Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Collection and Preparation
2.2. Preparation of the Algal Extract
2.3. Fractionation of Extract with Column Chromatography
2.4. Microorganisms
2.5. Disc Diffusion Method
2.6. Fish and Experimental Setup
2.7. Blood Samples
2.8. Non-Specific Immune Assays
2.8.1. Bactericidal Activity
2.8.2. Superoxide Anion Production
2.8.3. Lysozyme Activity
2.9. Analysis of Serum Biochemical Parameters
2.10. Determination of Haematological Parameters
2.11. Growth Measurements
2.12. Challenge of Fish
2.13. Statistical Analysis
3. Results
3.1. Screening of Antibacterial Activity
3.2. Immunological Indices
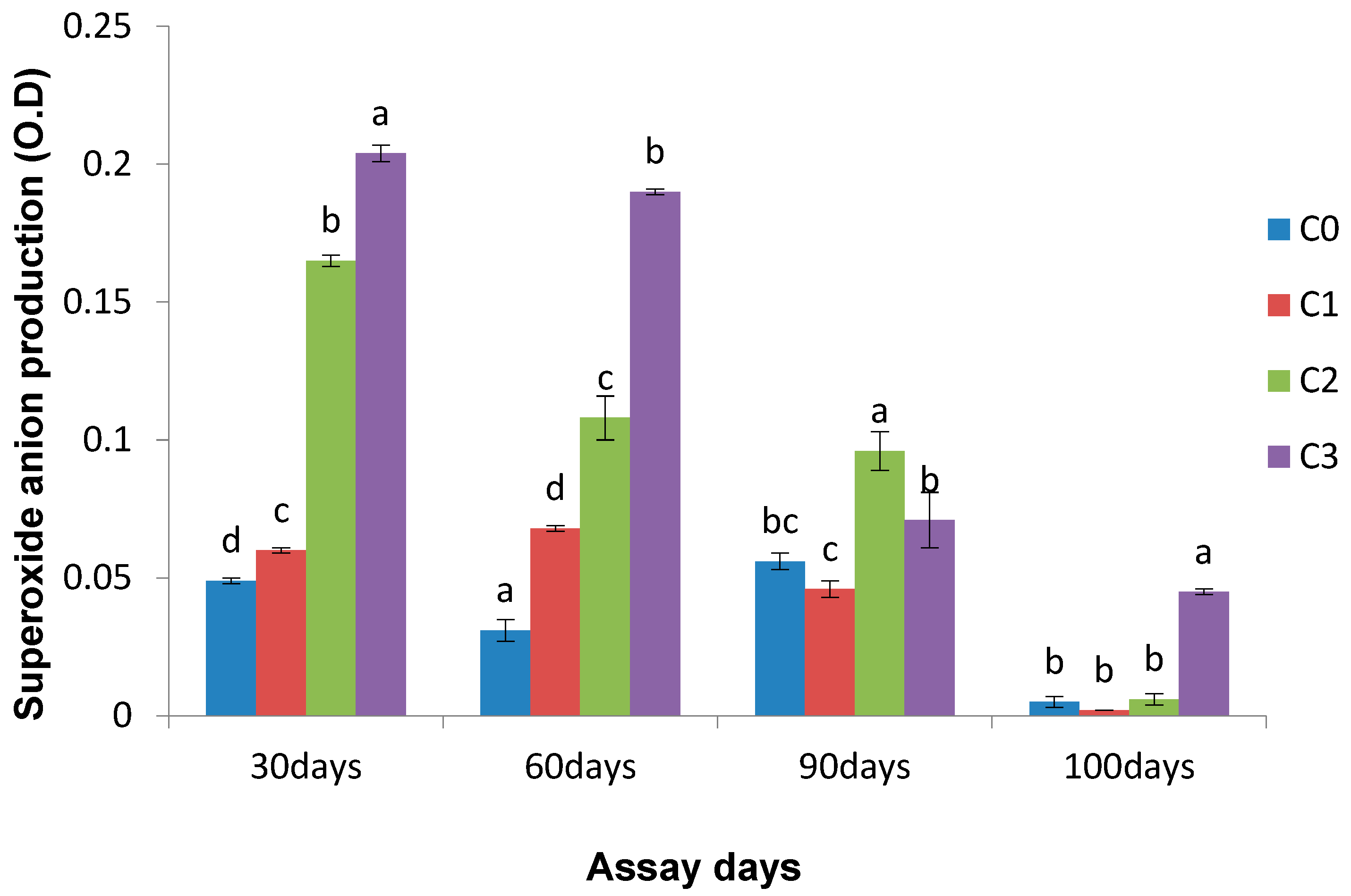
3.2.1. Superoxide Anion Production
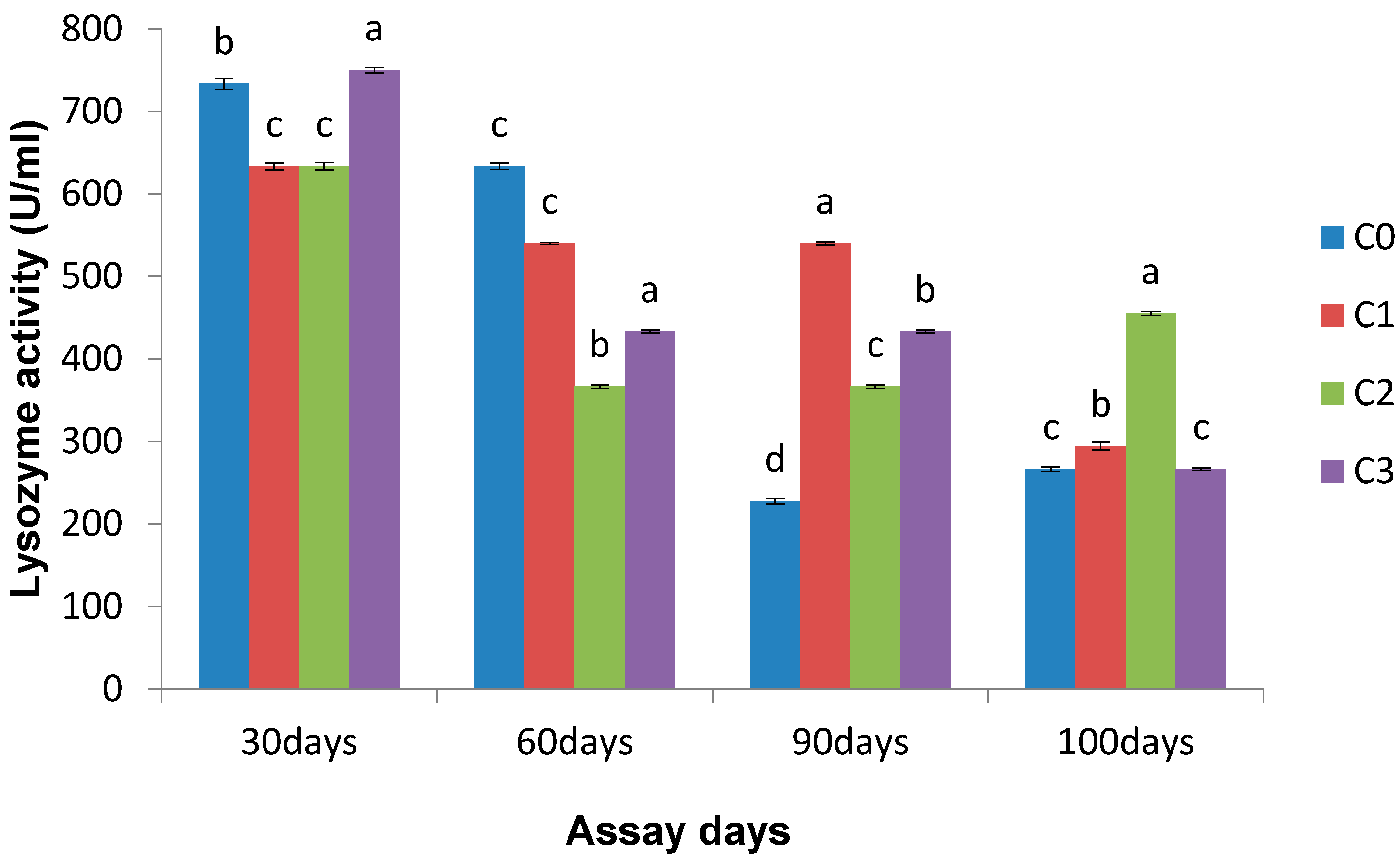
3.2.2. Lysozyme Activity
3.2.3. Bactericidal Activity
3.3. Serum Biochemical Indices
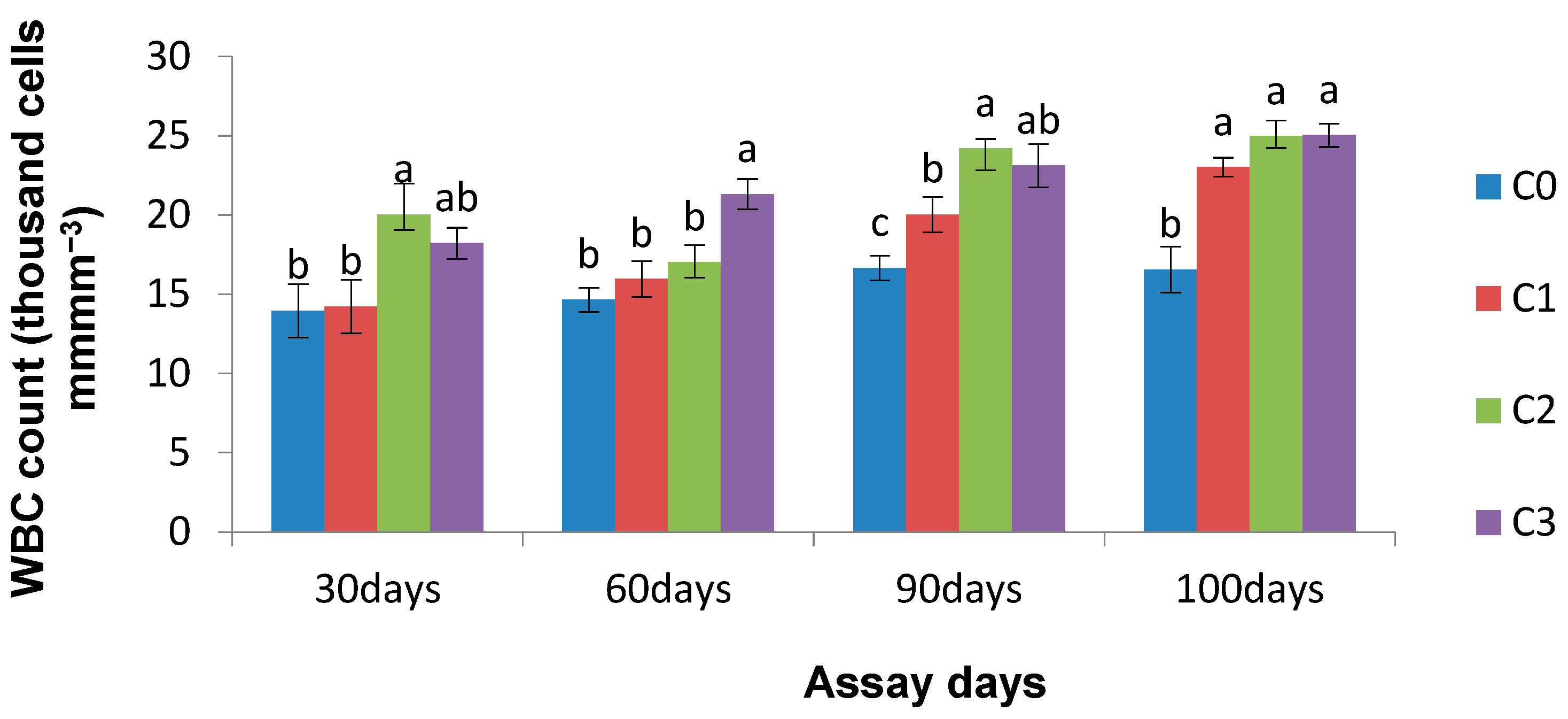
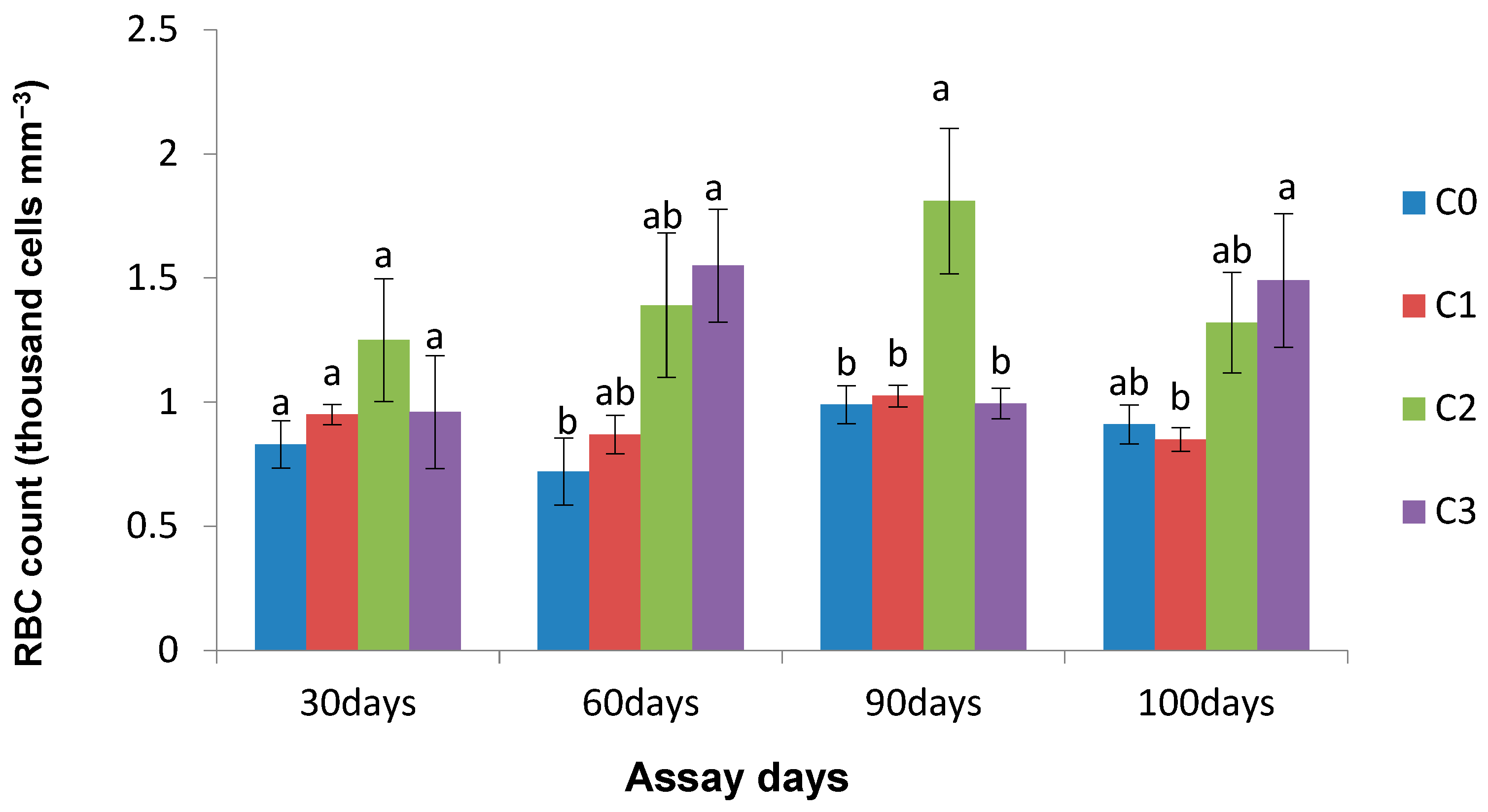
3.4. Effect of Chlorella on Haematological Parameters
3.5. Growth and Feed Efficiency
3.6. Fish Survivability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.S.; Rakesh, D.; Dhiman, M.; Choudhary, P.; Debbarma, J.; Sahoo, S.N.; Mishra, C.K. Present Status of Fish Disease Management in Freshwater Aquaculture in India: State-of-the-Art-Review. J. Aquac. Fish. 2017, 1, 14. [Google Scholar]
- Frerichs, G.N. Isolation and Identification of Fish Bacterial Pathogens; Institute of Aquaculture, University of Stirling: Stirling, UK, 1984; ISBN 0901636606. [Google Scholar]
- Janda, J.M.; Abbott, S.L. Evolving Concepts Regarding the Genus Aeromonas: An Expanding Panorama of Species, Disease Presentations, and Unanswered Questions. Clin. Infect. Dis. 1998, 27, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Amiri, M.S. A Review of the Use of Prebiotic in Aquaculture for Fish and Shrimp. Afr. J. Biotechnol. 2009, 8, 7313–7318. [Google Scholar]
- Sharma, S.; Shah, E.; Davla, D.; Dixit, G.; Patel, A.; Kumar, A.K. Effect of Microalga-Based Diet on Oxidative Stress Enzymes of African Catfish, Clarias Gariepinus. Int. Aquat. Res. 2019, 11, 377–387. [Google Scholar] [CrossRef]
