Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
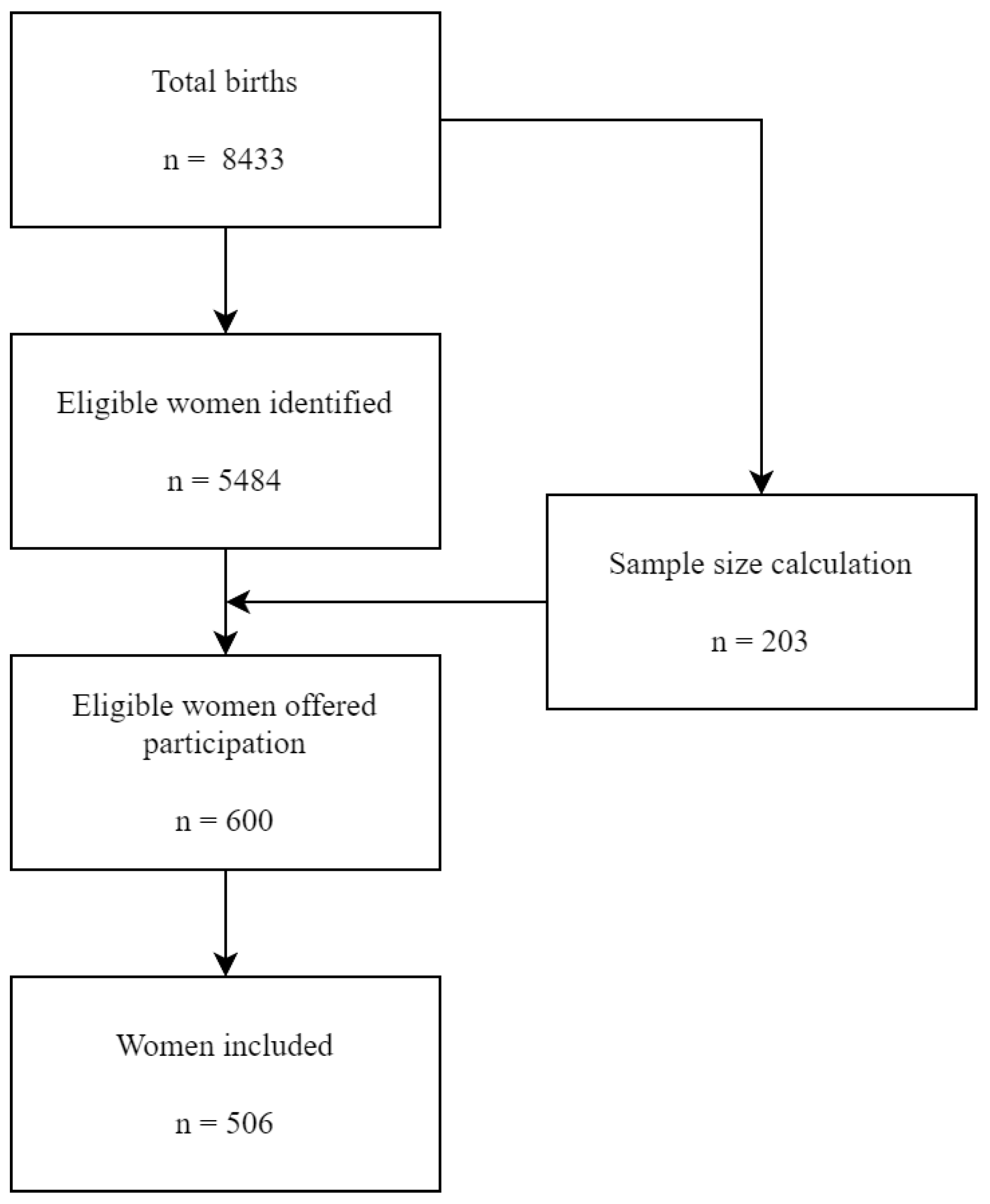
2.2. Study Population
2.3. Data Collection
2.3.1. Personal and Medical Data
2.3.2. Adherence to the Mediterranean Dietary Pattern
2.4. Statistical Analysis
3. Results
3.1. Mediterranean Dietary Pattern Adherence
3.2. Maternal Sociodemographic and Anthropometric Characteristics
3.3. Newborn Characteristics
3.4. Iron-Related Biochemical Parameters
4. Discussion
4.1. Iron Deficiency
4.2. Anemia
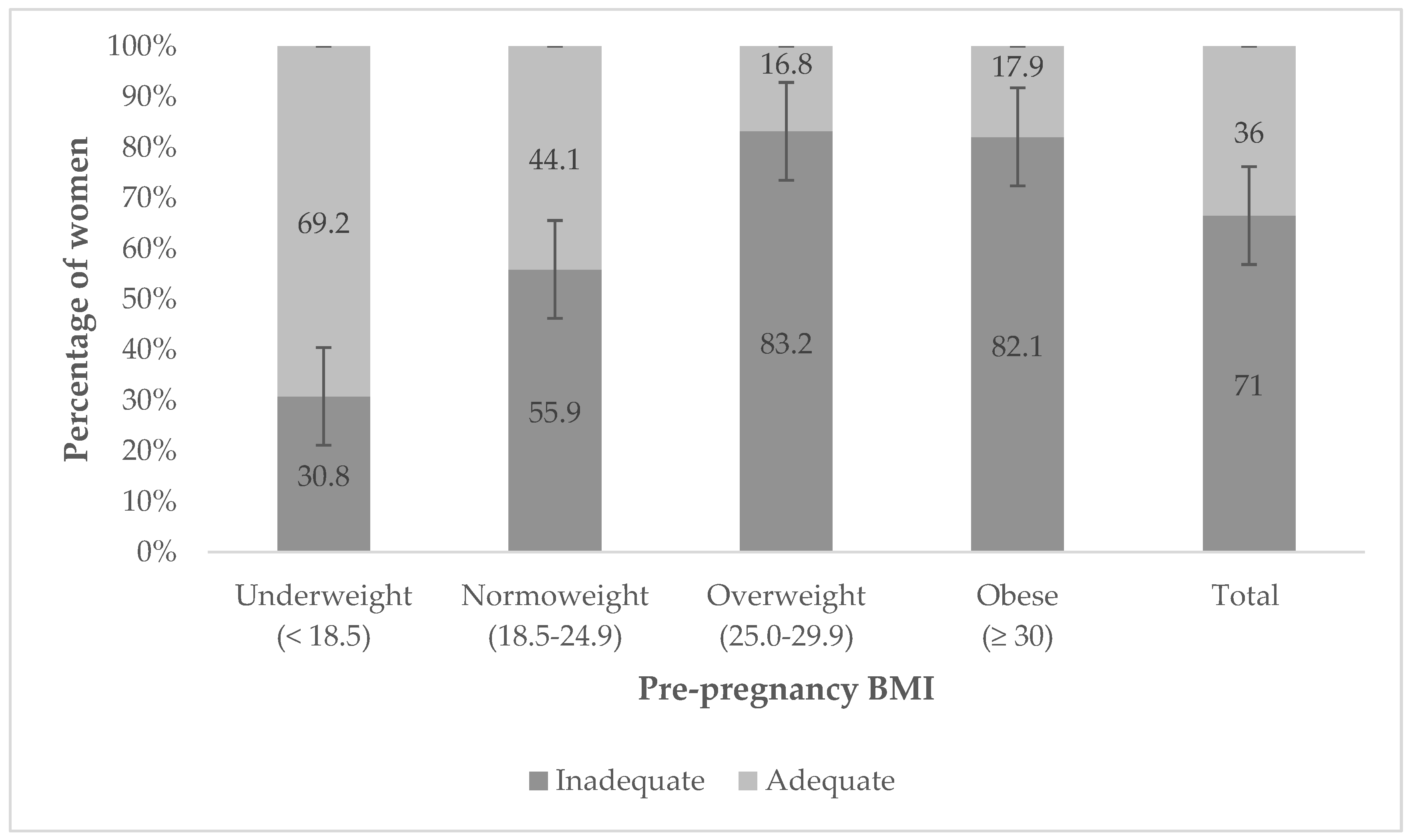
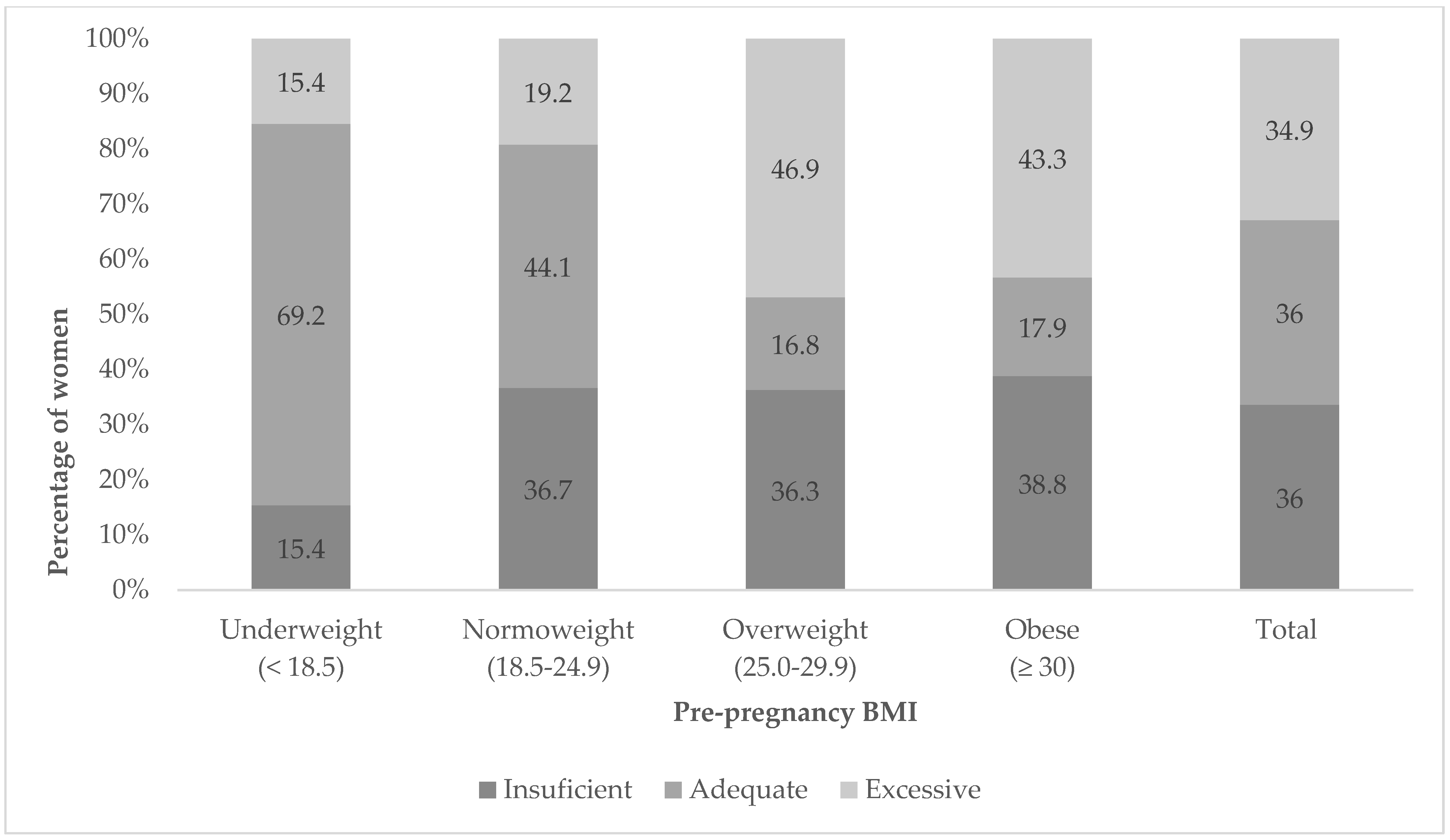
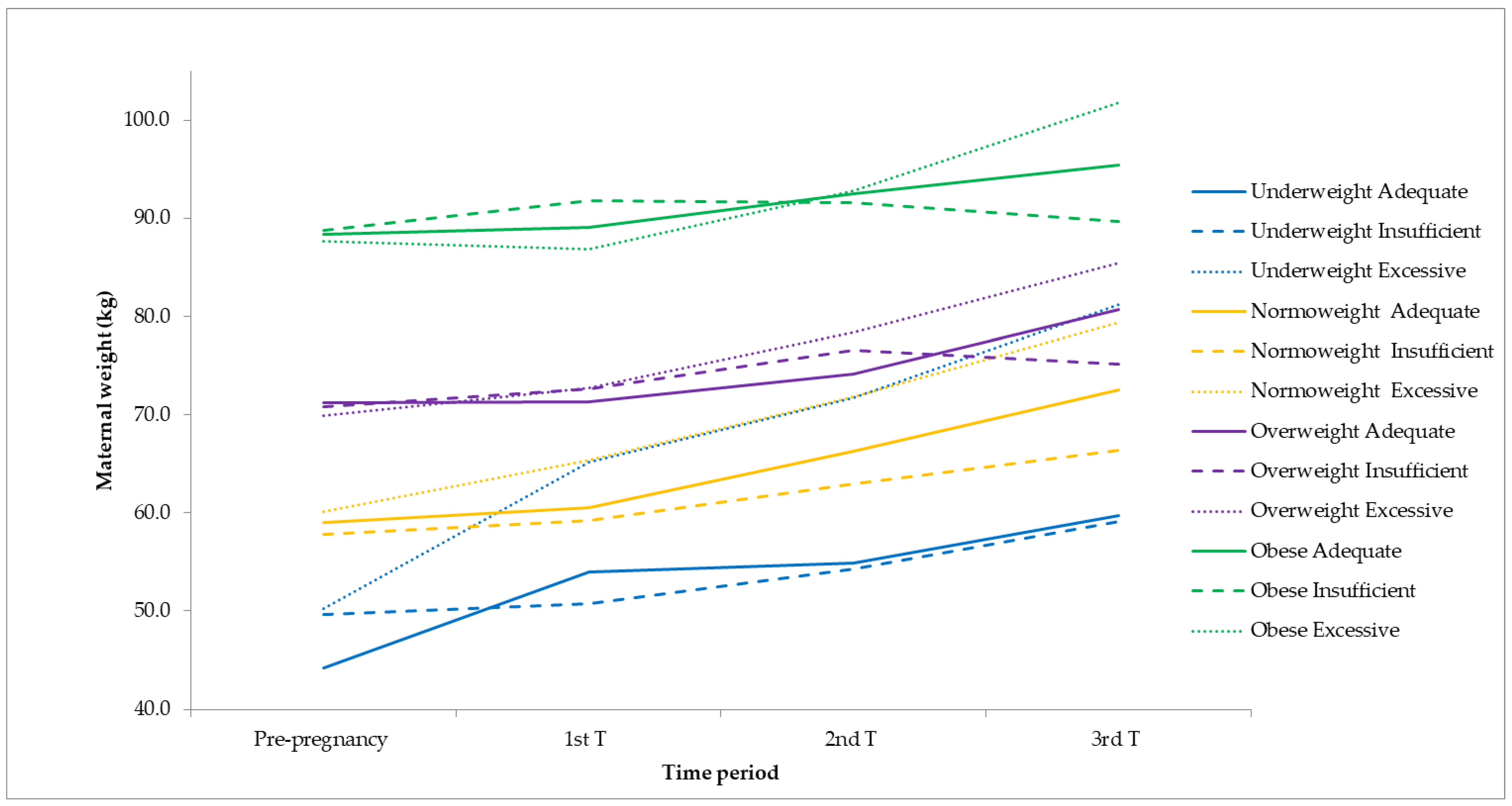
4.3. Gestational Weight Gain
4.4. Interventions
4.5. Limitations
4.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2019, 4, CD004905. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. In Utero Programming of Chronic Disease. J. Womens Med. 2009, 2, 48–53. [Google Scholar]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E.; Paules, C.; Nakaki, A. Effects of Mediterranean Diet Or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to at-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Biesalski, H.K.