Modern Trends in Natural Antibiotic Discovery
Abstract
1. Introduction
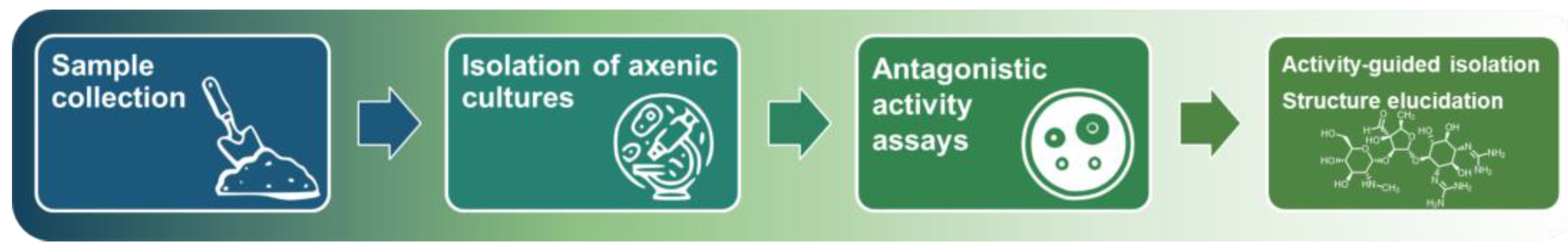
- Most of the soil microbiome is unculturable in standard lab conditions. Even for actinomycetes, classical isolation methods yield a large number of Streptomyces colonies, but other species of the class are underrepresented. As a result, we cannot evaluate the biosynthetic potential of the majority of microorganisms (the microbial dark matter problem).
- Screening of cultures with broad antimicrobial activity often yields toxic and/or well-known compounds (the re-isolation problem).
- The screening requires prolonged cultivations (to isolate axenic cultures, for test fermentations, etc.) and resource-consuming activity-guided isolation of antibiotics. In general, it cannot be adapted for fast and high-throughput screening.
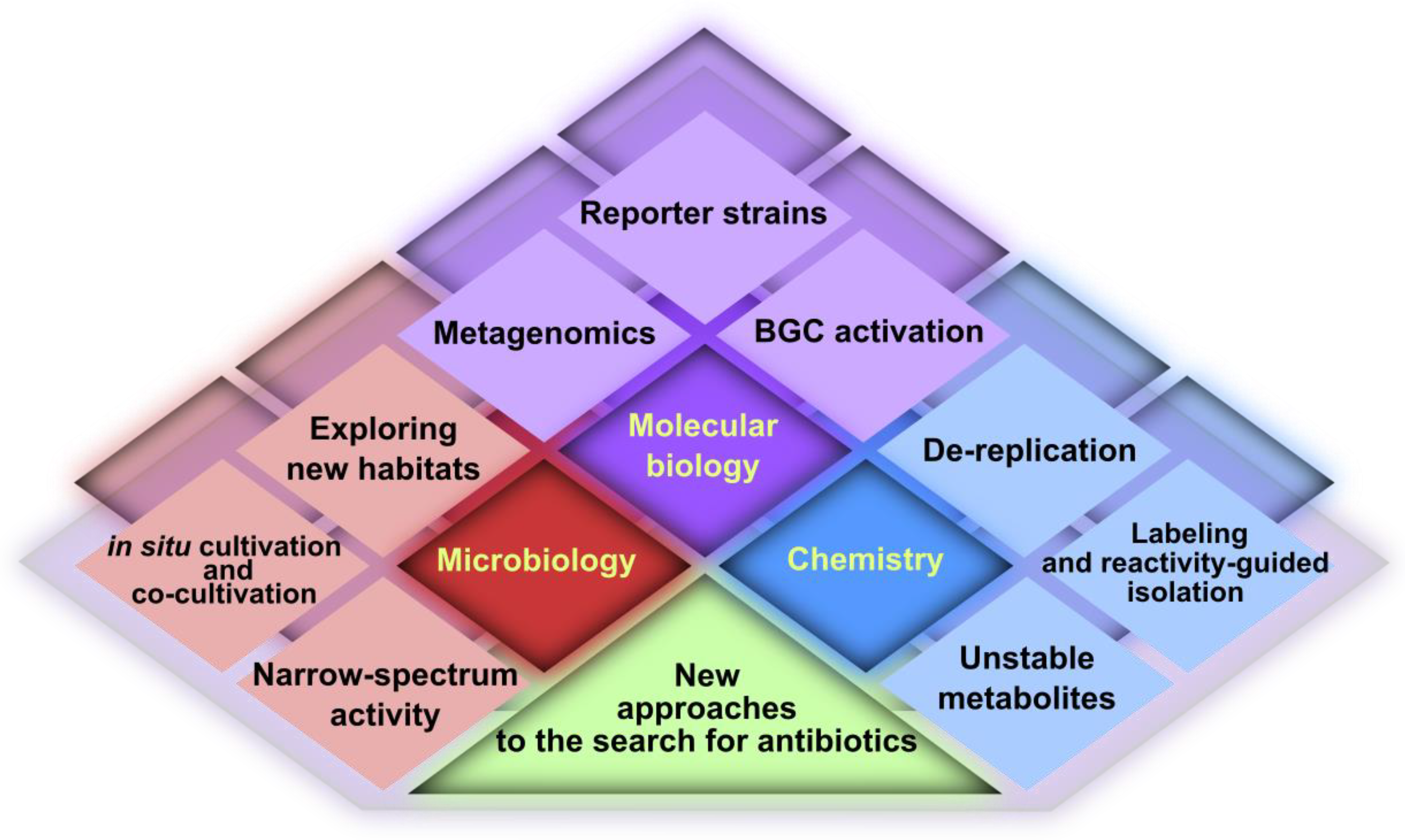
2. Microbiology
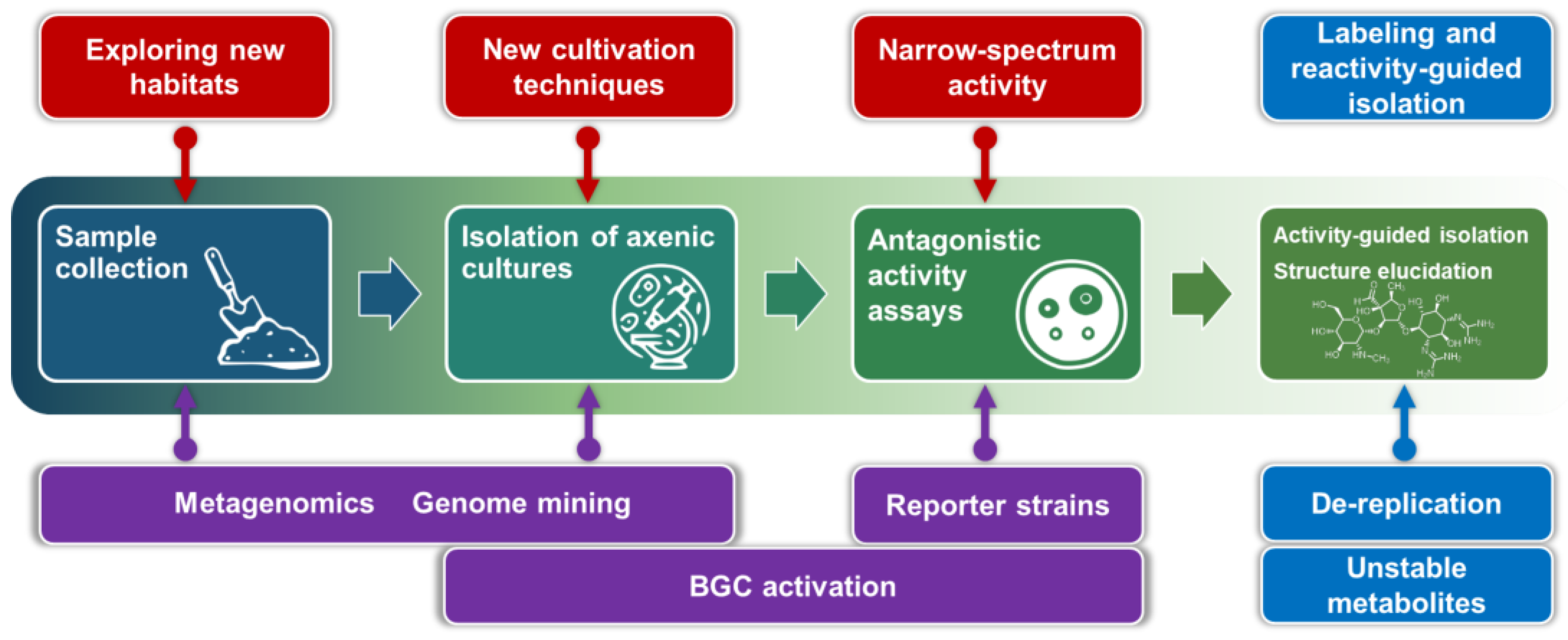
2.1. Exploring New Habitats
2.2. New Cultivation Techniques
2.2.1. The Co-Cultivation Approach
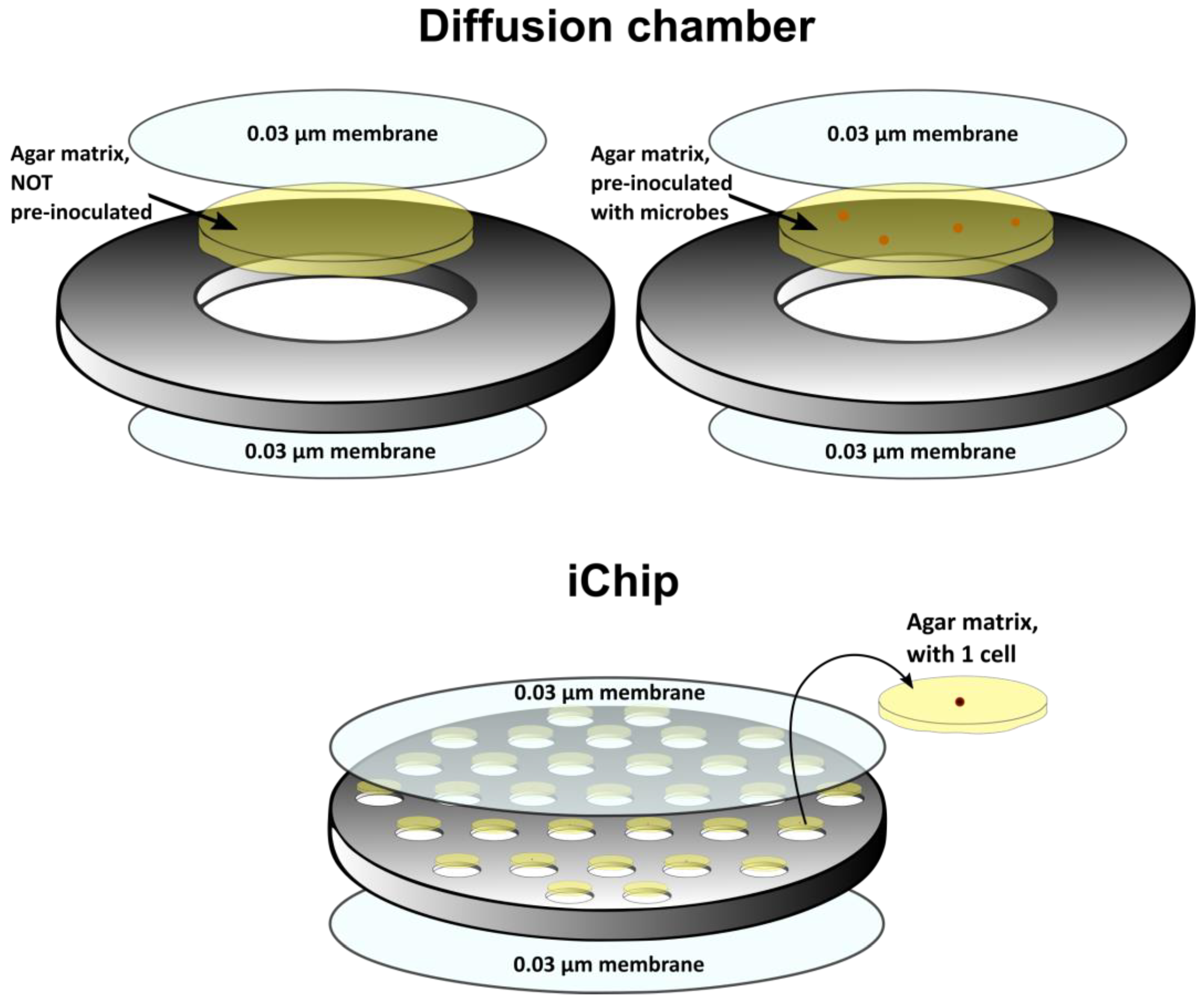
2.2.2. In Situ Cultivation
2.2.3. Microtechnology
2.3. New Approaches to Phenotypic Screening (Narrow-Spectrum Activity)
3. Molecular Biology
3.1. Metagenomic Screening and Genome Mining
3.2. Biosynthetic Gene Cluster Activation
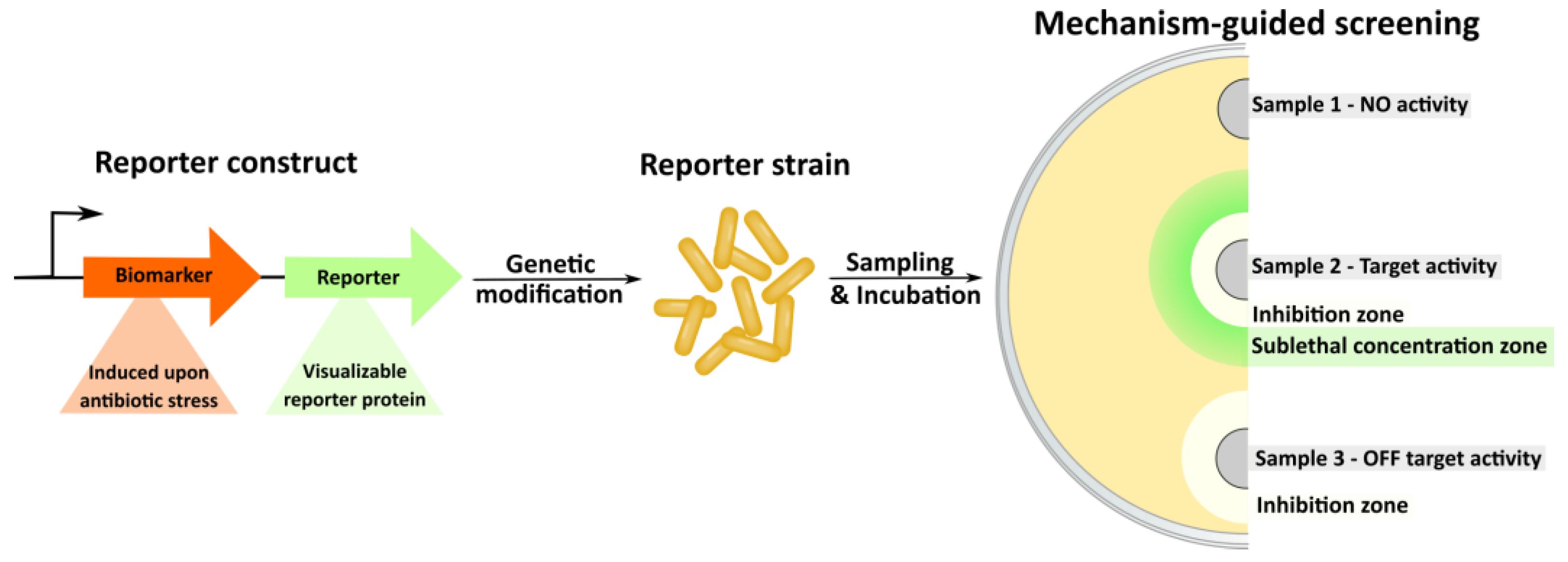
3.3. Reporter Strains and Mechanism-Guided Isolation
4. Chemistry
4.1. Dereplication
- The DEREP-NP (https://github.com/clzani/DEREP-NP accessed on 20 April 2023) platform has been developed for structural feature search in the UNPD public NMR database [186]. Later, diffusion-ordered NMR spectroscopy (DOSY)-related functionality was implemented [187].
