Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy
Abstract
1. Introduction
2. Methods
3. Frequency and Type of Cardiovascular Comorbidities in COPD
3.1. Heart Failure (HF)
3.2. Ischemic Heart Disease (IHD) and Coronary Artery Disease (CAD)
3.3. Arrhythmia
3.4. Systemic Hypertension and Stroke
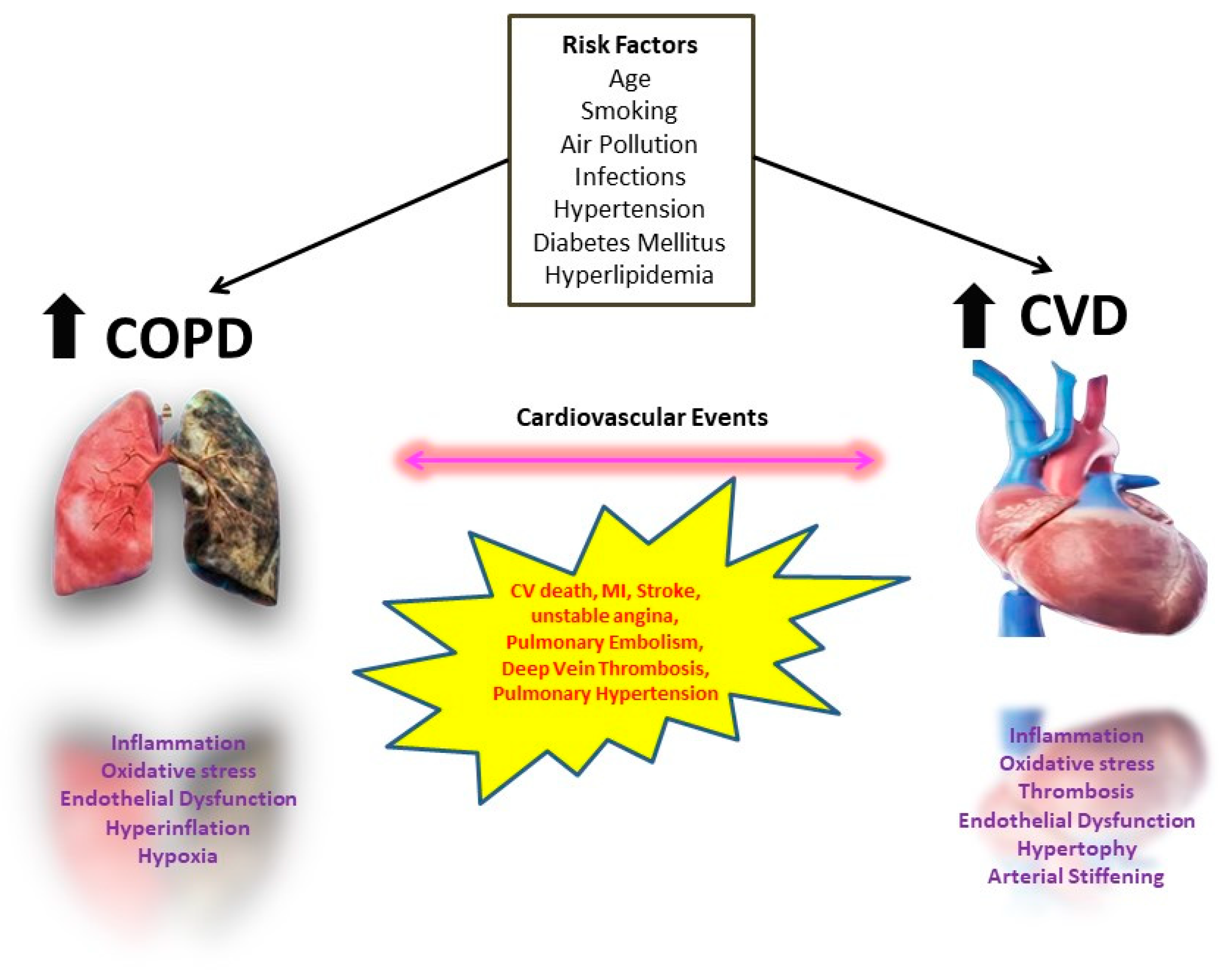
4. Interplay between CVD and COPD
5. Effect of Treatment for CVD on COPD and Vice Versa
5.1. B- Blockers
5.2. Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin II Receptor Blockers (ARBs)
5.3. Diuretics
5.4. Antiplatelets
5.5. Statins
5.6. COPD Medication
6. COPD Exacerbations Course and Treatment in Patients with CVDs
6.1. Pathophysiology
6.2. Treatment
7. Life Expectancy and Quality of Life
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes de Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Fabbri, L.M.; Aaron, S.D.; Agusti, A.; Brook, R.; Criner, G.J.; Franssen, F.M.E.; Humbert, M.; Hurst, J.R.; O’Donnell, D.; et al. An Updated Definition and Severity Classification of Chronic Obstructive Pulmonary Disease Exacerbations: The Rome Proposal. Am. J. Respir. Crit. Care Med. 2021, 204, 1251–1258. [Google Scholar] [CrossRef]
- Sin, D.D.; Stafinski, T.; Ng, Y.C.; Bell, N.R.; Jacobs, P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am. J. Respir. Crit. Care Med. 2002, 165, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Guarascio, A.J.; Ray, S.M.; Finch, C.K.; Self, T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clin. Outcomes Res. 2013, 5, 235–245. [Google Scholar] [CrossRef]
- Chatila, W.M.; Thomashow, B.M.; Minai, O.A.; Criner, G.J.; Make, B.J. Comorbidities in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 549–555. [Google Scholar] [CrossRef]
- Putcha, N.; Drummond, M.B.; Wise, R.A.; Hansel, N.N. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin. Respir. Crit. Care Med. 2015, 36, 575–591. [Google Scholar] [CrossRef]
- Rennard, S.I. Looking at the patient—Approaching the problem of COPD. N. Engl. J. Med. 2004, 350, 965–966. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Recio Iglesias, J.; Diez-Manglano, J.; Lopez Garcia, F.; Diaz Peromingo, J.A.; Almagro, P.; Varela Aguilar, J.M. Management of the COPD Patient with Comorbidities: An Experts Recommendation Document. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1015–1037. [Google Scholar] [CrossRef]
- Magnussen, H.; Disse, B.; Rodriguez-Roisin, R.; Kirsten, A.; Watz, H.; Tetzlaff, K.; Towse, L.; Finnigan, H.; Dahl, R.; Decramer, M.; et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014, 371, 1285–1294. [Google Scholar] [CrossRef]
- European Heart Network. European Cardiovascular Disease Statistics 2017 Edition. 2017. Available online: http://www.ehnheart.org/cvd-statistics.htm (accessed on 1 February 2023).
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin. Sci. 2019, 133, 885–904. [Google Scholar] [CrossRef]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Curkendall, S.M.; DeLuise, C.; Jones, J.K.; Lanes, S.; Stang, M.R.; Goehring, E., Jr.; She, D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann. Epidemiol. 2006, 16, 63–70. [Google Scholar] [CrossRef]