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in Pregnancy: Current Knowledge and Unresolved Questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West Jr, K.P.; Christian, P. Micronutrient Deficiencies in Pregnancy Worldwide: Health Effects and Prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean Diet and Incidence of and Mortality from Coronary Heart Disease and Stroke in Women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus Nutrition Intervention Randomized Trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines. 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK32813/ (accessed on 15 June 2022).
- Martínez-Hortelano, J.A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Garrido-Miguel, M.; Soriano-Cano, A.; Martínez-Vizcaíno, V. Monitoring Gestational Weight Gain and Prepregnancy BMI Using the 2009 IOM Guidelines in the Global Population: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2020, 20, 649. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P. A Systematic Analysis of Global Anemia Burden from 1990 to 2010. Blood J. Am. Soc. Hematol. 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Mégier, C.; Peoc’h, K.; Puy, V.; Cordier, A. Iron Metabolism in Normal and Pathological Pregnancies and Fetal Consequences. Metabolites 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Brown, W.J.; Powers, J.R.; Roberts, D.C. Iron Deficiency, General Health and Fatigue: Results from the Australian Longitudinal Study on Women’s Health. Qual. Life Res. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; von Haehling, S.; Doehner, W.; Banasiak, W.; et al. Iron Deficiency Predicts Impaired Exercise Capacity in Patients with Systolic Chronic Heart Failure. J. Card. Fail. 2011, 17, 899–906. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency Across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef]
- Ludwig, H.; Aapro, M.; Bokemeyer, C.; Glaspy, J.; Hedenus, M.; Littlewood, T.J.; Österborg, A.; Rzychon, B.; Mitchell, D.; Beguin, Y. A European Patient Record Study on Diagnosis and Treatment of Chemotherapy-Induced Anaemia. Support. Care Cancer 2014, 22, 2197–2206. [Google Scholar] [CrossRef]
- Comín-Colet, J.; Martín Lorenzo, T.; González-Domínguez, A.; Oliva, J.; Jiménez Merino, S. Impact of Non-Cardiovascular Comorbidities on the Quality of Life of Patients with Chronic Heart Failure: A Scoping Review. Health Qual. Life Outcomes 2020, 18, 329. [Google Scholar] [CrossRef]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Anaemia Estimates, 2021 Edition. Anaemia in Women and Children. 2021. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 18 February 2022).