- To decipher complex mixtures using 13C-NMR data, MixONat (https://sourceforge.net/projects/mixonat/ accessed on 20 April 2023) open-source software was developed [188].
- The MADByTE data analysis platform (Metabolomics and Dereplication by Two-Dimensional Experiments, https://github.com/liningtonlab/MADByTE accessed on 20 April 2023) for complex mixture analysis was developed. This platform employs a combination of TOCSY and HSQC spectra to identify spin system features within complex mixtures and create a chemical similarity network [189].
- Poor compatibility with the main methods of mixture separation: LC-NMR is an exotic combination, unlike LC-MS.
- Limited throughput due to the significant duration of registration of the spectra.
- Distinguishing the components of complex mixtures is difficult: the characteristic spectral range for natural compounds (0–10 ppm for 1H signals) is very narrow and it takes time to register reliable signal at a sufficient resolution in mixtures with additional correlations and/or additional computation [190,191].
4.2. Chemical Labeling and Reactivity-Guided Isolation
4.3. Methods for Detection and Isolation of Unstable Metabolites
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- World Health Organization Prioritization of Pathogenes to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, including Tuberculosis. 2017. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 20 April 2023).
- Waksman, S.A. What is an antibiotic or an antibiotic substance? Mycologia 1947, 39, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.; Bennett, J.W. What is an antibiotic? Revisited. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 52, pp. 303–331. ISBN 978-0-12-002654-8. [Google Scholar]
- Davies, J. Are antibiotics naturally antibiotics? J. Ind. Microbiol. Biotechnol. 2006, 33, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Schatz, A.; Bugie, E.; Waksman, S.A.; Hanssen, A.D.; Patel, R.; Osmon, D.R. The classic: Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Clin. Orthopaed. Rel. Res. 2005, 437, 3–6. [Google Scholar] [CrossRef]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug. Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Bernal, F.A.; Hammann, P.; Kloss, F. Natural products in antibiotic development: Is the success story over? Curr. Opin. Biotechnol. 2022, 78, 102783. [Google Scholar] [CrossRef]
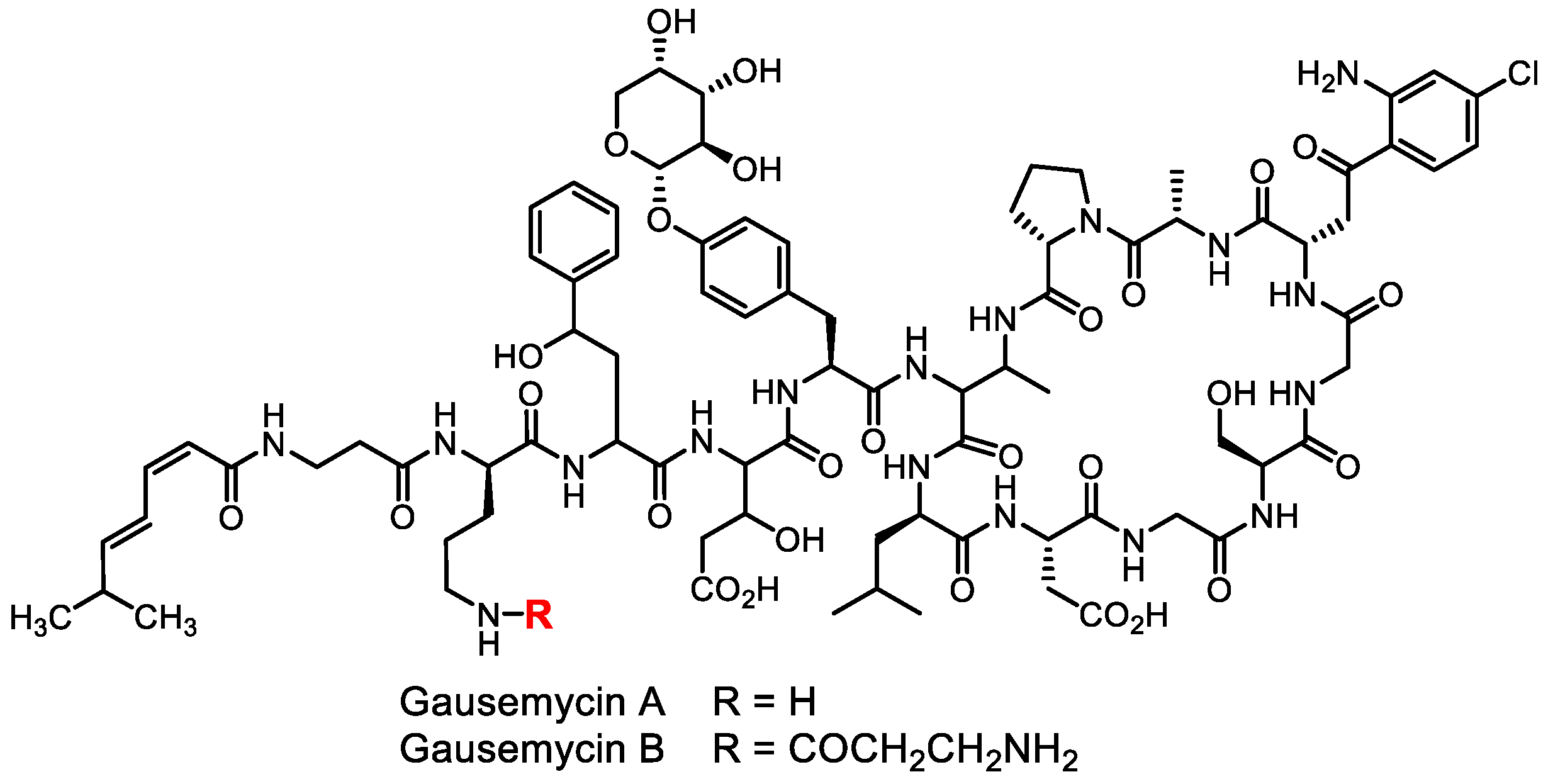
- Tyurin, A.P.; Alferova, V.A.; Paramonov, A.S.; Shuvalov, M.V.; Kudryakova, G.K.; Rogozhin, E.A.; Zherebker, A.Y.; Brylev, V.A.; Chistov, A.A.; Baranova, A.A.; et al. Gausemycins A,B: Cyclic lipoglycopeptides from Streptomyces sp. Angew. Chem. Int. Ed. 2021, 60, 18694–18703. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Diamond, S.; Butterfield, C.N.; Thomas, B.C.; Banfield, J.F. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature 2018, 558, 440–444. [Google Scholar] [CrossRef]
- Monciardini, P.; Iorio, M.; Maffioli, S.; Sosio, M.; Donadio, S. Discovering new bioactive molecules from microbial sources. Microb. Biotechnol. 2014, 7, 209–220. [Google Scholar] [CrossRef]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Ahmad, W.; Zhu, X.-Y.; Chen, J.; Austin, B. Viable but nonculturable bacteria and their resuscitation: Implications for cultivating uncultured marine microorganisms. Mar. Life Sci. Technol. 2021, 3, 189–203. [Google Scholar] [CrossRef]
- Salam, N.; Xian, W.-D.; Asem, M.D.; Xiao, M.; Li, W.-J. From ecophysiology to cultivation methodology: Filling the knowledge gap between uncultured and cultured microbes. Mar. Life Sci. Technol. 2021, 3, 132–147. [Google Scholar] [CrossRef]
- Mu, D.-S.; Ouyang, Y.; Chen, G.-J.; Du, Z.-J. Strategies for culturing active/dormant marine microbes. Mar. Life Sci. Technol. 2021, 3, 121–131. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Erdeiné Kis, Á.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- De Simeis, D.; Serra, S. Actinomycetes: A never-ending source of bioactive compounds—An overview on antibiotics production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef]
- Ezeobiora, C.E.; Igbokwe, N.H.; Amin, D.H.; Enwuru, N.V.; Okpalanwa, C.F.; Mendie, U.E. Uncovering the biodiversity and biosynthetic potentials of rare actinomycetes. Fut. J. Pharm. Sci. 2022, 8, 23. [Google Scholar] [CrossRef]
- Subramani, R.; Sipkema, D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef]
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, A.M.; Ismail, M.M.; Elsayed, T.R.; Ramadan, M.A. Recent methods for discovering novel bioactive metabolites, specifically antimicrobial agents, from marine-associated micro-organisms. Lett. Appl. Microbiol. 2022, 75, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.B.; Balachandran, L. Sources of antibiotics: Hot springs. Biochem. Pharmacol. 2017, 134, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Uma, G.; Babu, M.M.; Prakash, V.S.G.; Nisha, S.J.; Citarasu, T. Nature and bioprospecting of haloalkaliphilics: A review. World J. Microbiol. Biotechnol. 2020, 36, 66. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, S.; Hattiholi, A.; Chavadar, M.; Dodamani, S. Psychrophiles: A journey of hope. J. Biosci. 2021, 46, 64. [Google Scholar] [CrossRef]
- Baranova, A.A.; Alferova, V.A.; Korshun, V.A.; Tyurin, A.P. Antibiotics from extremophilic micromycetes. Russ. J. Bioorg. Chem. 2020, 46, 903–971. [Google Scholar] [CrossRef]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 2021, 7, e06362. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Brown, A.M.V. Deep learning approaches for natural product discovery from plant endophytic microbiomes. Environ. Microbiome 2021, 16, 6. [Google Scholar] [CrossRef]
- Jiang, Z.; Tuo, L.; Huang, D.; Osterman, I.A.; Tyurin, A.P.; Liu, S.; Lukyanov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Korshun, V.A.; et al. Diversity, novelty, and antimicrobial activity of endophytic actinobacteria from mangrove plants in Beilun Estuary National Nature Reserve of Guangxi, China. Front. Microbiol. 2018, 9, 868. [Google Scholar] [CrossRef]