- Sidney, S.; Sorel, M.; Quesenberry, C.P., Jr.; DeLuise, C.; Lanes, S.; Eisner, M.D. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005, 128, 2068–2075. [Google Scholar] [CrossRef]
- Almagro, P.; Cabrera, F.J.; Diez, J.; Boixeda, R.; Alonso Ortiz, M.B.; Murio, C.; Soriano, J.B.; Working Group on, COPD, Spanish Society of Internal Medicine. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: The EPOC en Servicios de medicina interna (ESMI) study. Chest 2012, 142, 1126–1133. [Google Scholar] [CrossRef]
- Mentz, R.J.; Fiuzat, M.; Wojdyla, D.M.; Chiswell, K.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: Findings from OPTIMIZE-HF. Eur. J. Heart Fail. 2012, 14, 395–403. [Google Scholar] [CrossRef]
- Matamis, D.; Tsagourias, M.; Papathanasiou, A.; Sineffaki, H.; Lepida, D.; Galiatsou, E.; Nakos, G. Targeting occult heart failure in intensive care unit patients with acute chronic obstructive pulmonary disease exacerbation: Effect on outcome and quality of life. J. Crit. Care 2014, 29, 315.e7–315.e14. [Google Scholar] [CrossRef]
- Lee, H.; Jhun, B.W.; Cho, J.; Yoo, K.H.; Lee, J.H.; Kim, D.K.; Lee, J.D.; Jung, K.S.; Lee, J.Y.; Park, H.Y. Different impacts of respiratory symptoms and comorbidities on COPD-specific health-related quality of life by COPD severity. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3301–3310. [Google Scholar] [CrossRef]
- Almagro, P.; Calbo, E.; Ochoa de Echaguen, A.; Barreiro, B.; Quintana, S.; Heredia, J.L.; Garau, J. Mortality after hospitalization for COPD. Chest 2002, 121, 1441–1448. [Google Scholar] [CrossRef]
- Axson, E.L.; Ragutheeswaran, K.; Sundaram, V.; Bloom, C.I.; Bottle, A.; Cowie, M.R.; Quint, J.K. Hospitalisation and mortality in patients with comorbid COPD and heart failure: A systematic review and meta-analysis. Respir. Res. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.J.; Kim, D.B.; Jang, S.W.; Yoo, K.D.; Moon, K.W.; Shim, B.J.; Ahn, S.H.; Cho, E.J.; Rho, T.H.; Kim, J.H. Prognosis of heart failure patients with reduced and preserved ejection fraction and coexistent chronic obstructive pulmonary disease. Eur. J. Heart Fail. 2010, 12, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Canepa, M.; Straburzynska-Migaj, E.; Drozdz, J.; Fernandez-Vivancos, C.; Pinilla, J.M.G.; Nyolczas, N.; Temporelli, P.L.; Mebazaa, A.; Lainscak, M.; Laroche, C.; et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2018, 20, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Mapel, D. Insights into COPD comorbidities from the OLIN study and other large databases. COPD 2011, 8, 397–399. [Google Scholar] [CrossRef]
- Mapel, D.W.; Dedrick, D.; Davis, K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991–1999. COPD 2005, 2, 35–41. [Google Scholar] [CrossRef]
- Almagro, P.; Lopez Garcia, F.; Cabrera, F.J.; Montero, L.; Morchon, D.; Diez, J.; Soriano, J.B.; Grupo Epoc De La Sociedad Espanola De Medicina, I. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir. Med. 2010, 104, 253–259. [Google Scholar] [CrossRef]
- Patel, A.R.C.; Donaldson, G.C.; Mackay, A.J.; Wedzicha, J.A.; Hurst, J.R. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest 2012, 141, 851–857. [Google Scholar] [CrossRef]
