; Goris, A.; Bosch, L.V.D.; Depreitere, B.; Claeys, K.G.; et al. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathol. Appl. Neurobiol. 2018, 45, 291–304. [Google Scholar] [CrossRef]
- Barceló, M.A.; Povedano, M.; Vázquez-Costa, J.F.; Franquet, Á.; Solans, M.; Saez, M. Estimation of the prevalence and incidence of motor neuron diseases in two Spanish regions: Catalonia and Valencia. Sci. Rep. 2021, 11, 6207. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; BDNF Study Group. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Balendra, R.; Al Khleifat, A.; Fang, T.; Al-Chalabi, A. A standard operating procedure for King’s ALS clinical staging. Amyotroph Lateral Scler Front. Degener 2019, 20, 159–164. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- van Rheenen, W.; van Blitterswijk, M.; Huisman, M.H.B.; Vlam, L.; van Doormaal, P.T.C.; Seelen, M.; Van Den Berg, L.H. Hexanucleotide repeat expansions in C9ORF72 in the spectrum of motor neuron diseases. Neurology 2012, 79, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Nagy, P.L.; Gennings, C.; Murphy, J.; Andrews, H.; Goetz, R.; Floeter, M.K.; Hupf, J.; Singleton, J.; Barohn, R.J.; et al. Phenotypic and molecular analyses of primary lateral sclerosis. Neurol. Genet. 2015, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Liewluck, T.; Saperstein, D.S. Progressive Muscular Atrophy. Neurol. Clin. 2015, 33, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; de Jong JM BVianney de Visser, M. The history of progressive muscular atrophy: Syndrome or disease? Neurology 2008, 70, 723–727. [Google Scholar] [CrossRef]
- van Blitterswijk, M.; Vlam, L.; van Es, M.A.; van der Pol, W.-L.; Hennekam, E.A.M.; Dooijes, D.; Schelhaas, H.J.; van der Kooi, A.J.; de Visser, M.; Veldink, J.H.; et al. Genetic overlap between apparently sporadic motor neuron diseases. PLoS ONE 2012, 7, e48983. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Monaghan, T.F.; Rahman, S.N.; Agudelo, C.W.; Wein, A.J.; Lazar, J.M.; Everaert, K.; Dmochowski, R.R. Foundational Statistical Principles in Medical Research: Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value. Medicina 2021, 57, 503. [Google Scholar] [CrossRef]
- Abdelhak, A.; Kuhle, J.; Green, A.J. Challenges and Opportunities for the Promising Biomarker Blood Neurofilament Light Chain. JAMA Neurol. 2023. [Google Scholar] [CrossRef]
- Thompson, A.G.; Gray, E.; Verber, N.; Bobeva, Y.; Lombardi, V.; Shepheard, S.R.; Yildiz, O.; Feneberg, E.; Farrimond, L.; Dharmadasa, T.; et al. Multicentre appraisal of amyotrophic lateral sclerosis biofluid biomarkers shows primacy of blood neurofilament light chain. Brain Commun. 2022, 4, fcac029. [Google Scholar] [CrossRef]
- van Eenennaam, R.M.; Kruithof, W.J.; van Es, M.A.; Kruitwagen-van Reenen, E.T.; Westeneng, H.; Visser-Meily, J.M.A.; van den Berg, L.H.; Beelen, A. Discussing personalized prognosis in amyotrophic lateral sclerosis: Development of a communication guide. BMC Neurol. 2020, 20, 446–448. [Google Scholar] [CrossRef] [PubMed]