- Milman, N.; Taylor, C.L.; Merkel, J.; Brannon, P.M. Iron Status in Pregnant Women and Women of Reproductive Age in Europe. Am. J. Clin. Nutr. 2017, 106, 1655S–1662S. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Api, O.; Breyman, C.; Çetiner, M.; Demir, C.; Ecder, T. Diagnosis and Treatment of Iron Deficiency Anemia during Pregnancy and the Postpartum Period: Iron Deficiency Anemia Working Group Consensus Report. Turk. J. Obstet. Gynecol. 2015, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M. A Short Screener is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- World Health Organization. Iron Deficiency Anemia. Assessment, Prevention, and Control. In A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2001; pp. 47–62. [Google Scholar]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Haslam, N.; Lock, R.J.; Unsworth, D.J. Coeliac Disease, Anaemia and Pregnancy. Clin. Lab. 2001, 47, 467–469. [Google Scholar] [PubMed]
- Auerbach, M.; Abernathy, J.; Juul, S.; Short, V.; Derman, R. Prevalence of Iron Deficiency in First Trimester, Nonanemic Pregnant Women. J. Matern.-Fetal Neonatal Med. 2021, 34, 1002–1005. [Google Scholar] [CrossRef]
- Hollowell, J.G.; van Assendelft, O.W.; Gunter, E.W.; Lewis, B.G.; Najjar, M.; Pfeiffer, C. Hematological and Iron-Related Analytes—Reference Data for Persons Aged 1 Year and Over: United States, 1988-94. In Vital and Health Statistics. Series 11, Data from the National Health Survey; US Department of Health and Human Services, Public Health Service, National Center for Health Statistics: Washington, DC, USA, 2005; pp. 1–156. [Google Scholar]
- Teichman, J.; Nisenbaum, R.; Lausman, A.; Sholzberg, M. Suboptimal Iron Deficiency Screening in Pregnancy and the Impact of Socioeconomic Status in a High-Resource Setting. Blood Adv. 2021, 5, 4666–4673. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Fisher, A.L.; Nemeth, E. Iron Homeostasis during Pregnancy. Am. J. Clin. Nutr. 2017, 106, 1567S–1574S. [Google Scholar] [CrossRef]
- Cao, C.; O’Brien, K.O. Pregnancy and Iron Homeostasis: An Update. Nutr. Rev. 2013, 71, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lou, J.; Rao, R.; Georgieff, M.K.; Kaciroti, N.; Felt, B.T.; Zhao, Z.; Lozoff, B. Maternal Serum Ferritin Concentration is Positively Associated with Newborn Iron Stores in Women with Low Ferritin Status in Late Pregnancy. J. Nutr. 2012, 142, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient Requirements during Pregnancy and Lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Milman, N. Postpartum Anemia II: Prevention and Treatment. Ann. Hematol. 2012, 91, 143–154. [Google Scholar] [CrossRef]
- Abu-Ouf, N.M.; Jan, M.M. The Impact of Maternal Iron Deficiency and Iron Deficiency Anemia on Child’s Health. Saudi Med. J. 2015, 36, 146. [Google Scholar] [CrossRef]
- Lee, K.A.; Zaffke, M.E.; Baratte-Beebe, K. Restless Legs Syndrome and Sleep Disturbance during Pregnancy: The Role of Folate and Iron. J. Women’s Health Gend.-Based Med. 2001, 10, 335–341. [Google Scholar] [CrossRef]
- Azami, M.; Badfar, G.; Khalighi, Z.; Qasemi, P.; Shohani, M.; Soleymani, A.; Abbasalizadeh, S. The Association between Anemia and Postpartum Depression: A Systematic Review and Meta-Analysis. Casp. J. Intern. Med. 2019, 10, 115. [Google Scholar]
- Sentilhes, L.; Maillard, F.; Brun, S.; Madar, H.; Merlot, B.; Goffinet, F.; Deneux-Tharaux, C. Risk Factors for Chronic Post-Traumatic Stress Disorder Development One Year after Vaginal Delivery: A Prospective, Observational Study. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Darmstadt, G.L.; Hasan, B.S.; Haws, R.A. Community-Based Interventions for Improving Perinatal and Neonatal Health Outcomes in Developing Countries: A Review of the Evidence. Pediatrics 2005, 115, 519–617. [Google Scholar] [CrossRef]