- Dalal, A.A.; Shah, M.; Lunacsek, O.; Hanania, N.A. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir. Med. 2011, 105, 1516–1522. [Google Scholar] [CrossRef]
- Soriano Ortiz, J.B.; Almagro, P.; Sauleda Roig, J. Causes of mortality in COPD. Arch. Bronconeumol. 2009, 45 (Suppl. S4), 8–13. [Google Scholar] [CrossRef]
- MacDonald, M.I.; Shafuddin, E.; King, P.T.; Chang, C.L.; Bardin, P.G.; Hancox, R.J. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir. Med. 2016, 4, 138–148. [Google Scholar] [CrossRef]
- Rothnie, K.J.; Smeeth, L.; Herrett, E.; Pearce, N.; Hemingway, H.; Wedzicha, J.; Timmis, A.; Quint, J.K. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart 2015, 101, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Mullerova, H.; Agusti, A.; Erqou, S.; Mapel, D.W. Cardiovascular comorbidity in COPD: Systematic literature review. Chest 2013, 144, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Houben-Wilke, S.; Jorres, R.A.; Bals, R.; Franssen, F.M.; Glaser, S.; Holle, R.; Karch, A.; Koch, A.; Magnussen, H.; Obst, A.; et al. Peripheral Artery Disease and Its Clinical Relevance in Patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am. J. Respir. Crit. Care Med. 2017, 195, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Lehman, E.B. Preexisting Conditions Determine the Occurrence of Unplanned Readmissions after Procedures for Treatment of Peripheral Arterial Disease. Ann. Vasc. Surg. 2018, 50, 60–72. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Lip, G.Y.; Tse, H.F.; Lane, D.A. Atrial fibrillation. Lancet 2012, 379, 648–661. [Google Scholar] [CrossRef]
- Roversi, S.; Fabbri, L.M.; Sin, D.D.; Hawkins, N.M.; Agusti, A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am. J. Respir. Crit. Care Med. 2016, 194, 1319–1336. [Google Scholar] [CrossRef]
- Konecny, T.; Park, J.Y.; Somers, K.R.; Konecny, D.; Orban, M.; Soucek, F.; Parker, K.O.; Scanlon, P.D.; Asirvatham, S.J.; Brady, P.A.; et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am. J. Cardiol. 2014, 114, 272–277. [Google Scholar] [CrossRef]
- Terzano, C.; Romani, S.; Gaudio, C.; Pelliccia, F.; Serao, M.; Vitarelli, A. Right heart functional changes in the acute, hypercapnic exacerbations of COPD. BioMed Res. Int. 2014, 2014, 596051. [Google Scholar] [CrossRef]
- Steer, J.; Gibson, J.; Bourke, S.C. The DECAF Score: Predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012, 67, 970–976. [Google Scholar] [CrossRef]
- Echevarria, C.; Steer, J.; Heslop-Marshall, K.; Stenton, S.C.; Hickey, P.M.; Hughes, R.; Wijesinghe, M.; Harrison, R.N.; Steen, N.; Simpson, A.J.; et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax 2016, 71, 133–140. [Google Scholar] [CrossRef]
- Onishi, K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J. Cardiol. 2017, 70, 128–134. [Google Scholar] [CrossRef]
- Barrett, T.W.; Self, W.H.; Jenkins, C.A.; Storrow, A.B.; Heavrin, B.S.; McNaughton, C.D.; Collins, S.P.; Goldberger, J.J. Predictors of regional variations in hospitalizations following emergency department visits for atrial fibrillation. Am. J. Cardiol. 2013, 112, 1410–1416. [Google Scholar] [CrossRef]
- GOLD Guidelines. 2023. Available online: www.goldcopd.org (accessed on 20 February 2023).
