Postprandial Inflammatory and Metabolic Responses Induced by Authentic Mytilinis Cheese: A Preliminary, Crossover, Nutritional Intervention in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Meal Challenge Procedure
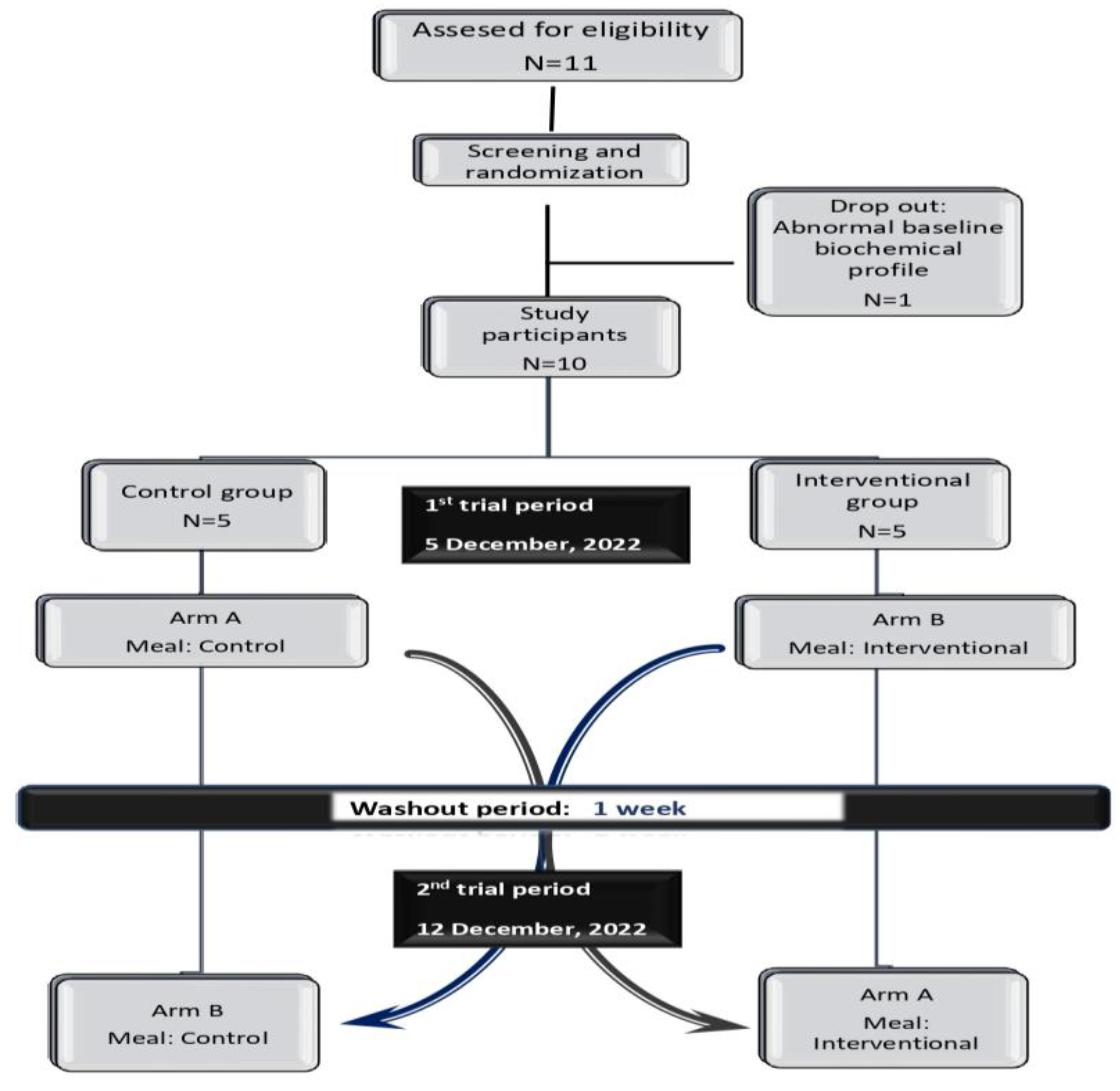
2.3. Study Design
2.4. Blood Sampling and Analysis
2.5. Data Analysis
3. Results
3.1. Preliminary In Vitro Measurements
3.2. Study Population Characteristics
3.3. Baseline and Postprandial Responses of Test Meals on Metabolic Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skalkos, D.; Kalyva, Z. Exploring the impact of COVID-19 pandemic on Food Choice Motives: A systemtic review. Sustainability 2023, 15, 1606. [Google Scholar] [CrossRef]
- Skalkos, D.; Kosma, I.S.; Chasioti, E.; Bintsis, T.; Karantonis, H.C. Consumers’ perception on traceability of greek traditional foods in the post-COVID-19 era. Sustainability 2021, 13, 12687. [Google Scholar] [CrossRef]
- Papagianni, O.; Loukas, T.; Magkoutis, A.; Kagoudi, M.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Responses of Serum Cholesterol, Glucose and Plasma Antioxidant Activity, after Intake of an Innovative High Fat Mayonnaise-Based Appetizer, Enhanced with Olive Paste, in Healthy Volunteers. Life 2022, 12, 1385. [Google Scholar] [CrossRef] [PubMed]
- Skalkos, D.; Kosma, I.S.; Chasioti, E.; Skendi, A.; Papageorgiou, M.; Guiné, R.P.F. Consumers’ Attitude and Perception toward Traditional Foods of Northwest Greece during the COVID-19 Pandemic. Appl. Sci. 2021, 11, 4080. [Google Scholar] [CrossRef]
