Abstract
Inflammatory bowel disease (IBD) is a prominent global public health issue. Anti-inflammatory medications, immunosuppressants, and biological therapies are currently used as treatments. However, they are often unsuccessful and have negative consequences on human health. Thus, there is a tremendous demand for using natural substances, such as seaweed polysaccharides, to treat IBD’s main pathologic treatment targets. The cell walls of marine algae are rich in sulfated polysaccharides, including carrageenan in red algae, ulvan in green algae, and fucoidan in brown algae. These are effective candidates for drug development and functional nutrition products. Algal polysaccharides treat IBD through therapeutic targets, including inflammatory cytokines, adhesion molecules, intestinal epithelial cells, and intestinal microflora. This study aimed to systematically review the potential therapeutic effects of algal polysaccharides on IBD while providing the theoretical basis for a nutritional preventive mechanism for IBD and the restoration of intestinal health. The results suggest that algal polysaccharides have significant potential in complementary IBD therapy and further research is needed for fully understanding their mechanisms of action and potential clinical applications.
1. Introduction
The intestine is the largest surface that creates a connection between the body and the external environment. In addition to being a significant entry point for dangerous infections, it carries food antigens as well as a large, varied bacterial flora that must be tolerated. The collaboration of several regulatory systems stops the immune system from responding toward innocuous external antigens found in the colon, and it maintains the balance between the host and flora [1]. Intestinal inflammation occurs due to the increase in proinflammatory stimuli, the disruption of the intestinal barrier, defective immunoregulatory mechanisms, or excessive immune effector activity [2]. In humans, the most frequent cause of chronic intestinal inflammation is inflammatory bowel disease (IBD) [3].
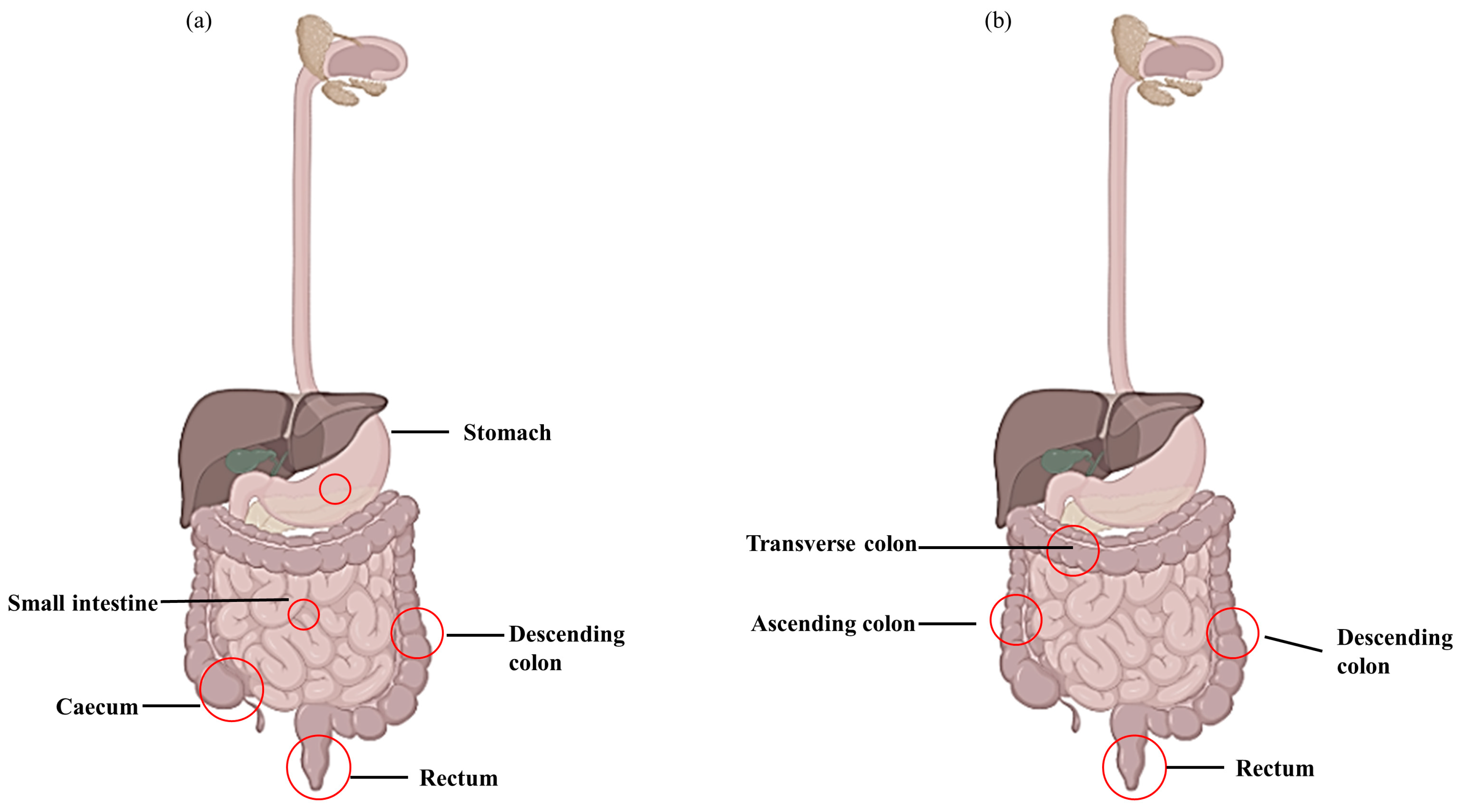
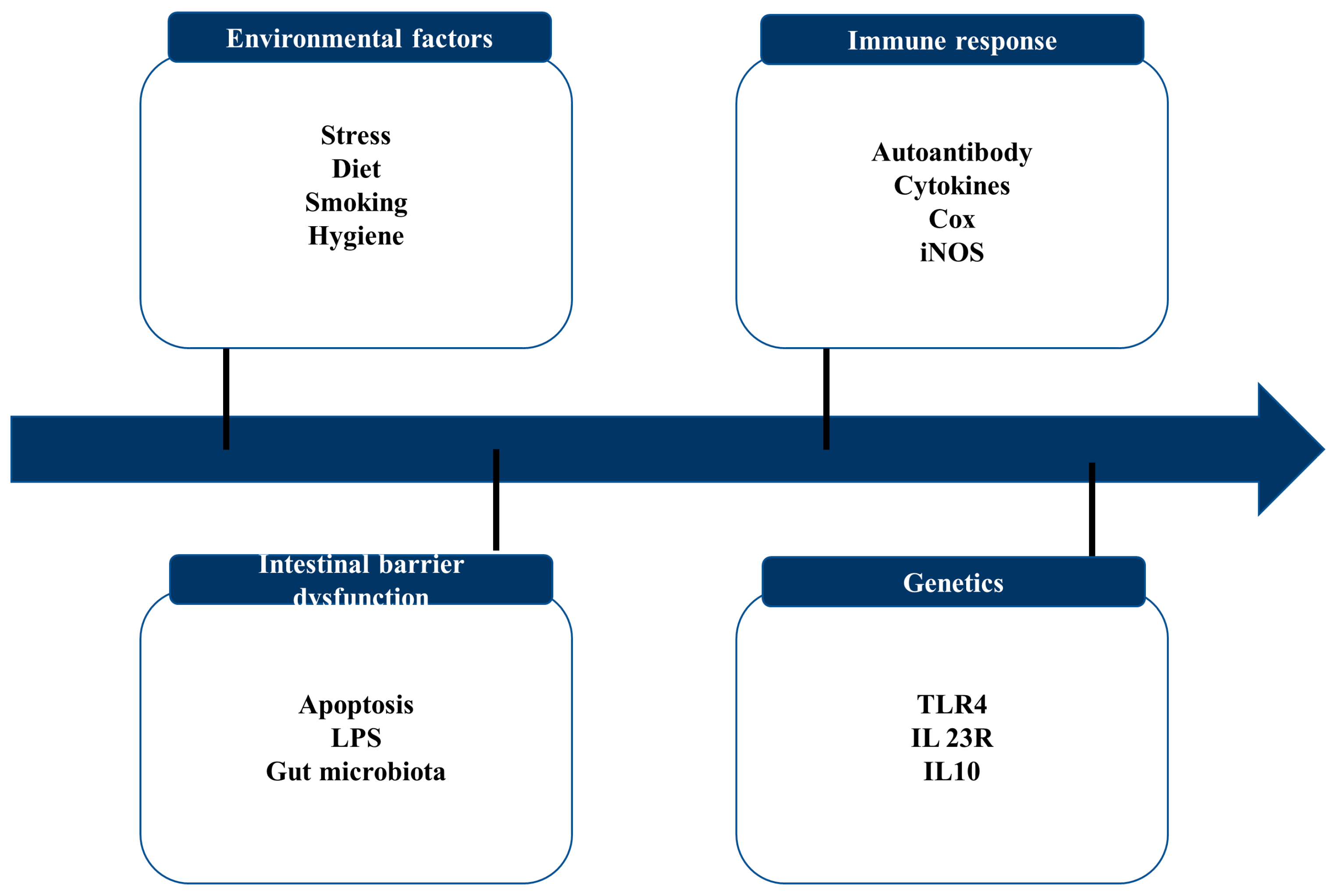