- Scholl, T.O.; Johnson, W.G. Folic Acid: Influence on the Outcome of Pregnancy. Am. J. Clin. Nutr. 2000, 71, 1295S–1303S. [Google Scholar] [CrossRef] [PubMed]
- Gambling, L.; Danzeisen, R.; Fosset, C.; Andersen, H.S.; Dunford, S.; Srai, S.K.S.; MCArdle, H.J. Iron and Copper Interactions in Development and the Effect on Pregnancy Outcome. J. Nutr. 2003, 133, 1554S–1556S. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F. Low Birth Weight in the United States. Am. J. Clin. Nutr. 2007, 85, 584S–590S. [Google Scholar] [CrossRef] [PubMed]
- Felt, B.T.; Lozoff, B. Brain Iron and Behavior of Rats are Not Normalized by Treatment of Iron Deficiency Anemia during Early Development. J. Nutr. 1996, 126, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Kamal, M.; Bener, H.Z.; Bhugra, D. Higher Prevalence of Iron Deficiency as Strong Predictor of Attention Deficit Hyperactivity Disorder in Children. Ann. Med. Health Sci. Res. 2014, 4, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, J. Mentoring and Precepting Lactation Consultants. J. Hum. Lact. 2007, 23, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Simpong, N.L.; Afefa, C.T.; Yimpuri, L.; Akum, B.; Safo, A.; Edziah, S.; Simpong, D.L.; Adu, P. Establishing Pregnancy-Specific Haematological Reference Intervals in Ghana; a Three-Center Cross-Sectional Study. PLoS ONE 2023, 18, e0274422. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.M.; Billah, S.M.; Lyons, G.R.; Siraj, M.S.; Rahman, Q.S.; Thorsten, V.; McClure, E.M.; Haque, R.; Petri, W.A. U-Shaped Association between Maternal Hemoglobin and Low Birth Weight in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2022, 106, 424. [Google Scholar] [CrossRef]
- Dewey, K.G.; Oaks, B.M. U-Shaped Curve for Risk Associated with Maternal Hemoglobin, Iron Status, Or Iron Supplementation. Am. J. Clin. Nutr. 2017, 106, 1694S–1702S. [Google Scholar] [CrossRef]
- Abeysena, C.; Jayawardana, P.; Seneviratne, R.D.A. Maternal Haemoglobin Level at Booking Visit and its Effect on Adverse Pregnancy Outcome. Aust. New Zealand J. Obstet. Gynaecol. 2010, 50, 423–427. [Google Scholar] [CrossRef]
- Díaz-López, A.; Ribot, B.; Basora, J.; Arija, V. High and Low Haemoglobin Levels in Early Pregnancy are Associated to a Higher Risk of Miscarriage: A Population-Based Cohort Study. Nutrients 2021, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, A.; Suzuki, E.; Takayama, Y. Post-partum Anemia and Factors that Work against Alleviation of the Anemia. Jpn. J. Nurs. Sci. 2015, 12, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Arnedillo-Sánchez, S.; de la Osa, R.M.; Arnedillo-Sánchez, I. Unhealthy Gestational Weight Gain: Are We Neglecting Inadequate Gestational Weight Gain? Midwifery 2022, 107, 103277. [Google Scholar] [CrossRef]
- Dude, A.M.; Grobman, W.; Haas, D.; Mercer, B.M.; Parry, S.; Silver, R.M.; Wapner, R.; Wing, D.; Saade, G.; Reddy, U. Gestational Weight Gain and Pregnancy Outcomes among Nulliparous Women. Am. J. Perinatol. 2021, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.G.; Bhatia, V.; Nayak, R. Maternal Nutrition and Inadequate Gestational Weight Gain in Relation to Birth Weight: Results from a Prospective Cohort Study in India. Clin. Nutr. Res. 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Padro, L.; Benacer, R.; Foix, S.; Maestre, E.; Murillo, S.; Sanvicens, E.; Somoza, D.; Ngo, J.; Cervera, P. Assessment of Dietary Adequacy for an Elderly Population Based on a Mediterranean Model. J. Nutr. Health Aging 2002, 6, 31–33. [Google Scholar] [PubMed]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean Diet and Nutritional Adequacy: A Review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Feart, C.; Alles, B.; Merle, B.; Samieri, C.; Barberger-Gateau, P. Adherence to a Mediterranean Diet and Energy, Macro-, and Micronutrient Intakes in Older Persons. J. Physiol. Biochem. 2012, 68, 691–700. [Google Scholar] [CrossRef]