- Spilioti, M.; Stahtiaris, S.; Kominakis, A.; Karanikolas, P.; Tsiboukas, K. Advancing the value of local livestock breeds: The case of greek cheese ladotyri mytilinis. In Proceedings of the CEUR Workshop, Bologna, Italy, 14–16 September 2020; Volume 2761, pp. 309–316. [Google Scholar]
- Tsorotioti, S.E.; Nasopoulou, C.; Detopoulou, M.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. In vitro anti-atherogenic properties of traditional Greek cheese lipid fractions. Dairy Sci. Technol. 2014, 94, 269–281. [Google Scholar] [CrossRef]
- Schmid, A.; Petry, N.; Walther, B.; Bütikofer, U.; Luginbüh, W.; Gille, D.; Chollet, M.; McTernan, P.G.; Gijs, M.A.M.; Vionnet, N.; et al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br. J. Nutr. 2015, 113, 1853–1861. [Google Scholar] [CrossRef]
- Papagianni, O.; Argyri, K.; Loukas, T.; Magkoutis, A.; Biagki, T.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Bioactivity of a Spread Cheese Enriched with Mountain Tea and Orange Peel Extract in Plasma Oxidative Stress Status, Serum Lipids and Glucose Levels: An Interventional Study in Healthy Adults. Biomolecules 2021, 11, 1241. [Google Scholar] [CrossRef]
- Papagianni, O.; Moulas, I.; Loukas, T.; Magkoutis, A.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Trends in food innovation: An interventional study on the benefits of consuming novel functional cookies enriched with olive paste. Sustainability 2021, 13, 11472. [Google Scholar] [CrossRef]
- Argyri, E.; Piromalis, S.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Nasopoulou, C.; Dimou, C.; Karantonis, H.C. Olive Paste-Enriched Cookies Exert Increased Antioxidant Activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Tremblay, A.J.; Maltais-Giguère, J.; Charest, A.; Guinot, L.; Rioux, L.E.; Labrie, S.; Britten, M.; Lamarche, B.; Turgeon, S.L.; et