Photoluminescent Scaffolds Based on Natural and Synthetic Biodegradable Polymers for Bioimaging and Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Upconversion Nanoparticles
2.3. Electrospinning
2.4. Extrusion 3D Printing with Simultaneous Photocuring
2.5. Antisolvent 3D Printing
2.6. Microscopy
2.7. Analysis of Photoluminescent Properties of Polymer Scaffolds
2.8. In Vivo Mice Imaging Study
2.9. Histological Assays
2.10. Statistical Analysis
3. Results and Discussion
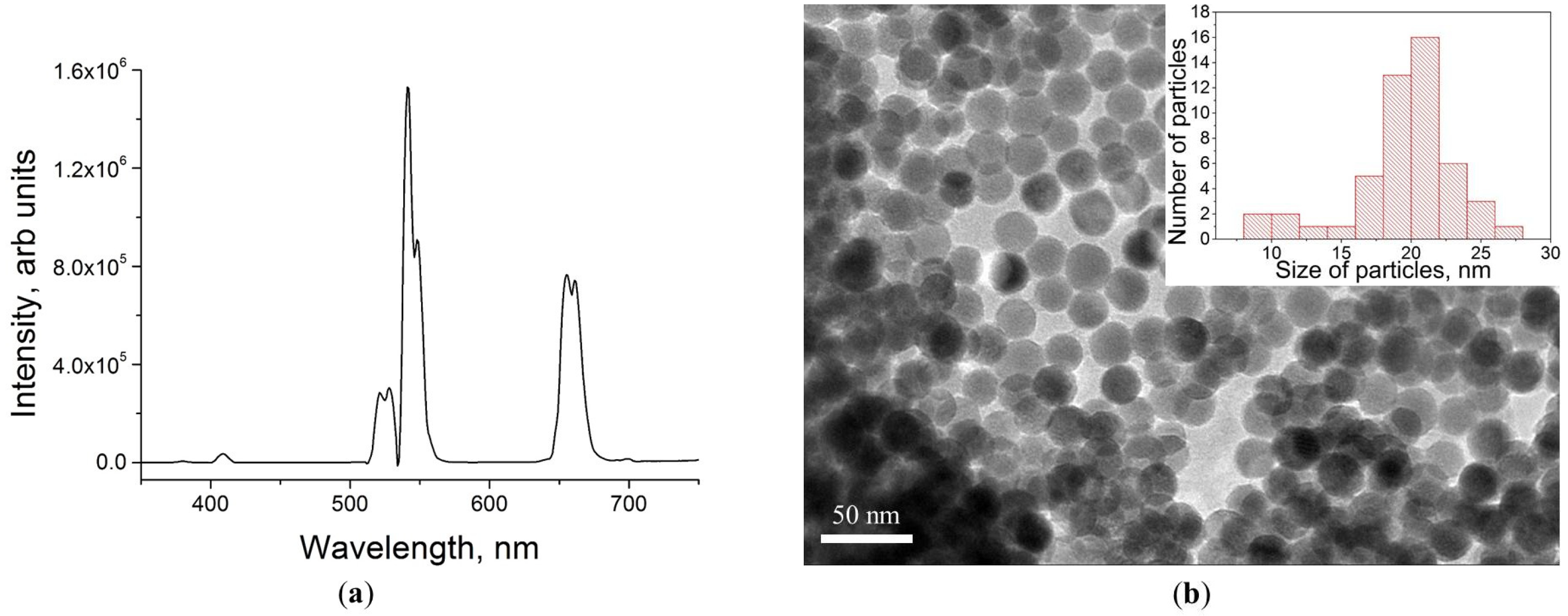
3.1. UCPNs
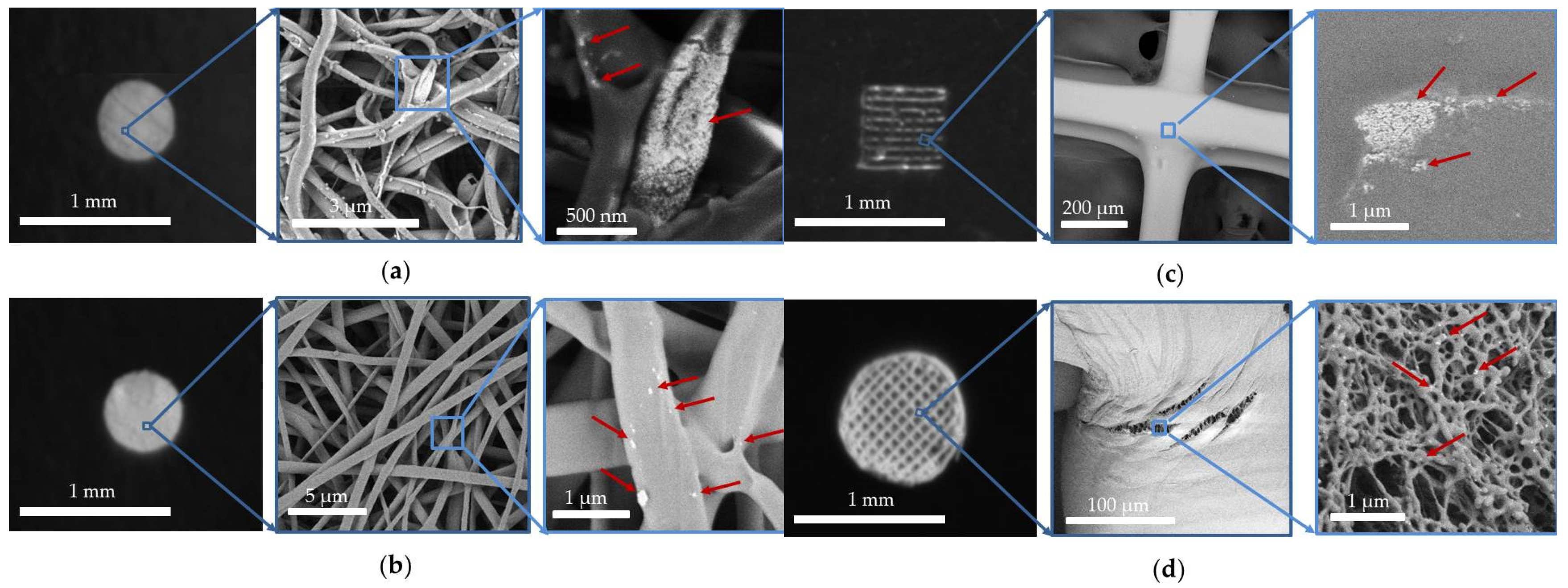
3.2. UCNP-Loaded Polymer Scaffolds
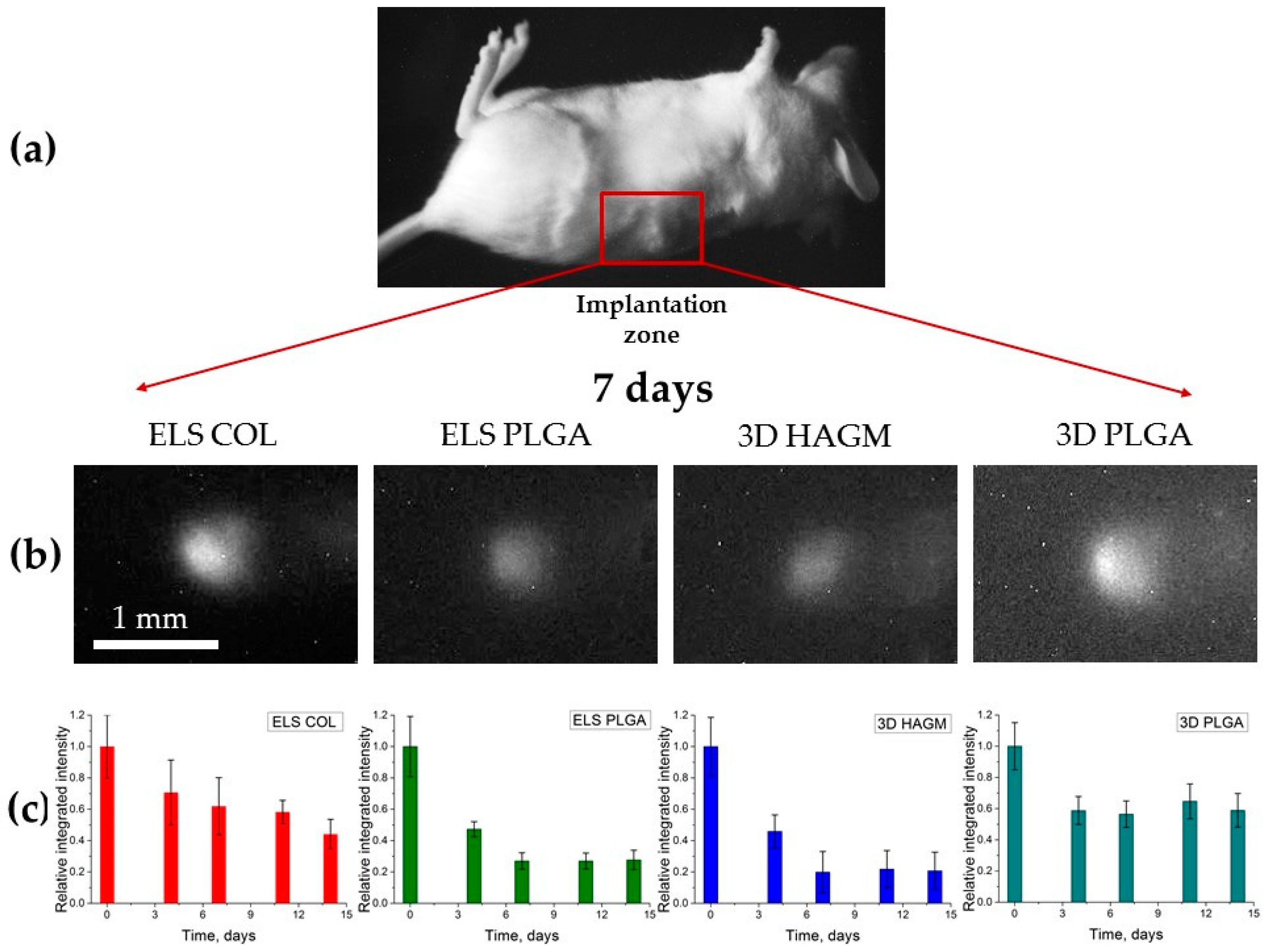
3.3. In Vivo Mice Imaging Study
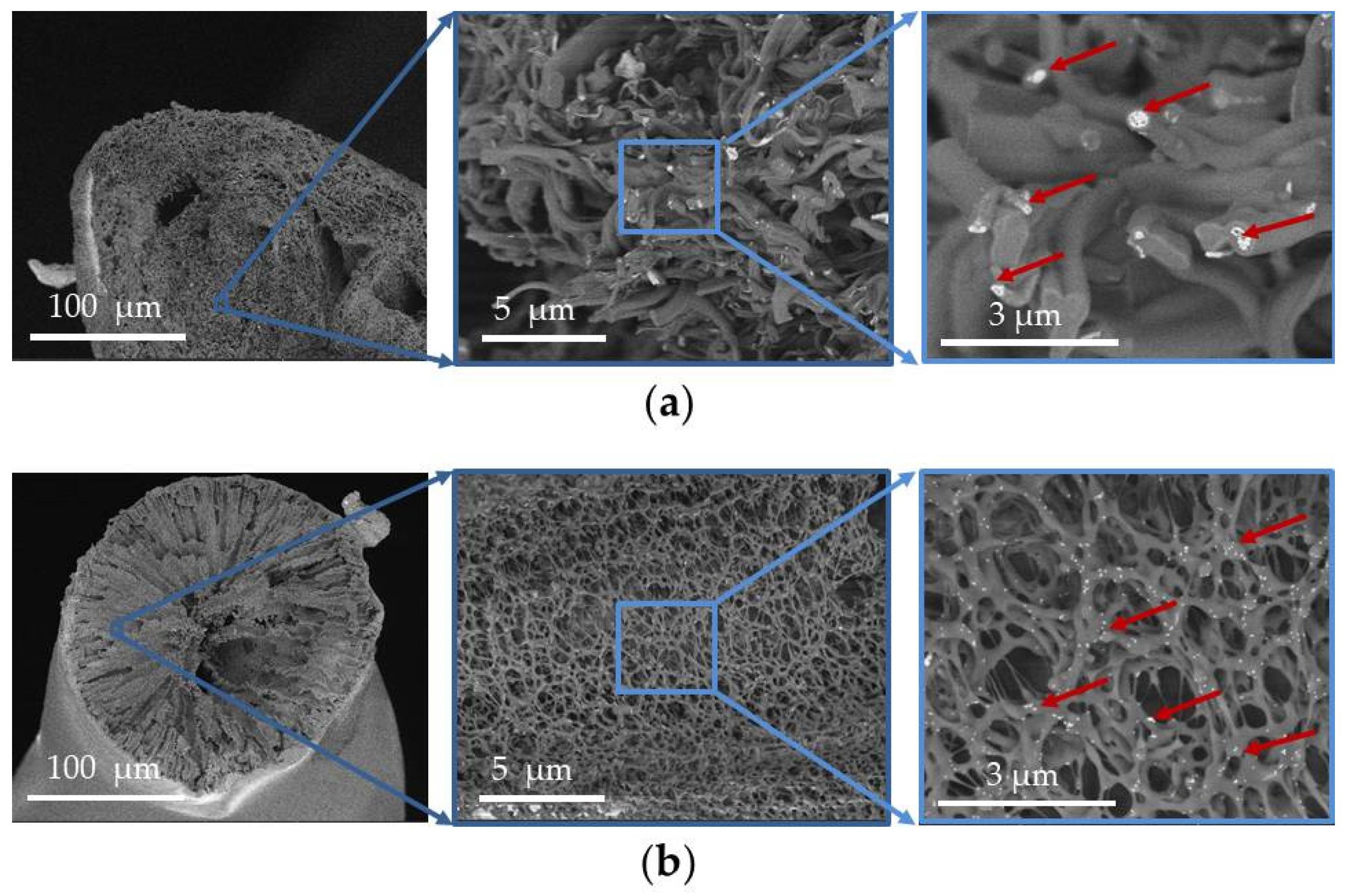
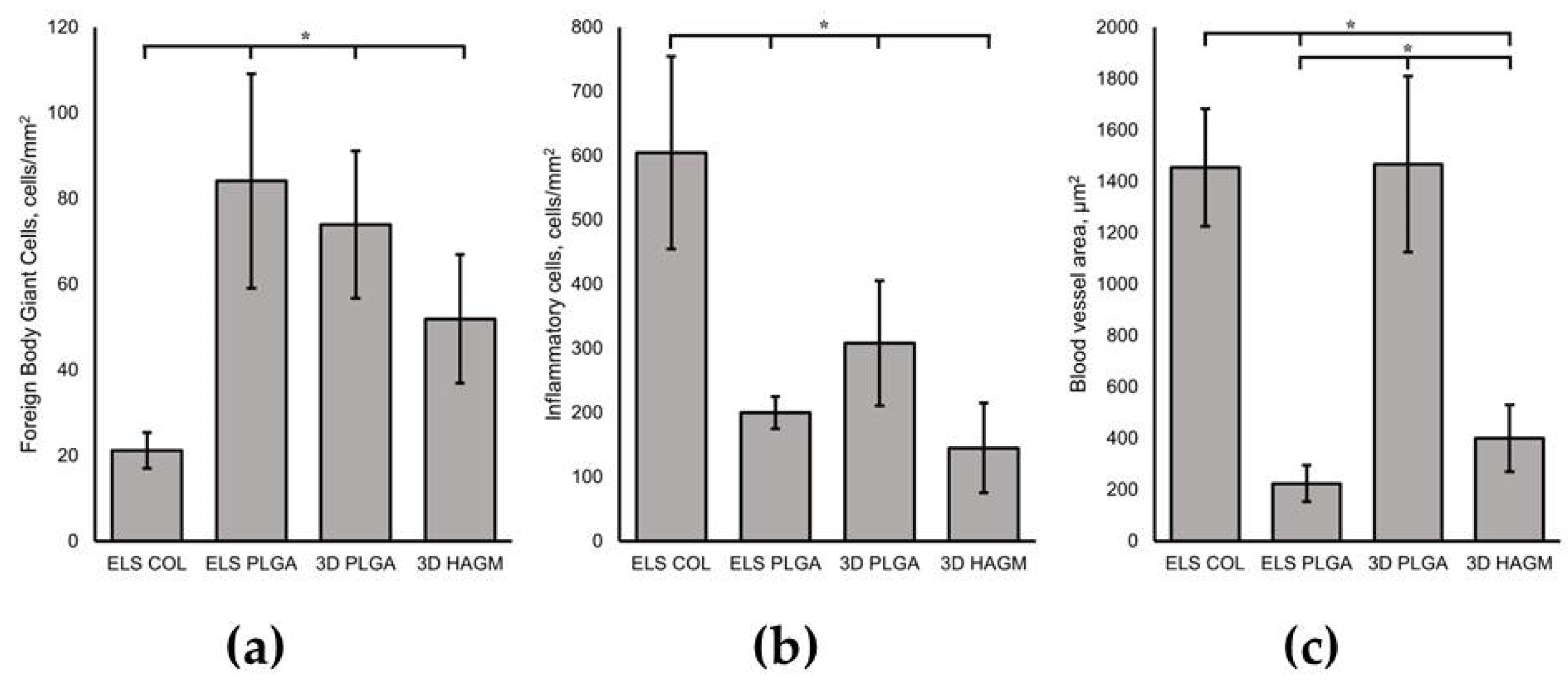
3.4. In Vivo Biocompatibility Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Hutmacher, D.W.; Tandon, B.; Dalton, P.D. Scaffold Design and Fabrication. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 355–385. [Google Scholar]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Agrawal, V.; Prasad, B.L.V. Development of a Smart Scaffold for Sequential Cancer Chemotherapy and Tissue Engineering. ACS Omega 2020, 5, 20724–20733. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, N.; Okkelman, I.A.; Timashev, P.; Gromovykh, T.I.; Papkovsky, D.B.; Dmitriev, R.I. Cellulose-Based Scaffolds for Fluorescence Lifetime Imaging-Assisted Tissue Engineering. Acta Biomater. 2018, 80, 85–96. [Google Scholar] [CrossRef]
