Preliminary In Vitro and In Vivo Insights of In Silico Candidate Repurposed Drugs for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro BACE1 Inhibitor Screening Assay
2.2. In Vivo Testing in Mice
2.2.1. Drug Delivery in 5xFAD Mice
2.2.2. Y-Maze Test in 5xFAD Mice
2.2.3. Brain Dissection of 5xFAD Mice
2.2.4. ELISA
2.3. Statistical Analysis
2.4. Bioinformatics Functional Analysis
2.4.1. Gene Ontology Analysis
2.4.2. PPI Network Construction
3. Results
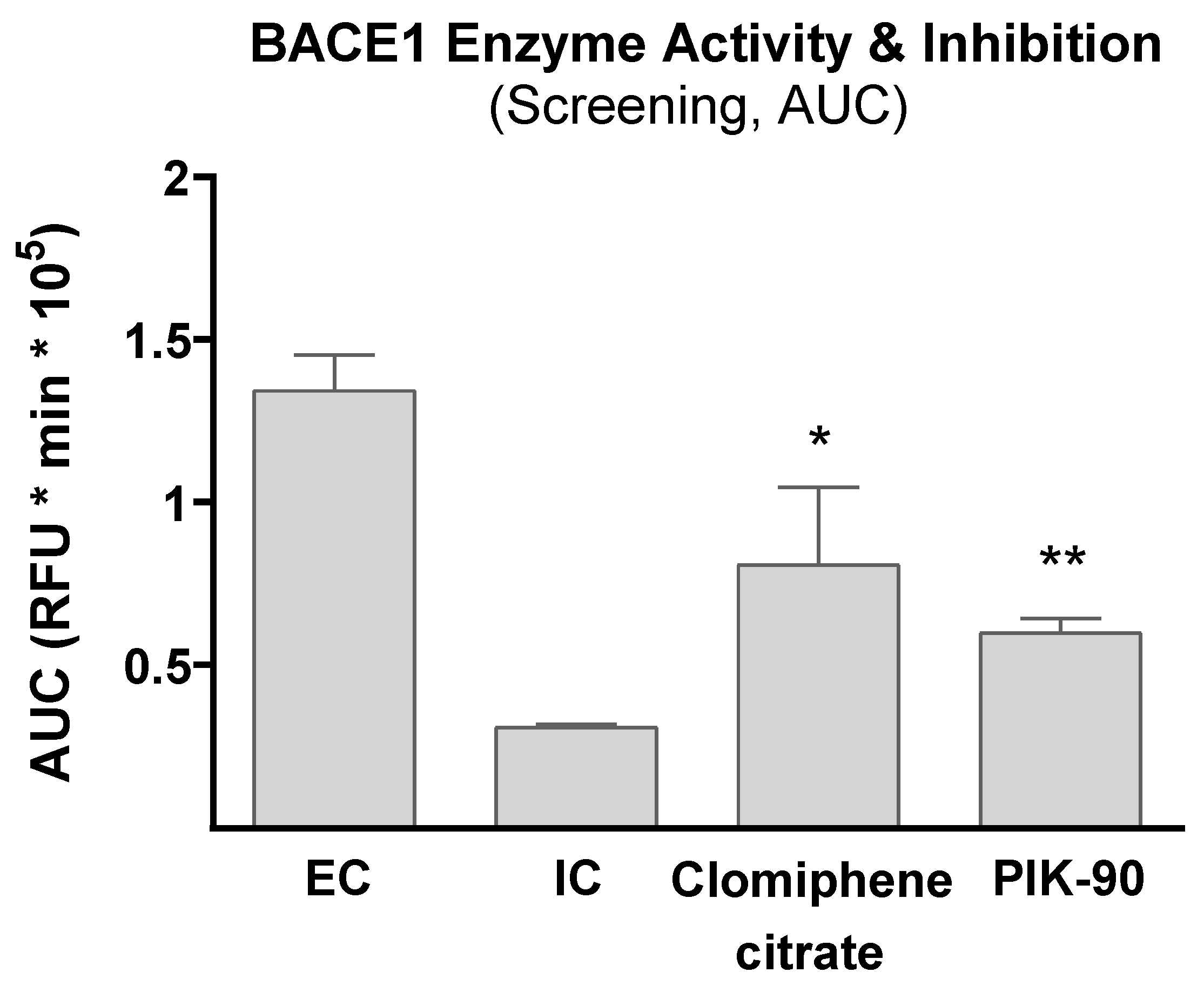
3.1. Effect of Tested Compounds on BACE1 Enzyme Activity
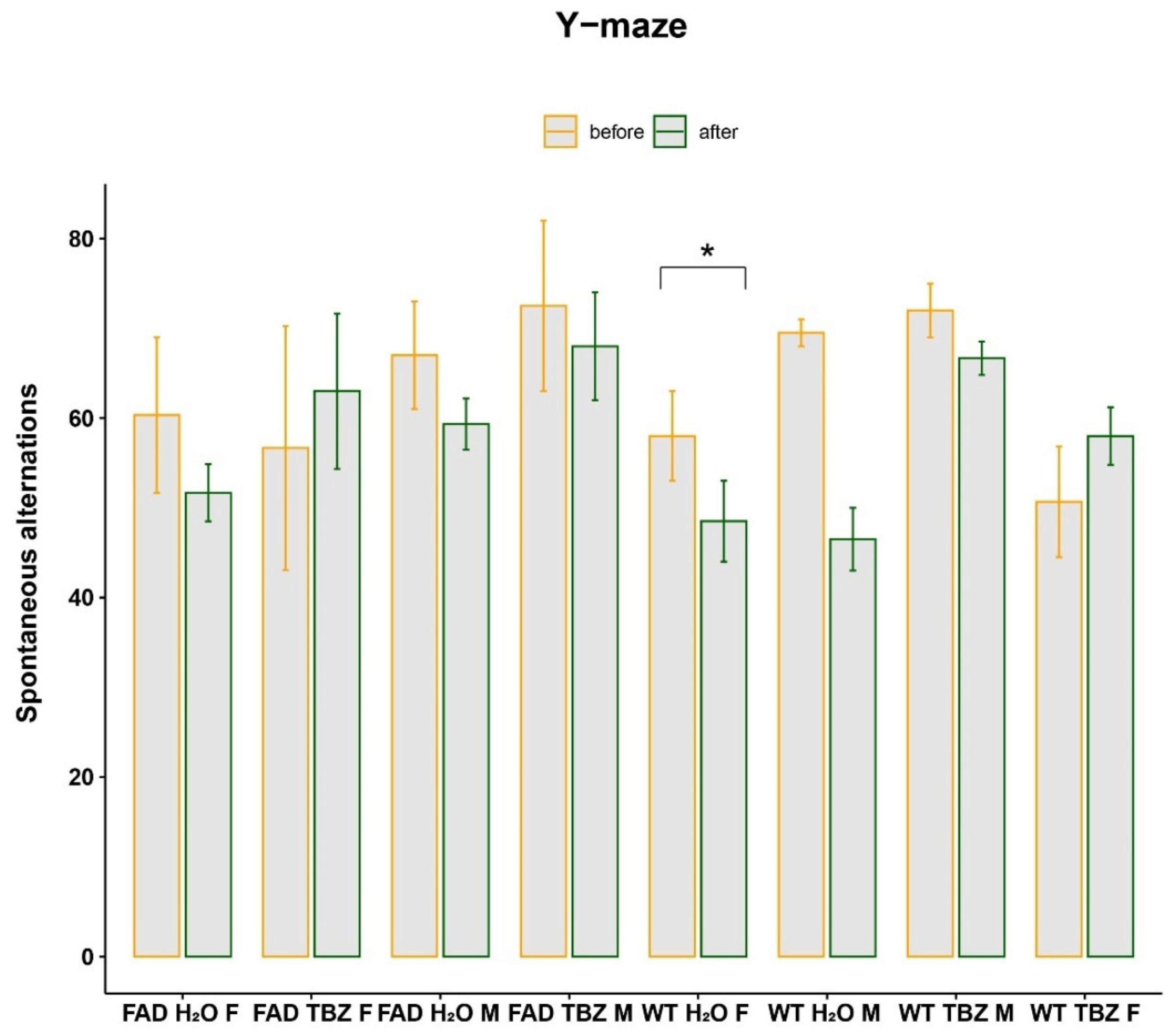
3.2. Y-Maze Test before and after Tetrabenazine Treatment in 5XFAD Mice
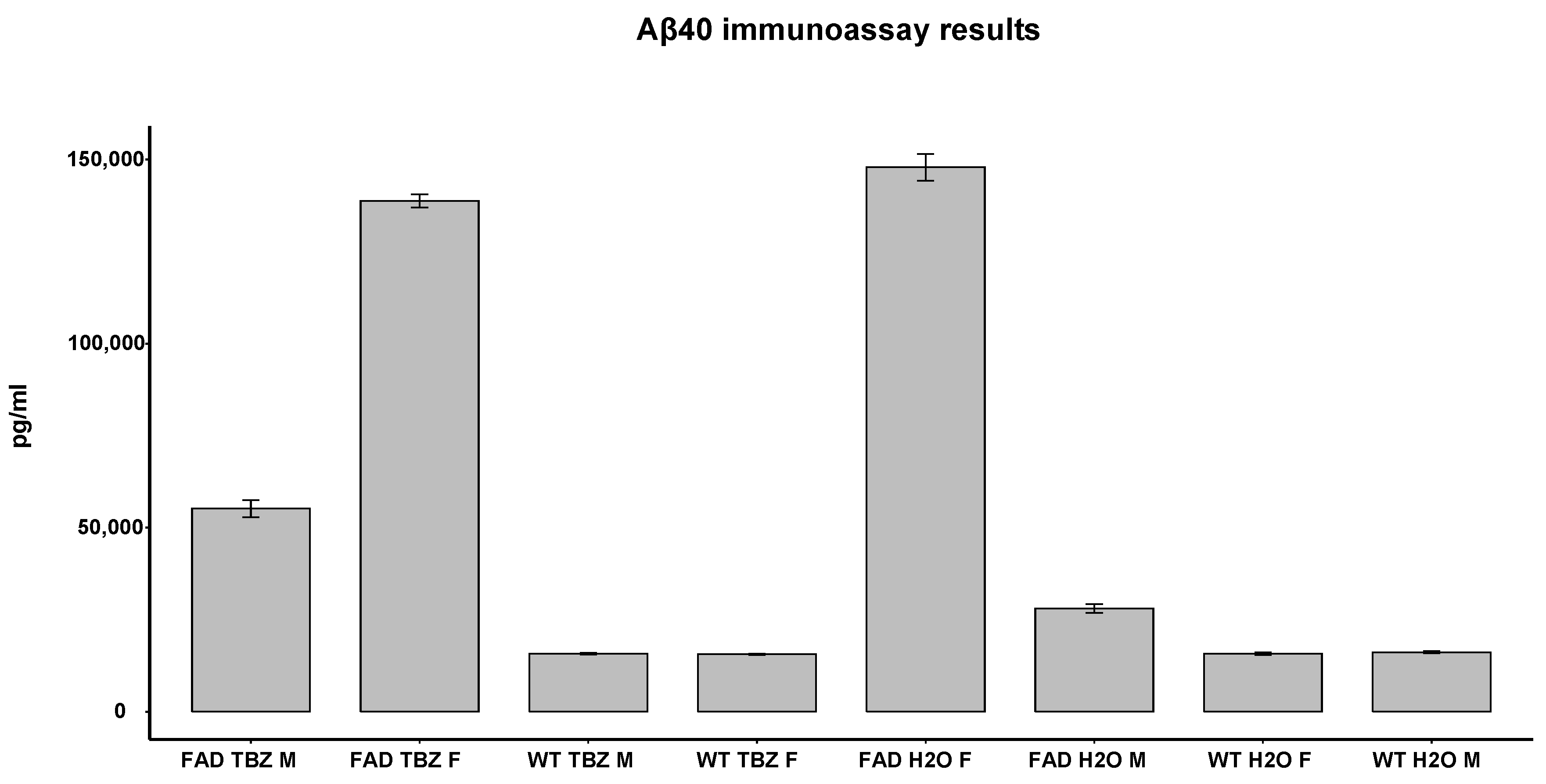
3.3. ELISA Test of Aβ Peptide in 5XFAD Mice
3.4. Bioinformatics Functional Analysis
3.4.1. Gene Ontology Analysis
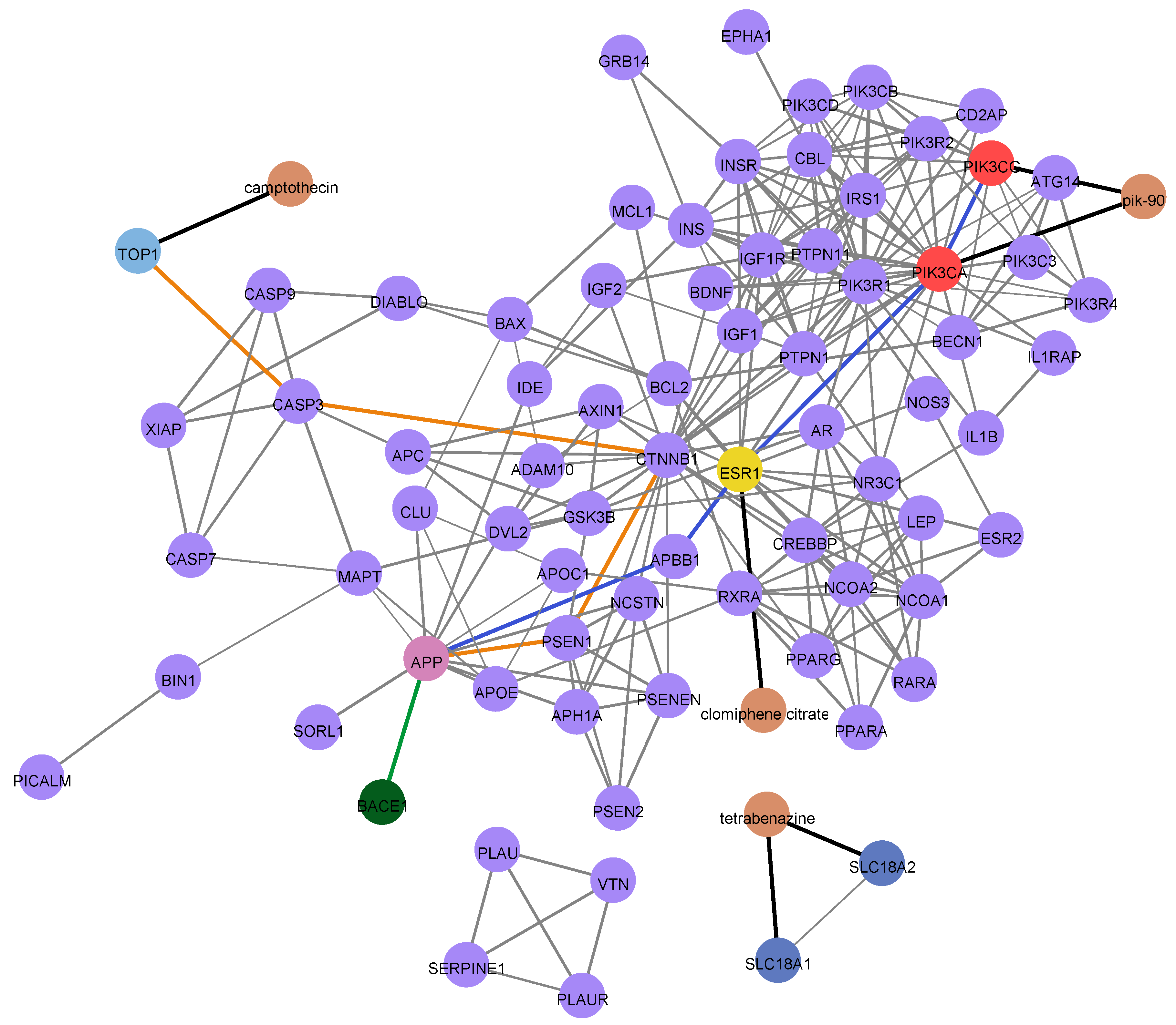
3.4.2. PPI Network Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Kiso, Y. Discovery of BACE1 Inhibitors for the Treatment of Alzheimer’s Disease. In Quantitative Structure-Activity Relationship; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Dominguez, D.; Tournoy, J.; Hartmann, D.; Huth, T.; Cryns, K.; Deforce, S.; Serneels, L.; Camacho, I.E.; Marjaux, E.; Craessaerts, K.; et al. Phenotypic and Biochemical Analyses of BACE1- and BACE2-deficient Mice. J. Biol. Chem. 2005, 280, 30797–30806. [Google Scholar] [CrossRef]