al. Differential impact of the cheese matrix on the postprandial lipid response: A randomized, crossover, controlled trial. Am. J. Clin. Nutr. 2017, 106, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.; Holven, K.B.; Øyri, L.K.L.; Brekke, H.K.; Biong, A.S.; Gjevestad, G.O.; Raza, G.S.; Herzig, K.H.; Thoresen, M.; Ulven, S.M. Meals with similar fat content from different dairy products induce different postprandial triglyceride responses in healthy adults: A randomized controlled cross-over trial. J. Nutr. 2019, 149, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hjerpsted, J.; Leedo, E.; Tholstrup, T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011, 94, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Murillo, S.; Mallol, A.; Adot, A.; Juárez, F.; Coll, A.; Gastaldo, I.; Roura, E. Culinary strategies to manage glycemic response in people with type 2 diabetes: A narrative review. Front. Nutr. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Klisović, D.; Koprivnjak, O.; Novoselić, A.; Pleadin, J.; Lešić, T.; Brkić Bubola, K. Compositional Changes in the Extra Virgin Olive Oil Used as a Medium for Cheese Preservation. Foods 2022, 11, 2329. [Google Scholar] [CrossRef]
- Feeney, E.L.; Barron, R.; Dible, V.; Hamilton, Z.; Power, Y.; Tanner, L.; Flynn, C.; Bouchier, P.; Beresford, T.; Noronha, N.; et al. Dairy matrix effects: Response to consumption of dairy fat differs when eaten within the cheese matrix—A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 1–8. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. Microbiological characteristics of Greek traditional cheeses. Small Rumin. Res. 2011, 101, 17–32. [Google Scholar] [CrossRef]