- Costa, A.C.; Alves, P.M.; Monteiro, F.J.; Salgado, C. Interactions between Dental MSCs and Biomimetic Composite Scaffold during Bone Remodeling Followed by In Vivo Real-Time Bioimaging. Int. J. Mol. Sci. 2023, 24, 1827. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, W. Functional Electrospun Fibers for Local Therapy of Cancer. Adv. Fiber Mater. 2020, 2, 229–245. [Google Scholar] [CrossRef]
- Kim, H.; Rao, B.A.; Jeong, J.; Angupillai, S.; Choi, J.S.; Nam, J.-O.; Lee, C.-S.; Son, Y.-A. A Rhodamine Scaffold Immobilized onto Mesoporous Silica as a Fluorescent Probe for the Detection of Fe (III) and Applications in Bio-Imaging and Microfluidic Chips. Sens. Actuators B Chem. 2016, 224, 404–412. [Google Scholar] [CrossRef]
- Tian, R.; Ren, X.; Niu, P.; Yang, L.; Sun, A.; Li, Y.; Liu, X.; Wei, L. Development of Chromenoquinoline-Fused Coumarin Dyes and Their Application in Bioimaging. Dye. Pigment. 2022, 205, 110530. [Google Scholar] [CrossRef]
- Feng, G.; Fang, Y.; Liu, J.; Geng, J.; Ding, D.; Liu, B. Multifunctional Conjugated Polymer Nanoparticles for Image-Guided Photodynamic and Photothermal Therapy. Small 2017, 13, 1602807. [Google Scholar] [CrossRef]