IBD includes various types of idiopathic chronic inflammatory diseases. Due to their severity, frequency of complications, and mortality, Crohn’s disease (CD) and ulcerative colitis (UC) are considered the main disease categories in IBD (Figure 1) [4]. While studies have shown that the etiology of IBD is complicated, the key contributors include genetic predisposition, epithelial barrier abnormalities, dysregulated immunological responses, and environmental variables (Figure 2) [5]. The immunological dysregulation in IBD occurs due to the effector and regulatory cell dysfunction in innate immunity, leading to the uncontrolled release of soluble inflammatory mediators [6]. CD and UC result in the activation of T cells, but differ in terms of the differentiation and activation of T cell immunity. CD is described as a Th1 and Th17 condition with an increased production of IL-12, IL-23, IFN-ɤ, and IL-17, whereas UC is characterized as a Th2 condition with increased IL-12, IL-5, and IL-9 production [6]. Recently, much information has been gathered on malfunctioning innate immunity as the primary mechanism involved in IBD development. Innate immunity recognizes pathogen-associated molecular structures (PAMPs). These include NOD-like receptors (NLRs), mannose receptors, and Toll-like receptors (TLRs). The ongoing inflammatory process occurs from the inappropriate control of these signaling pathways during both the acute and chronic phases of intestinal inflammation, resulting in the pathogenesis of IBD [7]. Modern medical therapy, including anti-inflammatory drugs (e.g., corticosteroids), biological drugs (antibiotics, adhesion molecule antagonists), and immunosuppressants such as thiopurine and calcineurin inhibitors, are used to treat IBD [8]. However, severe adverse effects are prominent when using synthetic drugs. Several studies have reported that aminosalicylate causes severe headaches and nausea, while corticosteroids lead to obesity, hypertension, and diabetes [9]. Moreover, glucocorticoids have been proven to work best for short-term treatments, and their long-term application has been reported to cause moon face and weakened immunity [10]. Therefore, finding novel sources of medications to lessen the severe side effects of traditional therapy is crucial for treating the clinical symptoms of IBD.
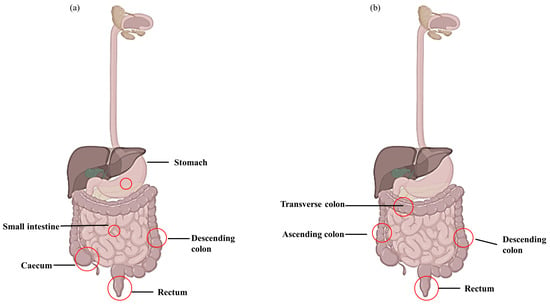
Figure 1.
Lesions appear during Crohn’s disease (CD) and ulcerative colitis (UC). (a) CD lesion involving entire digestive tract and (b) UC lesion located in large intestine and rectum.
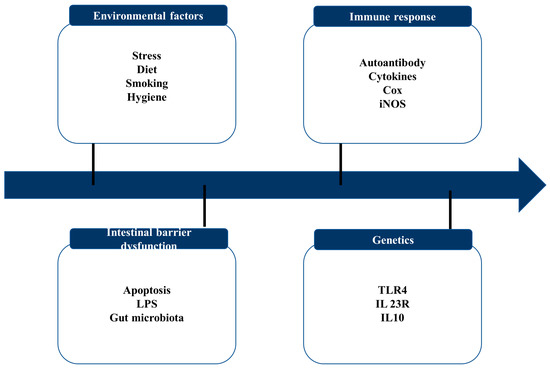
Figure 2.
Key contributing factors to IBD.