- Ohno, M.; Cole, S.L.; Yasvoina, M.; Zhao, J.; Citron, M.; Berry, R.; Disterhoft, J.F.; Vassar, R. BACE1 Gene Deletion Prevents Neuron Loss and Memory Deficits in 5XFAD APP/PS1 Transgenic Mice. Neurobiol. Dis. 2007, 26, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.F.; Maloney, J.; et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.A.; Bainbridge, T.; Gustafson, A.; Zhang, S.; Kyauk, R.; Steiner, P.; van der Brug, M.; Liu, Y.; Ernst, J.A.; Watts, R.J.; et al. Molecular Mechanisms of Alzheimer Disease Protection by the A673T Allele of Amyloid Precursor Protein. J. Biol. Chem. 2014, 289, 30990–31000. [Google Scholar] [CrossRef] [PubMed]
- Chatila, Z.K.; Kim, E.; Berlé, C.; Bylykbashi, E.; Rompala, A.; Oram, M.K.; Gupta, D.; Kwak, S.S.; Kim, Y.H.; Kim, D.Y.; et al. BACE1 Regulates Proliferation and Neuronal Differentiation of Newborn Cells in the Adult Hippocampus in Mice. eNeuro 2018, 5, ENEURO.0067-18.2018. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Qu, X.A.; Gudivada, R.C.; Jegga, A.G.; Neumann, E.K.; Aronow, B.J. Inferring novel disease indications for known drugs by semantically linking drug action and disease mechanism relationships. BMC Bioinform. 2009, 10, S4. [Google Scholar] [CrossRef]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.-H.D. Drug repositioning and repurposing: Terminology and definitions in literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Boguski, M.S.; Mandl, K.D.; Sukhatme, V.P. Repurposing with a Difference. Science 2009, 324, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Savva, K.; Zachariou, M.; Bourdakou, M.M.; Dietis, N.; Spyrou, G.M. Network-based stage-specific drug repurposing for Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2022, 20, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Leavitt, B.R.; Yasothan, U.; Kirkpatrick, P. Tetrabenazine. Nat. Rev. Drug Discov. 