- Ogier de Baulny, M.; Quentel, C.; Fournier, V.; Lamour, F.; Le Gouvello, R. Effect of Long-Term Oral Administration of Beta-Glucan as an Immunostimulant or an Adjuvant on Some Non-Specific Parameters of the Immune Response of Turbot Scophthalmus Maximus. Dis. Aquat. Org. 1996, 26, 139–147. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in Aquafeeds for a Sustainable Aquaculture Industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Yamaguchi, K. Recent Advances in Microalgal Bioscience in Japan, with Special Reference to Utilization of Biomass and Metabolites: A Review. J. Appl. Phycol. 1996, 8, 487–502. [Google Scholar] [CrossRef]
- Enyidi, U.D. Chlorella Vulgaris as Protein Source in the Diets of African Catfish Clarias Gariepinus. Fishes 2017, 2, 17. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella Vulgaris: A Perspective on Its Potential for Combining High Biomass with High Value Bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz Bda, S.; de Morais, E.G.; Costa, J.A. Biologically Active Metabolites Synthesized by Microalgae. Biomed. Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sweet, B.V. Lutein and zeaxanthin for macular degeneration. Am. J. Health-Syst. Pharm. 2008, 65, 1232–1238. [Google Scholar] [CrossRef]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and Immunomodulatory Activities of Polysaccharide from Chlorella Stigmatophora and Phaeodactylum Tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Pradhan, J. Antibacterial Properties of Selected Freshwater Microalgae against Pathogenic Bacteria. Indian J. Fish. 2010, 57, 61–66. [Google Scholar]