- van Eenennaam, R.M.; Koppenol, L.S.; Kruithof, W.J.; Reenen, E.T.K.-V.; Pieters, S.; van Es, M.A.; Berg, L.H.v.D.; Visser-Meily, J.M.A.; Beelen, A. Discussing Personalized Prognosis Empowers Patients with Amyotrophic Lateral Sclerosis to Regain Control over Their Future: A Qualitative Study. Brain Sci. 2021, 11, 1597. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Petrillo, M.; Spellman, R.; Garner, R.; Zhang, R.; Kiefer, M.; Simeone, S.; Sohn, J.; Eichelberger, E.J.; Rodrigues, E.; et al. Implications of circulating neurofilaments for spinal muscular atrophy treatment early in life: A case series. Mol. Ther. Methods Clin. Dev. 2021, 23, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Crawford, T.O.; Finkel, R.S.; Mercuri, E.; De Vivo, D.C.; Oskoui, M.; Tizzano, E.F.; Ryan, M.M.; Muntoni, F.; Zhao, G.; et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 932–944. [Google Scholar] [CrossRef]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Masullo, M.; Quadalti, C.; Avoni, P.; Polischi, B.; Cherici, A.; Capellari, S.; Salvi, F.; et al. Plasma and CSF Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: A Cross-Sectional and Longitudinal Study. Front. Aging Neurosci. 2021, 13, 753242. [Google Scholar] [CrossRef]
- Gendron, T.F.; Daughrity, L.M.; Heckman, M.G.; Diehl, N.N.; Wuu, J.; Miller, T.M.; Pastor, P.; Trojanowski, J.Q.; Grossman, M.; Berry, J.D.; et al. Phosphorylated neurofilament heavy chain: A biomarker of survival for C9ORF 72 -associated amyotrophic lateral sclerosis. Ann. Neurol. 2017, 82, 139–146. [Google Scholar] [CrossRef]
- Rich, K.A.; Fox, A.; Yalvac, M.; Heintzman, S.; Tellez, M.; Bartlett, A.; Severyn, S.; Linsenmayer, M.; Kelly, K.; Reynolds, J.; et al. Neurofilament Levels in CSF and Serum in an Adult SMA Cohort Treated with Nusinersen. J. Neuromuscul. Dis. 2022, 9, 111–119. [Google Scholar] [CrossRef]
- Lombardi, V.; Querin, G.; Ziff, O.J.; Zampedri, L.; Martinelli, I.; Heller, C.; Foiani, M.; Bertolin, C.; Lu, C.-H.; Malik, B.; et al. Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology 2019, 92, e1205–e1211. [Google Scholar] [CrossRef]
- Lombardi, V.; Bombaci, A.; Zampedri, L.; Lu, C.; Malik, B.; Zetterberg, H.; Heslegrave, A.J.; Rinaldi, C.; Greensmith, L.; Hanna, M.G.; et al. Plasma pNfH levels differentiate SBMA from ALS. J. Neurol. Neurosurg Psychiatry 2020, 91, 215–217. [Google Scholar] [CrossRef]
- Millere, E.; Rots, D.; Glazere, I.; Taurina, G.; Kurjane, N.; Priedite, V.; Gailite, L.; Blennow, K.; Zetterberg, H.; Kenina, V. Clinical Phenotyping and Biomarkers in Spinal and Bulbar Muscular Atrophy. Front. Neurol. 2021, 11, 586610. [Google Scholar] [CrossRef]
- Sandelius, Å.; Zetterberg, H.; Blennow, K.; Adiutori, R.; Malaspina, A.; Laura, M.; Reilly, M.M.; Rossor, A.M. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 2018, 90, e518–e524. [Google Scholar] [CrossRef] [PubMed]
- Bischof, A.; Manigold, T.; Barro, C.; Heijnen, I.; Berger, C.T.; Derfuss, T.; Kuhle, J.; Daikeler, T. Serum neurofilament light chain: A biomarker of neuronal injury in vasculitic neuropathy. Ann. Rheum. Dis. 2017, 77, 1093–1094. [Google Scholar] [CrossRef] [PubMed]
| MND | Disease Controls | Healthy Controls | p Value | ||
|---|---|---|---|---|---|
| Number of patients | 47 | 34 | 33 | ||
| Gender | Male (%) | 28 (59.6) | 22 (64.7) | 13 (39.4) | 0.085 |
| Female (%) | 19 (40.4) | 12 (35.3) | 20 (60.6) | ||