- Wakabayashi, K.; Gonzalez, M.A.; Delhaye, C.; Ben-Dor, I.; Maluenda, G.; Collins, S.D.; Syed, A.I.; Gaglia, M.A., Jr.; Torguson, R.; Xue, Z.; et al. Impact of chronic obstructive pulmonary disease on acute-phase outcome of myocardial infarction. Am. J. Cardiol. 2010, 106, 305–309. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Yamagata, T.; Maeda, K.; Honda, N.; Sano, A.; Nishiyama, O.; Sano, H.; Iwanaga, T.; Chiba, Y.; Fukuda, K.; et al. Influence of comorbidities on the efficacy of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 2016, 16, 934–941. [Google Scholar] [CrossRef]
- Battaglia, S.; Basile, M.; Scichilone, N.; Bellia, V. Prevalence of Co-morbidities and Severity of COPD. COPD 2015, 12, 390–394. [Google Scholar] [CrossRef]
- Yin, L.; Lensmar, C.; Ingelsson, E.; Back, M. Differential association of chronic obstructive pulmonary disease with myocardial infarction and ischemic stroke in a nation-wide cohort. Int. J. Cardiol. 2014, 173, 601–603. [Google Scholar] [CrossRef]
- Portegies, M.L.; Lahousse, L.; Joos, G.F.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.; Brusselle, G.G.; Ikram, M.A. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2016, 193, 251–258. [Google Scholar] [CrossRef]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef]
- Campo, G.; Pavasini, R.; Malagu, M.; Mascetti, S.; Biscaglia, S.; Ceconi, C.; Papi, A.; Contoli, M. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: Overview of mechanisms and clinical management. Cardiovasc. Drugs Ther. 2015, 29, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I. Clinical approach to patients with chronic obstructive pulmonary disease and cardiovascular disease. Proc. Am. Thorac. Soc. 2005, 2, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Maclay, J.D.; MacNee, W. Cardiovascular disease in COPD: Mechanisms. Chest 2013, 143, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Man, S.F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003, 107, 1514–1519. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Linden, F.; Domschke, G.; Erbel, C.; Akhavanpoor, M.; Katus, H.A.; Gleissner, C.A. Inflammatory therapeutic targets in coronary atherosclerosis-from molecular biology to clinical application. Front. Physiol. 2014, 5, 455. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons. Eur. Respir. Rev. 2018, 27, 185057. [Google Scholar] [CrossRef]
- Boschetto, P.; Beghe, B.; Fabbri, L.M.; Ceconi, C. Link between chronic obstructive pulmonary disease and coronary artery disease: Implication for clinical practice. Respirology 2012, 17, 422–431. [Google Scholar] [CrossRef]
- Pinto-Plata, V.M.; Livnat, G.; Girish, M.; Cabral, H.; Masdin, P.; Linacre, P.; Dew, R.; Kenney, L.; Celli, B.R. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest 2007, 131, 37–43. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 51–57. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Tamisier, R.; Baguet, J.P.; Borel, J.C.; Levy, P.; Pepin, J.L. Arterial stiffness in COPD. Chest 2014, 145, 861–875. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.; Spruit, M.A.; Groenen, M.T.; Bruijnzeel, P.L.; Taib, Z.; Rutten, E.P.; Roodt, J.O.; Akkermans, M.A.; Wouters, E.F.; Franssen, F.M. Arterial stiffness in patients with COPD: The role of systemic inflammation and the effects of pulmonary rehabilitation. Eur. Respir. J. 2014, 43, 1306–1315. [Google Scholar] [CrossRef]
- Sabit, R.; Bolton, C.E.; Edwards, P.H.; Pettit, R.J.; Evans, W.D.; McEniery, C.M.; Wilkinson, I.B.; Cockcroft, J.R.; Shale, D.J. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 1259–1265. [Google Scholar] [CrossRef]
- Husain, K.; Hernandez, W.; Ansari, R.A.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015, 6, 209–217. [Google Scholar] [CrossRef]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Chronic obstructive pulmonary disease and atherosclerosis: Common mechanisms and novel therapeutics. Clin. Sci. 2022, 136, 405–423. [Google Scholar] [CrossRef]
- Eickhoff, P.; Valipour, A.; Kiss, D.; Schreder, Z.M.; Cekici, L.; Geyer, K.; Kohansal, R.; Burghuber, O.C. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 1211–1218. [Google Scholar] [CrossRef]
- De Moraes, M.R.; da Costa, A.C.; Correa Kde, S.; Junqueira-Kipnis, A.P.; Rabahi, M.F. Interleukin-6 and interleukin-8 blood levels’ poor association with the severity and clinical profile of ex-smokers with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 735–743. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arter. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Meyer, T.; Kretschmar, G.; Kirsten, A.; Claussen, M.; Magnussen, H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: Role of hyperinflation. Chest 2010, 138, 32–38. [Google Scholar] [CrossRef]
- Ferguson, G.T. Why does the lung hyperinflate? Proc. Am. Thorac. Soc. 2006, 3, 176–179. [Google Scholar] [CrossRef]
- Shekerdemian, L.; Bohn, D. Cardiovascular effects of mechanical ventilation. Arch. Dis. Child. 1999, 80, 475–480. [Google Scholar] [CrossRef]
- Tzani, P.; Aiello, M.; Elia, D.; Boracchia, L.; Marangio, E.; Olivieri, D.; Clini, E.; Chetta, A. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir. Res. 2011, 12, 150. [Google Scholar] [CrossRef]
- Lukacsovits, J.; Szollosi, G.; Varga, J.T. Cardiovascular effects of exercise induced dynamic hyperinflation in COPD patients-Dynamically hyperinflated and non-hyperinflated subgroups. PLoS ONE 2023, 18, e0274585. [Google Scholar] [CrossRef]
- Vassaux, C.; Torre-Bouscoulet, L.; Zeineldine, S.; Cortopassi, F.; Paz-Diaz, H.; Celli, B.R.; Pinto-Plata, V.M. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur. Respir. J. 2008, 32, 1275–1282. [Google Scholar] [CrossRef]
- Zangiabadi, A.; De Pasquale, C.G.; Sajkov, D. Pulmonary hypertension and right heart dysfunction in chronic lung disease. BioMed Res. Int. 2014, 2014, 739674. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A. The shrinking heart in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 362, 267–268. [Google Scholar] [CrossRef]
- Su, V.Y.; Yang, Y.H.; Perng, D.W.; Tsai, Y.H.; Chou, K.T.; Su, K.C.; Su, W.J.; Chen, P.C.; Yang, K.Y. Real-world effectiveness of medications on survival in patients with COPD-heart failure overlap. Aging 2019, 11, 3650–3667. [Google Scholar] [CrossRef]
- NICE. Cardiovascular Disease Risk Assessment and Prevention. 2020. Available online: https://bnf.nice.org.uk/treatment-summary/cardiovascular-disease-risk-assessmentand-prevention.htm (accessed on 1 March 2023).
