Results of Nailfold Videocapillaroscopy in Patients with Pseudoexfoliative Glaucoma
Abstract
1. Introduction
2. Material and Methods
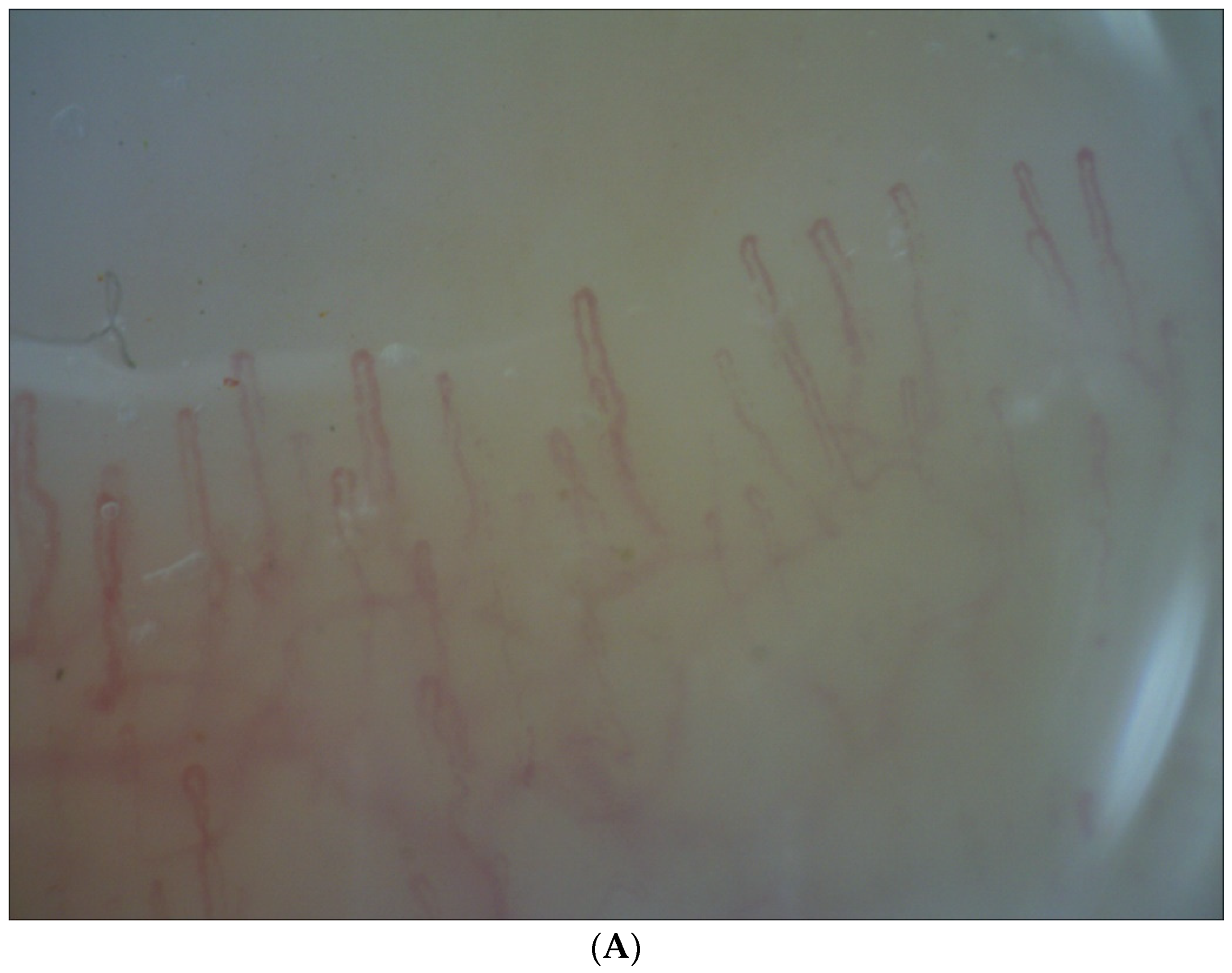
- Normal pattern (a–c), i.e., within normal limits with or without the presence of tortuous capillaries;
- Abnormal pattern (d–e), i.e., with features of microangiopathy.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elhawy, E.; Kamthan, G.; Dong, C.Q.; Danias, J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum. Genom. 2012, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zenkel, M.; Schlötzer-Schrehardt, U. The Composition of Exfoliation Material and the Cells Involved in Its Production. Eur. J. Gastroenterol. Hepatol. 2014, 23, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Hassanee, K.; Ahmed, I. Pseudoexfoliation syndrome in cataract surgery ophthalmol. Clin. N. Am. 2006, 19, 507–519. [Google Scholar]
- Łukasik, U.; Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Kustra, A.; Milanowski, P.; Żarnowski, T. Clinical Features of Pseudoexfoliative Glaucoma in Treated Polish Patients. Clin. Ophthalmol. 2020, 14, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Wang, J.J.; Smith, W. Association of Pseudoexfoliation Syndrome with Increased Vascular Risk. Am. J. Ophthalmol. 1997, 124, 685–687. [Google Scholar] [CrossRef]
- Linnér, E.; Popovic, V.; Gottfries, C.-G.; Jonsson, M.; Sjögren, M.; Wallin, A. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer’s type. Acta Ophthalmol. Scand. 2001, 79, 283–285. [Google Scholar] [CrossRef]
- Schumacher, S.; Schlötzer-Schrehardt, U.; Martus, P.; Lang, W.; Naumann, G. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet 2001, 357, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Puustjärvi, T.; Blomster, H.; Kontkanen, M.; Punnonen, K.; Teräsvirta, M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 749–754. [Google Scholar] [CrossRef]
- Cousins, C.C.; Kang, J.H.; Bovee, C.; Wang, J.; Greenstein, S.H.; Turalba, A.; Shen, L.Q.; Brauner, S.; Boumenna, T.; Blum, S.; et al. Nailfold capillary morphology in exfoliation syndrome. Eye 2017, 31, 698–707. [Google Scholar] [CrossRef]
- Cutolo, M.; Pizzorni, C.; Secchi, M.E.; Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Nailfold video-capillaroscopy in systemic sclerosis. Z. Rheumatol. 2004, 63, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.L.; Park, S.-H.; Oh, Y.-S.; Park, C.K. Nail Bed Hemorrhage: A clinical marker of optic disc hemorrhage in patients with glaucoma. Arch. Ophthalmol. 2011, 129, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Święch-Zubilewicz, A.; Śniegocki, T.; Dolar-Szczasny, J.; Pizoń, M. Determination of Tryptophan and Its Major Metabolites in Fluid from the Anterior Chamber of the Eye in Diabetic Patients with Cataract by Liquid Chromotography Mass Spectrometry (LC-MS/MS). Molecules 2018, 23, 3012. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Drance, S. Nocturnal Hypotension: Role in Glaucoma Progression. Surv. Ophthalmol. 1999, 43 (Suppl. S1), S10–S16. [Google Scholar] [CrossRef]