Recent developments in understanding the pathogenesis of IBD have created opportunities for developing novel therapeutic approaches focused on therapeutic targets and involving safe and efficient natural compounds that can normalize the intestinal microflora, maintain clinical remission, and speed up the healing of intestinal mucus layers [11,12,13]. Marine seaweed serves as a significant bioresource of natural compounds with beneficial activities. Seaweed consists of high amounts of polysaccharides, proteins, and polyphenols. Polyphenols from marine seaweed have been studied for their therapeutic activities, such as anti-viral, antioxidant, and photoprotective activities [14,15,16]. Research on the potential for employing complex marine polysaccharides as medicinal agents has been explored extensively. In addition to serving as supporting elements for tissues and stores for nutrients in both plants and animals, polysaccharides have a wide range of physiological functions, such as immune regulation as well as anti-inflammatory, antioxidant, and anti-viral effects [17,18]. According to a significant amount of experimental evidence, algal polysaccharides and algae extracts appear to have anti-inflammatory and gastroprotective actions, making them extremely promising for treating and preventing gastrointestinal illnesses [19,20,21]. Algal polysaccharides are excellent therapeutic agents for treating intestinal inflammatory diseases such as IBD due to their resistance to the action of the gastric juice and enzymes of the host and their ability to serve as a fermentation substrate to beneficial intestinal microbes [22,23]. Therefore, the present study mainly focused on polysaccharides isolated from marine seaweeds and their usage for treating IBD.
2. Methodology
The following methodology section explains the methods used to conduct the review. For the identification of relevant studies, a comprehensive search of electronic databases, including PubMed, Scopus, and Web of Science, was performed. Keywords such as “seaweed”, “polysaccharides”, “inflammatory bowel disease”, “ulcerative colitis”, and “Crohn’s disease” were used. In addition, a manual search of the reference list of relevant studies was performed to identify additional studies. Studies that examined the effect of polysaccharides on IBD in human and animals were included in the review, while studies that focused on other seaweed components were excluded. The data were extracted from eligible studies, including study design, sample, intervention type, outcome, and statistical significance. The data were synthesized using a narrative approach, which involved summarizing the finding of each study and pattern recognition.
3. IBD Mechanism
IBD is marked by recurring chronic intestinal inflammation and a poorly understood pathophysiology. Current research has demonstrated the four pathogeneses of IBD, including intestinal flora disorders, immunological responses, environmental factors, and genetic vulnerability [24].
3.1. Intestinal Epithelium
The first line of defense of the body against invading pathogens is the intestinal barrier. The mucus secreted by epithelial cells protects the cells from invading pathogens by creating a physical barrier. The epithelial cells in the intestine renew rapidly, with the cells shedding onto the intestinal lumen. Stem cells proliferate in the intestinal crypt to compensate for the ongoing cell loss. A failure in this process leads to severe damage to the barrier, resulting in extreme intra-luminal antigen invasion and inflammation [25]. An epithelial cell abscission due to tight junction protein (TJ proteins) rearrangement is another negative effect leading to colitis. As reported by Martini et al., 2017, an increased expression of Myosin light chain kinase resulted in tight junction dysregulation and the loss of epithelial barrier function in mice [26]. The main factor causing reduced barrier function and the increased permeability of colonic mucosa is the abnormal TJ protein expression. This allows the entry of toxic substances and pathogen microbes into the gut, leading to IBD. The attachment of pathogenic bacteria to the intestinal epithelium reduces the intestine’s integrity by withdrawing occluding ZO-1 and claudin from tight junctions [27].
The beneficial intestinal microflora in the gut produces a series of secondary metabolites containing short-chain fatty acids (SCFA). These encourage mucus secretion by goblet cells and promote antimicrobial peptide production, increasing intestinal integrity. These processes eliminate the harmful bacteria in the gut, which leads to IBD [28]. The intestinal epithelium cells regulate the intestinal mucosal innate immune defense and microbiome balance. Moreover, they work as a center for internal and external signaling pathways. Individuals with IBD drop their tolerance to symbiotic bacteria and produce increased inflammatory responses [29].
3.2. Intestinal Microflora
The incidence and progression of IBD are closely correlated with the gut flora’s homeostasis [30]. Several reports have been published regarding intestinal microbiotas’ contribution to IBD [31]. The intestinal microenvironment is composed of bacteria infiltrating intestinal lymphatic tissues. The decrease in anti-inflammatory microbes, such as Clostridium and Bacteroides, and the increase in colitis stains, such as Escherichia coli, occur due to the imbalance and irregular changes in the abundance and diversity of intestinal bacteria [32]. Studies reported that in CD patients, Veillonellaceae and Enterobacteriaceae are abundant in their mucus [33]. The primary initiator of intestinal damage in the pathogenesis of IBD is the fast rise in the generation of free radicals induced by phagocyte infiltration and the imbalance of intestinal homeostasis caused by the damage to the antioxidant defense system [34]. Increasing reactive oxygen species (ROS) production is one of the critical factors in intestinal tissue injury and inflammation. The SCFA produced by intestinal microflora lowers the intestine’s pH, inhibiting pathogenic bacterial growth and allowing the proliferation of beneficial bacteria. This results in the improvement of the homeostasis of intestinal flora [35].
3.3. Immune System
The intestine’s immune system tolerates dietary antigens while protecting the mucosal surface from infections and injuries. This necessitates a careful balance between regulatory T cells and effector T cells, and it is believed that the disruption of this equilibrium leads to CD and UC development [36]. The immune system depends heavily on helper T cells (Th), which include Th1, Th2, Th17, and regulatory T cells (Treg). In mice with UC, it has been discovered that Treg cell deficiency worsens IBD symptoms. It was proposed that the Th1 response regulates CD, while UC is tightly linked to an atypical Th2 response accompanied by the IL-5 and IL-3 cytokines [37]. Apart from this, the stimulated Th17 cells stimulate the production of IL-17, GM-CFS, IFN-ɤ, TNF-ɑ, and IL-22 from the inflamed intestinal tract. Although Treg cells serve a crucial anti-inflammatory and immune regulatory role, Th17 cells exert a pathogenic effect [38]. Studies have shown that transferring the two Treg cells CD4+ and CD25+ into colitis-affected mice can lessen the infiltration of inflammatory cells in the lamina propria and return the intestinal wall to its normal state, reversing the inflammatory response and healing the colitis [39].
The macrophages gather around the IBD lesions in the intestine and secrete anti-inflammatory cytokines following biological effects. The pro-inflammatory cytokines IL-1, IL-12, IL-23, TNF-ɑ, and many others are secreted by M1-type macrophages, and they also break down the NO and ROS that L-arginine produces. The Th1 and Th17 cell-mediated immune responses are overexpressed by inducible nitric oxide synthase (iNOS), which results in inflammatory injuries in the intestinal tissues [40]. Gut microbes digest lipopolysaccharide (LPS) and release it into the blood through the intestinal barrier. LPS activated the Toll-like receptor 4 (TLR-4) pathway, promoting pro-inflammatory cytokine production. The functions of TLR4 include initiating an innate immune response in the early stages of pathogen infection, promoting the expression of pertinent immune molecules, and stimulating the maturation and activation of immune cells, which bridges the gap between innate immunity and adaptive immunity [41]. Since numerous signaling pathways and cytokines are simultaneously stimulated in IBD, a multi-target treatment is more likely to reverse the illness successfully.