- Mascitelli, L.; Goldstein, M.R.; Zacharski, L.R. The Mediterranean Diet and body iron stores. In The Mediterranean Diet; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–269. [Google Scholar]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns during Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; Serrano Delgado, V.D.L.C.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Rawal, S. Dietary Iron Intake, Iron Status, and Gestational Diabetes. Am. J. Clin. Nutr. 2017, 106, 1672S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Agustina, R.; Black, R.E.; Christian, P.; Dewey, K.G.; Kraemer, K.; Shankar, A.H.; Smith, E.R.; Thorne-Lyman, A.; Tumilowicz, A. Multiple Micronutrient Supplements Versus Iron-folic Acid Supplements and Maternal Anemia Outcomes: An Iron Dose Analysis. Ann. N. Y. Acad. Sci. 2022, 1512, 114–125. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Composition of a Multi-Micronutrient Supplement to Be Used in Pilot Programmes among Pregnant Women in Developing Countries; UNICEF: New York, NY, USA, 1999. [Google Scholar]
- Yakoob, M.Y.; Khan, Y.P.; Bhutta, Z.A. Maternal Mineral and Vitamin Supplementation in Pregnancy. Expert Rev. Obstet. Gynecol. 2010, 5, 241–256. [Google Scholar] [CrossRef]
- Braithwaite, V.S.; Crozier, S.R.; D’Angelo, S.; Prentice, A.; Cooper, C.; Harvey, N.C.; Jones, K.S.; MAVIDOS Trial Group. The Effect of Vitamin D Supplementation on Hepcidin, Iron Status, and Inflammation in Pregnant Women in the United Kingdom. Nutrients 2019, 11, 190. [Google Scholar] [CrossRef]
- Ladipo, O.A. Nutrition in Pregnancy: Mineral and Vitamin Supplements. Am. J. Clin. Nutr. 2000, 72, 280S–290S. [Google Scholar] [CrossRef]
- Suharno, D.; Karyadi, D.; West, C.E.; Hautvast, J.G. Supplementation with Vitamin A and Iron for Nutritional Anaemia in Pregnant Women in West Java, Indonesia. Lancet 1993, 342, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
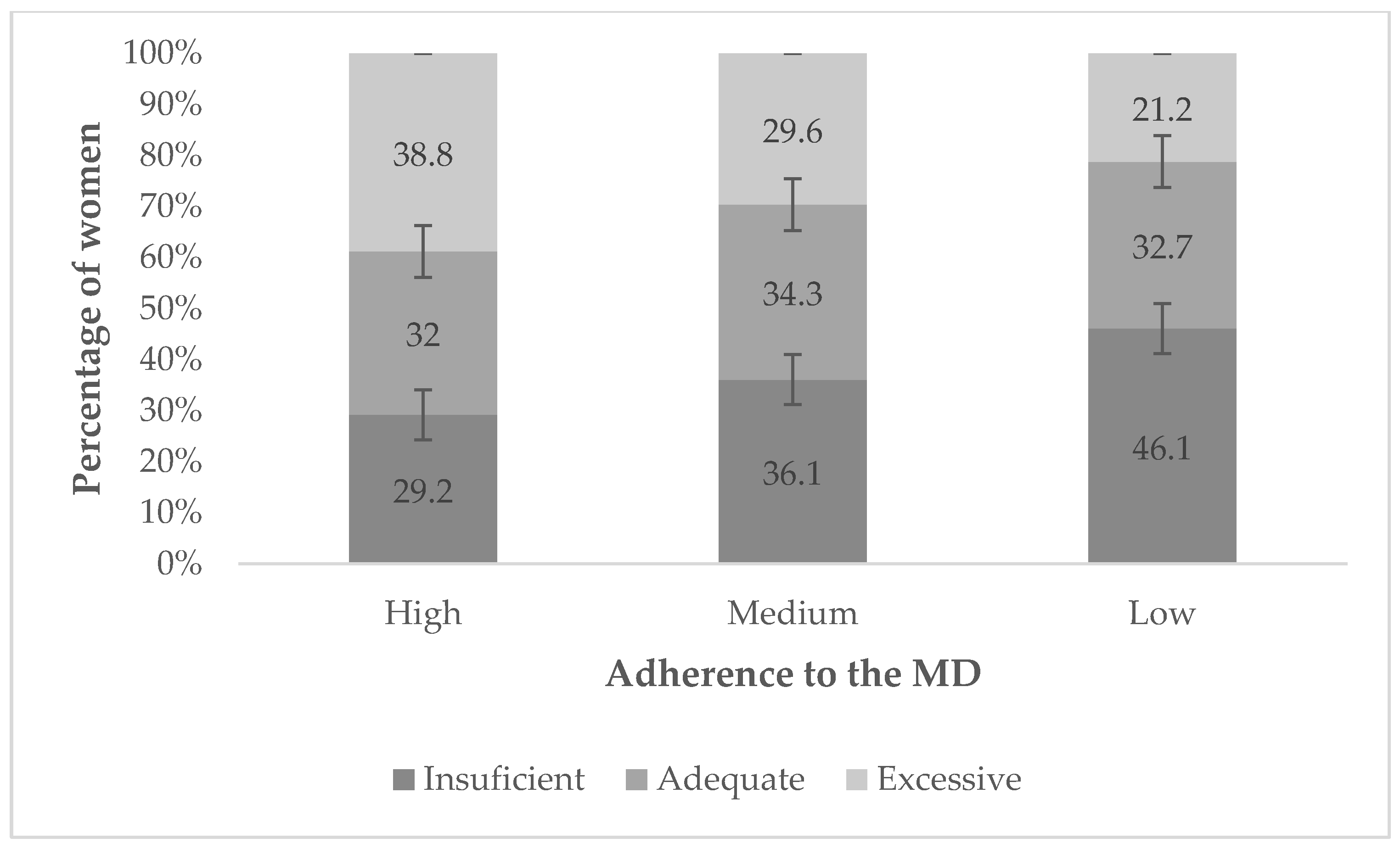
| High Adherence (Score ≥ 10) n = 116 (22.9%) | Medium Adherence (Score 6–9) n = 277 (54.7%) | Low Adherence (Score 0–5) n = 113 (22.3%) | ||
|---|---|---|---|---|
| n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | p-Value * | |
| Age (years) | 34.19 ± 5.38 | 32.36 ± 5.58 | 31.42 ± 6.23 | 0.001 |
| <35 years | 65 (56.5%) | 192 (69.3%) | 77 (68.1%) | 0.049 |
| ≥35 years | 50 (43.5%) | 85 (30.7%) | 36 (31.9%) | 0.049 |
| Origin | 0.057 | |||
| European | 100 (86.2%) | 216 (78.0%) | 99 (87.6%) | 0.043 |
| American | 8 (6.9%) | 61 (22.0%) | 14 (12.4%) | 0.004 |
| Asian | 8 (6.9%) | 0 (0.0%) | 0 (0.0%) | - |
| Educational Level | 0.001 | |||
| No studies | 6 (5.2%) | 9 (3.2%) | 1 (0.9%) | 0.178 |
| Primary | 12 (10.3%) | 34 (12.3%) | 21 (18.6%) | 0.143 |
| Secondary | 37 (31.9%) | 134 (48.4%) | 52 (46.0%) | 0.009 |
| Tertiary | 43 (37.1%) | 90 (32.5%) | 36 (31.9%) | 0.629 |
| Post-graduate | 18 (15.5%) | 10 (3.6%) | 3 (2.7%) | 0.001 |
| Height (m) | 1.64 ± 0.06 | 1.62 ± 0.61 | 1.62 ±0.58 | 0.707 |
| Pre-pregnancy weight (kg) | 64.32 ± 11.85 | 63.87 ± 13.08 | 64.92 ± 13.09 | 0.762 |
| Weight first trimester (kg) | 63.63 ± 13.00 | 65.70 ± 13.68 | 66.92 ± 12.33 | 0.360 |
| Weight second trimester (kg) | 68.61 ± 10.98 | 70.23 ± 13.76 | 72.00 ± 11.42 | 0.349 |
| Weight third trimester (kg) | 75.42 ± 12.44 | 75.76 ± 13.57 | 77.89 ± 12.68 | 0.283 |
| Gestational weight gain (kg) | 11.14 ± 4.67 | 11.89 ± 4.85 | 12.49 ± 4.92 | 0.122 |
| Insufficient | 34 (29.2%) | 100 (36.1%) | 52 (46.1%) | 0.030 |
| Adequate | 37 (32.0%) | 95 (34.3%) | 37 (32.7%) | 0.887 |
| Excessive | 45 (38.8%) | 82 (29.6%) | 24 (21.2%) | 0.014 |
| Pre-pregnancy BMI | 23.81 ± 3.74 | 24.03 ± 5.29 | 24.48 ± 5.37 | 0.589 |