- Radhakrishnan, S.; Bhavan, P.S.; Seenivasan, C.; Shanthi, R.; Muralisankar, T. Replacement of Fishmeal with Spirulina Platensis, Chlorella Vulgaris and Azolla Pinnata on Non-Enzymatic and Enzymatic Antioxidant Activities of Macrobrachium Rosenbergii. J. Basic Appl. Zool. 2014, 67, 25–33. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; El-Sayed, B.M.; Mahsoub, Y.H.; Neamat-Allah, A.N.F. Effect of Chlorella Vulgaris Enriched Diet on Growth Performance, Hemato-Immunological Responses, Antioxidant and Transcriptomics Profile Disorders Caused by Deltamethrin Toxicity in Nile Tilapia (Oreochromis Niloticus). Fish Shellfish Immunol. 2020, 102, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, K.; Sirakov, I.; Denev, S. In Vitro Antibacterial Effect of Lemna Minuta, Chlorella Vulgaris and Spirulina sp. Extracts against Fish Pathogen Aeromonas Hydrophila. Aquac. Aquar. Conserv. Legis. 2019, 12, 936–940. [Google Scholar]
- Das, B.K.; Pradhan, J.; Sahu, S.; Marhual, N.P.; Mishra, B.K.; Eknath, A.E. Microcystis aeruginosa (K Ütz) Incorporated Diets Increase Immunity and Survival of Indian Major Carp Labeo Rohita (H Am.) against Aeromonas Hydrophila Infection. Aquac. Res. 2013, 44, 918–927. [Google Scholar] [CrossRef]
- Bai, S.C.; Koo, J.; Kim, K.; Kim, S. Effects of Chlorella Powder as a Feed Additive on Growth Performance in Juvenile Korean Rockfish, Sebastes Schlegeli (Hilgendorf). Aquac. Res. 2001, 32, 92–98. [Google Scholar] [CrossRef]
- Khani, M.; Soltani, M.; Mehrjan, M.S.; Foroudi, F.; Ghaeni, M. The Effect of Chlorella Vulgaris (Chlorophyta, Volvocales) Microalga on Some Hematological and Immune System Parameters of Koi Carp (Cyprinus Carpio). Iran. J. Ichthyol. 2017, 4, 62–68. [Google Scholar]
- Cho, S.H.; Hur, S.B.; Jo, J. Effect of Enriched Live Feeds on Survival and Growth Rates in Larval Korean Rockfish, Sebastes Schlegeli Hilgendorf. Aquac. Res. 2001, 32, 199–208. [Google Scholar] [CrossRef]
- Kim, K.; Bai, S.C.; Koo, J.; Wang, X.; Kim, S. Effects of Dietary Chlorella Ellipsoidea Supplementation on Growth, Blood Characteristics, and Whole-body Composition in Juvenile Japanese Flounder Paralichthys Olivaceus. J. World Aquac. Soc. 2002, 33, 425–431. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.; Shao, R. Effect of Dietary Chlorella on the Growth Performance and Physiological Parameters of Gibel Carp, Carassius Auratus Gibelio. Turk. J. Fish. Aquat. Sci. 2014, 14, 53–57. [Google Scholar] [CrossRef]