4. Marine Polysaccharides
Seaweeds are mainly categorized as brown, green, and red. Polysaccharides obtained from seaweeds vary greatly depending on their taxonomic classification [42,43]. Seaweed’s structural polysaccharides are the most abundant, and their composition changes with species and environmental factors [44].
4.1. Brown Seaweeds
Brown seaweeds contain alginates and fucoidan, proven clinically and experimentally to possess pharmacological effects. Fucoidan is a sulfated polysaccharide consisting mainly of ʟ-fucose and sulfate ester groups [45]. Apart from fucose, it contains glucose, galactose, xylose, and uronic acids [46]. Fucoidans are mainly derived from the cell wall matrix of many kinds of brown algae, serving important defensive and structural purposes [47]. The ionic nature of fucoidans (negative charge) resulting from the presence of sulfate in both the second and fourth carbon positions makes them more suitable for applications in pharmaceutical technology. It allows them to form complexes with other charged molecules [48]. The low toxicity, biodegradability, and biocompatibility of fucoidans make them safe as food ingredients [48]. Alginic acids are found in brown seaweeds, such as kelp, Gulfweed, Ascophyllum, and macroalgae. The alginic acid in the cytoplasm is vital in strengthening the cell wall [49]. It is found in 18–40% of the plant’s total mass [50]. Similarly to fucoidan, alginic acids are non-toxic, biocompatible, and non-immunogenic. Apart from alginate’s ability to be a stabilizing agent, alginates are used in the biomedicine and food industries [51].
Laminarin is another underexploited polysaccharide found in brown seaweeds. Laminarins typically consist of low-molecular-weight (5–10 kDa) β-glucans with β-(1-3)-linked d-glucose residues [52,53]. These polysaccharides have been reported to possess distinct therapeutic roles, such as anti-inflammatory, antioxidant, anti-apoptotic, and anti-coagulant effects [54,55,56,57].
4.2. Green Algal Polysaccharides
Ulvan is a water-soluble sulfated polysaccharide found in green algae, which acts as a structural component of an algal cell wall. The structure represents a sequence of disaccharide units comprising two different types of aldobiuronic acid, ulvanobiuronic acid 3- sulfate type A and type B [58]. The structure of ulvan disaccharide moieties is similar to that of animal connective tissue glycosaminoglycans found in the extracellular matrix [59]. Ulvan has shown considerable biological activity in both animal and plant systems. Ulvan has been reported and proven to contain anti-coagulant, immunomodulating, anti-cancer, antioxidant, anti-viral, anti-hyperlipidemic, and anti-inflammatory activities [60,61,62,63].
4.3. Red Algal Polysaccharides
In contrast to the other two kinds of green and brown seaweed, which include polysaccharides, red seaweed is an essential source of many beneficial bioactive compounds. The main structural component of red algae is sulfated polysaccharides. Carrageenan, agar, and xylan are common polysaccharides found in red algae. The protein content of red algae is around 10–50% of its dry weight [64]. Carrageenan is an anionic sulfated polysaccharide commonly found in red algae, such as Chondrus, Gigartina, Hypnea, and Eucheuma [65,66]. It is a component of the outer cell wall and intracellular matrix and is composed of linear polysaccharide chains with sulfate half-esters attached to the sugar units. The three general forms of carrageenan are kappa, lambda, and iota. This classification is based on the availability of 3,6- anhydrogalactopyranose and the allocation of sulfate groups on the main structure [67]. Carrageenans are the algal polysaccharides most thoroughly researched regarding their toxicity, pyrogenicity, allergenicity, food safety, and medicinal usage [68]. Carrageenans are potential immunomodulating, antioxidant, anti-inflammatory, and anti-viral agents [69,70].
5. Therapeutic Targets of Algal Polysaccharides against IBD
5.1. Intracellular Adhesion Molecules
Selectins, integrins, and cadherin-like adhesion molecules are vital in migrating leukocytes in inflammatory areas, and these are potential therapeutic targets for chronic intestinal inflammation [71]. Selectin is bound to mannose, and fucose slows the movement of leukocytes and platelets to the endothelium surface, improving transendothelial transmission. The physiological effects of the interaction between fucoidan and selectins have potential therapeutic benefits. In 1999, Semenov et al. reported that rats with experimental peritonitis received fucoidan intravenously after receiving peptone intraperitoneally, significantly suppressing the neutrophils discharge into the abdominal cavity [72]. According to the authors, the interaction between fucoidan and P-selectin inhibits inflammation at an early stage of its development. Another study reported that the intravenous administration of fucoidan led to a reduction in colonic mucosal injury and crypt damage in dextran sodium sulfate (DSS)-induced mice. They further stated that this effect was due to the reduction in abolishing venular leukocyte rolling [73]. These findings demonstrate that sulfated polysaccharides, which exhibit the characteristics of glycosaminoglycan mimics, block adhesion molecules and restrict leukocyte migration, providing beneficial relief for inflammatory bowel illnesses.
5.2. Intestinal Epithelial Cells
Reducing the efficacy of the epithelial barrier is believed to have a significant role in the etiology of IBD. The epithelial barrier comprises an intact epithelial monolayer and tight junctions by which the membranes of the neighboring cells are brought together. They are cross-linked with two proteins, namely occluding and claudin [74]. Previously reported studies have proven that tight junction abnormalities and excess paracellular permeability result in the increased stimulation of antigens, leading to the development of IBD. Therefore, the maintenance of remission in people with IBD or the prevention of illness in at-risk persons may benefit from restoring intestinal barrier function [75]. A study on the potential of fucoidan against H2O2-induced epithelial barrier function damage reported that the treatment of fucoidan dose-dependently increased the transepithelial resistance and protected the intestinal epithelium from paracellular permeability. Fucoidan treatment was also reported to increase claudin-1 expression. According to the authors, fucoidan, which boosts epithelium protective function and encourages epithelial regeneration, may be a suitable therapy for managing IBD [76]. A group of researchers who investigated the anti-inflammatory activity of Laminaria japonica against intestinal inflammation reported that the seaweed improved the intestinal barrier function and prevented tight-junction-related protein inhibition. It also downregulated the cells’ IL-6 and nitric oxide levels [77].