| Median age at sampling years (IQR) | 65 (58, 75) | 56 (40.8, 66.3) | 59 (51, 68.5) | <0.001 | |
| Disease subcategories | 34 ALS | 8 Myopathies | |||
| 8 PMA | 7 SMA | ||||
| 5 PLS | 6 Neuropathies | ||||
| 6 PD | |||||
| 5 SBMA | |||||
| 2 MG | |||||
| ALS | PMA | PLS | p Value | |||
|---|---|---|---|---|---|---|
| Number | 34 | 8 | 5 | |||
| Median age at sampling years(IQR) | 64 (56.8, 75.3) | 68 (64,74.3) | 67 (57.5, 78) | 0.683 | ||
| Gender | Male (%) | 18 (52.9) | 8 (100) | 2 (40) | 0.033 | |
| Female (%) | 16 (47.1) | 0 (0) | 3 (60) | |||
| Site of onset | Bulbar (%) | 8 (23.5) | 1 (12.5) | 3 (60) | 0.142 | |
| Spinal (%) | 26 (76.5) | 7 (87.5) | 2 (40) | |||
| Familial (%) | 8 (23.5) | 0 (0) | 0 (0) | 0.158 | ||
| Median disease duration months | 26.5 (18.8, 45) | 58 (28.5, 103) | 175 (109, 213) | 0.008 | ||
| Median ALSFRS-r | 37 (28.8, 40.3) | 35 (26, 41.8) | 34 (31, 36.5) | 0.935 | ||
| Median DeltaFS | 0.47 (0.25, 0.64) | 0.19 (0.17, 0.28) | 0.08 (0.07, 0.14) | <0.001 | ||
| King’s staging | Stage 1 | 4 | 3 | 0 | 0.069 | |
| Stage 2 | 10 | 2 | 1 | |||
| Stage 3 | 14 | 0 | 4 | |||
| Stage 4 | 6 | 3 | 0 | |||
| Riluzole use (%) | 30 (88.2) | 6 (75) | 2 (40) | 0.034 | ||
| Gastrostomy (%) | 7 (20.6) | 1 (12.5) | 0 (0) | 0.485 | ||
| Non-invasive ventilation (%) | 1 (2.9) | 3 (37.5) | 0 (0) | 0.005 | ||
| Tracheostomy (%) | 0 (0) | 0 (0) | 0 (0) | |||
| Disease | Number of Patients | NFL (pg/mL) | NFH (pg/mL) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| All MND | 47 | 314.0 (227.1) | 60.3 (74.7) | |
| Type of MND | ALS | 34 | 369.9 (232.4) | 62.6 (75.4) |
| PMA | 8 | 176.5 (153.5) | 42.6 (54.4) | |
| PLS | 5 | 154.5 (102.5) | 72.3 (105.9) | |
| All Disease Controls | 34 | 67.7 (59.9) | 22.9 (30.5) | |
| Disease controls | Neuropathy | 6 | 79.9 (46.9) | 39.1 (35.8) |
| Myopathy | 8 | 106.6 (59.9) | 35.1 (38.9) | |
| MG | 2 | 18.8 (1.7) | 1.0 (1.2) | |
| PD | 6 | 102.7 (83.0) | 26.3 (32.9) | |
| SBMA | 5 | 29.4 (7.6) | 4.3 (2.7) | |
| SMA | 7 | 23.9 (16.8) | 11.9 (17.6) | |
| Healthy Controls | 33 | 73.0 (95.6) | 17.4 (33.6) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCluskey, G.; Morrison, K.E.; Donaghy, C.; McConville, J.; McCarron, M.O.; McVerry, F.; Duddy, W.; Duguez, S. Serum Neurofilaments in Motor Neuron Disease and Their Utility in Differentiating ALS, PMA and PLS. Life 2023, 13, 1301. https://doi.org/10.3390/life13061301
McCluskey G, Morrison KE, Donaghy C, McConville J, McCarron MO, McVerry F, Duddy W, Duguez S. Serum Neurofilaments in Motor Neuron Disease and Their Utility in Differentiating ALS, PMA and PLS. Life. 2023; 13(6):1301. https://doi.org/10.3390/life13061301
Chicago/Turabian StyleMcCluskey, Gavin, Karen E. Morrison, Colette Donaghy, John McConville, Mark O. McCarron, Ferghal McVerry, William Duddy, and Stephanie Duguez. 2023. "Serum Neurofilaments in Motor Neuron Disease and Their Utility in Differentiating ALS, PMA and PLS" Life 13, no. 6: 1301. https://doi.org/10.3390/life13061301
APA StyleMcCluskey, G., Morrison, K. E., Donaghy, C., McConville, J., McCarron, M. O., McVerry, F., Duddy, W., & Duguez, S. (2023). Serum Neurofilaments in Motor Neuron Disease and Their Utility in Differentiating ALS, PMA and PLS. Life, 13(6), 1301. https://doi.org/10.3390/life13061301