- Huang, J.-C.; Wang, Y.; Sandmann, G.; Chen, F. Isolation and Characterization of a Carotenoid Oxygenase Gene from Chlorella Zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2006, 71, 473–479. [Google Scholar] [CrossRef]
- Suárez, E.R.; Syvitski, R.; Kralovec, J.A.; Noseda, M.D.; Barrow, C.J.; Ewart, H.S.; Lumsden, M.D.; Grindley, T.B. Immunostimulatory Polysaccharides from Chlorella p Yrenoidosa. A New Galactofuranan. Measurement of Molecular Weight and Molecular Weight Dispersion by DOSY NMR. Biomacromolecules 2006, 7, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- El-Habashi, N.; Fadl, S.E.; Farag, H.F.; Gad, D.M.; Elsadany, A.Y.; El Gohary, M.S. Effect of Using Spirulina and Chlorella as Feed Additives for Elevating Immunity Status of Nile Tilapia Experimentally Infected with Aeromonas Hydrophila. Aquac. Res. 2019, 50, 2769–2781. [Google Scholar] [CrossRef]
- Stahl, E. Apparatus and General Techniques in TLC. In Thin-Layer Chromatography; Springer: Berlin/Heidelberg, Germany, 1969; pp. 52–86. [Google Scholar]
- Das, B.K.; Pradhan, J.; Sahu, S. The Effect of Euglena Viridis on Immune Response of Rohu, Labeo Rohita (Ham.). Fish Shellfish Immunol. 2009, 26, 871–876. [Google Scholar] [CrossRef]
- Das, B.K.; Pradhan, J.; Pattnaik, P.; Samantaray, B.R.; Samal, S.K. Production of Antibacterials from the Freshwater Alga Euglena Viridis (Ehren). World J. Microbiol. Biotechnol. 2005, 21, 45–50. [Google Scholar] [CrossRef]
- Izzo, A.A.; Carlo, I.; Biscardi, G.; Fusco, D.; Mascolo, R.; Borreli, N.; Capasso, F.; Fasulo, F.; Autore, M.P. Biological Screening of Italian Medicinal Plants for Antibacterial Activity. Phytother. Res. 1995, 9, 281–286. [Google Scholar] [CrossRef]