- Alter, P.; Mayerhofer, B.A.; Kahnert, K.; Watz, H.; Waschki, B.; Andreas, S.; Biertz, F.; Bals, R.; Vogelmeier, C.F.; Jorres, R.A. Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: Results from the COSYCONET cohort. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2163–2172. [Google Scholar] [CrossRef]
- Baker, J.G.; Wilcox, R.G. beta-Blockers, heart disease and COPD: Current controversies and uncertainties. Thorax 2017, 72, 271–276. [Google Scholar] [CrossRef]
- Li, X.F.; Mao, Y.M. Beta-blockers in COPD: A systematic review based on recent research. Life Sci. 2020, 252, 117649. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Voelker, H.; Bhatt, S.P.; Brenner, K.; Casaburi, R.; Come, C.E.; Cooper, J.A.D.; Criner, G.J.; Curtis, J.L.; Han, M.K.; et al. Metoprolol for the Prevention of Acute Exacerbations of COPD. N. Engl. J. Med. 2019, 381, 2304–2314. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Yang, Y.L.; Xiang, Z.J.; Yang, J.H.; Wang, W.J.; Xu, Z.C.; Xiang, R.L. Association of beta-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 4415–4422. [Google Scholar] [CrossRef]
- Parikh, M.A.; Aaron, C.P.; Hoffman, E.A.; Schwartz, J.E.; Madrigano, J.; Austin, J.H.M.; Kalhan, R.; Lovasi, G.; Watson, K.; Stukovsky, K.H.; et al. Angiotensin-Converting Inhibitors and Angiotensin II Receptor Blockers and Longitudinal Change in Percent Emphysema on Computed Tomography. The Multi-Ethnic Study of Atherosclerosis Lung Study. Ann. Am. Thorac. Soc. 2017, 14, 649–658. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, Y.H.; Wang, C.Y.; Wang, H.C.; Yu, C.J.; Chen, L. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 867–874. [Google Scholar] [CrossRef]
- Meysman, M. Angiotensin II blockers in obstructive pulmonary disease: A randomised controlled trial. Eur. Respir. J. 2006, 28, 670; author reply 670–671. [Google Scholar] [CrossRef]
- Ehteshami-Afshar, S.; Mooney, L.; Dewan, P.; Desai, A.S.; Lang, N.N.; Lefkowitz, M.P.; Petrie, M.C.; Rizkala, A.R.; Rouleau, J.L.; Solomon, S.D.; et al. Clinical Characteristics and Outcomes of Patients With Heart Failure With Reduced Ejection Fraction and Chronic Obstructive Pulmonary Disease: Insights From PARADIGM-HF. J. Am. Heart Assoc. 2021, 10, e019238. [Google Scholar] [CrossRef]
- Brijker, F.; Heijdra, Y.F.; van den Elshout, F.J.; Folgering, H.T. Discontinuation of furosemide decreases PaCO(2) in patients with COPD. Chest 2002, 121, 377–382. [Google Scholar] [CrossRef]
- Light, R.W.; George, R.B. Serial pulmonary function in patients with acute heart failure. Arch. Intern. Med. 1983, 143, 429–433. [Google Scholar] [CrossRef]
- Herrin, M.A.; Feemster, L.C.; Crothers, K.; Uman, J.E.; Bryson, C.L.; Au, D.H. Combination antihypertensive therapy among patients with COPD. Chest 2013, 143, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Vozoris, N.T.; Wang, X.; Austin, P.C.; O’Donnell, D.E.; Aaron, S.D.; To, T.M.; Gershon, A.S. Incident diuretic drug use and adverse respiratory events among older adults with chronic obstructive pulmonary disease. Br. J. Clin. Pharmacol. 2018, 84, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Biscaglia, S.; d’Ascenzo, F.; Del Franco, A.; Contoli, M.; Zaraket, F.; Guerra, F.; Ferrari, R.; Campo, G. Antiplatelet Treatment Reduces All-Cause Mortality in COPD Patients: A Systematic Review and Meta-Analysis. COPD 2016, 13, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, M.P.; Hermansson, A.B.; Strom, K.E. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 715–720. [Google Scholar] [CrossRef]
- Andell, P.; James, S.K.; Cannon, C.P.; Cyr, D.D.; Himmelmann, A.; Husted, S.; Keltai, M.; Koul, S.; Santoso, A.; Steg, P.G.; et al. Ticagrelor Versus Clopidogrel in Patients With Acute Coronary Syndromes and Chronic Obstructive Pulmonary Disease: An Analysis From the Platelet Inhibition and Patient Outcomes (PLATO) Trial. J. Am. Hear. Assoc. 2015, 4, e002490. [Google Scholar] [CrossRef]
- Kunadian, V.; Wilson, N.; Stocken, D.D.; Ali, H.; McColl, E.; Burns, G.; Howe, N.; Fisher, A.; De Soyza, A. Antiplatelet therapy in the primary prevention of cardiovascular disease in patients with chronic obstructive pulmonary disease: A randomised controlled proof-of-concept trial. ERJ Open Res. 2019, 5, 00110–2019. [Google Scholar] [CrossRef]
- Balbirsingh, V.; Mohammed, A.S.; Turner, A.M.; Newnham, M. Cardiovascular disease in chronic obstructive pulmonary disease: A narrative review. Thorax 2022, 77, 939–945. [Google Scholar] [CrossRef]