2009, 8, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Li, Y.; Tang, T.-S.; Bezprozvanny, I. Tetrabenazine is neuroprotective in Huntington’s disease mice. Mol. Neurodegener. 2010, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.D.; Schafer, M.K.; Bonner, T.I.; Eiden, L.E.; Weihe, E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl. Acad. Sci. USA 1996, 93, 5166–5171. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Hoggatt, A.F.; Hoggatt, J.; Honerlaw, M.; Pelus, L.M. A spoonful of sugar helps the medicine go down: A novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 329–334. [Google Scholar]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Arendash, G.W.; Gordon, M.N.; Diamond, D.M.; Austin, L.A.; Hatcher, J.M.; Jantzen, P.T.; DiCarlo, G.; Wilcock, D.M.; Morgan, D.; Hallé, M.; et al. Behavioral Assessment of Alzheimer’s Transgenic Mice Following Long-Term Ab Vaccination: Task Specificity and Correlations between Ab Deposition and Spatial Memory. DNA Cell Biol. 2001, 20, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sadleir, K.R.; Eimer, W.A.; Cole, S.L.; Vassar, R. Aβ reduction in BACE1 heterozygous null 5XFAD mice is associated with transgenic APP level. Mol. Neurodegener. 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, C.L.; Kokjohn, T.A.; Whiteside, C.M.; Macias, M.P.; Kalback, W.M.; Sabbagh, M.N.; Beach, T.G.; Vassar, R.; Roher, A.E. Molecular Differences and Similarities between Alzheimer’s Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis. Biochem. Insights 2013, 6, 1–10. [Google Scholar] [CrossRef]