- Khademi, S.; Sarkar, S.; Shakeri-Zadeh, A.; Attaran, N.; Kharrazi, S.; Ay, M.R.; Ghadiri, H. Folic Acid-Cysteamine Modified Gold Nanoparticle as a Nanoprobe for Targeted Computed Tomography Imaging of Cancer Cells. Mater. Sci. Eng. C 2018, 89, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure-Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.-F. A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Gao, P.F.; Zou, H.Y.; Huang, C.Z. Precision Improvement in Dark-Field Microscopy Imaging by Using Gold Nanoparticles as an Internal Reference: A Combined Theoretical and Experimental Study. Nanoscale 2016, 8, 8729–8736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong Two-Photon-Induced Fluorescence from Photostable, Biocompatible Nitrogen-Doped Graphene Quantum Dots for Cellular and Deep-Tissue Imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum Dots: Prospectives, Toxicity, Advances and Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Generalova, A.N.; Rocheva, V.V.; Nechaev, A.V.; Khochenkov, D.A.; Sholina, N.V.; Semchishen, V.A.; Zubov, V.P.; Koroleva, A.V.; Chichkov, B.N.; Khaydukov, E.V. PEG-Modified Upconversion Nanoparticles for in Vivo Optical Imaging of Tumors. RSC Adv. 2016, 6, 30089–30097. [Google Scholar] [CrossRef]
- Trifanova, E.M.; Khvorostina, M.A.; Mariyanats, A.O.; Sochilina, A.V.; Nikolaeva, M.E.; Khaydukov, E.V.; Akasov, R.A.; Popov, V.K. Natural and Synthetic Polymer Scaffolds Comprising Upconversion Nanoparticles as a Bioimaging Platform for Tissue Engineering. Molecules 2022, 27, 6547. [Google Scholar] [CrossRef]