| Underweight (≤18.4) | 8 (7.0%) | 17 (6.2%) | 5 (4.5%) | 0.713 |
| Normal weight (18.5–24.9) | 69 (60.5%) | 165 (59.8%) | 63 (56.3%) | 0.806 |
| Overweight (25.0–29.9) | 29 (25.4%) | 60 (21.7%) | 28 (25.0%) | 0.691 |
| Obesity (≥30) | 8 (7.0%) | 34 (12.3%) | 16 (14.3%) | 0.137 |
| BMI first trimester | 27.88 ± 3.87 | 28.43 ± 5.63 | 29.45 ± 5.20 | 0.104 |
| BMI second trimester | 25.58 ± 3.56 | 26.84 ± 5.27 | 27.41 ± 4.33 | 0.092 |
| BMI third trimester | 27.69 ± 4.60 | 28.01 ± 5.63 | 28.17 ± 3.55 | 0.961 |
| Glucose (mg/dL) first trimester | 74.22 ± 9.52 | 77.65 ± 6.26 | 82.31 ± 10.14 | 0.028 |
| Glucose (mg/dL) second trimester | 69.71 ± 9.62 | 74.33 ± 14.65 | 75.16 ± 8.51 | 0.611 |
| Glucose (mg/dL) third trimester | 74.50 ± 14.47 | 79.95 ± 15.68 | 75.10 ± 6.45 | 0.172 |
| O’Sullivan glucose (mg/dL) first trimester | 141.33 ± 30.87 | 119.07 ± 3249 | 129.28 ± 33.09 | 0.067 |
| O´Sullivan glucose (mg/dL) second trimester | 145.38 ± 33.80 | 142.58 ± 48.81 | 141.67 ± 36.24 | 0.980 |
| Physical activity (leisure) | 0.005 | |||
| None | 20 (17.2%) | 83 (30.0%) | 42 (37.8%) | 0.001 |
| Light | 76 (65.5%) | 162 (58.5%) | 60 (53.2%) | 0.158 |
| Moderate | 20 (17.2%) | 27 (9.7%) | 8 (7.2%) | 0.031 |
| Intense | 0 (0.0%) | 5 (1.8%) | 1 (0.9%) | 0.828 |
| Physical activity (work) | 0.523 | |||
| None | 31 (26.7%) | 88 (31.8%) | 38 (33.9%) | 0.488 |
| Light | 85 (73.3%) | 187 (67.5%) | 74 (66.1%) | 0.451 |
| Moderate | 0 (0.0%) | 2 (0.7%) | 0 (0.0%) | - |
| Intense | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Tobacco | 11 (9.6%) | 49 (18.2%) | 12 (11.0%) | 0.046 |
| Alcohol | 0 (0.0%) | 9 (3.3%) | 6 (5.3%) | 0.160 |
| Folic acid supplement | 70 (60.3%) | 169 (61.2%) | 56 (50.0%) | 0.114 |
| Iron supplement | 12 (85.7%) | 57 (90.5%) | 30 (93.8%) | 0.678 |
| Vitamin supplement | 98 (86.0%) | 201 (73.6%) | 87 (77.7%) | 0.030 |
| Nutritional supplement | 64 (62.1%) | 125 (56.1%) | 56 (59.6%) | 0.563 |
| Previous pregnancy loss | 31 (27.0%) | 94 (34.2%) | 44 (39.3%) | 0.065 |
| Nulliparous | 59 (51.3%) | 146 (53.3%) | 51 (45.5%) | 0.798 |
| Time between pregnancies (months) | 59.83 ± 6.67 | 53.24 ± 4.2 | 56.65 ± 5.8 | 0.939 |
| High Adherence (Score ≥ 10) n = 116 (22.9%) | Medium Adherence (Score 6–9) n = 277 (54.7%) | Low Adherence (Score 0–5) n = 113 (22.3%) | ||
|---|---|---|---|---|
| n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | p-Value * | |
| Sex | 0.280 | |||
| Male | 67 (57.8%) | 141 (50.9%) | 50 (44.2%) | |
| Female | 49 (42.2%) | 136 (49.1%) | 63 (55.8%) | |
| Gestational age (weeks) | 39.24 ± 1.39 | 39.14 ± 2.04 | 39.10 ± 1.71 | 0.821 |
| Birth weight (g) | 3270.75 ± 400.69 | 3155.38 ± 487.54 | 3203.29 ± 593.79 | 0.247 |
| Low <2500 g | 0 (0.0%) | 18 (6.5%) | 7 (6.2%) | 0.911 |
| Average 2500–4000 g | 110 (94.8%) | 254 (91.7%) | 98 (86.7%) | 0.089 |
| High >4000 g | 6 (5.2%) | 5 (1.8%) | 8 (7.1%) | 0.029 |
| Length | 50.80 ± 1.47 | 50.2 1± 1.92 | 50.22 ± 2.33 | 0.247 |
| Head circumference | 34.07 ± 1.31 | 33.97 ± 1.35 | 34.09 ± 1.38 | 0.776 |
| Newborn classification | 0.659 | |||
| Small (SGA) | 8 (6.9%) | 31 (11.2%) | 12 (10.6%) | 0.425 |
| Adequate (AGA) | 95 (81.9%) | 223 (80.5%) | 86 (76.1%) | 0.506 |
| Large (LGA) | 13 (11.2%) | 23 (8.3%) | 15 (13.3%) | 0.301 |
| Vaginal delivery | 83 (71.6%) | 130 (46.9%) | 39 (34.5%) | 0.001 |
| Cesarean section | 33 (28.4%) | 147 (53.1%) | 74 (65.5%) | 0.001 |
| Admission to NICU | 2 (1.7%) | 2 (0.7%) | 0 (0.0%) | 0.338 |
| Admission to SCNU | 11 (9.5%) | 16 (5.8%) | 7 (6.2%) | 0.407 |
| High Adherence (Score ≥ 10) n = 116 (22.9%) | Medium Adherence (Score 6–9) n = 277 (54.7%) | Low Adherence (Score 0–5) n = 113 (22.3%) | ||
|---|---|---|---|---|
| n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | p-Value * | |
| First trimester | ||||
| Hematocrit % | 38.85 ± 2.96 | 37.73 ± 2.59 | 37.97 ± 3.22 | 0.548 |