- Axenov-Gibanov, D.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rebets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria isolated from an underground lake and moonmilk speleothem from the biggest conglomeratic karstic cave in siberia as sources of novel biologically active compounds. PLoS ONE 2016, 11, e0149216. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Swaileh, Z.; Ammar, M.; Jaghama, W.; Yousef, M.; Karaman, R.; Bufo, S.A.; Scrano, L. Antifungal and antibacterial activities of isolated marine compounds. Toxins 2023, 15, 93. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Esteves, A.C.; Alves, A. Marine fungi: Opportunities and challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Giddings, L.-A.; Newman, D.J. Extremophilic fungi from marine environments: Underexplored sources of antitumor, anti-infective and other biologically active agents. Mar. Drugs 2022, 20, 62. [Google Scholar] [CrossRef]
- Siro, G.; Pipite, A.; Christi, K.; Srinivasan, S.; Subramani, R. Marine actinomycetes associated with stony corals: A potential hotspot for specialized metabolites. Microorganisms 2022, 10, 1349. [Google Scholar] [CrossRef]
- Ruwandeepika, H.A.D.; Fernando, G.C.P.; Jayaweera, T.S.P. An overview of biomedical, biotechnological, and industrial applications of actinomycetes. In Natural Products from Actinomycetes; Rai, R.V., Bai, J.A., Eds.; Springer: Singapore, 2022; pp. 475–508. ISBN 9789811661310. [Google Scholar]
- Lu, Q.-P.; Huang, Y.-M.; Liu, S.-W.; Wu, G.; Yang, Q.; Liu, L.-F.; Zhang, H.-T.; Qi, Y.; Wang, T.; Jiang, Z.-K.; et al. Metabolomics tools assisting classic screening methods in discovering new antibiotics from mangrove actinomycetia in Leizhou Peninsula. Mar. Drugs 2021, 19, 688. [Google Scholar] [CrossRef]
- Shi, T.; Wang, Y.-F.; Wang, H.; Wang, B. Genus nocardiopsis: A prolific producer of natural products. Mar. Drugs 2022, 20, 374. [Google Scholar] [CrossRef]
- Gogineni, V.; Chen, X.; Hanna, G.; Mayasari, D.; Hamann, M.T. Role of symbiosis in the discovery of novel antibiotics. J. Antibiot. 2020, 73, 490–503. [Google Scholar] [CrossRef]
- Baranova, A.A.; Zakalyukina, Y.V.; Ovcharenko, A.A.; Korshun, V.A.; Tyurin, A.P. Antibiotics from insect-associated actinobacteria. Biology 2022, 11, 1676. [Google Scholar] [CrossRef]
- Masson, F.; Lemaitre, B. Growing ungrowable bacteria: Overview and perspectives on insect symbiont culturability. Microbiol. Mol. Biol. Rev. 2020, 84, e00089-20. [Google Scholar] [CrossRef]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.H.; Doyle, M.T.; Bernstein, H.D. Small molecule antibiotics inhibit distinct stages of bacterial outer membrane protein assembly. mBio 2022, 13, e02286-22. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hauk, G.; Quigley, J.; Liang, L.; Son, S.; Ghiglieri, M.; Gates, M.F.; Morrissette, M.; Shahsavari, N.; Niles, S.; et al. Evybactin is a DNA gyrase inhibitor that selectively kills Mycobacterium tuberculosis. Nat. Chem. Biol. 2022, 18, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Ossai, J.; Khatabi, B.; Nybo, S.E.; Kharel, M.K. Renewed interests in the discovery of bioactive actinomycete metabolites driven by emerging technologies. J. Appl. Microbiol. 2022, 132, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.N.; Vimala, R.T.V.; Ramachandran, G.; Kanisha, C.C.; Manoharan, N.; Li, W.-J. An overview on natural product from endophytic actinomycetes. In Natural Products from Actinomycetes; Rai, R.V., Bai, J.A., Eds.; Springer: Singapore, 2022; pp. 151–165. ISBN 9789811661310. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.-X.; Ng, S.B. Fungal endophytes: A promising frontier for discovery of novel bioactive compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef]
- Khan, S.; Verma, V.; Rasool, S. Diversity and the role of endophytic bacteria: A review. Bot. Serb. 2020, 44, 103–120. [Google Scholar] [CrossRef]
- dos Santos, R.M.; Desoignies, N.; Rigobelo, E.C. The bacterial world inside the plant. Front. Sustain. Food Syst. 2022, 6, 830198. [Google Scholar] [CrossRef]
- Boruta, T. A Bioprocess perspective on the production of secondary metabolites by Streptomyces in submerged co-cultures. World J. Microbiol. Biotechnol. 2021, 37, 171. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of co-culture on the metabolism of marine microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef]
- Peng, X.-Y.; Wu, J.-T.; Shao, C.-L.; Li, Z.-Y.; Chen, M.; Wang, C.-Y. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2021, 3, 363–374. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal–fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Ye, X.; Wei, B.; Emam, M.; Zhang, H.; Wang, H. The structural diversity of marine microbial secondary metabolites based on co-culture strategy: 2009–2019. Mar. Drugs 2020, 18, 449. [Google Scholar] [CrossRef]
- Jung, D.; Liu, L.; He, S. Application of in situ cultivation in marine microbial resource mining. Mar. Life Sci. Technol. 2021, 3, 148–161. [Google Scholar] [CrossRef]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef]
- Gavrish, E.; Bollmann, A.; Epstein, S.; Lewis, K. A Trap for in situ cultivation of filamentous actinobacteria. J. Microbiol. Meth. 2008, 72, 257–262. [Google Scholar] [CrossRef]
- Ben-Dov, E.; Kramarsky-Winter, E.; Kushmaro, A. An in situ method for cultivating microorganisms using a double encapsulation technique: In situ method for cultivating microorganisms. FEMS Microbiol. Ecol. 2009, 68, 363–371. [Google Scholar] [CrossRef]
- Jung, D.; Liu, B.; He, X.; Owen, J.S.; Liu, L.; Yuan, Y.; Zhang, W.; He, S. Accessing previously uncultured marine microbial resources by a combination of alternative cultivation methods. Microb. Biotechnol. 2021, 14, 1148–1158. [Google Scholar] [CrossRef]
- Berdy, B.; Spoering, A.L.; Ling, L.L.; Epstein, S.S. In situ cultivation of previously uncultivable microorganisms using the ichip. Nat. Protoc. 2017, 12, 2232–2242. [Google Scholar] [CrossRef]
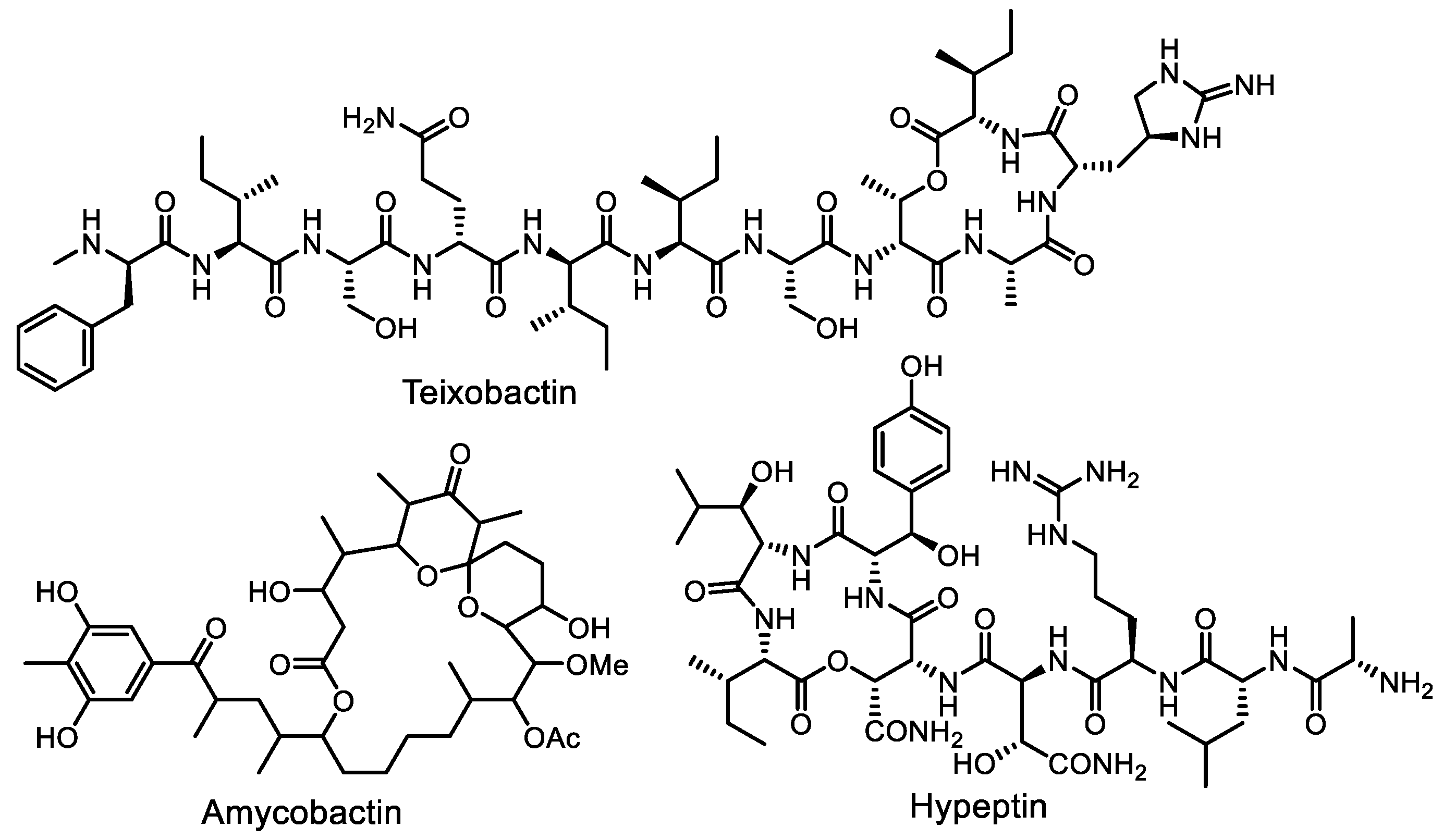