- He, X.-L.; Yan, N.; Chen, X.-S.; Qi, Y.-W.; Yan, Y.; Cai, Z. Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol. Rep. 2016, 68, 975–982. [Google Scholar] [CrossRef]
- Rosenberger, A.F.N.; Hilhorst, R.; Coart, E.; Barrado, L.G.; Naji, F.; Rozemuller, A.J.M.; van der Flier, W.M.; Scheltens, P.; Hoozemans, J.J.M.; van der Vies, S.M. Protein Kinase Activity Decreases with Higher Braak Stages of Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2016, 49, 927–943. [Google Scholar] [CrossRef]
- Nasseri, S.; Ledger, W.L. Clomiphene citrate in the twenty-first century. Hum. Fertil. 2001, 4, 145–151. [Google Scholar] [CrossRef]
- Chen, J.S.; Zhou, L.J.; Entin-Meer, M.; Yang, X.; Donker, M.; Knight, Z.A.; Weiss, W.; Shokat, K.M.; Haas-Kogan, D.; Stokoe, D. Characterization of structurally distinct, isoform-selective phosphoinositide 3′-kinase inhibitors in combination with radiation in the treatment of glioblastoma. Mol. Cancer Ther. 2008, 7, 841–850. [Google Scholar] [CrossRef]
- Yaffe, K.; Krueger, K.; Cummings, S.R.; Blackwell, T.; Henderson, V.W.; Sarkar, S.; Ensrud, K.; Grady, D. Effect of Raloxifene on Prevention of Dementia and Cognitive Impairment in Older Women: The Multiple Outcomes of Raloxifene Evaluation (MORE) Randomized Trial. Am. J. Psychiatry 2005, 162, 683–690. [Google Scholar] [CrossRef]
- Henderson, V.W.; Ala, T.; Sainani, K.L.; Bernstein, A.L.; Stephenson, B.S.; Rosen, A.C.; Farlow, M.R. Raloxifene for women with Alzheimer disease. Neurology 2015, 85, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, X.; Cai, C.; Zeng, S.; Bai, J.; Guo, K.; Fang, M.; Hu, J.; Liu, H.; Zhu, L.; et al. FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Exp. Neurol. 