- Cursiefen, C.; Wisse, M.; Cursiefen, S.; Jünemann, A.; Martus, P.; Korth, M. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am. J. Ophthalmol. 2000, 129, 102–104. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.M.; Sung, K.R.; Park, K.H. Association Between Platelet Function and Disc Hemorrhage in Patients with Normal-Tension Glaucoma: A Prospective Cross-Sectional Study. Am. J. Ophthalmol. 2016, 166, 209–210. [Google Scholar] [CrossRef]
- Caprioli, J.; Coleman, A.L. Blood Flow in Glaucoma Discussion Blood Pressure, Perfusion Pressure, and Glaucoma. Am. J. Ophthalmol. 2010, 149, 704–712. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U. Genetics in exfoliation syndrome and exfoliative glaucoma. In Exfoliation Syndrome and Exfoliative Glaucoma, 3rd ed.; Holló, G., Konstas, A.G.P., Eds.; PubliComm: Savona, Italy, 2015; pp. 57–78. [Google Scholar]
- Pasquale, L.R. Environmental factors in realtion to exfoliation syndrome and exfoliative glaucoma. In Exfoliation Syndrome and Exfoliative Glaucoma, 3rd ed.; Holló, G., Konstas, A.G.P., Eds.; PubliComm: Savona, Italy, 2015; pp. 95–106. [Google Scholar]
- Holló, G.; Konstas, A.G.P. Exfoliation syndrome: A systemic disease. In Exfoliation Syndrome and Exfoliative Glaucoma, 3rd ed.; Holló, G., Konstas, A.G.P., Eds.; PubliComm: Savona, Italy, 2015; pp. 121–130. [Google Scholar]
- Yaz, Y.A.; Yıldırım, N.; Yaz, Y.; Tekin, N.; Inal, M.; Şahin, F.M. Role of Oxidative Stress in Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Turk. J. Ophthalmol. 2019, 49, 61–67. [Google Scholar] [CrossRef]
- Kanthan, G.L.; Mitchell, P.; Burlutsky, G.; Rochtchina, E.; Wang, J.J. Pseudoexfoliation Syndrome and the Long-Term Incidence of Cataract and Cataract Surgery: The Blue Mountains Eye Study. Am. J. Ophthalmol. 2013, 155, 83–88.e1. [Google Scholar] [CrossRef]
- Ritch, R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. Eur. J. Gastroenterol. Hepatol. 1994, 3, 176–177. [Google Scholar] [CrossRef]
- Parodi, M.B.; Bondel, E.; Saviano, S.; Ravalico, G. Iris indocyanine green angiography in pseudoexfoliation syndrome and capsular glaucoma. Acta Ophthalmol. Scand. 2000, 78, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.B.; Bondel, E.; Saviano, S.; Ravalico, G. Fluorescein angiography and indocyanine green videoangiography in the iris of pseudoexfoliation syndrome. Metab. Pediatr. Syst. Ophthalmol. 1998, 21, 7–13. [Google Scholar]