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Nuxoll, A.; Gandt, A.B.; Ebner, P.; Engels, I.; Schneider, T.; Götz, F.; Lewis, K.; Conlon, B.P. Dual targeting of cell wall precursors by teixobactin leads to cell lysis. Antimicrob. Agents Chemother. 2016, 60, 6510–6517. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.; Peoples, A.; Sarybaeva, A.; Hughes, D.; Ghiglieri, M.; Achorn, C.; Desrosiers, A.; Felix, C.; Liang, L.; Malveira, S.; et al. Novel antimicrobials from uncultured bacteria acting against Mycobacterium tuberculosis. mBio 2020, 11, e01516-20. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, D.A.; Ludwig, K.C.; Arts, M.; Marx, C.E.; Krannich, S.; Barac, P.; Kehraus, S.; Josten, M.; Henrichfreise, B.; Müller, A.; et al. Biosynthesis and mechanism of action of the cell wall targeting antibiotic hypeptin. Angew. Chem. Int. Ed. 2021, 60, 13579–13586. [Google Scholar] [CrossRef]
- Davidson, S.-L.; Niepa, T.H.R. Micro-technologies for assessing microbial dynamics in controlled environments. Front. Microbiol. 2022, 12, 745835. [Google Scholar] [CrossRef]
- Pope, E.; Cartmell, C.; Haltli, B.; Ahmadi, A.; Kerr, R.G. Microencapsulation and in situ incubation methodology for the cultivation of marine bacteria. Front. Microbiol. 2022, 13, 958660. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Eliseev, I.E.; Ovchinnikova, L.A.; Kabilov, M.R.; Prjibelski, A.D.; Tupikin, A.E.; Smirnov, I.V.; Belogurov, A.A.; Severinov, K.V.; Lomakin, Y.A.; et al. Liquid drop of DNA libraries reveals total genome information. Proc. Natl. Acad. Sci. USA 2020, 117, 27300–27306. [Google Scholar] [CrossRef]
- Payne, E.M.; Holland-Moritz, D.A.; Sun, S.; Kennedy, R.T. High-throughput screening by droplet microfluidics: Perspective into key challenges and future prospects. Lab Chip 2020, 20, 2247–2262. [Google Scholar] [CrossRef]
- Hu, B.; Xu, P.; Ma, L.; Chen, D.; Wang, J.; Dai, X.; Huang, L.; Du, W. One cell at a time: Droplet-based microbial cultivation, screening and sequencing. Mar. Life Sci. Technol. 2021, 3, 169–188. [Google Scholar] [CrossRef]
- Ning, R.; Fan, J.; Kong, L.; Jiang, X.; Qian, Y.; Du, T.; Zhang, G.; Wu, W. Recent developments of droplets-based microfluidics for bacterial analysis. Chin. Chem. Lett. 2022, 33, 2243–2252. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, H.; Li, S.; Cao, H.; Li, X.; Ma, Z.; She, X.; Zhou, L.; Huang, S. Emerging microfluidic technologies for microbiome research. Front. Microbiol. 2022, 13, 906979. [Google Scholar] [CrossRef]
- Burmeister, A.; Grünberger, A. Microfluidic cultivation and analysis tools for interaction studies of microbial co-cultures. Curr. Opin. Biotechnol. 2020, 62, 106–115. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Nazarov, A.S.; Mokrushina, Y.A.; Baranova, M.N.; Potapova, N.A.; Malakhova, M.V.; Ilina, E.N.; Smirnov, I.V.; Gabibov, A.G. Deep functional profiling facilitates the evaluation of the antibacterial potential of the antibiotic amicoumacin. Antibiotics 2020, 9, 157. [Google Scholar] [CrossRef]
- Shim, J.; Ranasinghe, R.T.; Smith, C.A.; Ibrahim, S.M.; Hollfelder, F.; Huck, W.T.S.; Klenerman, D.; Abell, C. Ultrarapid generation of femtoliter microfluidic droplets for single-molecule-counting immunoassays. ACS Nano 2013, 7, 5955–5964. [Google Scholar] [CrossRef]
- Mahler, L.; Niehs, S.P.; Martin, K.; Weber, T.; Scherlach, K.; Hertweck, C.; Roth, M.; Rosenbaum, M.A. Highly parallelized droplet cultivation and prioritization of antibiotic producers from natural microbial communities. eLife 2021, 10, e64774. [Google Scholar] [CrossRef]
- Mahler, L.; Wink, K.; Beulig, R.J.; Scherlach, K.; Tovar, M.; Zang, E.; Martin, K.; Hertweck, C.; Belder, D.; Roth, M. Detection of antibiotics synthetized in microfluidic picolitre-droplets by various Actinobacteria. Sci. Rep. 2018, 8, 13087. [Google Scholar] [CrossRef]
- Oberpaul, M.; Brinkmann, S.; Marner, M.; Mihajlovic, S.; Leis, B.; Patras, M.A.; Hartwig, C.; Vilcinskas, A.; Hammann, P.E.; Schäberle, T.F.; et al. Combination of high-throughput microfluidics and FACS technologies to leverage the numbers game in natural product discovery. Microb. Biotechnol. 2022, 15, 415–430. [Google Scholar] [CrossRef]
- Baranova, M.N.; Babikova, P.A.; Kudzhaev, A.M.; Mokrushina, Y.A.; Belozerova, O.A.; Yunin, M.A.; Kovalchuk, S.; Gabibov, A.G.; Smirnov, I.V.; Terekhov, S.S. Live biosensors for ultrahigh-throughput screening of antimicrobial activity against Gram-negative bacteria. Antibiotics 2021, 10, 1161. [Google Scholar] [CrossRef]
- Wollein Waldetoft, K.; Brown, S.P. Evolving antibiotic spectrum. Proc. Natl. Acad. Sci. USA 2022, 119, e2214267119. [Google Scholar] [CrossRef]
- Johnston, C.W.; Badran, A.H. Natural and engineered precision antibiotics in the context of resistance. Curr. Opin. Chem. Biol. 2022, 69, 102160. [Google Scholar] [CrossRef] [PubMed]
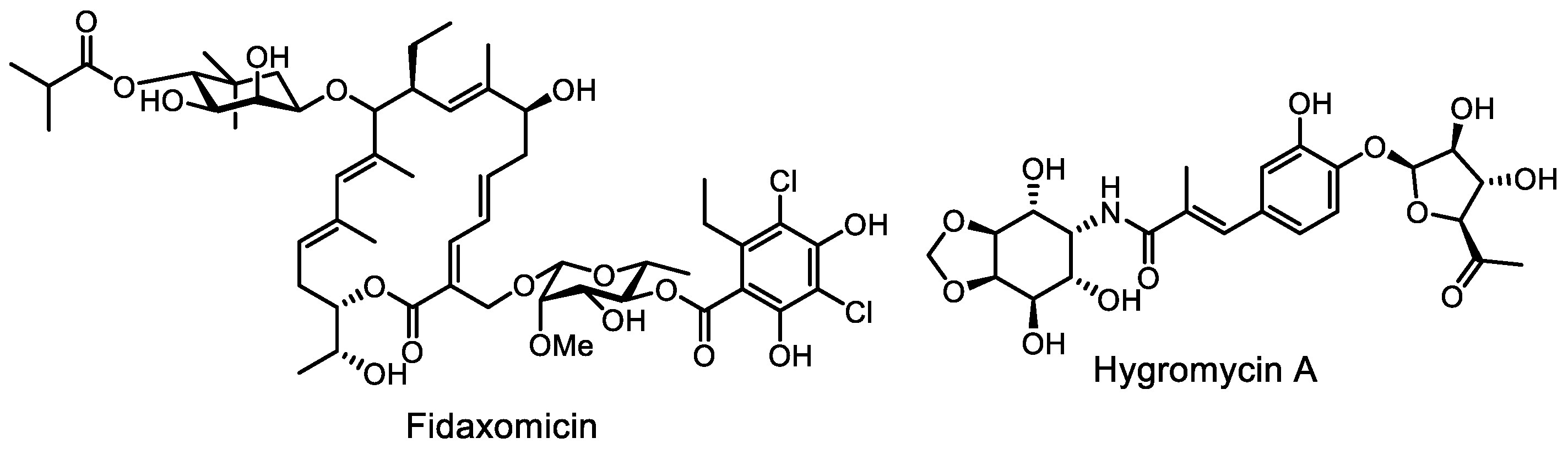
- Sears, P.; Ichikawa, Y.; Ruiz, N.; Gorbach, S. Advances in the treatment of Clostridium difficile with fidaxomicin: A narrow spectrum antibiotic. Ann. N. Y. Acad. Sci. 2013, 1291, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Boyaci, H.; Chen, J.; Bao, Y.; Landick, R.; Campbell, E.A. Basis of narrow-spectrum activity of fidaxomicin on Clostridioides difficile. Nature 2022, 604, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Leimer, N.; Wu, X.; Imai, Y.; Morrissette, M.; Pitt, N.; Favre-Godal, Q.; Iinishi, A.; Jain, S.; Caboni, M.; Leus, I.V.; et al. A selective antibiotic for Lyme disease. Cell 2021, 184, 5405–5418.e16. [Google Scholar] [CrossRef]
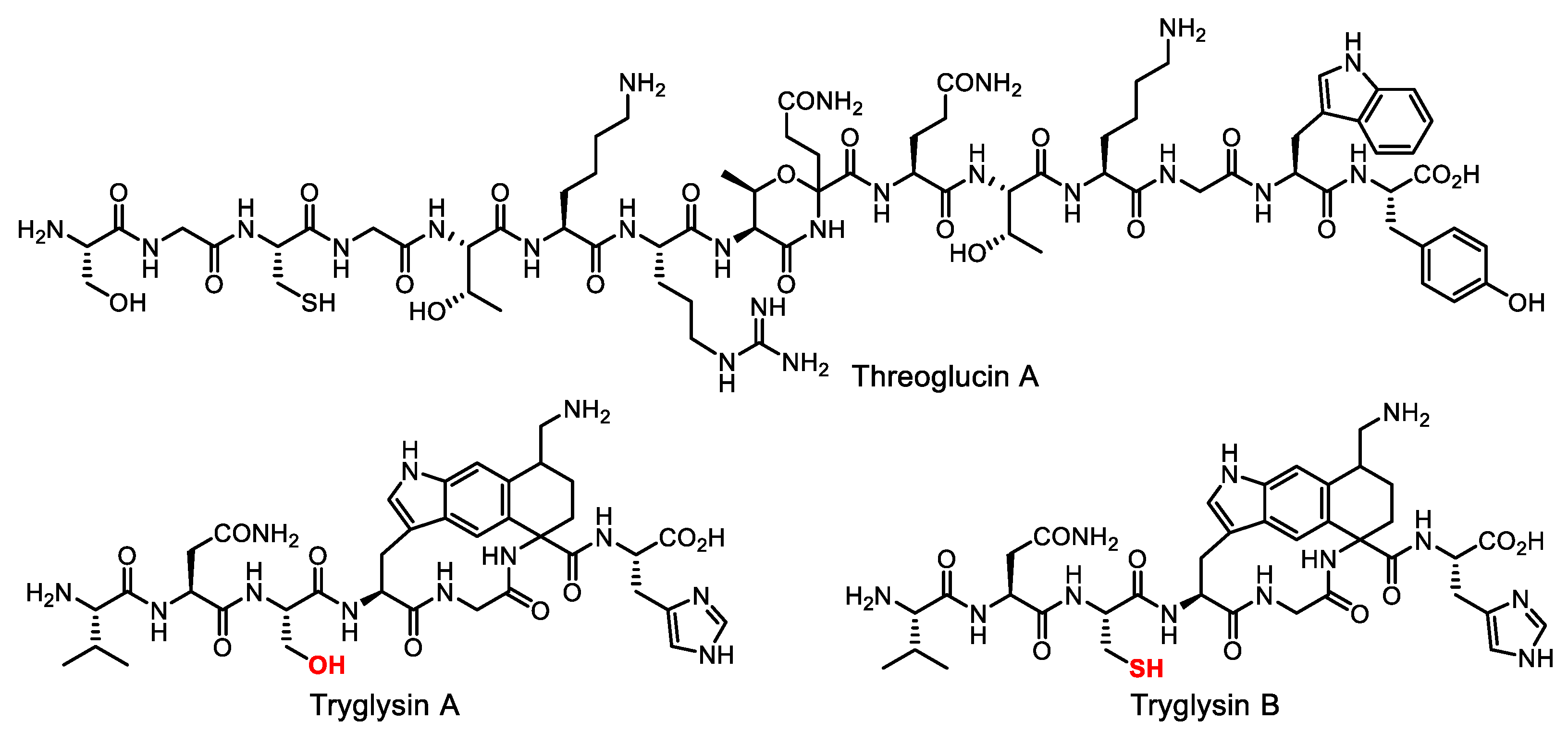
- Covington, B.C.; Seyedsayamdost, M.R. Vitamin B3 triggers biosynthesis of secondary metabolite dormancy signals in Streptococcus suis. J. Am. Chem. Soc. 2022, 144, 14997–15001. [Google Scholar] [CrossRef]
- Rued, B.E.; Covington, B.C.; Bushin, L.B.; Szewczyk, G.; Laczkovich, I.; Seyedsayamdost, M.R.; Federle, M.J. Quorum sensing in Streptococcus mutans regulates production of tryglysin, a novel RaS-RiPP antimicrobial compound. mBio 2021, 12, e02688-20. [Google Scholar] [CrossRef]
- Avis, T.; Wilson, F.X.; Khan, N.; Mason, C.S.; Powell, D.J. Targeted microbiome-sparing antibiotics. Drug Discov. Today 2021, 26, 2198–2203. [Google Scholar] [CrossRef]
- Bauman, K.D.; Butler, K.S.; Moore, B.S.; Chekan, J.R. Genome mining methods to discover bioactive natural products. Nat. Prod. Rep. 2021, 38, 2100–2129. [Google Scholar] [CrossRef]
- Schorn, M.A.; Verhoeven, S.; Ridder, L.; Huber, F.; Acharya, D.D.; Aksenov, A.A.; Aleti, G.; Moghaddam, J.A.; Aron, A.T.; Aziz, S.; et al. A community resource for paired genomic and metabolomic data mining. Nat. Chem. Biol. 2021, 17, 363–368. [Google Scholar] [CrossRef]