| Red blood cells 1012/L | 4.55 ± 0.46 | 4.21 ± 0.32 | 4.26 ± 0.36 | 0.030 |
| Hemoglobin g/dL | 12.81 ± 1.00 | 12.61 ± 095 | 12.80 ± 1.03 | 0.727 |
| Hemoglobin level < 11 g/dL | 0 (0.0%) | 22 (7.9%) | 5 (4.4%) | 0.594 |
| Iron mcg/dL | 96.50 ± 47.39 | 88.60 ± 34.36 | 89.00 ± 33.16 | 0.825 |
| Ferritin g/L | 61.70 ± 6.03 | 55.96 ± 3. 68 | 41.17 ± 2.20 | 0.303 |
| Ferritin level < 30 g/L | 12 (10.3%) | 71 (25.6%) | 38 (33.6%) | 0.396 |
| Second trimester | ||||
| Hematocrit % | 34.73 ± 2.08 | 34.29 ± 2.41 | 34.61 ± 2.42 | 0.793 |
| Red blood cells 1012/L | 3.99 ± 0.28 | 3.72 ± 0.25 | 3.78 ± 0.30 | 0.017 |
| Hemoglobin g/dL | 11.45 ± 0.90 | 11.48 ± 0.82 | 11.59 ± 0.82 | 0.837 |
| Hemoglobin level < 10.5 g/dL | 23 (19.8%) | 47 (16.9%) | 9 (8.0%) | 0.030 |
| Iron mcg/dL | 87.67 ± 57.78 | 87.72 ± 36.6 | 80.40 ± 2806 | 0.720 |
| Ferritin g/L | 31.42 ± 4.20 | 25.03 ± 15.70 | 25.90 ± 18.80 | 0.703 |
| Ferritin level < 30 g/L | 81 (69.8%) | 206 (74.3%) | 81 (71.7%) | 0.994 |
| Third trimester | ||||
| Hematocrit % | 36.91 ± 2.78 | 36.46 ± 2.25 | 35.45 ± 3.14 | 0.131 |
| Red blood cells 1012/L | 4.22 ± 0.29 | 3.96 ± 0.26 | 3.92 ± 0.37 | 0.007 |
| Hemoglobin g/dL | 12.14 ± 1.27 | 12.18 ± 0.86 | 11.83 ± 1.19 | 0.304 |
| Hemoglobin level < 11 g/dL | 17 (14.7%) | 22 (7.9%) | 23 (20.4%) | 0.228 |
| Iron mcg/dL | 92.64 ± 6.47 | 90.32 ± 4.13 | 78.33 ± 4.37 | 0.549 |
| Ferritin g/L | 27.29 ± 22.50 | 22.31 ± 11.73 | 22.67 ± 19.09 | 0.796 |
| Ferritin level < 30 g/L | 83 (71.6%) | 225 (81.2%) | 85 (75.2%) | 0.855 |
| High Adherence (Score ≥ 10) n = 116 (22.9%) | Medium Adherence (Score 6–9) n = 277 (54.7%) | Low Adherence (Score 0–5) n = 113 (22.3%) | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | Attributable Fraction | Odds Ratio | Attributable Fraction (%) | Odds Ratio | Attributable Fraction (%) | |
| First trimester | Ref | Ref | 2.99 (1.55–5.75) | 66.6 (35.5–82.6) | 4.39 (2.15–8.96) | 77.2 (53.5–88.8) |
| 1.40 (0.09–22.15) * | 28.5 (0.0–95.5) * | 2.36 (0.12–45.75) * | 57.6 (0.0–97.8) * | |||
| 2.92 (0.32–26.70) ‡ | 65.8 (0.0–96.3) ‡ | 3.86 (0.38–38.98) * | 74.1 (0.0–97.4) ‡ | |||
| Second trimester | Ref | Ref | 1.25 (0.78–2.03) | 20.2 (0.0–50.6) | 1.09 (0.62–1.93) | 8.6 (0.0–48.3) |
| 4.13 (0.40–43.07) * | 75.8 (0.0–97.7) * | 3.09 (0.20–47.40) * | 67.6 (0.0–97.9) * | |||
| 1.23 (0.26–5.70) ‡ | 18.7 (0.0–82.5) ‡ | 1.06 (0.20–5.64) ‡ | 5.7 (0.0–82.3) ‡ | |||
| Third trimester | Ref | Ref | 1.72 (1.04–2.85) | 41.9 (3.8–64.9) | 1.21 (0.67–2.17) | 17.1 (0.0–54.0) |
| 0.03 (0.00–3002.03) * | 0.0 (0.0–100.0) * | 19,687.84 (0.32–1,205,893,473.80) * | 100.0 (0.0–100.0) * | |||
| 2.68 (0.28–25.87) ‡ | 62.7 (0.0–96.1) ‡ | 1.83 (0.19–17.66) ‡ | 45.4 (0.0–94.3) ‡ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Suárez-Varela, M.; Peraita-Costa, I.; Perales-Marín, A.; Marcos Puig, B.; Llopis-Morales, J.; Picó, Y. Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain. Life 2023, 13, 1138. https://doi.org/10.3390/life13051138
Morales-Suárez-Varela M, Peraita-Costa I, Perales-Marín A, Marcos Puig B, Llopis-Morales J, Picó Y. Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain. Life. 2023; 13(5):1138. https://doi.org/10.3390/life13051138
Chicago/Turabian StyleMorales-Suárez-Varela, María, Isabel Peraita-Costa, Alfredo Perales-Marín, Beatriz Marcos Puig, Juan Llopis-Morales, and Yolanda Picó. 2023. "Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain" Life 13, no. 5: 1138. https://doi.org/10.3390/life13051138
APA StyleMorales-Suárez-Varela, M., Peraita-Costa, I., Perales-Marín, A., Marcos Puig, B., Llopis-Morales, J., & Picó, Y. (2023). Effect of Adherence to the Mediterranean Diet on Maternal Iron Related Biochemical Parameters during Pregnancy and Gestational Weight Gain. Life, 13(5), 1138. https://doi.org/10.3390/life13051138