- Parodi, M.B.; Bondel, E.; Saviano, S.; Ravalico, G. Iris fluorescein angiography and iris indocyanine green videoangiography in pseudoexfoliation syndrome. Eur. J. Ophthalmol. 1999, 9, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.M.; Gillies, W. The Development of Microneovascular Changes in the Iris in Pseudoexfoliation of the Lens Capsule. Ophthalmology 1987, 94, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.M.; Gillies, W.E. Fluorescein angiography and fluorophotometry of the iris in pseudoexfoliation of the lens capsule. Br. J. Ophthalmol. 1983, 67, 249–254. [Google Scholar] [CrossRef]
- Holló, G. Vascular Dysfunction in Exfoliation Syndrome. Eur. J. Gastroenterol. Hepatol. 2018, 27, S72–S74. [Google Scholar] [CrossRef]
- Holló, G. Exfoliation Syndrome and Systemic Cardiovascular Diseases. Eur. J. Gastroenterol. Hepatol. 2014, 23, S9–S11. [Google Scholar] [CrossRef]
- Andrikopoulos, G.K.; Alexopoulos, D.K.; Gartaganis, S.P. Pseudoexfoliation syndrome and cardiovascular diseases. World J. Cardiol. 2014, 6, 847–854. [Google Scholar] [CrossRef]
- Drankowska, J.; Kos, M.; Kościuk, A.; Marzęda, P.; Boguszewska-Czubara, A.; Tylus, M.; Święch-Zubilewicz, A. MMP targeting in the battle for vision: Recent developments and future prospects in the treatment of diabetic retinopathy. Life Sci. 2019, 229, 149–156. [Google Scholar] [CrossRef]
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation syndrome. Surv. Ophthalmol. 2001, 45, 265–315. [Google Scholar] [CrossRef]
- Visontai, Z.; Merisch, B.; Kollai, M.; Holló, G. Increase of carotid artery stiffness and decrease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br. J. Ophthalmol. 2006, 90, 563–567. [Google Scholar] [CrossRef]
- Ritch, R.; Prata, T.S.; De Moraes, C.G.V.; Vessani, R.; Costa, V.P.; Konstas, A.G.P.; Liebmann, J.M.; Schlötzer-Schrehardt, U. Association of exfoliation syndrome and central retinal vein occlusion: An ultrastructural analysis. Acta Ophthalmol. 2010, 88, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Helbig, H.; Schlötzer-Schrehardt, U.; Noske, W.; Kellner, U.; Foerster, M.H.; Naumann, G.O. Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger. J. Ophthalmol. 1994, 3, 148–153. [Google Scholar] [PubMed]
- Ritch, R. Systemic Associations of Exfoliation Syndrome. Asia-Pac. J. Ophthalmol. 2016, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, M.; Zhou, M.; Zhang, X. Ocular Pseudoexfoliation Syndrome and Vascular Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e92767. [Google Scholar] [CrossRef]
- Levin, L.A.; Albert, D.M. Exfoliation (psuedoexfoliation) syndrome. In Ocular Disease: Mechanisms and Management; Gabbedy, R., Davie, B., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2010; pp. 212–222. [Google Scholar]