- Aliwarga, L.; Christianti, E.N.; Lazarus, C. Development of parmesan cheese production from local cow milk. AIP Conf. Proc. 2017, 1840, 060003. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free. Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L. Dairy products and plasma cholesterol levels. Food Nutr. Res. 2010, 54, 5124. [Google Scholar] [CrossRef] [PubMed]
- Cortés, B.; Núñez, I.; Cofán, M.; Gilabert, R.; Pérez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute Effects of High-Fat Meals Enriched With Walnuts or Olive Oil on Postprandial Endothelial Function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Selby-Pham, S.; Colton, B.L.; Bennett, L.; Williamson, G.; Dordevic, A.L. Does timing of phytonutrient intake influence the suppression of postprandial oxidative stress? A systematic literature review. Redox Biol. 2021, 46, 102123. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Control | Interventional |
|---|---|---|
| Total weight (g) | 180 | 180 |
| Energy (kcal) | 615.6 | 596.6 |
| Fat, total (g) | 32.57 | 35.87 |
| Saturated fat (g) | 20.13 | 19.33 |
| Carbohydrates (g) | 39.36 | 40.06 |
| Protein (g) | 39.94 | 28.24 |
| Salt | 2.64 | 5.54 |
| TAC * | 0.96 | 0.76 |
| TPC * | 0.33 | 0.32 |
| Volunteers Baseline Characteristics | |
|---|---|
| Volunteers (number) | 10 |
| Men (number) | 5 |
| Women (number) | 5 |
| Dietary supplementation (number of participants) | 0 |
| Physical activity medium or high (number of participants) | 5 |
| Age (years) | 23 ± 3.01 |
| Weight (kg) | 72.18 ± 10.3 |
| Height (cm) | 170.2 ± 7.2 |