2019, 317, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Tahirovic, S.; Bradke, F. Neuronal Polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a001644. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. PI3-kinase/Akt/mTOR signaling: Impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp. Gerontol. 2013, 48, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperon. 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Chiang, H.-C.; Wang, L.; Xie, Z.; Yau, A.; Zhong, Y. PI3 kinase signaling is involved in Aβ-induced memory loss in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 7060–7065. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.-Q.; Zhang, M.; Xin, G.-Z.; Pang, T.; Zhou, P.; Chen, J.; Qi, L.-W.; Yang, H.; Xu, X.; et al. Camptothecin and its Analogs Reduce Amyloid-β Production and Amyloid-β42-Induced IL-1β Production. J. Alzheimers Dis. 2015, 43, 465–477. [Google Scholar] [CrossRef]
- Reeves, R.R. Gatifloxacin Precipitation of Psychosis in Alzheimer Disease. Am. J. Geriatr. Psychiatry 2003, 11, 470–471. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Campbell, B. Gatifloxacin-induced delirium and psychosis in an elderly demented woman. J. Am. Geriatr. Soc. 2006, 54, 871. [Google Scholar] [CrossRef]
- Oblak, A.L.; Lin, P.B.; Kotredes, K.P.; Pandey, R.S.; Garceau, D.; Williams, H.M.; Uyar, A.; O’Rourke, R.; O’Rourke, S.; Ingraham, C.; et al. Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Front. Aging Neurosci. 2021, 13, 713726. [Google Scholar] [CrossRef]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramár, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef] [PubMed]