A study in 2014 conducted by Wu et al. reported that bacterial-derived lipopolysaccharides cause intestinal barrier function defectives [78]. They reported that sulfonate fucoidan nanoparticles coated with berberine inhibit the redistribution of tight junction proteins ZO-1 and ameliorate LPS-induced intestinal epithelial tight junction disruption. In dietary-fiber-deficient mice, feeding them Scytosiphon lomentaria was proven to induce epithelial cell layer integrity and the loss of goblet cells as well as inhibit inflammatory cell infiltration, leading to the inhibition of colon damage [79]. The illustrations of their results demonstrate how algal polysaccharides might influence intestinal health by reducing intestinal inflammation and improving barrier integrity by partially controlling dense substances and related proteins (claudine, occludine).
5.3. Pro-Inflammatory Cytokines
It has been undeniably proven that cytokine responses are essential components for managing the inflammatory processes underlying IBD [11]. In addition to causing intestinal inflammation and diarrhea in IBD, cytokines control systemic effects and extra-intestinal disease symptoms (such as arthralgia or arthritis). Moreover, cytokines appear to be a significant factor in developing IBD complications, such as intestinal stenosis, fistula formation, and colitis-associated neoplasias [80]. The cytokine spectrum, including pro-inflammatory cytokines IL-6, IL-12, IL-23, and IL-21 as well as anti-inflammatory cytokines such as IL-10 and TGF-β, has been identified as possible novel targets for treating intestinal inflammation in studies using tissues from individuals with IBD and animal models of IBD [81,82]. Among the targets, IL-17 has been recognized as the main pathogenic factor stimulating IBD [83]. The fact that TNF blocking is now a popular treatment option for IBD in clinics emphasizes cytokines’ crucial function in IBD [84].
Due to the modulation of several pro-inflammatory processes and mediators, including the control of the gene expression of pro- and anti-inflammatory cytokines associated with UC, brown algae polysaccharides are a potential therapeutic option for patients with IBD [18,85]. A study conducted on laminarin and fucoidan on the pathology and inflammation in pigs resulting due to DSS showed that a combination treatment prevents weight loss and diarrhea. Moreover, the expression of IL-6 was also downregulated with the co-treatment of laminarin and fucoidan [86]. In 2018, Sudirman et al. evaluated the potential of Eucheuma cottonii polysaccharides against the inflammatory response in mice induced with DSS [87]. The polysaccharides were orally treated, ameliorating the weight loss in DSS-induced mice while decreasing their colon weight. The administration of polysaccharides was reported to down-regulate the expression of pro-inflammatory cytokines TNF-ɑ, IL-1β, IL-6, and IL-10.
In vitro studies using the colon epithelial cell line CMT-93 stimulated by lipopolysaccharides and in vivo studies using mice with chronic colitis brought on by sodium dextran were conducted by Matsumoto et al. in 2004 to examine the effects of different types of fucoidans as inhibitors of IL-6 production. It was demonstrated that the fucoidans from Kjellmaniella crassifolia and Cladosiphon okamuranus Tokida reduced IL-6 production in CMT-93 cells and decreased NF-kB nuclear translocation. Fucoidan from Cladosiphon okamuranus also decreased the expression level of IL-6 mRNA in mouse epithelial cells compared to animals fed a regular diet, while boosting the synthesis of IL-10 and TGF-β and inhibiting the synthesis of IFNɤ and IL-6. A study on β-glucan obtained from Laminaria hyperborean and L. digitata proved that the expression of Th-17-associated cytokines, such as IL-17a, IL-17F, and IL-22, decreased. Moreover, the expression of IL-23R and IL-6 receptors also decreased. No alterations of T regulatory cell (Treg)-related targets were observed in the study [88]. In 2015, Lean et al. reported the potential activity of a fucoidan–polyphenol complex obtained from Fucus vesiculosus on a DSS-induced mouse model of acute colitis [89]. According to their results, the oral administration of polysaccharides considerably reduced diarrhea, fecal blood loss, weight retention, and other colitis symptoms compared to the colitis group that did not receive treatment.
Furthermore, in mice given oral fucoidan, the weights of their colon and spleen were likewise much lower, indicating lessened inflammation and edema. The reduced production of inflammatory cytokines in the colin tissues resulted in reduced macroscopic alterations. Notably, the intraperitoneal administration of deproteinized fucoidan increased the animals’ condition and the expression of pro-inflammatory cytokines in their colon tissues. The study proposed the usage of fucoidan as an oral drug to decrease inflammation and preserve the integrity of the intestinal epithelium.
5.4. Intestinal Microbiome
The most important development in IBD research over the past ten years has been the idea of dysbiosis or changed gut flora. Modern molecular and genetic diagnostic methods have revealed the deficits of innate immunity and unique alterations in the gut microbiota of IBD patients [90]. IBD has started to be understood as an immunodeficiency disease, with compromised microbiota significantly contributing to the long duration of inflammation. In light of this, altering the intestinal microbiome through antibiotics, prebiotics, and probiotics is a promising approach for preventative and therapeutic IBD intervention [91,92,93,94].
The most recent definition of prebiotics includes “substrates that host bacteria employ specifically to produce health benefits”. Three requirements must be met for substances to be considered prebiotics: they must be resistant to digestion and the action of upper gastrointestinal tract enzymes; they must be fermented by colon microflora; and they must also be a selective substrate for the growth of beneficial bacteria and have local or systemic effects that are advantageous to the host [93]. According to experimental and clinical studies, prebiotics inhibit potentially pathogenic bacteria, lessen mucous membrane inflammation, and lower the risk of subsequent clinical relapses of IBD. They also promote the growth of probiotic strains in the intestine and inhibit potentially pathogenic bacteria [95,96,97]. As they may be added to food, feed, or taken as tablets, algal polysaccharides and oligosaccharides have an advantage over other sources for the manufacture of prebiotics. These dietary fibers are different from fibers of terrestrial origin in terms of their chemical and physicochemical characteristics. Their quantity is more significant than that in most fruits and vegetables and varies from 33 to 50 g per 100 g of algae [98]. Several polysaccharides are currently used as prebiotics and in IBD treatment [99,100]. Data on the potential for fucoidan to be fermented by gut bacteria are conflicting. Sulfated polysaccharides have been shown to have prebiotic action in vitro, although there is an apparent delay in using them as prebiotics. This situation can be due to the need for clinical trials and insufficient in vivo research on the prebiotic potential of algal polysaccharides [101,102].