- Louwen, J.J.R.; Medema, M.H.; van der Hooft, J.J.J. Enhanced correlation-based linking of biosynthetic gene clusters to their metabolic products through chemical cass matching. Microbiome 2023, 11, 13. [Google Scholar] [CrossRef]
- Muller, E.; Algavi, Y.M.; Borenstein, E. The gut microbiome-metabolome dataset collection: A curated resource for integrative meta-analysis. npj Biofilms Microbiom. 2022, 8, 79. [Google Scholar] [CrossRef]
- Hou, P.; Nowak, V.V.; Taylor, C.J.; Calcott, M.J.; Knight, A.; Owen, J.G. A genomic survey of the natural product biosynthetic potential of actinomycetes isolated from New Zealand lichens. mSystems 2023, 8, e01030-22. [Google Scholar] [CrossRef]
- Tenebro, C.P.; Trono, D.J.V.L.; Balida, L.A.P.; Bayog, L.K.A.; Bruna, J.R.; Sabido, E.M.; Caspe, D.P.C.; de Los Santos, E.L.C.; Saludes, J.P.; Dalisay, D.S. Synergy between genome mining, metabolomics, and bioinformatics uncovers antibacterial chlorinated carbazole alkaloids and their biosynthetic gene cluster from Streptomyces tubbatahanensis sp. Nov., a novel actinomycete isolated from Sulu Sea, Philippines. Microbiol. Spectr. 2023, 11, e03661-22. [Google Scholar] [CrossRef]
- Milshteyn, A.; Colosimo, D.A.; Brady, S.F. Accessing bioactive natural products from the human microbiome. Cell Host Microbe 2018, 23, 725–736. [Google Scholar] [CrossRef]
- Chiumento, S.; Roblin, C.; Kieffer-Jaquinod, S.; Tachon, S.; Leprètre, C.; Basset, C.; Aditiyarini, D.; Olleik, H.; Nicoletti, C.; Bornet, O.; et al. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci. Adv. 2019, 5, eaaw9969. [Google Scholar] [CrossRef]
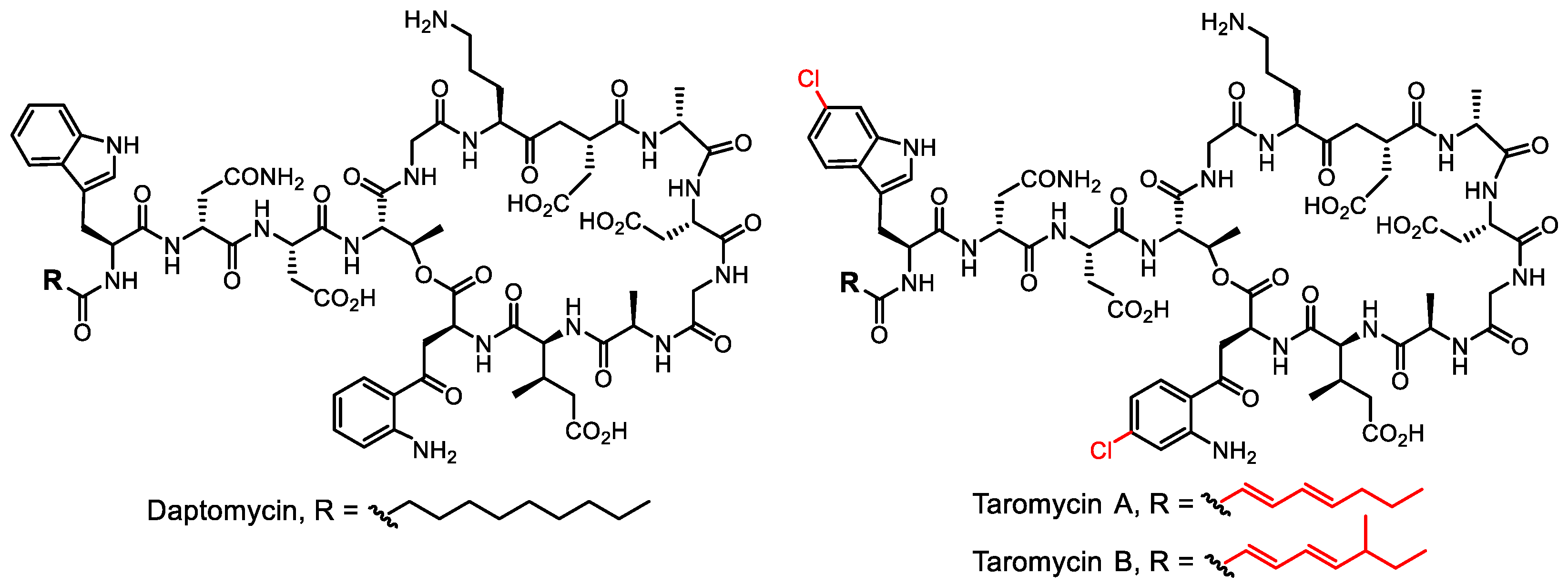
- Yamanaka, K.; Reynolds, K.A.; Kersten, R.D.; Ryan, K.S.; Gonzalez, D.J.; Nizet, V.; Dorrestein, P.C.; Moore, B.S. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA 2014, 111, 1957–1962. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Luhavaya, H.; Li, J.; Dahesh, S.; Nizet, V.; Yamanaka, K.; Moore, B.S. Isolation and structure elucidation of lipopeptide antibiotic taromycin B from the activated taromycin biosynthetic gene cluster. J. Antibiot. 2018, 71, 333–338. [Google Scholar] [CrossRef]
- Luhavaya, H.; Sigrist, R.; Chekan, J.R.; McKinnie, S.M.K.; Moore, B.S. Biosynthesis of l-4-chlorokynurenine, an antidepressant prodrug and a non-proteinogenic amino acid found in lipopeptide antibiotics. Angew. Chem. Int. Ed. 2019, 58, 8394–8399. [Google Scholar] [CrossRef]
- Hover, B.M.; Kim, S.-H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef]
- Wu, C.; Shang, Z.; Lemetre, C.; Ternei, M.A.; Brady, S.F. Cadasides, calcium-dependent acidic lipopeptides from the soil metagenome that are active against multidrug-resistant bacteria. J. Am. Chem. Soc. 2019, 141, 3910–3919. [Google Scholar] [CrossRef]
- Li, L.; Koirala, B.; Hernandez, Y.; MacIntyre, L.W.; Ternei, M.A.; Russo, R.; Brady, S.F. Identification of structurally diverse menaquinone-binding antibiotics with in vivo activity against multidrug-resistant pathogens. Nat. Microbiol. 2021, 7, 120–131. [Google Scholar] [CrossRef] [PubMed]
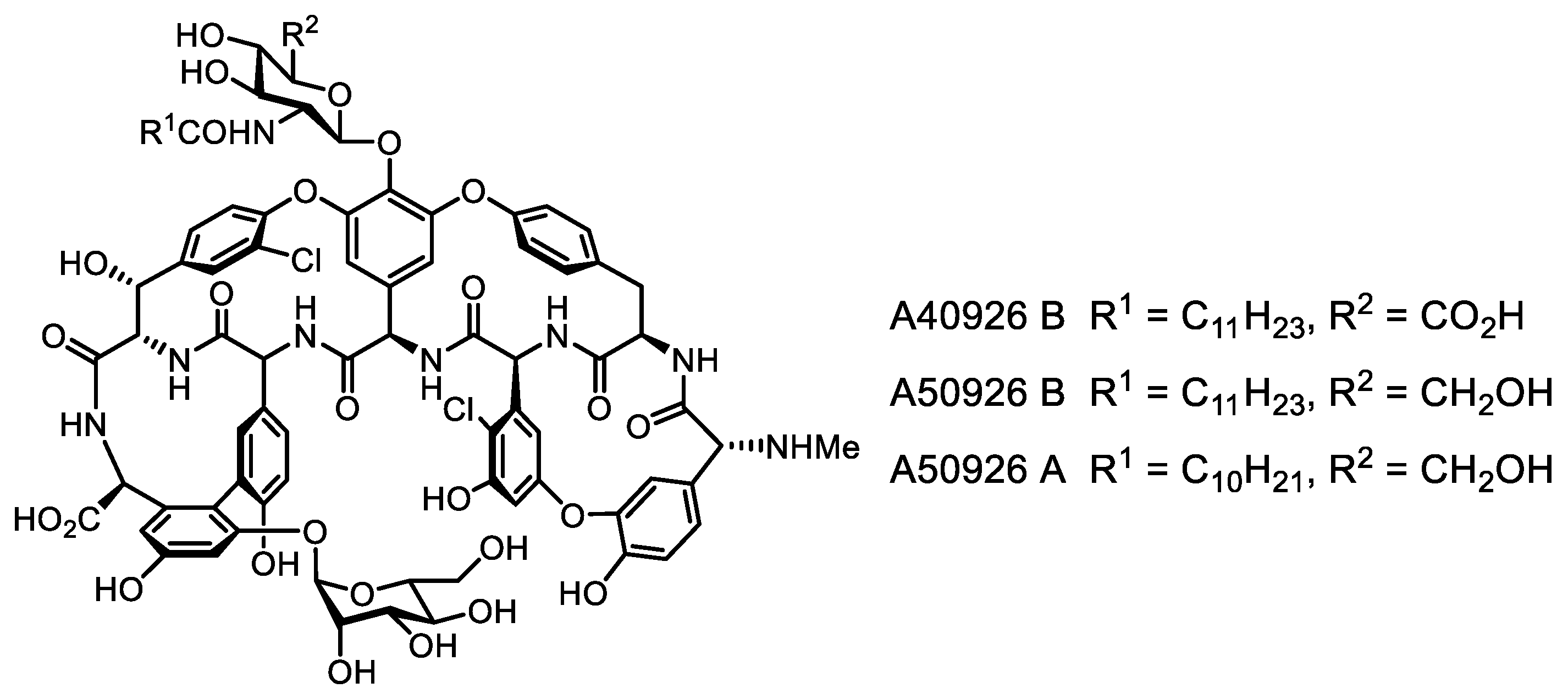
- Yushchuk, O.; Vior, N.M.; Andreo-Vidal, A.; Berini, F.; Rückert, C.; Busche, T.; Binda, E.; Kalinowski, J.; Truman, A.W.; Marinelli, F. Genomic-led discovery of a novel glycopeptide antibiotic by Nonomuraea coxensis DSM 45129. ACS Chem. Biol. 2021, 16, 915–928. [Google Scholar] [CrossRef] [PubMed]
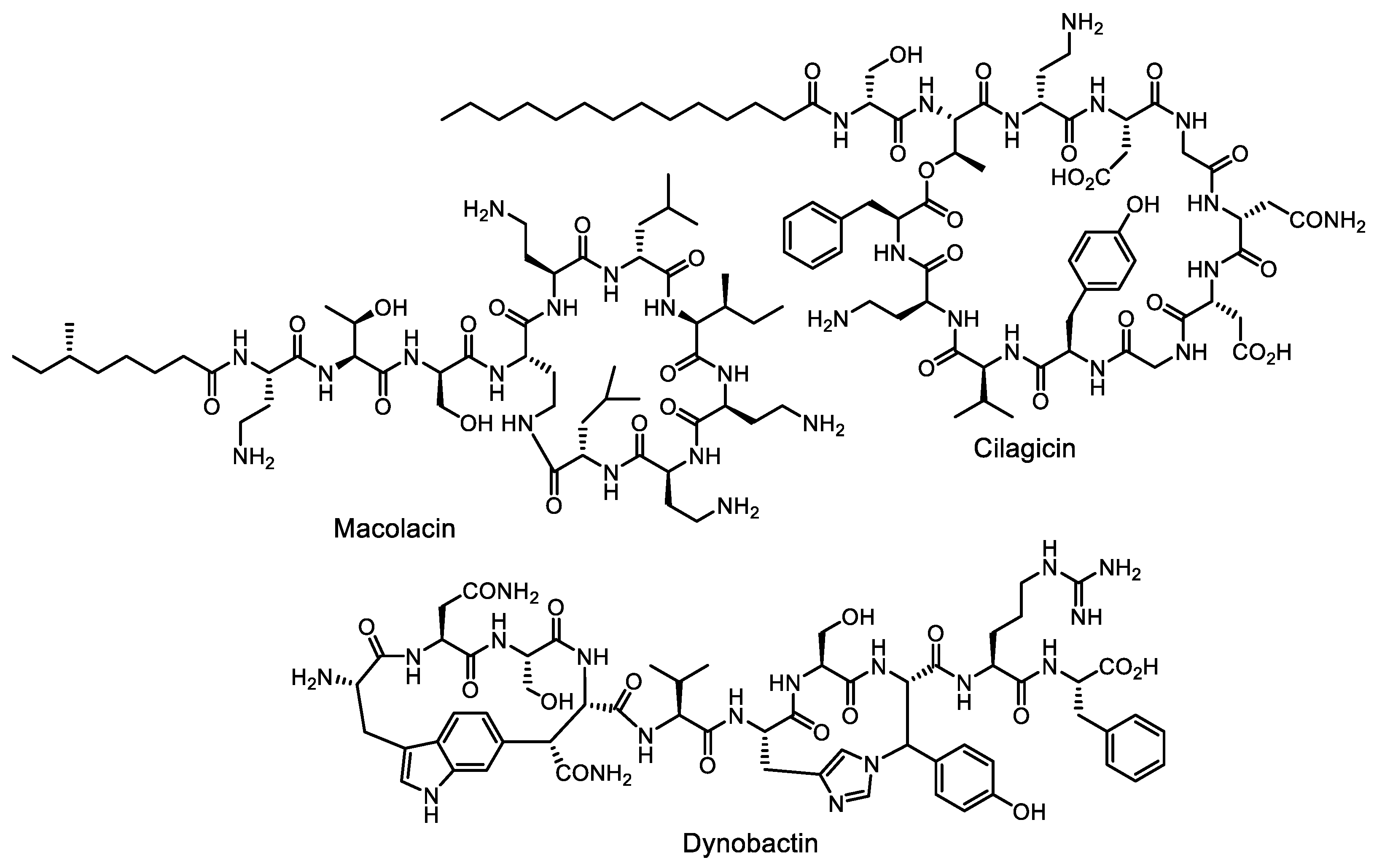
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Brady, S.F. Bioinformatic prospecting and synthesis of a bifunctional lipopeptide antibiotic that evades resistance. Science 2022, 376, 991–996. [Google Scholar] [CrossRef]
- Miller, R.D.; Iinishi, A.; Modaresi, S.M.; Yoo, B.-K.; Curtis, T.D.; Lariviere, P.J.; Liang, L.; Son, S.; Nicolau, S.; Bargabos, R.; et al. Computational identification of a systemic antibiotic for Gram-negative bacteria. Nat. Microbiol. 2022, 7, 1661–1672. [Google Scholar] [CrossRef]
- Behsaz, B.; Bode, E.; Gurevich, A.; Shi, Y.-N.; Grundmann, F.; Acharya, D.; Caraballo-Rodríguez, A.M.; Bouslimani, A.; Panitchpakdi, M.; Linck, A.; et al. Integrating genomics and metabolomics for scalable non-ribosomal peptide discovery. Nat. Commun. 2021, 12, 3225. [Google Scholar] [CrossRef]
- Russell, A.H.; Truman, A.W. Genome mining strategies for ribosomally synthesised and post-translationally modified peptides. Comput. Struct. Biotechnol. J. 2020, 18, 1838–1851. [Google Scholar] [CrossRef]
- Major, D.; Flanzbaum, L.; Lussier, L.; Davies, C.; Caldo, K.M.P.; Acedo, J.Z. Transporter protein-guided genome mining for head-to-tail cyclized bacteriocins. Molecules 2021, 26, 7218. [Google Scholar] [CrossRef]
- Costa, S.S.; da Silva Moia, G.; Silva, A.; Baraúna, R.A.; de Oliveira Veras, A.A. BADASS: BActeriocin-Diversity ASsessment software. BMC Bioinform. 2023, 24, 24. [Google Scholar] [CrossRef]
- Wambui, J.; Stevens, M.J.A.; Sieber, S.; Cernela, N.; Perreten, V.; Stephan, R. Targeted genome mining reveals the psychrophilic clostridium estertheticum complex as a potential source for novel bacteriocins, including cesin A and estercticin A. Front. Microbiol. 2022, 12, 801467. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Im, J.H.; Kang, I.; Kim, E.; Jang, S.C.; Cho, E.; Shin, D.; Hwang, S.; Du, Y.E.; Huynh, T.-H.; et al. Genomic and spectroscopic signature-based discovery of natural macrolactams. J. Am. Chem. Soc. 2023, 145, 1886–1896. [Google Scholar] [CrossRef]
- Malmierca, M.G.; González-Montes, L.; Pérez-Victoria, I.; Sialer, C.; Braña, A.F.; García Salcedo, R.; Martín, J.; Reyes, F.; Méndez, C.; Olano, C.; et al. Searching for glycosylated natural products in actinomycetes and identification of novel macrolactams and angucyclines. Front. Microbiol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, C.; Zhang, L. Investigation of the molecular landscape of bacterial aromatic polyketides by global analysis of type II polyketide synthases. Angew. Chem. Int. Ed. 2022, 61, e202202286. [Google Scholar] [CrossRef]
- Hemmerling, F.; Piel, J. Strategies to access biosynthetic novelty in bacterial genomes for drug discovery. Nat. Rev. Drug Discov. 2022, 21, 359–378. [Google Scholar] [CrossRef]