| Body Mass Index | 24.86 ± 3.01 |
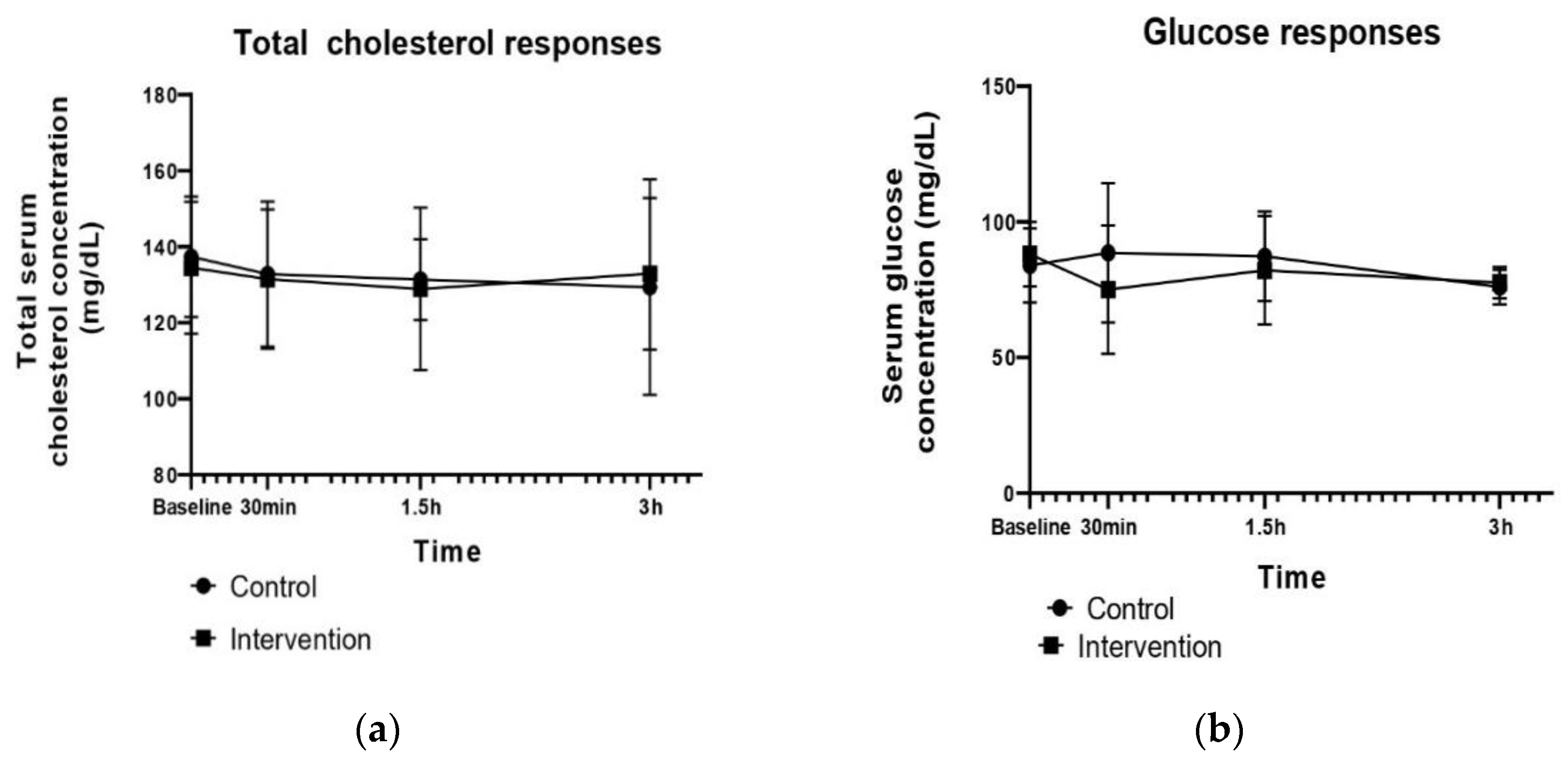
| Total Cholesterol at Baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
|---|---|---|---|---|---|---|---|
| Control | 137.33 ± 15.82 | −4.56 ± 3.2 | −1.44 ± 1 | −2 ± 1.4 | 0.871 | 0.322 | 0.621 |
| Intervention | 134.44 ± 17.38 | −3 ± 2.1 | −2.56 ± 1.8 | 4 ± 2.8 | |||
| Glucose at baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 83.92 ± 13.64 | 3.6 ± 2.5 | −2.3 ± 1.6 | −10.2 ± 7.2 | 0.817 | 0.232 | 0.516 |
| Intervention | 88.07 ± 11.9 | −8.9 ± 6.3 | 1.7 ± 1.2 | −5.9 ± 4.2 | |||
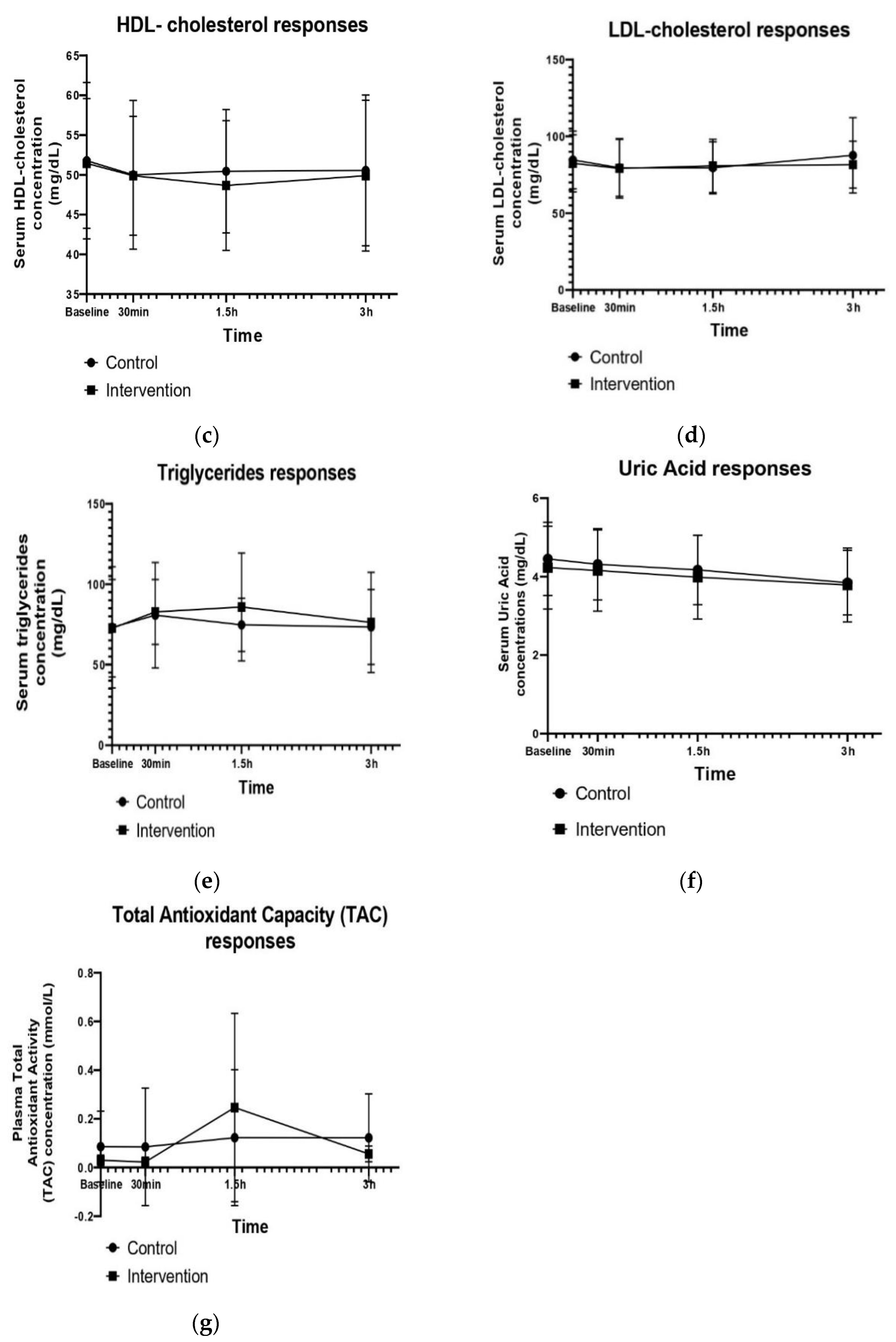
| HDL cholesterol at baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 51.77 ± 9.83 | −1.78 ± 1.3 | 0.44 ± 0.3 | 0.11 ± 0.1 | 0.527 | 0.050 | 0.682 |
| Intervention | 51.44 ± 8.15 | −1.56 ± 1.1 | −1.22 ± 0.9 | 1.22 ± 0.9 | |||
| LDL cholesterol at baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 82.45 ± 18.58 | −4.64 ± 3.3 | 0.34 ± 0.2 | 7.72 ± 5.5 | 0.335 | 0.0004 | 0.454 |
| Intervention | 82.46 ± 18.58 | −3.3 ± 2.3 | 1.89 ± 1.3 | 2.21 ± 1.6 | |||
| Triglycerides at baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 73.22 ± 37.7 | 7.6 ± 5.3 | −6 ± 4.2 | −1.3 ± 0.9 | 0.759 | 0.220 | 0.536 |
| Intervention | 72.66 ± 30.38 | 10.1 ± 7.1 | 3.1 ± 2.2 | −9.7 ± 6.8 | |||
| Uric acid at baseline (mg/dL) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 4.45 ± 0.93 | −0.14 ± 0.1 | −0.15 ± 0.1 | 0.32 ± 0.2 | 0.441 | 0.0001 | 0.348 |
| Intervention | 4.22 ± 1.05 | −0.07 ± 0.05 | −0.17 ± 0.02 | −0.2 ± 0.04 | |||
| Total Plasma Antioxidant Capacity (TAC) (mmol/L) | Δ30 min | Δ1.5 h | Δ3 h | p-Value Group Effect | p-Value Time Effect | p-Value Group × Time Interaction | |