The beneficial bacterial species in the intestine are known as “probiotics”, which include Bifidobacteria, Lactobacillus, and Faecalibacterium spp. These bacteria possess immune regulatory potentials, such as Lactobacillus up-regulating the dendritic cell maturation, resulting in the production of IL-12, IL-18, and IL-23 and contributing to the Th1 responses. They also produce IL-4 and IL-10, contributing to the Th2 responses [103,104]. Bifidobacteria can boost IL-10 release in DC and decrease IFN production via activated CD4+ T cells, while Faecalibacteria increases the IL-10 production in DC and reduces IFN-ɤ production via activated CD4+ T cells [105]. In 2012, Kuznetsova et al. showed that cultivating bifidobacteria on a nutrient medium enriched with Fucus evanescens fucoidan increased their growth and the accumulation of biomass [106]. Likewise, polysaccharides from F. evanescens were also proven to have prebiotic activity on B. bifidum by testing in vivo a drug dysbacteriosis mice model after one month of treatment [107]. In 2016, Shang et al. reported that the oral treatment of fucoidan isolated from Ascophyllum nodosum and L. japonica increased the abundance of Lactobacillus and Ruminococcacea-like beneficial bacteria.
Moreover, dietary fucoidan also dramatically lowered the antigen load and the inflammatory response in the host, as seen by the decreased blood lipopolysaccharide-binding protein levels, by maintaining a more balanced composition of gut microbiota [108]. The in vitro fermentation of sulfated polysaccharides from Enteromorpha prolifera and L. japonica was reported to be effective as prebiotics in a study conducted by Kong et al. in 2016. The fermentation increased SCFA, such as acetic, butyric, and lactic acids, while modulating the microflora balance in the gut [109].
The information mentioned above demonstrates that algae polysaccharides have tremendous potential for use as prebiotics in IBD and can achieve health effects, such as regulating the composition and functions of microbiota, lowering the pH in the colon lumen, preventing intestinal colonization by pathogens, and reducing the production of reactive oxygen species, which are a source of energy for colonocytes and activate free fatty acid receptors. More health benefits of algal polysaccharides against IBD are given in Table 1.

Table 1.
Algal derived-polysaccharides and their protective effect and mechanism on intestinal health.
6. Algal Polysaccharides in Drug Delivery Systems
To treat IBD, medications are desired to be taken orally to reach the colon [122]. It is challenging to provide oral medications to the colon at the distal end of the gastrointestinal system due to physiological issues, biochemical obstacles, and environmental barriers, such as those brought on by mucus and the epithelium [123]. Several polymer designs (including linear and branched leading chains) and polymer combinations are currently suggested as carrier systems [124]. Certain polysulfated fucoidan chains can bind to TLR4, CD14, scavenger receptors, the mitogen-activated protein kinase receptor, and receptors specialized for mannose, fucose, galactose, and N-acetylglucosamine residues. They can also influence the effects of signaling molecules on cells [125]. Sulfated polysaccharides can therefore be utilized as precise recognition signals to target immune system cells and encourage drug accumulation in the inflamed gut.
Polysaccharides have the potential to not only alleviate inflammatory bowel disease on their own but also to act as a drug carrier for additional potent medications that can be employed to treat IBD specifically. Other beneficial factors include the capacity to chemically modify polysaccharides to have the following properties: high stability, biodegradability, safety, non-toxicity, hydrophilicity, and gel-forming. Recent preclinical investigations have demonstrated the promise of algal polysaccharide-based nanoparticle drug delivery systems as future therapeutics for IBD [126]. Most frequently, chitosan, carrageenan, and fucoidan have been indicated as matrix materials in the creation of nanoparticles. The sulfate group of polysaccharides interacts with the amino group of chitosan to create nanoparticles and regulate medication release [115]. Moreover, the fucoidan–chitosan nanoparticles’ pH-responsive profile prevents the gastrointestinal tract’s acidic conditions from degrading them and permits medicine to be absorbed in the gut. For this reason, the oral administration of active medicinal substances using fucoidan and chitosan nanoparticles has been thoroughly explored [126,127].
In order to create a compound with intense anti-inflammatory action and minimal toxicity, in 2017, Zhu et al. coated selenium nanoparticles with a polysaccharide from the green alga Ulva lactuca [128]. The complex was proven to have high anti-inflammatory action and minimal toxicity. In mice with DSS-induced acute colitis, supplementation with ULP-SeNPs significantly reduced body weight loss and colonic inflammatory damage, among other adverse effects. The reduced CD68 values in the colon tissue sections proved that the ULP-SeNPs reduced macrophage infiltration. The plasma levels of TNF-ɑ and IL-6, COX-2, and iNOS were reduced in complex-treated animals compared to control animals. The ULP-SeNPs acted by blocking the nuclear translocation of NF-κB, which activates these pro-inflammatory cytokines. These studies demonstrate the efficacy of drug delivery methods based on polysaccharides from seaweed for IBD therapy. However, more thorough research on the patterns of material accumulation in the intestinal tissues is required to implement this approach in the treatment of IBD successfully.
7. Conclusions
Due to its serious outcomes, IBD has become a popular research topic in recent years. Algal polysaccharides contain valuable anti-inflammatory, antioxidant, anti-tumor, and other physiological activities, which are especially useful in preventing IBD. Polysaccharides isolated from seaweed can inhibit IBD directly as well as indirectly. Due to their structural characteristics, seaweed polysaccharides have a pleiotropic effect that allows them to influence therapeutic targets for IBD, such as inflammatory cytokines, adhesion molecules, intestinal microbiota, and intestinal epithelial cells. Algal polysaccharides also have a lot of promise for creating drug delivery systems because of their physicochemical characteristics, which allow for interactions with many substances, including medicines, proteins, and other polymers. However, the bulk of the investigations into using algal polysaccharides in IBD have been conducted ex vivo or in trials on animals. While preclinical studies have shown promising results, there is a lack of clinical trials investigating their efficacy in humans with IBD. Clinical trials are necessary to establish the safety and effectiveness of algal polysaccharides in treating IBD. Moreover, most studies investigating algal polysaccharides have compared them to a placebo or control group. More research is needed to compare their effectiveness to standard treatments in IBD, such as anti-inflammatory drugs or immunosuppressive agents. Unquestionably, the use of algal polysaccharides in medicine will increase annually as science advances and the possibilities for generating standardized medicines based on these chemicals increase. The shortcomings of the present review include limited data availability and the exclusion of certain studies due to language and publication biases. However, to improve the review, the search criteria were expanded and multiple databases were used.