- Thaker, M.N.; Wang, W.; Spanogiannopoulos, P.; Waglechner, N.; King, A.M.; Medina, R.; Wright, G.D. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat. Biotechnol. 2013, 31, 922–927. [Google Scholar] [CrossRef]
- Hobson, C.; Chan, A.N.; Wright, G.D. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef]
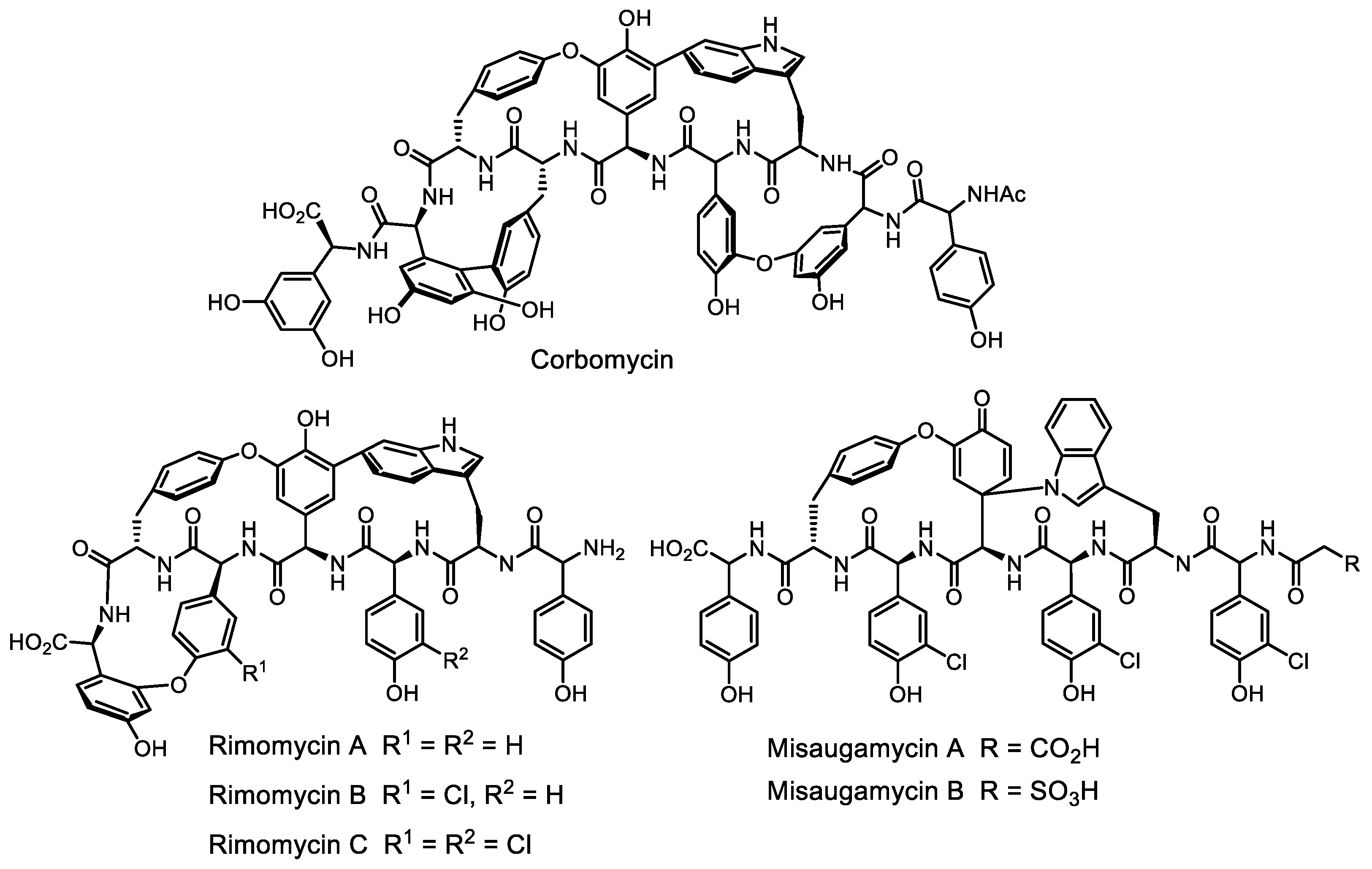
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.-P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Waglechner, N.; Culp, E.J.; Guitor, A.K.; Wright, G.D. Phylogeny-informed synthetic biology reveals unprecedented structural novelty in type V glycopeptide antibiotics. ACS Cent. Sci. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Tang, Y. Recent developments in self-resistance gene directed natural product discovery. Nat. Prod. Rep. 2020, 37, 879–892. [Google Scholar] [CrossRef]
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.; Philmus, B.; Ziemert, N. The Antibiotic Resistant Target Seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucl. Acids Res. 2017, 45, W42–W48. [Google Scholar] [CrossRef]
- Mungan, M.D.; Alanjary, M.; Blin, K.; Weber, T.; Medema, M.H.; Ziemert, N. ARTS 2.0: Feature updates and expansion of the antibiotic resistant target seeker for comparative genome mining. Nucl. Acids Res. 2020, 48, W546–W552. [Google Scholar] [CrossRef] [PubMed]
- Mungan, M.D.; Blin, K.; Ziemert, N. ARTS-DB: A database for antibiotic resistant targets. Nucl. Acids Res. 2022, 50, D736–D740. [Google Scholar] [CrossRef] [PubMed]
- Handel, F.; Kulik, A.; Wex, K.W.; Berscheid, A.; Saur, J.S.; Winkler, A.; Wibberg, D.; Kalinowski, J.; Brötz-Oesterhelt, H.; Mast, Y. Ψ-Footprinting approach for the identification of protein synthesis inhibitor producers. NAR Genom. Bioinformat. 2022, 4, lqac055. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, L.; Xu, F.; Zeng, X.; Wang, R.; Yang, J.; Wang, W.; Karthik, L.; Liu, J.; Yang, Z.; et al. Activating cryptic biosynthetic gene cluster through a CRISPR–Cas12a-mediated direct cloning approach. Nucl. Acids Res. 2022, 50, 3581–3592. [Google Scholar] [CrossRef] [PubMed]
- Enghiad, B.; Huang, C.; Guo, F.; Jiang, G.; Wang, B.; Tabatabaei, S.K.; Martin, T.A.; Zhao, H. Cas12a-assisted precise targeted cloning using in vivo Cre-lox recombination. Nat. Commun. 2021, 12, 1171. [Google Scholar] [CrossRef]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and genomics in natural products research: Complementary tools for targeting new chemical entities. Nat. Prod. Rep. 2021, 38, 2041–2065. [Google Scholar] [CrossRef]
- McCaughey, C.S.; van Santen, J.A.; van der Hooft, J.J.J.; Medema, M.H.; Linington, R.G. An isotopic labeling approach linking natural products with biosynthetic gene clusters. Nat. Chem. Biol. 2022, 18, 295–304. [Google Scholar] [CrossRef]
- Chanana, S.; Thomas, C.S.; Zhang, F.; Rajski, S.R.; Bugni, T.S. hcapca: Automated hierarchical clustering and principal component analysis of large metabolomic datasets in R. Metabolites 2020, 10, 297. [Google Scholar] [CrossRef]
- Wu, Q.; Bell, B.A.; Yan, J.-X.; Chevrette, M.G.; Brittin, N.J.; Zhu, Y.; Chanana, S.; Maity, M.; Braun, D.R.; Wheaton, A.M.; et al. Metabolomics and genomics enable the discovery of a new class of nonribosomal peptidic metallophores from a marine Micromonospora. J. Am. Chem. Soc. 2023, 145, 58–69. [Google Scholar] [CrossRef]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic dialogues: Induction of silent biosynthetic gene clusters by exogenous small molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef]
- Zarins-Tutt, J.S.; Barberi, T.T.; Gao, H.; Mearns-Spragg, A.; Zhang, L.; Newman, D.J.; Goss, R.J.M. Prospecting for new bacterial metabolites: A glossary of approaches for inducing, activating and upregulating the biosynthesis of bacterial cryptic or silent natural products. Nat. Prod. Rep. 2016, 33, 54–72. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef]
- Zong, G.; Fu, J.; Zhang, P.; Zhang, W.; Xu, Y.; Cao, G.; Zhang, R. Use of elicitors to enhance or activate the antibiotic production in Streptomyces. Crit. Rev. Biotechnol. 2022, 42, 1260–1283. [Google Scholar] [CrossRef]
- Tyurin, A.; Alferova, V.; Korshun, V. Chemical elicitors of antibiotic biosynthesis in actinomycetes. Microorganisms 2018, 6, 52. [Google Scholar] [CrossRef]
- Covington, B.C.; Seyedsayamdost, M.R. MetEx, a metabolomics explorer application for natural product discovery. ACS Chem. Biol. 2021, 16, 2825–2833. [Google Scholar] [CrossRef]
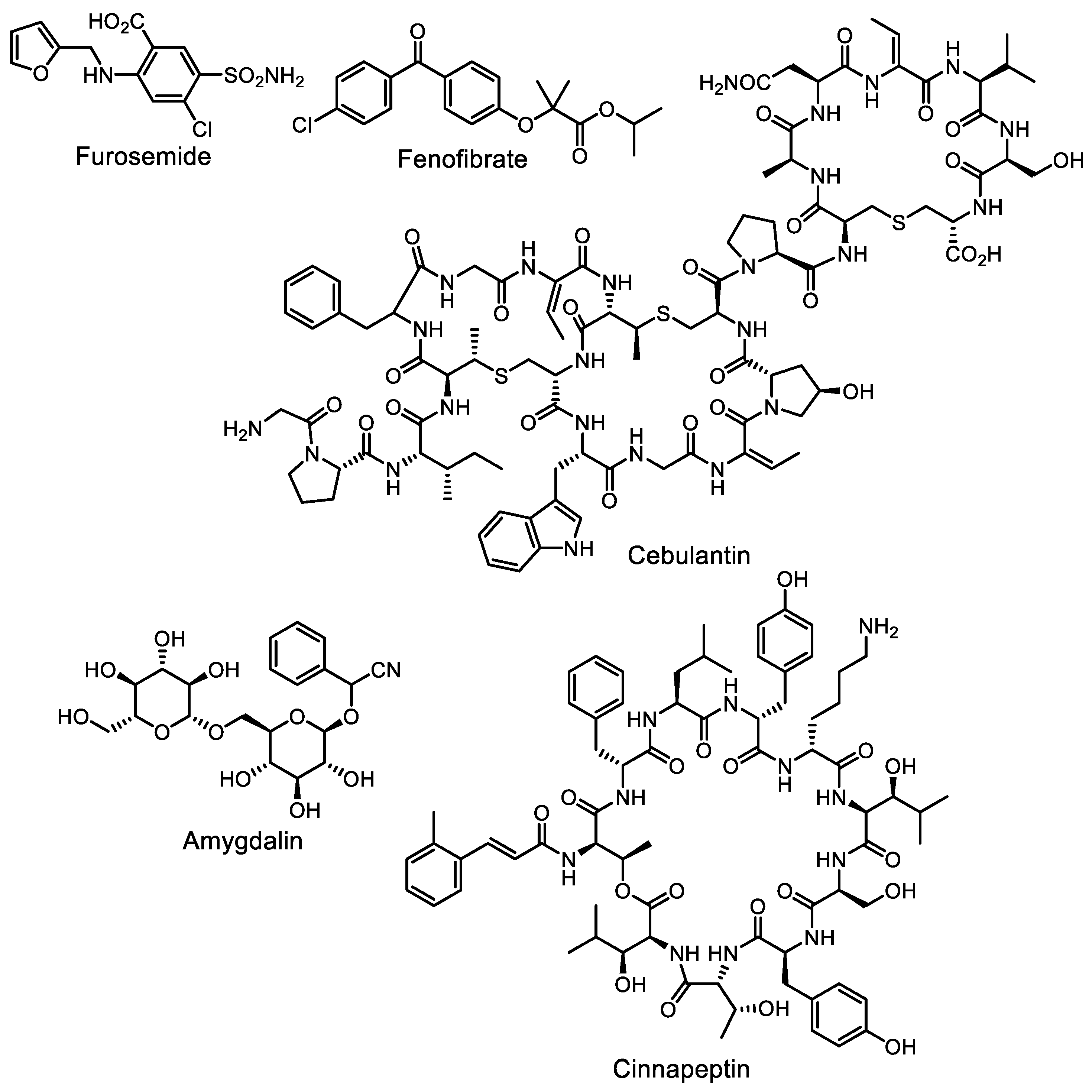
- Moon, K.; Xu, F.; Seyedsayamdost, M.R. Cebulantin, a cryptic lanthipeptide antibiotic uncovered using bioactivity-coupled HiTES. Angew. Chem. Int. Ed. 2019, 58, 5973–5977. [Google Scholar] [CrossRef]
- Zhang, C.; Seyedsayamdost, M.R. Discovery of a cryptic depsipeptide from Streptomyces ghanaensis via MALDI-MS-guided high-throughput elicitor screening. Angew. Chem. Int. Ed. 2020, 59, 23005–23009. [Google Scholar] [CrossRef]
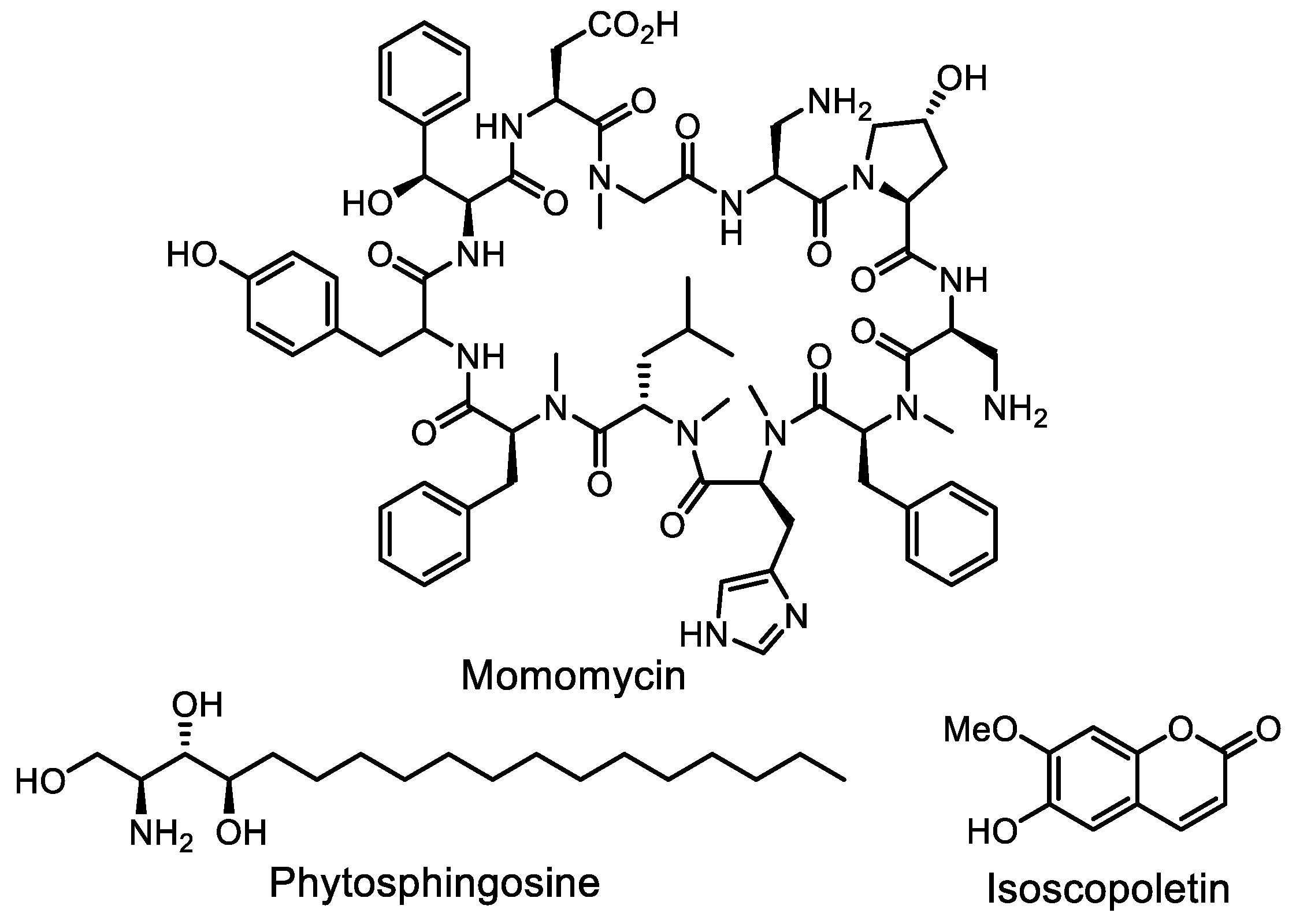
- Li, Y.; Lee, S.R.; Han, E.J.; Seyedsayamdost, M.R. Momomycin, an antiproliferative cryptic metabolite from the oxytetracycline producer Streptomyces rimosus. Angew. Chem. Int. Ed. 2022, 61, e202208573. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, S.R.; Hoshino, S.; Seyedsayamdost, M.R. Targeted discovery of cryptic metabolites with antiproliferative activity. ACS Chem. Biol. 2022, 17, 3121–3130. [Google Scholar] [CrossRef]
- Arakawa, K. Manipulation of metabolic pathways controlled by signaling molecules, inducers of antibiotic production, for genome mining in Streptomyces spp. Antonie Leeuwenhoek 2018, 111, 743–751. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Schneider, O.; Zotchev, S.B. Novel bioactive natural Products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb. Biotechnol. 2019, 12, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Akhgari, A.; Metsä-Ketelä, M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth. Syst. Biotechnol. 2018, 3, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Deng, Z.; Gao, J. CRISPR/Cas-based strategy for unearthing hidden chemical space from microbial genomes. Tr. Chem. 2021, 3, 997–1001. [Google Scholar] [CrossRef]