| Control | 0.086 ± 0.14 | 0.012 ± 0.008 | 0.056 ± 0.04 | −0.05 ± 0.03 | 0.378 | 0.256 | 0.720 |
| Intervention | 0.029 ± 0.021 | −0.01 ± 0.007 | 0.155 ± 0.08 | −0.12 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagianni, O.; Voutsa, A.; Katira, O.; Potsaki, P.; Almpounioti, K.; Tzitziri, K.; Skalkos, D.; Koutelidakis, A.E. Postprandial Inflammatory and Metabolic Responses Induced by Authentic Mytilinis Cheese: A Preliminary, Crossover, Nutritional Intervention in Healthy Adults. Life 2023, 13, 923. https://doi.org/10.3390/life13040923
Papagianni O, Voutsa A, Katira O, Potsaki P, Almpounioti K, Tzitziri K, Skalkos D, Koutelidakis AE. Postprandial Inflammatory and Metabolic Responses Induced by Authentic Mytilinis Cheese: A Preliminary, Crossover, Nutritional Intervention in Healthy Adults. Life. 2023; 13(4):923. https://doi.org/10.3390/life13040923
Chicago/Turabian StylePapagianni, Olga, Angeliki Voutsa, Olga Katira, Panagiota Potsaki, Kalliopi Almpounioti, Konstantina Tzitziri, Dimitrios Skalkos, and Antonios E. Koutelidakis. 2023. "Postprandial Inflammatory and Metabolic Responses Induced by Authentic Mytilinis Cheese: A Preliminary, Crossover, Nutritional Intervention in Healthy Adults" Life 13, no. 4: 923. https://doi.org/10.3390/life13040923
APA StylePapagianni, O., Voutsa, A., Katira, O., Potsaki, P., Almpounioti, K., Tzitziri, K., Skalkos, D., & Koutelidakis, A. E. (2023). Postprandial Inflammatory and Metabolic Responses Induced by Authentic Mytilinis Cheese: A Preliminary, Crossover, Nutritional Intervention in Healthy Adults. Life, 13(4), 923. https://doi.org/10.3390/life13040923