- Maric, V.; Grgurevic, A.; Cirkovic, A.; Stankovic, S.; Marjanovic, I.; Milovanovic, J.; Milovanovic, A.; Bozic, M. Nailfold capillary morphology and platelet function in patients with exfoliative glaucoma. PLoS ONE 2019, 14, e0219505. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Najafi, A.; Tantraworasin, A.; Pasquale, L.R.; Ritch, R. Nailfold Capillaroscopy of Resting Peripheral Blood Flow in Exfoliation Glaucoma and Primary Open-Angle Glaucoma. JAMA Ophthalmol. 2019, 137, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-B.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Homocysteine, small-vessel disease, and atherosclerosis: An MRI study of 825 stroke patients. Neurology 2014, 83, 695–701. [Google Scholar] [CrossRef]
- Lai, W.K.C.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Pasquale, L.R. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 94–101. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Borrás, T.; Fingert, J.H.; Wiggs, J.L.; Ritch, R. Exfoliation syndrome: Assembling the puzzle pieces. Acta Ophthalmol. 2016, 94, e505–e512. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, L.; Li, M. Plasma homocysteine, serum folic acid, serum vitamin B12, serum vitamin B6, MTHFR and risk of pseudoexfoliation glaucoma: A meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1067–1074. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic fiber homeostasis requires lysyl oxidase–like 1 protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, Y.; Fu, S.; Zhao, W.; Liu, P. LOXL1 Gene Polymorphism with Exfoliation Syndrome/Exfoliation Glaucoma: A me-ta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 25, 62–94. [Google Scholar] [CrossRef]
- Feher, J. Regulation of perfusion. In Quantitative Human Physiology; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar] [CrossRef]
- Wirostko, B.M.; Curtin, K.; Ritch, R.; Thomas, S.; Allen-Brady, K.; Smith, K.R.; Hageman, G.S.; Allingham, R.R. Risk for Exfoliation Syndrome in Women with Pelvic Organ Prolapse: A Utah Project on Exfoliation Syndrome (UPEXS) study. JAMA Ophth. 2016, 134, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Besch, B.M.; Curtin, K.; Ritch, R.; Allingham, R.R.; Wirostko, B.M. Association of Exfoliation Syndrome with Risk of Indirect Inguinal Hernia: The Utah Project on Exfoliation Syndrome. JAMA Ophthalmol. 2018, 136, 1368–1374. [Google Scholar] [CrossRef]
- Holló, G.; Lakatos, P.; Farkas, K. Cold pressor test and plasma endothelin-1 concentration in primary open-angle and capsular glaucoma. Eur. J. Gastroenterol. Hepatol. 1998, 7, 105–110. [Google Scholar] [CrossRef]
- Visontai, Z.; Horváth, T.; Kollai, M.; Holló, G. Decreased Cardiovagal Regulation in Exfoliation Syndrome. Eur. J. Gastroenterol. Hepatol. 2008, 17, 133–138. [Google Scholar] [CrossRef]