- Lim, Y.H.; Wong, F.T.; Yeo, W.L.; Ching, K.C.; Lim, Y.W.; Heng, E.; Chen, S.; Tsai, D.-J.; Lauderdale, T.-L.; Shia, K.-S.; et al. Auroramycin: A potent antibiotic from Streptomyces roseosporus by CRISPR-Cas9 activation. ChemBioChem 2018, 19, 1716–1719. [Google Scholar] [CrossRef]
- Yeo, W.L.; Heng, E.; Tan, L.L.; Lim, Y.W.; Ching, K.C.; Tsai, D.-J.; Jhang, Y.W.; Lauderdale, T.-L.; Shia, K.-S.; Zhao, H.; et al. Biosynthetic engineering of the antifungal, anti-MRSA auroramycin. Microb. Cell Fact. 2020, 19, 3. [Google Scholar] [CrossRef]
- Wang, B.; Guo, F.; Dong, S.-H.; Zhao, H. Activation of silent biosynthetic gene clusters using transcription factor decoys. Nat. Chem. Biol. 2019, 15, 111–114. [Google Scholar] [CrossRef]
- Tojo, S.; Tanaka, Y.; Ochi, K. Activation of antibiotic production in Bacillus spp. by cumulative drug resistance mutations. Antimicrob. Agents Chemother. 2015, 59, 7799–7804. [Google Scholar] [CrossRef]
- Ochi, K. Insights into microbial cryptic gene activation and strain improvement: Principle, application and technical aspects. J. Antibiot. 2017, 70, 25–40. [Google Scholar] [CrossRef]
- Zhu, S.; Duan, Y.; Huang, Y. The application of ribosome engineering to natural product discovery and yield improvement in Streptomyces. Antibiotics 2019, 8, 133. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, J.-W.; Wang, W.; Zhai, J.; Yang, J.; Liu, N.; Huang, Y.; Chen, Y.; Pan, G.; Fan, K. A versatile transcription–translation in one approach for activation of cryptic biosynthetic gene clusters. ACS Chem. Biol. 2020, 15, 2551–2557. [Google Scholar] [CrossRef]
- Guo, F.; Xiang, S.; Li, L.; Wang, B.; Rajasärkkä, J.; Gröndahl-Yli-Hannuksela, K.; Ai, G.; Metsä-Ketelä, M.; Yang, K. Targeted activation of silent natural product biosynthesis pathways by reporter-guided mutant selection. Metabol. Eng. 2015, 28, 134–142. [Google Scholar] [CrossRef]
- Mao, D.; Yoshimura, A.; Wang, R.; Seyedsayamdost, M.R. Reporter-guided transposon mutant selection for activation of silent gene clusters in Burkholderia thailandensis. ChemBioChem 2020, 21, 1826–1831. [Google Scholar] [CrossRef]
- Yoshimura, A.; Covington, B.C.; Gallant, É.; Zhang, C.; Li, A.; Seyedsayamdost, M.R. Unlocking cryptic metabolites with mass spectrometry-guided transposon mutant selection. ACS Chem. Biol. 2020, 15, 2766–2774. [Google Scholar] [CrossRef]
- Covington, B.C.; Seyedsayamdost, M.R. Guidelines for metabolomics-guided transposon mutagenesis for microbial natural product discovery. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 665, pp. 305–323. [Google Scholar] [CrossRef]
- Hudson, M.A.; Lockless, S.W. Elucidating the mechanisms of action of antimicrobial agents. mBio 2022, 13, e02240-21. [Google Scholar] [CrossRef]
- Rütten, A.; Kirchner, T.; Musiol-Kroll, E.M. Overview on strategies and assays for antibiotic discovery. Pharmaceuticals 2022, 15, 1302. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Osterman, I.A.; Golovina, A.Y.; Andreyanova, E.S.; Laptev, I.G.; Pletnev, P.I.; Evfratov, S.A.; Marusich, E.I.; Leonov, S.V.; Ivanenkov, Y.A.; et al. Application of reporter strains for screening of new antibiotics. Biochem. Moscow Suppl. Ser. B 2016, 10, 293–299. [Google Scholar] [CrossRef]
- Osterman, I.A.; Komarova, E.S.; Shiryaev, D.I.; Korniltsev, I.A.; Khven, I.M.; Lukyanov, D.A.; Tashlitsky, V.N.; Serebryakova, M.V.; Efremenkova, O.V.; Ivanenkov, Y.A.; et al. Sorting out antibiotics’ mechanisms of action: A double fluorescent protein reporter for high-throughput screening of ribosome and DNA biosynthesis inhibitors. Antimicrob. Agents Chemother. 2016, 60, 7481–7489. [Google Scholar] [CrossRef]
- Osterman, I.A.; Wieland, M.; Maviza, T.P.; Lashkevich, K.A.; Lukianov, D.A.; Komarova, E.S.; Zakalyukina, Y.V.; Buschauer, R.; Shiriaev, D.I.; Leyn, S.A.; et al. Tetracenomycin X inhibits translation by binding within the ribosomal exit tunnel. Nat. Chem. Biol. 2020, 16, 1071–1077. [Google Scholar] [CrossRef]
- Volynkina, I.A.; Zakalyukina, Y.V.; Alferova, V.A.; Belik, A.R.; Yagoda, D.K.; Nikandrova, A.A.; Buyuklyan, Y.A.; Udalov, A.V.; Golovin, E.V.; Kryakvin, M.A.; et al. Mechanism-based approach to new antibiotic producers screening among actinomycetes in the course of the citizen science project. Antibiotics 2022, 11, 1198. [Google Scholar] [CrossRef]
- Wex, K.W.; Saur, J.S.; Handel, F.; Ortlieb, N.; Mokeev, V.; Kulik, A.; Niedermeyer, T.H.J.; Mast, Y.; Grond, S.; Berscheid, A.; et al. Bioreporters for direct mode of action-informed screening of antibiotic producer strains. Cell Chem. Biol. 2021, 28, 1242–1252.e4. [Google Scholar] [CrossRef]
- Johnson, E.O.; LaVerriere, E.; Office, E.; Stanley, M.; Meyer, E.; Kawate, T.; Gomez, J.E.; Audette, R.E.; Bandyopadhyay, N.; Betancourt, N.; et al. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 2019, 571, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.S.; Kumar, G.; Yokubynas, N.A.; Côté, J.-P.; Wang, W.; French, S.; MacNair, C.R.; Wright, G.D.; Brown, E.D. Exploiting the sensitivity of nutrient transporter deletion strains in discovery of natural product antimetabolites. ACS Infect. Dis. 2017, 3, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; French, S.; Brown, E.D. Systems-level chemical biology to accelerate antibiotic drug discovery. Acc. Chem. Res. 2021, 54, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. 2014, 67, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. CRC Handbook of Antibiotic Compounds; CRC Press: Boca Raton, FL, USA, 1980; ISBN 978-0-8493-3450-4. [Google Scholar]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- van Santen, J.A.; Jacob, G.; Singh, A.L.; Aniebok, V.; Balunas, M.J.; Bunsko, D.; Neto, F.C.; Castaño-Espriu, L.; Chang, C.; Clark, T.N.; et al. The natural products atlas: An open access knowledge base for microbial natural products discovery. ACS Cent. Sci. 2019, 5, 1824–1833. [Google Scholar] [CrossRef]
- van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The natural products atlas 2.0: A database of microbially-derived natural products. Nucl. Acids Res. 2022, 50, D1317–D1323. [Google Scholar] [CrossRef]
- van Santen, J.A.; Kautsar, S.A.; Medema, M.H.; Linington, R.G. Microbial natural product databases: Moving forward in the multi-omics era. Nat. Prod. Rep. 2021, 38, 264–278. [Google Scholar] [CrossRef]