| Drug | AD Braak Stage | Main Indication |
|---|---|---|
| Tetrabenazine | V–VI | Huntington’s disease symptoms and other hyperkinetic movement disorders |
| Clomiphene citrate | I–II | Infertility |
| Camptothecin | I–II | Cancer treatment |
| Homoharringtonine | III–IV | Chronic myeloid leukaemia |
| Paroxetine | III–IV | Major depressive disorder, obsessive-compulsive disorder, social anxiety disorder, panic disorder, posttraumatic stress disorder, generalized anxiety disorder and premenstrual dysphoric disorder |
| V–VI | ||
| Gatifloxacin | III–IV | Bacterial infections |
| Iloperidone | V–VI | Schizophrenia |
| BI-2536 | V–VI | Experimental drug |
| Piperidolate hydrochloride | III–IV | Convulsive pain in gastrointestinal diseases |
| V–VI | ||
| Emetine (hydrochloride hydrate) | I–II | Emesis induction in acute oral poisonings |
| III–IV | ||
| Pik-90 | I–II | Experimental drug |
| Scoulerine | V–VI | Experimental drug |
| Ro-04-5595 | III–IV | Experimental drug |
| Section | Analysis | Categories | Statistical Methods |
|---|---|---|---|
| Effect of tested compounds on BACE1 enzyme activity | Comparison of the response of the top 13 drugs against IC and EC |
| One-Way ANOVA with Bonferroni multiple comparison |
| Comparison of AUC means overall inhibition of the clomiphene citrate and Pik-90 against IC and EC |
| One-Way ANOVA with Bonferroni multiple comparison | |
| Y-maze test before and after tetrabenazine treatment in 5XFAD mice | Comparison of the performance in Y-maze before and after treatment within the same group and across different groups |