- Aaron, C.P.; Schwartz, J.E.; Hoffman, E.A.; Angelini, E.; Austin, J.H.M.; Cushman, M.; Jacobs, D.R., Jr.; Kaufman, J.D.; Laine, A.; Smith, L.J.; et al. A Longitudinal Cohort Study of Aspirin Use and Progression of Emphysema-like Lung Characteristics on CT Imaging: The MESA Lung Study. Chest 2018, 154, 41–50. [Google Scholar] [CrossRef]
- Fawzy, A.; Putcha, N.; Aaron, C.P.; Bowler, R.P.; Comellas, A.P.; Cooper, C.B.; Dransfield, M.T.; Han, M.K.; Hoffman, E.A.; Kanner, R.E.; et al. Aspirin Use and Respiratory Morbidity in COPD: A Propensity Score-Matched Analysis in Subpopulations and Intermediate Outcome Measures in COPD Study. Chest 2019, 155, 519–527. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.; Eaton, T.E. Pharmacological actions of statins: Potential utility in COPD. Eur. Respir. Rev. 2009, 18, 222–232. [Google Scholar] [CrossRef]
- Soyseth, V.; Brekke, P.H.; Smith, P.; Omland, T. Statin use is associated with reduced mortality in COPD. Eur. Respir. J. 2007, 29, 279–283. [Google Scholar] [CrossRef]
- Walsh, A.; Perrem, L.; Khashan, A.S.; Henry, M.T.; Ni Chroinin, M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 7, CD011959. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.S.; Marott, J.L.; Nordestgaard, B.G.; Lange, P.; Hallas, J.; Vestbo, J. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax 2015, 70, 33–40. [Google Scholar] [CrossRef]
- Criner, G.J.; Connett, J.E.; Aaron, S.D.; Albert, R.K.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N. Engl. J. Med. 2014, 370, 2201–2210. [Google Scholar] [CrossRef]
- Wu, W.T.; Chen, C.Y. Protective Effect of Statins on Pulmonary Hypertension in Chronic Obstructive Pulmonary Disease Patients: A Nationwide Retrospective, Matched Cohort Study. Sci. Rep. 2020, 10, 3104. [Google Scholar] [CrossRef]
- Gupta, P.; O’Mahony, M.S. Potential adverse effects of bronchodilators in the treatment of airways obstruction in older people: Recommendations for prescribing. Drugs Aging 2008, 25, 415–443. [Google Scholar] [CrossRef]
- Vestbo, J.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.; Celli, B.R.; Crim, C.; Martinez, F.; Yates, J.; Newby, D.E.; Investigators, S. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet 2016, 387, 1817–1826. [Google Scholar] [CrossRef]
- Almagro, P.; Boixeda, R.; Diez-Manglano, J.; Gomez-Antunez, M.; Lopez-Garcia, F.; Recio, J. Insights into Chronic Obstructive Pulmonary Disease as Critical Risk Factor for Cardiovascular Disease. Int. J. Chron Obstr. Pulm. Dis. 2020, 15, 755–764. [Google Scholar] [CrossRef]
- Oba, Y.; Sarva, S.T.; Dias, S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: A network meta-analysis. Thorax 2016, 71, 15–25. [Google Scholar] [CrossRef]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A Systematic Review With Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Concurrent use of long-acting bronchodilators in COPD and the risk of adverse cardiovascular events. Eur. Respir. J. 2017, 49, 1602245. [Google Scholar] [CrossRef] [PubMed]
- Parkin, L.; Williams, S.; Sharples, K.; Barson, D.; Horsburgh, S.; Jackson, R.; Wu, B.; Dummer, J. Dual versus single long-acting bronchodilator use could raise acute coronary syndrome risk by over 50%: A population-based nested case-control study. J. Intern. Med. 2021, 290, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Jiang, Y.; Guo, S.; He, J.Q.; Sin, D.D. Combination therapy with long-acting bronchodilators and the risk of major adverse cardiovascular events in patients with COPD: A systematic review and meta-analysis. Eur. Respir. J. 2023, 61, 2200302. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Yates, J.C.; Vestbo, J.; Investigators, T. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007, 356, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St Rose, E.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef]
- Hillas, G.; Perlikos, F.; Tzanakis, N. Acute exacerbation of COPD: Is it the “stroke of the lungs”? Int. J. Chron Obstr. Pulm. Dis. 2016, 11, 1579–1586. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Hurst, J.R.; Smith, C.J.; Hubbard, R.B.; Wedzicha, J.A. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 2010, 137, 1091–1097. [Google Scholar] [CrossRef]
- Reilev, M.; Pottegard, A.; Lykkegaard, J.; Sondergaard, J.; Ingebrigtsen, T.S.; Hallas, J. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology 2019, 24, 1183–1190. [Google Scholar] [CrossRef]