- Jarmusch, S.A.; van der Hooft, J.J.J.; Dorrestein, P.C.; Jarmusch, A.K. Advancements in capturing and mining mass spectrometry data are transforming natural products research. Nat. Prod. Rep. 2021, 38, 2066–2082. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Litaudon, M.; Touboul, D.; Queiroz, E.F. Innovative omics-based approaches for prioritisation and targeted isolation of natural products—New strategies for drug discovery. Nat. Prod. Rep. 2019, 36, 855–868. [Google Scholar] [CrossRef]
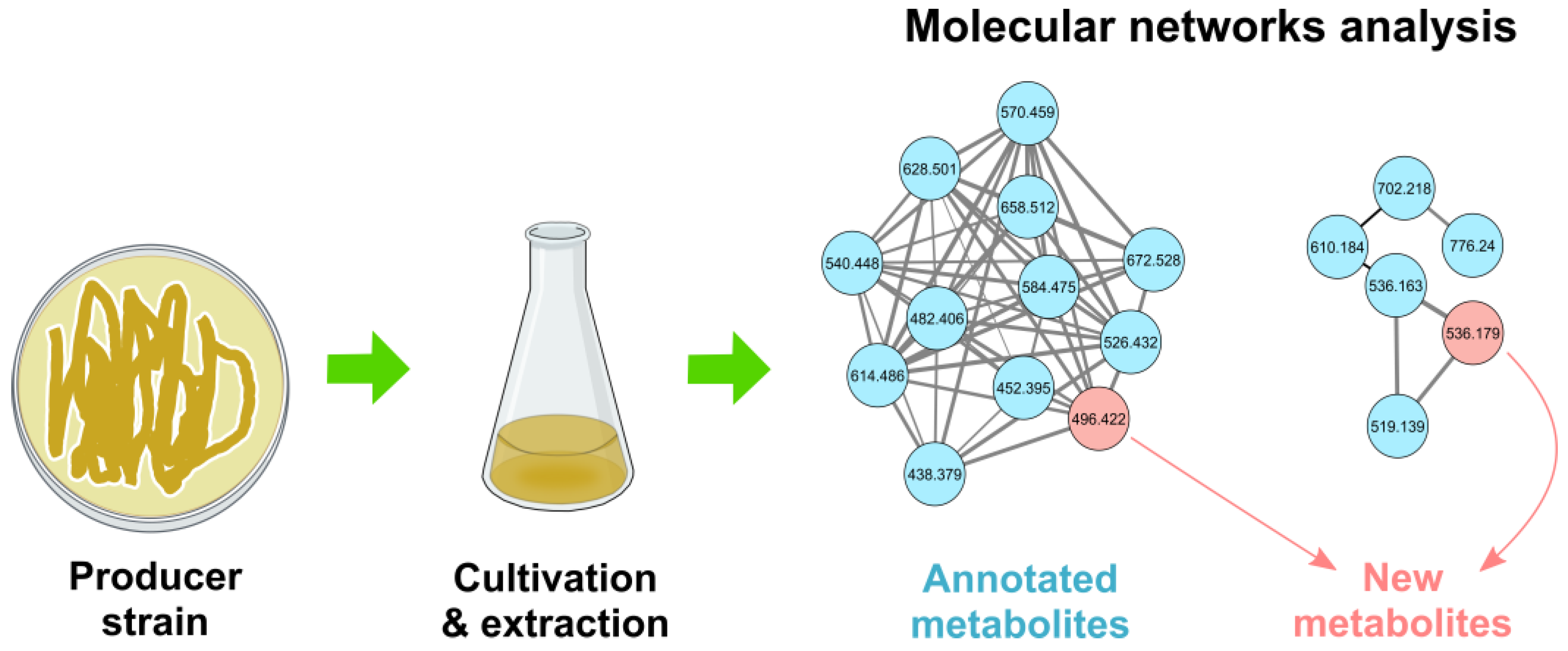
- Fox Ramos, A.E.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural products targeting strategies involving molecular networking: Different manners, one goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef]
- Gurevich, A.; Mikheenko, A.; Shlemov, A.; Korobeynikov, A.; Mohimani, H.; Pevzner, P.A. Increased diversity of peptidic natural products revealed by modification-tolerant database search of mass spectra. Nat. Microbiol. 2018, 3, 319–327. [Google Scholar] [CrossRef]
- Paulo, B.S.; Sigrist, R.; Angolini, C.F.F.; De Oliveira, L.G. New cyclodepsipeptide derivatives revealed by genome mining and molecular networking. ChemistrySelect 2019, 4, 7785–7790. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Q.; Sun, C.; Lukianov, D.; Osterman, I.A.; Sergiev, P.V.; Dontsova, O.A.; Hu, X.; You, X.; Liu, S.; et al. Hetiamacin E and F, new amicoumacin antibiotics from Bacillus subtilis PJS using MS/MS-based molecular networking. Molecules 2020, 25, 4446. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef]
- Schmid, R.; Petras, D.; Nothias, L.-F.; Wang, M.; Aron, A.T.; Jagels, A.; Tsugawa, H.; Rainer, J.; Garcia-Aloy, M.; Dührkop, K.; et al. Ion identity molecular networking for mass spectrometry-based metabolomics in the GNPS environment. Nat. Commun. 2021, 12, 3832. [Google Scholar] [CrossRef]
- Morehouse, N.J.; Clark, T.N.; McMann, E.J.; van Santen, J.A.; Haeckl, F.P.J.; Gray, C.A.; Linington, R.G. Annotation of natural product compound families using molecular networking topology and structural similarity fingerprinting. Nat. Commun. 2023, 14, 308. [Google Scholar] [CrossRef]
- Zani, C.L.; Carroll, A.R. Database for rapid dereplication of known natural products using data from MS and fast NMR experiments. J. Nat. Prod. 2017, 80, 1758–1766. [Google Scholar] [CrossRef]
- Kleks, G.; Holland, D.C.; Porter, J.; Carroll, A.R. Natural products dereplication by diffusion ordered NMR spectroscopy (DOSY). Chem. Sci. 2021, 12, 10930–10943. [Google Scholar] [CrossRef] [PubMed]
- Bruguière, A.; Derbré, S.; Dietsch, J.; Leguy, J.; Rahier, V.; Pottier, Q.; Bréard, D.; Suor-Cherer, S.; Viault, G.; Le Ray, A.-M.; et al. MixONat, a software for the dereplication of mixtures based on 13C NMR spectroscopy. Anal. Chem. 2020, 92, 8793–8801. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.M.; van Santen, J.A.; Liu, D.Y.; Linington, R.G. Development of an NMR-based platform for the direct structural annotation of complex natural products mixtures. J. Nat. Prod. 2021, 84, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Chen, S.-N.; Lankin, D.C.; Bisson, J.; Case, R.J.; Chadwick, L.R.; Gödecke, T.; Inui, T.; Krunic, A.; Jaki, B.U.; et al. Essential parameters for structural analysis and dereplication by 1H NMR spectroscopy. J. Nat. Prod. 2014, 77, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; O’Neil-Johnson, M.; Williams, A.J.; Wheeler, P.; Pol, R.; Moser, A. Dereplication of natural products using minimal NMR data inputs. Org. Biomol. Chem. 2015, 13, 9957–9962. [Google Scholar] [CrossRef]
- Wong, W.R.; Oliver, A.G.; Linington, R.G. Development of antibiotic activity profile screening for the classification and discovery of natural product antibiotics. Chem. Biol. 2012, 19, 1483–1495. [Google Scholar] [CrossRef]
- Cox, G.; Sieron, A.; King, A.M.; De Pascale, G.; Pawlowski, A.C.; Koteva, K.; Wright, G.D. A common platform for antibiotic dereplication and adjuvant discovery. Cell Chem. Biol. 2017, 24, 98–109. [Google Scholar] [CrossRef]
- Lee, S.; van Santen, J.A.; Farzaneh, N.; Liu, D.Y.; Pye, C.R.; Baumeister, T.U.H.; Wong, W.R.; Linington, R.G. NP Analyst: An open online platform for compound activity mapping. ACS Cent. Sci. 2022, 8, 223–234. [Google Scholar] [CrossRef]
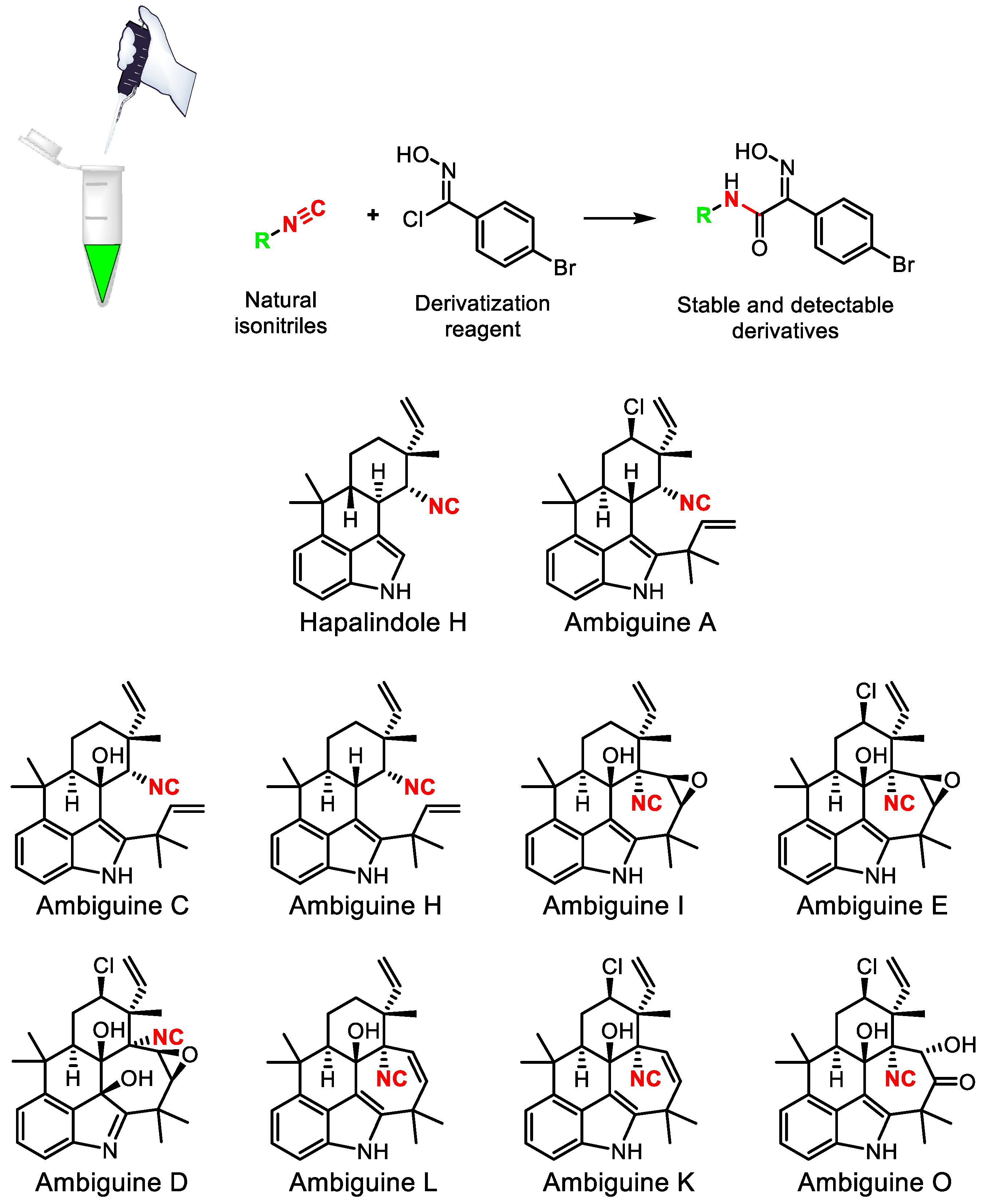
- Hughes, C.C. Chemical labeling strategies for small molecule natural product detection and isolation. Nat. Prod. Rep. 2021, 38, 1684–1705. [Google Scholar] [CrossRef]
- Schäfer, R.J.B.; Wilson, K.; Biedermann, M.; Moore, B.S.; Sieber, S.; Wennemers, H. Identification of isonitrile-containing natural products in complex biological matrices through ligation with chlorooximes. Chem. Eur. J. 2023, 29, e202203277. [Google Scholar] [CrossRef]
- Müller, M.J.; Dorst, A.; Paulus, C.; Khan, I.; Sieber, S. Catch-enrich-release approach for amine-containing natural products. Chem. Commun. 2022, 58, 12560–12563. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Monteiro, A.F.; Bertonha, A.F.; Bernardi, D.I.; Gubiani, J.R.; Slivinski, J.; Michaliski, L.F.; Tonon, L.A.C.; Venancio, V.A.; Freire, V.F. Approaches for the isolation and identification of hydrophilic, light-sensitive, volatile and minor natural products. Nat. Prod. Rep. 2019, 36, 981–1004. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Zhang, J.; Dong, Y.; Xu, S.; Bao, Y.; Chen, C.; Feng, Y.; Cui, Q.; Li, W. An effective strategy for identification of highly unstable bacillaenes. J. Nat. Prod. 2019, 82, 3340–3346. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, A.A.; Alferova, V.A.; Korshun, V.A.; Tyurin, A.P. Modern Trends in Natural Antibiotic Discovery. Life 2023, 13, 1073. https://doi.org/10.3390/life13051073
Baranova AA, Alferova VA, Korshun VA, Tyurin AP. Modern Trends in Natural Antibiotic Discovery. Life. 2023; 13(5):1073. https://doi.org/10.3390/life13051073
Chicago/Turabian StyleBaranova, Anna A., Vera A. Alferova, Vladimir A. Korshun, and Anton P. Tyurin. 2023. "Modern Trends in Natural Antibiotic Discovery" Life 13, no. 5: 1073. https://doi.org/10.3390/life13051073
APA StyleBaranova, A. A., Alferova, V. A., Korshun, V. A., & Tyurin, A. P. (2023). Modern Trends in Natural Antibiotic Discovery. Life, 13(5), 1073. https://doi.org/10.3390/life13051073