| Paired (between the same group) and non-paired t-test (between different groups) |
| ELISA test of Aβ peptide in 5XFAD mice | Comparison of Aβ levels in 5XFAD and WT mice, both treated with TBZ and water |
| Unpaired two samples Wilcoxon test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savva, K.; Zachariou, M.; Kynigopoulos, D.; Fella, E.; Vitali, M.-I.; Kosofidou, X.; Spyrou, M.; Sargiannidou, I.; Panayiotou, E.; Dietis, N.; et al. Preliminary In Vitro and In Vivo Insights of In Silico Candidate Repurposed Drugs for Alzheimer’s Disease. Life 2023, 13, 1095. https://doi.org/10.3390/life13051095
Savva K, Zachariou M, Kynigopoulos D, Fella E, Vitali M-I, Kosofidou X, Spyrou M, Sargiannidou I, Panayiotou E, Dietis N, et al. Preliminary In Vitro and In Vivo Insights of In Silico Candidate Repurposed Drugs for Alzheimer’s Disease. Life. 2023; 13(5):1095. https://doi.org/10.3390/life13051095
Chicago/Turabian StyleSavva, Kyriaki, Margarita Zachariou, Demos Kynigopoulos, Eleni Fella, Maria-Ioanna Vitali, Xeni Kosofidou, Michail Spyrou, Irene Sargiannidou, Elena Panayiotou, Nikolas Dietis, and et al. 2023. "Preliminary In Vitro and In Vivo Insights of In Silico Candidate Repurposed Drugs for Alzheimer’s Disease" Life 13, no. 5: 1095. https://doi.org/10.3390/life13051095
APA StyleSavva, K., Zachariou, M., Kynigopoulos, D., Fella, E., Vitali, M.-I., Kosofidou, X., Spyrou, M., Sargiannidou, I., Panayiotou, E., Dietis, N., & Spyrou, G. M. (2023). Preliminary In Vitro and In Vivo Insights of In Silico Candidate Repurposed Drugs for Alzheimer’s Disease. Life, 13(5), 1095. https://doi.org/10.3390/life13051095