- Halpin, D.M.; Decramer, M.; Celli, B.; Kesten, S.; Leimer, I.; Tashkin, D.P. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT(R) trial. Lung 2011, 189, 261–268. [Google Scholar] [CrossRef]
- Rothnie, K.J.; Connell, O.; Mullerova, H.; Smeeth, L.; Pearce, N.; Douglas, I.; Quint, J.K. Myocardial Infarction and Ischemic Stroke after Exacerbations of Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2018, 15, 935–946. [Google Scholar] [CrossRef]
- Goto, T.; Shimada, Y.J.; Faridi, M.K.; Camargo, C.A., Jr.; Hasegawa, K. Incidence of Acute Cardiovascular Event After Acute Exacerbation of COPD. J. Gen. Intern. Med. 2018, 33, 1461–1468. [Google Scholar] [CrossRef]
- Wang, M.; Lin, E.P.; Huang, L.C.; Li, C.Y.; Shyr, Y.; Lai, C.H. Mortality of Cardiovascular Events in Patients With COPD and Preceding Hospitalization for Acute Exacerbation. Chest 2020, 158, 973–985. [Google Scholar] [CrossRef]
- Brekke, P.H.; Omland, T.; Holmedal, S.H.; Smith, P.; Soyseth, V. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur. Respir. J. 2008, 31, 563–570. [Google Scholar] [CrossRef]
- Sofia, M.; Maniscalco, M.; Celentano, L.; Faraone, S.; Mormile, M.; Alifano, M.; Carratu, L. Abnormalities of renal endothelin during acute exacerbation in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2001, 14, 321–327. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar]
- Ozben, B.; Eryuksel, E.; Tanrikulu, A.M.; Papila-Topal, N.; Celikel, T.; Basaran, Y. Acute exacerbation impairs endothelial function in patients with chronic obstructive pulmonary disease. Turk Kardiyol. Dern. Ars. 2010, 38, 1–7. [Google Scholar]
- Sinden, N.J.; Stockley, R.A. Systemic inflammation and comorbidity in COPD: A result of ’overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010, 65, 930–936. [Google Scholar] [CrossRef]
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of comorbidities. Eur. Respir. J. 2006, 28, 1245–1257. [Google Scholar] [CrossRef]
- Theodorakopoulou, M.P.; Bakaloudi, D.R.; Alexandrou, M.E.; Papakosta, D.; Pataka, A.; Kioumis, I.; Boutou, A.K. Endothelial Dysfunction during Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. COPD 2021, 18, 246–253. [Google Scholar] [CrossRef]
- Falk, J.A.; Kadiev, S.; Criner, G.J.; Scharf, S.M.; Minai, O.A.; Diaz, P. Cardiac disease in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 543–548. [Google Scholar] [CrossRef]
- Clarenbach, C.F.; Senn, O.; Sievi, N.A.; Camen, G.; van Gestel, A.J.; Rossi, V.A.; Puhan, M.A.; Thurnheer, R.; Russi, E.W.; Kohler, M. Determinants of endothelial function in patients with COPD. Eur. Respir. J. 2013, 42, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Labonte, L.E.; Bourbeau, J.; Daskalopoulou, S.S.; Zhang, M.; Coulombe, P.; Garland, K.; Baglole, C.J. Club Cell-16 and RelB as Novel Determinants of Arterial Stiffness in Exacerbating COPD Patients. PLoS ONE 2016, 11, e0149974. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Churg, A.; Wright, J.L.; Li, Y.; Tam, S.; Man, S.F.; Tashkin, D.; Wise, R.A.; Connett, J.E.; Sin, D.D. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Lomas, D.A.; Silverman, E.K.; Edwards, L.D.; Miller, B.E.; Coxson, H.O.; Tal-Singer, R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008, 63, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Labonte, L.; Coulombe, P.; Zago, M.; Bourbeau, J.; Baglole, C.J. Alterations in the expression of the NF-kappaB family member RelB as a novel marker of cardiovascular outcomes during acute exacerbations of chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e112965. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Lin, G.; Sun, L.; Li, H.; Xie, C. Decreased CD34+ cell number is correlated with cardiac dysfunction in patients with acute exacerbation of COPD. Heart Lung Circ. 2014, 23, 875–882. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Laveneziana, P.; Webb, K.; Neder, J.A. Chronic obstructive pulmonary disease: Clinical integrative physiology. Clin. Chest Med. 2014, 35, 51–69. [Google Scholar] [CrossRef]
- Strohmayer, E.A.; Krakoff, L.R. Glucocorticoids and cardiovascular risk factors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 409–417. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Ormiston, T.M.; Salpeter, E.E. Cardiovascular effects of beta-agonists in patients with asthma and COPD: A meta-analysis. Chest 2004, 125, 2309–2321. [Google Scholar] [CrossRef]