- Pradhan, J.; Sahu, S.; Nayak, K.K.; Das, B.K. Effect of Dietary Supplementation of Euglena Viridis on Growth Performance and Selected Serum Biochemical Parameters in Labeo Rohita (Hamilton, 1822) Fingerlings. Indian J. Fish. 2021, 68, 56–64. [Google Scholar] [CrossRef]
- Kajita, Y.; Sakai, M.; Atsuta, S.; Kobayashi, M. The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchus mykiss. Fish Pathol. 1990, 25, 93–98. [Google Scholar] [CrossRef]
- Chung, S.; Secombes, C.J. Analysis of Events Occurring within Teleost Macrophages during the Respiratory Burst. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 539–544. [Google Scholar] [CrossRef]
- Parry, R.M.; Chandan, R.; Shahani, K. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384. [Google Scholar] [CrossRef]
- Classics Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dumas, B.T.; Watson, W.A.; Biggs, H.G. Quantitative Colorimetric Determination of Albumin in Serum or Plasma. Clin. Chem. Acta 1971, 31, 87–91. [Google Scholar]
- Van Kampen, E.J.; Zijlstra, W.G. Recommendations for Haemoglobinometry in Human Blood. Br. J. Haematol. 1967, 13, 71. [Google Scholar]
- Hendricks, L.J. Erythrocyte Counts and Hemoglobin Determinations for Two Species of Suckers, Genus Catostomus, from Colorado. Copeia 1952, 1952, 265–266. [Google Scholar] [CrossRef]
- Ricker, W.E. Growth rate and models. In Fish Physiology; Hore, W.S., Brett, P.J., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 677–743. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Rairakhwada, D.; Pal, A.K.; Bhathena, Z.P.; Sahu, N.P.; Jha, A.; Mukherjee, S.C. Dietary Microbial Levan Enhances Cellular Non-Specific Immunity and Survival of Common Carp (Cyprinus Carpio) Juveniles. Fish Shellfish Immunol. 2007, 22, 477–486. [Google Scholar] [CrossRef]
- Ibrahem, M.D.; Mohamed, M.F.; Ibrahim, M.A. The Role of Spirulina Platensis (Arthrospira Platensis) in Growth and Immunity of Nile Tilapia (Oreochromis Niloticus) and Its Resistance to Bacterial Infection. J. Agric. Sci. 2013, 5, 109–117. [Google Scholar] [CrossRef]