- Atalar, P.T.; Atalar, E.; Kilic, H.; Abbasoglu, Ö.E.; Ozer, N.; Aksöyek, S.; Övünç, K.; Ozmen, F.; Gürsel, E. Impaired Systemic Endothelial Function in Patients with Pseudoexfoliation Syndrome. Int. Heart J. 2006, 47, 77–84. [Google Scholar] [CrossRef]
- Musch, D.C.; Shimizu, T.; Niziol, L.M.; Gillespie, B.W.; Cashwell, L.F.; Lichter, P.R. Clinical characteristics of newly diagnosed primary, pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative Initial Glaucoma Treatment Study. Br. J. Ophthalmol. 2012, 96, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, N.Z.; Baker, R.H.; Brubaker, R.F. Aqueous Dynamics in Exfoliation Syndrome. Am. J. Ophthalmol. 1992, 114, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Kosior-Jarecka, E.; Bartosińska, J.; Łukasik, U.; Wróbel-Dudzińska, D.; Krasowska, D.; Chodorowska, G.; Żarnowski, T. Results of Nailfold Capillaroscopy in Patients with Normal-Tension Glaucoma. Curr. Eye Res. 2018, 43, 747–753. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef]
- Flammer, J.; Mozaffarieh, M. What Is the Present Pathogenetic Concept of Glaucomatous Optic Neuropathy? Surv. Ophthalmol. 2007, 52, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, M.C.; Mozaffarieh, M.; Flammer, J. What Is the Link Between Vascular Dysregulation and Glaucoma? Surv. Ophthalmol. 2007, 52, S144–S154. [Google Scholar] [CrossRef] [PubMed]
- Božić, M.; Senćanić, P.-H.; Spahić, G.; Kontić, Đ.; Marković, V.; Marjanović, I.; Stojkovic, M.; Đorđević-Jocić, J. Is Nail Fold Capillaroscopy Useful in Normotensive and Primary Open Angle Glaucoma? A Pilot Study. Curr. Eye Res. 2010, 35, 1099–1104. [Google Scholar] [CrossRef]
- Nucci, C.; Osbornec, N.N.; Bagetta, G.; Cerulli, L. Glaucoma an Open-Window to Neurodegeneration and Neuroprotection Newnes. Prog. Brain Res. 2008, 173, xi. [Google Scholar] [CrossRef] [PubMed]
- Ocakoglu, O.; Koyluoglu, N.; Kayiran, A.; Tamcelik, N.; Ozkan, S. Microvascular blood flow of the optic nerve head and peripapillary retina in unilateral exfoliation syndrome. Acta Ophthalmol. Scand. 2004, 82, 49–53. [Google Scholar] [CrossRef]
- Zenkel, M. Extracellular Matrix Regulation and Dysregulation in Exfoliation Syndrome. Eur. J. Gastroenterol. Hepatol. 2018, 27 (Suppl. S1), S24–S28. [Google Scholar] [CrossRef]

| XFG (N = 39) | hXFG (N = 29) | nXFG (N = 10) | Control (N = 32) | p XFG vs. Control | p hXFG vs. nXFG | |
|---|---|---|---|---|---|---|
| Gender (Female:Male) | 66.7%:33.3% | 58.6%:41.4% | 80%:20% | 65.6%:34.4% | 0.8730 | 0.4048 |
| Age (years) | 75.46 ± 7.63 | 74.24 ± 7.91 | 78.39 ± 6.40 | 73.5 ± 5.21 | 0.748 | 0.053 |
| Best Corrected Visual Acuity | 0.59 ± 0.33 | 0.61 ± 0.34 | 0.56 ± 0.33 | 0.8 ± 0.15 | 0.3521 | 0.037 |
| Maximal Intraocular Pressure (mmHg) | 26.68 ± 10.74 | 29.89 ± 11.22 | 18.66 ± 2.38 | 15.41 ± 3.82 | 0.0001 * | 0.001 * |