- Hemmer, E.; Venkatachalam, N.; Hyodo, H.; Hattori, A.; Ebina, Y.; Kishimoto, H.; Soga, K. Upconverting and NIR Emitting Rare Earth Based Nanostructures for NIR-Bioimaging. Nanoscale 2013, 5, 11339. [Google Scholar] [CrossRef]
- Peng, H.-S.; Chiu, D.T. Soft Fluorescent Nanomaterials for Biological and Biomedical Imaging. Chem. Soc. Rev. 2015, 44, 4699–4722. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic Nanomaterials for Bioimaging, Targeted Drug Delivery and Therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Wan, B.; Gu, Y.; Li, X. Optically Active Nanomaterials for Bioimaging and Targeted Therapy. Front. Bioeng. Biotechnol. 2019, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold Nanoparticles in Biological Optical Imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-Based Nanomaterials for Bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef]
- Li, Y.; Bai, G.; Zeng, S.; Hao, J. Theranostic Carbon Dots with Innovative NIR-II Emission for In Vivo Renal-Excreted Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 4737–4744. [Google Scholar] [CrossRef]
- del Rosal, B.; Jaque, D. Upconversion Nanoparticles for In Vivo Applications: Limitations and Future Perspectives. Methods Appl. Fluoresc. 2019, 7, 022001. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and Therapeutic Applications of Persistent Luminescence Nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef]