- Engstad, R.E.; Robertsen, B.; Frivold, E. Yeast Glucan Induces Increase in Lysozyme and Complement-Mediated Haemolytic Activity in Atlantic Salmon Blood. Fish Shellfish Immunol. 1992, 2, 287–297. [Google Scholar] [CrossRef]
- Sahu, S.; Das, B.K.; Mishra, B.K.; Pradhan, J.; Sarangi, N. Effect of Allium Sativum on the Immunity and Survival of Labeo Rohita Infected with Aeromonas Hydrophila. J. Appl. Ichthyol. 2007, 23, 80–86. [Google Scholar] [CrossRef]
- Chen, J.; Seviour, R. Medicinal Importance of Fungal β-(1→3), (1→6)-Glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Kumar, S.; Raman, R.P.; Kumar, K.; Pandey, P.K.; Kumar, N.; Mallesh, B.; Mohanty, S.; Kumar, A. Effect of Azadirachtin on Haematological and Biochemical Parameters of Argulus-Infested Goldfish Carassius Auratus (Linn. 1758). Fish Physiol. Biochem. 2013, 39, 733–747. [Google Scholar] [CrossRef]
- Wiegertjes, G.F.; Stet, R.M.; Parmentier, H.K.; van Muiswinkel, W.B. Immunogenetics of Disease Resistance in Fish: A Comparative Approach. Dev. Comp. Immunol. 1996, 20, 365–381. [Google Scholar] [CrossRef]
- Lucía-Pavón, E.; Sarma, S.S.S.; Nandini, S. Effect of Different Densities of Live and Dead Chlorella Vulgaris on the Population Growth of Rotifers Brachionus Calyciflorus and Brachionus Patulus (Rotifera). Rev. Biología Trop. 2001, 49, 895–902. [Google Scholar]
- Rieger, A.M.; Barreda, D.R. Antimicrobial Mechanisms of Fish Leukocytes. Dev. Comp. Immunol. 2011, 35, 1238–1245. [Google Scholar] [CrossRef]
- Karuppiah, V. Evaluation of Antibacterial Activity and Immunostimulant of Red Seaweed Chondrococcus Hornemanni (Kuetzing, 1847) against Marine Ornamental Fish Pathogens. J. Coast. Life Med. 2014, 2, 64–69. [Google Scholar]
- Khani, M.; Soltani, M.; Shamsaie Mehrjan, M.; Foroudi, F.; Ghaeni, M. The effects of Chlorella vulgaris supplementation on growth performance, blood characteristics, and digestive enzymes in Koi (Cyprinus carpio). Iranian J. Fish. Sc. 2017, 16, 832–843. Available online: http://jifro.ir/article-1-2753-en.html (accessed on 25 November 2022).