Author Contributions
Y.-J.J., T.U.J., D.P.N. and N.M.L.; methodology: D.P.N. and N.M.L.; software, D.P.N. and N.M.L.; validation, D.P.N. and N.M.L.; formal analysis, D.P.N. and N.M.L.; investigation, T.U.J., D.P.N. and N.M.L.; resources, Y.-J.J. and T.U.J.; data curation D.P.N. and N.M.L.; writing—original draft preparation, D.P.N. and N.M.L.; writing—review and editing, Y.-J.J., T.U.J., D.P.N. and N.M.L.; visualization, D.P.N. and N.M.L.; supervision, Y.-J.J. and T.U.J.; project administration, Y.-J.J.; funding acquisition, Y.-J.J. and T.U.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2019R1A6A1A03033553).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory Lymphocytes and Intestinal Inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The Immunology of Mucosal Models of Inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- World Gastroenterology Organisation. Global Guardian of Digestive Health. Serving the World. 2022. [Google Scholar]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Lee, S.H.; eun Kwon, J.; Cho, M.-L. Immunological pathogenesis of inflammatory bowel disease. Intestig. Res. 2018, 16, 26. [Google Scholar] [CrossRef]
- Elia, P.P.; Tolentino, Y.F.M.; Bernardazzi, C.; de Souza, H.S.P. The Role of Innate Immunity Receptors in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2015, 2015, 936193. [Google Scholar] [CrossRef]
- Duff, W.; Haskey, N.; Potter, G.; Alcorn, J.; Hunter, P.; Fowler, S. Non-pharmacological therapies for inflammatory bowel disease: Recommendations for self-care and physician guidance. World J. Gastroenterol. 2018, 24, 3055. [Google Scholar] [CrossRef]
- Nielsen, O.H. New Strategies for Treatment of Inflammatory Bowel Disease. Front. Med. 2014, 1, 3. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.-M.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Catalan-Serra, I.; Brenna, Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum. Vaccines Immunother. 2018, 14, 2597–2611. [Google Scholar] [CrossRef]
- Dulai, P.S.; Siegel, C.A. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol. Clin. 2014, 43, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Je, J.-G.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Jeong, S.-H.; Kwon, H.-J.; Choi, C.S.; Jeon, Y.-J. Polyphenolic Compounds Isolated from Marine Algae Attenuate the Replication of SARS-CoV-2 in the Host Cell through a Multi-Target Approach of 3CLpro and PLpro. Mar. Drugs 2022, 20, 786. [Google Scholar] [PubMed]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Kim, Y.-S.; Jeon, Y.-J. Clionasterol-Rich Fraction of Caulerpa racemosa against Particulate Matter-Induced Skin Damage via Inhibition of Oxidative Stress and Apoptosis-Related Signaling Pathway. Antioxidants 2022, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Lee, H.-G.; Heo, M.-S.; Jeon, Y.-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. [Google Scholar]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Jayawardena, T.U.; Jeon, Y.-J. Anti-Fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Leaves in Macrophages via Inhibiting Inflammatory Signaling Pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [PubMed]
- Liyanage, N.M.; Lee, H.-G.; Nagahawatta, D.P.; Jayawardhana, H.H.A.C.K.; Ryu, B.; Jeon, Y.-J. Characterization and therapeutic effect of Sargassum coreanum fucoidan that inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophages by blocking NF-κB signaling. Int. J. Biol. Macromol. 2022, 223, 500–510. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar]
- Choi, J.-I.; Raghavendran, H.R.B.; Sung, N.-Y.; Kim, J.-H.; Chun, B.S.; Ahn, D.H.; Choi, H.-S.; Kang, K.-W.; Lee, J.-W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- Okolie, C.L.; CK Rajendran, S.R.; Udenigwe, C.C.; Aryee, A.N.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, P.; Li, X.; Guo, L.; Gao, W. The potential roles of natural plant polysaccharides in inflammatory bowel disease: A review. Carbohydr. Polym. 2022, 277, 118821. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. CMGH 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-strain probiotic supplement influences gut microbiota composition and gut wall function in patients with ulcerative colitis. Int. J. Pharm. 2020, 587, 119648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-Y.; Xiang, X.-W.; Du, M.; Zhang, L.-F.; Cheng, N.-X.; Liu, X.-L.; Zheng, B.; Wen, Z.-S. Protective effect of polysaccharides of sea cucumber Acaudina leucoprocta on hydrogen peroxide-induced oxidative injury in RAW264.7 cells. Int. J. Biol. Macromol. 2019, 139, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, X.; Luo, Y.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Huang, Y.; Ren, Z. Characterization of polysaccharides isolated from Hericium erinaceus and their protective effects on the DON-induced oxidative stress. Int. J. Biol. Macromol. 2020, 152, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. J. Nutr. Biochem. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, M.; Wei, J.; Yang, L.; Wu, G. Perioperative alterations in the intestinal microbiota and functional changes mediate innate immune activation after small bowel transplantation. Life Sci. 2021, 277, 119468. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shen, F.; Wang, W.; Qi, C.; Wang, C.; Shang, A.; Xuan, S. The effect of multispecies probiotics on cognitive reactivity to sad mood in patients with Crohn’s disease. J. Funct. Foods 2021, 82, 104431. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, R.; Cheng, L.; Yang, J.; Zhu, L. Peroxisome proliferator-activated receptor gamma activation promotes intestinal barrier function by improving mucus and tight junctions in a mouse colitis model. JGLD 2018, 50, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Huang, C.; Yao, R.; Zhu, Z.; Pang, D.; Cao, X.; Feng, B.; Paulsen, B.S.; Li, L.; Yin, Z.; Chen, X. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress. Int. J. Biol. Macromol. 2019, 130, 508–514. [Google Scholar] [CrossRef]
- Okada, S.; Puel, A.; Casanova, J.L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. CTI 2016, 5, e114. [Google Scholar] [CrossRef]