| Mean Deviation (dB) | −12.15 ± 10.73 | −11.49 ± 10.9 | −13.87 ± 10.74 | 0.36 ± 0.7 | 0.001 | 0.671 |
| n (%) | ||||
|---|---|---|---|---|
| NVC Feature | XFG N = 39 | hXFG N = 29 | nXFG N = 10 | Control N = 32 |
| Pale background | 7 (17.95) | 5 (17.24) | 1 (10.00) | 4 (12.50) |
| Architectual derangement | 13 (33.33) | 11 (37.93) | 3 (30.06) | 3 (9.38) |
| Decreased number of capillaries (<7 capillaries per millimeter) | 1 (2.56) | 0 | 1 (10.00) | 0 |
| Capillary dilatation (up to 35 µm) | 11 (28.21) | 8 (27.59) | 3 (30.00) | 12 (37.50) |
| Tortuous capillaries | 16 (41.03) | 13 (44.83) | 3 (30.00) | 17 (53.13) |
| Glomerular capillaries | 1 (2.56) | 0 | 1 (10.00) | 1 (3.13) |
| Neoangiogenesis | 12 (30.77) | 9 (31.03) | 3 (30.00) | 10 (31.25) |
| Microhaemorrhages | 7 (17.95) | 4 (13.79) | 3 (30.00) | 2 (6.25) |
| Aneurysmal dilatations | 5 (12.82) | 3 (10.34) | 2 (20.00) | 4 (12.5) |
| p | ||||
|---|---|---|---|---|
| NVC Feature | XFG vs. Control | nXFG vs. Control | hXFG vs. Control | hXFG vs. nXFG |
| Pale background | 0.4983 | 0.7502 | 0.5863 | 0.9333 |
| Architectural derangement | 0.0332 * | 0.7358 | 0.1251 | 0.7569 |
| Decreased number of capillaries (<7 capillaries per millimeter) | 0.3161 | 0.0297 * | 0.8261 | 0.0639 ^ |
| Capillary dilatation (up to 35 µm) | 0.4196 | 0.8472 | 0.4852 | 0.8092 |
| Tortuous capillaries | 0.0386 * | 0.0171 * | 0.3953 | 0.1722 |
| Glomerular capillaries | 0.8456 | 0.5886 | 0.6809 | 0.9097 |
| Neoangiogenesis | 0.9029 | 0.9508 | 0.8273 | 0.9670 |
| Microhaemorrhages | 0.1221 | 0.0520 * | 0.4927 | 0.3246 |
| Aneurysmal dilatations | 0.9813 | 0.6664 | 0.7671 | 0.6376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukasik, U.; Bartosińska, J.; Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Krasowska, D.; Żarnowski, T. Results of Nailfold Videocapillaroscopy in Patients with Pseudoexfoliative Glaucoma. Life 2023, 13, 967. https://doi.org/10.3390/life13040967
Łukasik U, Bartosińska J, Kosior-Jarecka E, Wróbel-Dudzińska D, Krasowska D, Żarnowski T. Results of Nailfold Videocapillaroscopy in Patients with Pseudoexfoliative Glaucoma. Life. 2023; 13(4):967. https://doi.org/10.3390/life13040967
Chicago/Turabian StyleŁukasik, Urszula, Joanna Bartosińska, Ewa Kosior-Jarecka, Dominika Wróbel-Dudzińska, Dorota Krasowska, and Tomasz Żarnowski. 2023. "Results of Nailfold Videocapillaroscopy in Patients with Pseudoexfoliative Glaucoma" Life 13, no. 4: 967. https://doi.org/10.3390/life13040967
APA StyleŁukasik, U., Bartosińska, J., Kosior-Jarecka, E., Wróbel-Dudzińska, D., Krasowska, D., & Żarnowski, T. (2023). Results of Nailfold Videocapillaroscopy in Patients with Pseudoexfoliative Glaucoma. Life, 13(4), 967. https://doi.org/10.3390/life13040967