- Lingeshwar Reddy, K.; Balaji, R.; Kumar, A.; Krishnan, V. Lanthanide Doped Near Infrared Active Upconversion Nanophosphors: Fundamental Concepts, Synthesis Strategies, and Technological Applications. Small 2018, 14, 1801304. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Oskoei, P.; Andresen, E.; Saleh, M.I.; Rühle, B.; Resch-Genger, U.; Oliveira, H. Stability, Dissolution, and Cytotoxicity of NaYF4-Upconversion Nanoparticles with Different Coatings. Sci. Rep. 2022, 12, 3770. [Google Scholar] [CrossRef]
- Guryev, E.L.; Shilyagina, N.Y.; Kostyuk, A.B.; Sencha, L.M.; Balalaeva, I.V.; Vodeneev, V.A.; Kutova, O.M.; Lyubeshkin, A.V.; Yakubovskaya, R.I.; Pankratov, A.A.; et al. Preclinical Study of Biofunctional Polymer-Coated Upconversion Nanoparticles. Toxicol. Sci. 2019, 170, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Osuchowski, F.; Latos, W.; Kawczyk-Krupka, A. The Use of Upconversion Nanoparticles in Prostate Cancer Photodynamic Therapy. Life 2021, 11, 360. [Google Scholar] [CrossRef]
- Sochilina, A.V.; Savelyev, A.G.; Akasov, R.A.; Zubov, V.P.; Khaydukov, E.V.; Generalova, A.N. Preparing Modified Hyaluronic Acid with Tunable Content of Vinyl Groups for Use in Fabrication of Scaffolds by Photoinduced Crosslinking. Russ. J. Bioorg. Chem. 2021, 47, 828–836. [Google Scholar] [CrossRef]
- Trifanova, E.M.; Nikolaeva, M.E.; Popov, V.K. Synthesis and Characterization of NaYF4:Yb3+:Er3+/NaYF4 Upconversion Nanophosphors. Inorg. Mater. Appl. Res. 2022, 13, 426–433. [Google Scholar] [CrossRef]