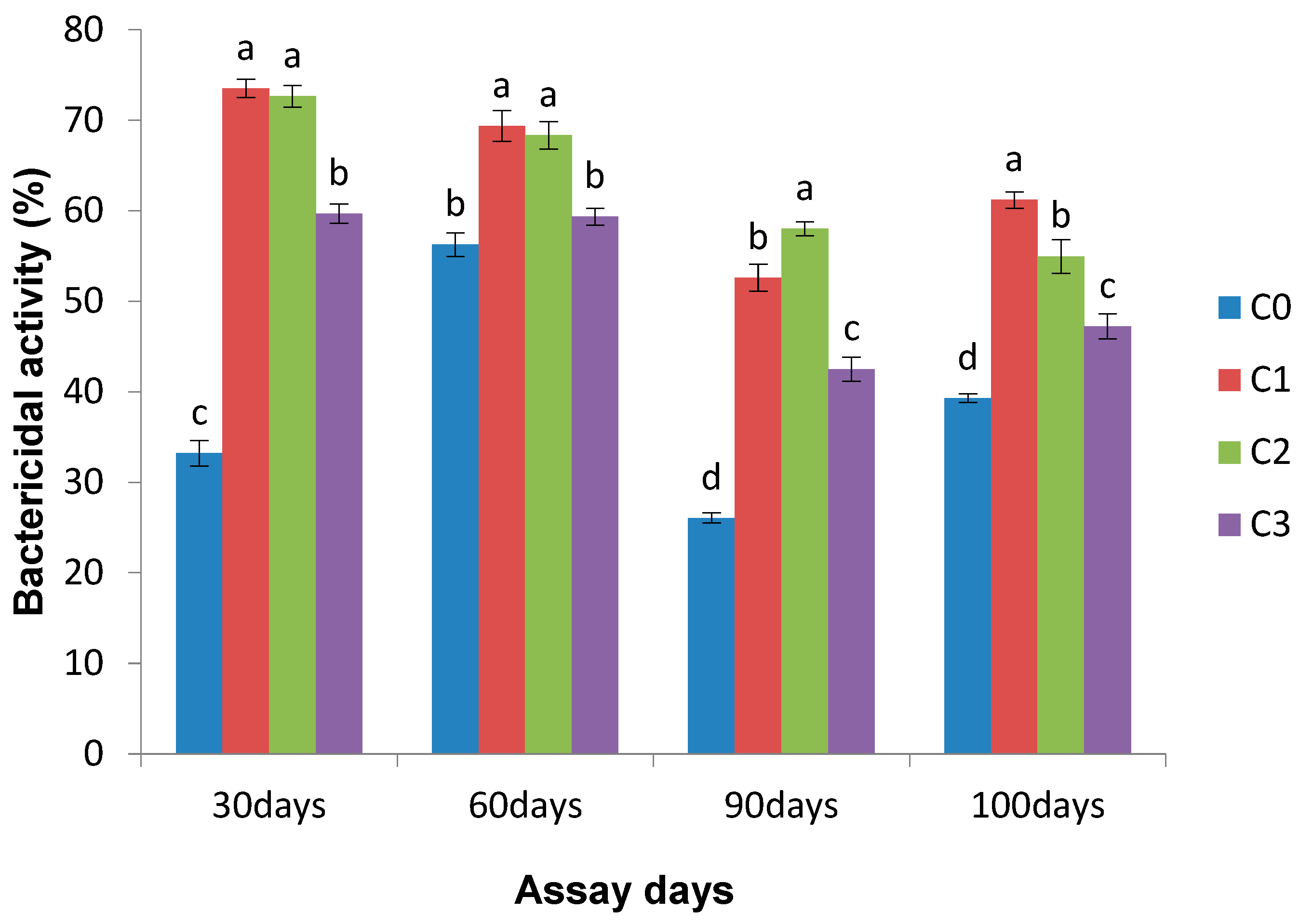

| Ingredients | Group C0 (Control) | Group C1 | Group C2 | Group C3 |
|---|---|---|---|---|
| Groundnut oil cake | 400 g | 400 g | 400 g | 400 g |
| Fish meal | 250 g | 250 g | 250 g | 250 g |
| Rice bran | 200 g | 199.9 g | 199.5 g | 199 g |
| Soyabean meal | 120 g | 120 g | 120 g | 120 g |
| Vitamin and mineral mixture | 20 g | 20 g | 20 g | 20 g |
| Starch | 10 g | 10 g | 10 g | 10 g |
| Chlorella powder | - | 0.1 | 0.5 | 1.0 |
| Fractions | A. hydrophila | P. putida | ||||||
|---|---|---|---|---|---|---|---|---|
| Sl. No. | Code No. | Disc Potency (mcg) | AH1 | AH2 | AH3 | AH4 | PP1 | PP2 |
| Zone of Inhibition (in mm) | ||||||||
| 1 | CF2 | 100 | 8.0 ± 0 | 12.33 ± 0.58 | 15.0 ± 0 | 15.67 ± 0.58 | 13.0 ± 0 | 12.33 ± 0.58 |
| 2 | CF4 | 85 | 9.33 ± 0.58 | 9.67 ± 0.58 | 8.0 ± 0 | 11.0 ± 1.0 | 12.33 ± 0.58 | 12.67 ± 0.58 |
| 3 | CF5 | 100 | 13.67 ± 0.58 | 12.0 ± 1.0 | 9.33 ± 0.58 | 11.67 ± 0.58 | 13.33 ± 0.58 | 14.0 ± 1.73 |
| 4 | Clotrimazole (10 mcg) | - | 11 | 11 | - | - | 10 | - |
| 5 | Tetracycline (25 mcg) | - | 20 | 29 | - | - | 15 | - |
| 6 | Furazolodone (50 mcg) | - | 23 | 23 | - | - | 15 | - |
| Parameters | Groups | Pre Challenge | Post Challenge | ||
|---|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | 10 Days | ||
| Total Protein (g dL−1) | C0 | 1.95 ± 0.05 b | 1.37 ± 0.17 b | 2.14 ± 0.20 c | 2.12 ± 0.52 b |
| C1 | 2.61 ± 0.21 a | 1.43 ± 0.16 b | 2.63 ± 0.11 bc | 2.32 ± 0.38 b | |
| C2 | 1.76 ± 0.15 b | 2.57 ± 0.21 a | 2.72 ± 0.06 b | 4.56 ± 0.24 a | |
| C3 | 1.38 ± 0.37 b | 1.66 ± 0.33 b | 3.66 ± 0.17 a | 2.01 ± 0.81 b | |
| Albumin (g dL−1) | C0 | 0.44 ± 0.01 a | 0.95 ± 0.02 a | 1.44 ± 0.10 a | 1.46 ± 0.13 a |
| C1 | 0.31 ± 0.01 b | 0.87 ± 0.02 a | 1.33 ± 0.31 a | 1.45 ± 0.09 a | |