- Berton, D.C.; Barbosa, P.B.; Takara, L.S.; Chiappa, G.R.; Siqueira, A.C.; Bravo, D.M.; Ferreira, L.F.; Neder, J.A. Bronchodilators accelerate the dynamics of muscle O2 delivery and utilisation during exercise in COPD. Thorax 2010, 65, 588–593. [Google Scholar] [CrossRef]
- Schembri, S.; Williamson, P.A.; Short, P.M.; Singanayagam, A.; Akram, A.; Taylor, J.; Singanayagam, A.; Hill, A.T.; Chalmers, J.D. Cardiovascular events after clarithromycin use in lower respiratory tract infections: Analysis of two prospective cohort studies. BMJ 2013, 346, f1235. [Google Scholar] [CrossRef]
- Berni, E.; de Voogd, H.; Halcox, J.P.; Butler, C.C.; Bannister, C.A.; Jenkins-Jones, S.; Jones, B.; Ouwens, M.; Currie, C.J. Risk of cardiovascular events, arrhythmia and all-cause mortality associated with clarithromycin versus alternative antibiotics prescribed for respiratory tract infections: A retrospective cohort study. BMJ Open 2017, 7, e013398. [Google Scholar] [CrossRef]
- Agarwal, S.; Rokadia, H.; Senn, T.; Menon, V. Burden of cardiovascular disease in chronic obstructive pulmonary disease. Am. J. Prev. Med. 2014, 47, 105–114. [Google Scholar] [CrossRef]
- Mannino, D.M.; Doherty, D.E.; Sonia Buist, A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir. Med. 2006, 100, 115–122. [Google Scholar] [CrossRef]
- McGarvey, L.P.; John, M.; Anderson, J.A.; Zvarich, M.; Wise, R.A.; Committee, T.C.E. Ascertainment of cause-specific mortality in COPD: Operations of the TORCH Clinical Endpoint Committee. Thorax 2007, 62, 411–415. [Google Scholar] [CrossRef]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef]
- Salisbury, A.C.; Reid, K.J.; Spertus, J.A. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am. J. Cardiol. 2007, 99, 636–641. [Google Scholar] [CrossRef]
- Stone, I.S.; Barnes, N.C.; Petersen, S.E. Chronic obstructive pulmonary disease: A modifiable risk factor for cardiovascular disease? Heart 2012, 98, 1055–1062. [Google Scholar] [CrossRef]
- Friedman, G.D.; Klatsky, A.L.; Siegelaub, A.B. Lung function and risk of myocardial infarction and sudden cardiac death. N. Engl. J. Med. 1976, 294, 1071–1075. [Google Scholar] [CrossRef]
- Ebi-Kryston, K.L.; Hawthorne, V.M.; Rose, G.; Shipley, M.J.; Gillis, C.R.; Hole, D.J.; Carmen, W.; Eshleman, S.; Higgins, M.W. Breathlessness, chronic bronchitis and reduced pulmonary function as predictors of cardiovascular disease mortality among men in England, Scotland and the United States. Int. J. Epidemiol. 1989, 18, 84–88. [Google Scholar] [CrossRef]
- Ebi-Kryston, K.L. Respiratory symptoms and pulmonary function as predictors of 10-year mortality from respiratory disease, cardiovascular disease, and all causes in the Whitehall Study. J. Clin. Epidemiol. 1988, 41, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Putcha, N.; Puhan, M.A.; Hansel, N.N.; Drummond, M.B.; Boyd, C.M. Impact of co-morbidities on self-rated health in self-reported COPD: An analysis of NHANES 2001–2008. COPD 2013, 10, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Comin-Colet, J.; Martin Lorenzo, T.; Gonzalez-Dominguez, A.; Oliva, J.; Jimenez Merino, S. Impact of non-cardiovascular comorbidities on the quality of life of patients with chronic heart failure: A scoping review. Health Qual. Life Outcomes 2020, 18, 329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaporfyriou, A.; Bartziokas, K.; Gompelmann, D.; Idzko, M.; Fouka, E.; Zaneli, S.; Bakakos, P.; Loukides, S.; Papaioannou, A.I. Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life 2023, 13, 1299. https://doi.org/10.3390/life13061299
Papaporfyriou A, Bartziokas K, Gompelmann D, Idzko M, Fouka E, Zaneli S, Bakakos P, Loukides S, Papaioannou AI. Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life. 2023; 13(6):1299. https://doi.org/10.3390/life13061299
Chicago/Turabian StylePapaporfyriou, Anastasia, Konstantinos Bartziokas, Daniela Gompelmann, Marco Idzko, Evangelia Fouka, Stavrina Zaneli, Petros Bakakos, Stelios Loukides, and Andriana I. Papaioannou. 2023. "Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy" Life 13, no. 6: 1299. https://doi.org/10.3390/life13061299
APA StylePapaporfyriou, A., Bartziokas, K., Gompelmann, D., Idzko, M., Fouka, E., Zaneli, S., Bakakos, P., Loukides, S., & Papaioannou, A. I. (2023). Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life, 13(6), 1299. https://doi.org/10.3390/life13061299