- Später, T.; Mariyanats, A.O.; Syachina, M.A.; Mironov, A.V.; Savelyev, A.G.; Sochilina, A.V.; Menger, M.D.; Vishnyakova, P.A.; Kananykhina, E.Y.; Fatkhudinov, T.K.; et al. In Vitro and in Vivo Analysis of Adhesive, Anti-Inflammatory, and Proangiogenic Properties of Novel 3D Printed Hyaluronic Acid Glycidyl Methacrylate Hydrogel Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 5744–5757. [Google Scholar] [CrossRef]
- Mironov, A.V.; Mironova, O.A.; Syachina, M.A.; Popov, V.K. 3D Printing of Polylactic-Co-Glycolic Acid Fiber Scaffolds Using an Antisolvent Phase Separation Process. Polymer 2019, 182, 121845. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Generalova, A.N.; Kochneva, I.K.; Khaydukov, E.V.; Semchishen, V.A.; Guller, A.E.; Nechaev, A.V.; Shekhter, A.B.; Zubov, V.P.; Zvyagin, A.V.; Deyev, S.M. Submicron Polyacrolein Particles in Situ Embedded with Upconversion Nanoparticles for Bioassay. Nanoscale 2015, 7, 1709–1717. [Google Scholar] [CrossRef]
- Aescht, E.; Büchl-Zimmermann, S.; Burmester, A.; Dänhardt-Pfeiffer, S.; Desel, C.; Hamers, C.; Jach, G.; Kässens, M.; Makovitzky, J.; Mulisch, M.; et al. Romeis Mikroskopische Technik; Mulisch, M., Welsch, U., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germnay, 2010; ISBN 978-3-8274-1676-6. [Google Scholar]