| C2 | 0.41 ± 0.02 a | 0.98 ± 0.05 a | 1.27 ± 0.35 a | 1.35 ± 0.09 a | |
| C3 | 0.26 ± 0.02 b | 0.60 ± 0.01 b | 1.54 ± 0.22 a | 0.97 ± 0.01 b | |
| Globulin (g dL−1) | C0 | 1.55 ± 0.10 ab | 0.44 ± 0.03 b | 0.70 ± 0.01 b | 0.65 ± 0.12 b |
| C1 | 2.30 ± 0.21 a | 0.55 ± 0.19 b | 1.30 ± 0.07 a | 0.87 ± 0.07 b | |
| C2 | 1.28 ± 0.51 ab | 1.60 ± 0.22 a | 1.44 ± 0.02 a | 2.45 ± 0.20 a | |
| C3 | 1.13 ± 0.28 b | 1.06 ± 0.30 ab | 1.52 ± 0.28 a | 1.03 ± 0.38 b | |
| A: G Ratio | C0 | 0.27 ± 0.06 b | 2.09 ± 0.09 a | 2.66 ± 0.31 a | 2.14 ± 0.20 a |
| C1 | 0.13 ± 0.02 b | 1.79 ± 0.32 a | 0.99 ± 0.28 b | 1.69 ± 0.19 a | |
| C2 | 0.45 ± 0.08 b | 1.24 ± 0.39 ab | 0.88 ± 0.29 b | 0.60 ± 0.23 b | |
| C3 | 0.23 ± 0.01 ab | 0.53 ± 0.04 b | 0.92 ± 0.24 b | 0.86 ± 0.11 b | |
| Group (Dose in g kg−1) | SGR | FCR | Survivability (%) |
|---|---|---|---|
| C0 (0) | 0.86 ± 0.016 b | 1.83 ± 0.027 a | 51.65 ± 1.65 c |
| C1 (0.1) | 0.88 ± 0.014 b | 1.81 ± 0.024 a | 68.30 ± 5.00 b |
| C2 (0.5) | 0.96 ± 0.016 a | 1.69 ± 0.029 b | 81.65 ± 1.65 a |
| C3 (1.0) | 0.98 ± 0.016 a | 1.61 ± 0.026 b | 86.50 ± 3.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, J.; Sahu, S.; Das, B.K. Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection. Life 2023, 13, 1028. https://doi.org/10.3390/life13041028
Pradhan J, Sahu S, Das BK. Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection. Life. 2023; 13(4):1028. https://doi.org/10.3390/life13041028
Chicago/Turabian StylePradhan, Jyotirmayee, Swagatika Sahu, and Basanta Kumar Das. 2023. "Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection" Life 13, no. 4: 1028. https://doi.org/10.3390/life13041028
APA StylePradhan, J., Sahu, S., & Das, B. K. (2023). Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection. Life, 13(4), 1028. https://doi.org/10.3390/life13041028