- Würth, C.; Kaiser, M.; Wilhelm, S.; Grauel, B.; Hirsch, T.; Resch-Genger, U. Excitation Power Dependent Population Pathways and Absolute Quantum Yields of Upconversion Nanoparticles in Different Solvents. Nanoscale 2017, 9, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Zhuohong, F.; Lin, L.; Zhezhe, W.; Zhiqiang, Z. NIR Optical Temperature Sensing with Efficiently Relative Sensitivity Based on β-NaYF4: Er3+ Nanoparticles. J. Lumin. 2020, 221, 117005. [Google Scholar] [CrossRef]
- Lee, H.; Woo, J.; Son, D.; Kim, M.; Choi, W.I.; Sung, D. Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers. Polymers 2020, 12, 2520. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-Spinning of Pure Collagen Nano-Fibres—Just an Expensive Way to Make Gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Savelyev, A.G.; Sochilina, A.V.; Akasov, R.A.; Mironov, A.V.; Kapitannikova, A.Y.; Borodina, T.N.; Sholina, N.V.; Khaydukov, K.V.; Zvyagin, A.V.; Generalova, A.N.; et al. Facile Cell-Friendly Hollow-Core Fiber Diffusion-Limited Photofabrication. Front. Bioeng. Biotechnol. 2021, 9, 783834. [Google Scholar] [CrossRef]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef]
- Arppe, R.; Hyppänen, I.; Perälä, N.; Peltomaa, R.; Kaiser, M.; Würth, C.; Christ, S.; Resch-Genger, U.; Schäferling, M.; Soukka, T. Quenching of the Upconversion Luminescence of NaYF4:Yb3+,Er3+ and NaYF4:Yb3+,Tm3+ Nanophosphors by Water: The Role of the Sensitizer Yb3+ in Non-Radiative Relaxation. Nanoscale 2015, 7, 11746–11757. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifanova, E.M.; Babayeva, G.; Khvorostina, M.A.; Atanova, A.V.; Nikolaeva, M.E.; Sochilina, A.V.; Khaydukov, E.V.; Popov, V.K. Photoluminescent Scaffolds Based on Natural and Synthetic Biodegradable Polymers for Bioimaging and Tissue Engineering. Life 2023, 13, 870. https://doi.org/10.3390/life13040870
Trifanova EM, Babayeva G, Khvorostina MA, Atanova AV, Nikolaeva ME, Sochilina AV, Khaydukov EV, Popov VK. Photoluminescent Scaffolds Based on Natural and Synthetic Biodegradable Polymers for Bioimaging and Tissue Engineering. Life. 2023; 13(4):870. https://doi.org/10.3390/life13040870
Chicago/Turabian StyleTrifanova, Ekaterina M., Gulalek Babayeva, Maria A. Khvorostina, Aleksandra V. Atanova, Maria E. Nikolaeva, Anastasia V. Sochilina, Evgeny V. Khaydukov, and Vladimir K. Popov. 2023. "Photoluminescent Scaffolds Based on Natural and Synthetic Biodegradable Polymers for Bioimaging and Tissue Engineering" Life 13, no. 4: 870. https://doi.org/10.3390/life13040870
APA StyleTrifanova, E. M., Babayeva, G., Khvorostina, M. A., Atanova, A. V., Nikolaeva, M. E., Sochilina, A. V., Khaydukov, E. V., & Popov, V. K. (2023). Photoluminescent Scaffolds Based on Natural and Synthetic Biodegradable Polymers for Bioimaging and Tissue Engineering. Life, 13(4), 870. https://doi.org/10.3390/life13040870