- Belkaid, Y.; Rouse, B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005, 6, 353–360. [Google Scholar] [CrossRef]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, S.; Ye, J.; Wang, X.; Zhang, X.; Shen, J.; Yuan, M.; Liao, W. Polysaccharide from flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020, 149, 1252–1261. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.D.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gurpilhares, D.D.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A., Jr.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Mao, X.; Ci, F. Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities, and Potential Applications. J. Ocean Univ. 2021, 20, 641–653. [Google Scholar] [CrossRef]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Graves, B.; Child, R.; Rice, P.J.; Ma, Z.; Lowman, D.W.; Ensley, H.E.; Ryter, K.T.; Evans, J.T.; Williams, D.L. Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties. J. Immun. 2018, 200, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Brunel, M.; Bonnin, E.J.C.R. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr. Res. 1997, 304, 325–333. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.S.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Liu, X.; Yu, K.; Cheng, S.; Ren, T.; Maitusong, M.; Liu, F.; Chen, J.; Qian, Y.; Xu, D.; Zhu, G.; et al. Ulvan mediated VE cadherin antibody and REDV peptide co-modification to improve endothelialization potential of bioprosthetic heart valves. Mater. Sci. Eng. C 2021, 128, 112337. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, antioxidant, and anti-tumor activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. 3 Biotech 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and emerging therapies for inflammatory bowel disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.V.; Mazurov, A.V.; Preobrazhenskaia, M.E.; Ushakova, N.A.; Mikhaĭlov, V.I.; Berman, A.E.; Usov, A.I.; Nifant’ev, N.E.; Bovin, N.V. Sulfated polysaccharides as inhibitors of receptor activity of P-selectin and P-selectin-dependent inflammation. Vopr. Med. Khimii 1998, 44, 135–144. [Google Scholar] [PubMed]
- Nuñez-Andrade, N.; Lamana, A.; Sancho, D.; Gisbert, J.P.; Gonzalez-Amaro, R.; Sanchez-Madrid, F.; Urzainqui, A. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J. Pathol. 2011, 224, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Yao, S.-J.; Das, S.; Guma, M. Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500. [Google Scholar] [CrossRef]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Don, T.-M.; Lin, C.-W.; Mi, F.-L. Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zheng, W.; Zhang, C.; Zhang, P.; Guo, X.; Song, S.; Ai, C. Fucoidan from Scytosiphon lomentaria protects against destruction of intestinal barrier, inflammation and lipid abnormality by modulating the gut microbiota in dietary fibers-deficient mice. Int. J. Biol. Macromol. 2023, 224, 556–567. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Wang, X.; Yu, Y.; Xie, J. Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms. Foods 2021, 10, 1288. [Google Scholar] [CrossRef]
- Rana, S.V.; Sharma, S.; Kaur, J.; Prasad, K.K.; Sinha, S.K.; Kochhar, R.; Malik, A.; Morya, R.K. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J. Crohns Colitis 2014, 8, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.; Nikfar, S.; Abdolghaffari, A.H.; Abdollahi, M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin. Biol. Ther. 2014, 14, 583–600. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2. [Google Scholar] [CrossRef]
- O’Shea, C.J.; O’Doherty, J.V.; Callanan, J.J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef]
- Sudirman, S.; Hsu, Y.-H.; He, J.-L.; Kong, Z.-L. Dietary polysaccharide-rich extract from Eucheuma cottonii modulates the inflammatory response and suppresses colonic injury on dextran sulfate sodium-induced colitis in mice. PLoS ONE 2018, 13, e0205252. [Google Scholar] [CrossRef]
- Ryan, M.T.; O’Shea, C.J.; Collins, C.B.; O’Doherty, J.V.; Sweeney, T. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J. Anim. Sci. 2012, 90 (Suppl. 4), 263–265. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Kiron, V.; Hayes, M.; Avni, D. Inflammatory bowel disease—A peek into the bacterial community shift and algae-based ‘biotic’ approach to combat the disease. Trends Food Sci. Technol. 2022, 129, 210–220. [Google Scholar] [CrossRef]
- Pan, X.; Yin, M.; Guo, M.; Niu, X.; Han, L. The latest progress of natural food polysaccharides preventing ulcerative colitis by regulating intestinal microbiota. J. Funct. Foods 2022, 96, 105201. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Al Mijan, M.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Kim, Y.-S.; Nagahawatta, D.P.; Jin, H.; Yang, H.-W.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Jeon, Y.-J. Sargassum horneri as a Prebiotic Dietary Supplement for Immunity Development in Streptococcus parauberis Infected Zebrafish Model. Front. Mar. Sci. 2022, 9, 1676. [Google Scholar] [CrossRef]
- Huang, W.; Tan, H.; Nie, S. Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chem. 2022, 373, 131608. [Google Scholar] [CrossRef]
- Nie, Y.; Lin, Q.; Luo, F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1372. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Goulart, R.d.A.; Aranão, A.L.d.C.; de Oliveira, P.G.C. Inflammatory bowel diseases and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols: An overview. J. Med. Food 2018, 21, 633–640. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.; De Morais, R.M. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, L.; Xia, C.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Sources, Processing-Related Transformation, and Gut Axis Regulation of Conventional and Potential Prebiotics. J. Agric. Food Chem. 2022, 70, 4509–4521. [Google Scholar] [CrossRef] [PubMed]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, Q.; Li, G.; Wang, X.; Yu, G. Degradation of Marine Algae-Derived Carbohydrates by Bacteroidetes Isolated from Human Gut Microbiota. Mar. Drugs 2017, 15, 92. [Google Scholar] [CrossRef]
- Martínez-Abad, B.; Garrote, J.A.; Bernardo, D.; Montalvillo, E.; Escudero-Hernández, C.; Vázquez, E.; Rueda, R.; Arranz, E. Differential immunomodulatory effects of Lactobacillus rhamnosus DR20, Lactobacillus fermentum CECT 5716 and Bifidobacterium animalis subsp. lactis on monocyte-derived dendritic cells. J. Funct. Foods 2016, 22, 300–312. [Google Scholar] [CrossRef]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; Kamm, M.A. The dendritic cell: Its role in intestinal inflammation and relationship with gut bacteria. Gut 2003, 52, 1522. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602. [Google Scholar] [CrossRef]
- Kusnetsova, T.; Zaporozhets, T.; Makarenkova, I.; Besednova, N.; Timchenko, N.; Zvyagintseva, T.; Shevchenko, N.; Mandrakova, N.; Melnikov, V. The prebiotic potential of polysaccharides from the brown alga Fucus evanescens and significance for the clinical use. Pac. Med. J. 2012, 1, 37–40. [Google Scholar]
- Zaporozhets, T.S.; Besednova, N.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Melnikov, V.G. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisić, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. FCT 2021, 151, 112145. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chang, Y.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; López Nadal, A.; Zaccaria, E.; Iha, M.; Kitazawa, H.; Kleerebezem, M.; Brugman, S. Intestinal Microbiota and Immune Modulation in Zebrafish by Fucoidan from Okinawa Mozuku (Cladosiphon okamuranus). Front. Nutr. 2020, 7, 67. [Google Scholar] [CrossRef]
- Brito, T.V.; Neto, J.P.R.P.; Prudêncio, R.S.; Batista, J.A.; Júnior, J.S.C.; Silva, R.O.; Franco, Á.X.; Aragão, K.S.; Soares, P.M.G.; Souza, M.H.L.P.; et al. Sulfated-polysaccharide fraction extracted from red algae Gracilaria birdiae ameliorates trinitrobenzenesulfonic acid-induced colitis in rats. J. Pharm. Pharmacol. 2014, 66, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Dutra, N.L.S.; de Brito, T.V.; Magalhães, D.d.A.; Sousa, S.G.; Batista, J.A.; Pereira, C.M.C.; Ferreira, J.d.S.; Rodrigues, L.d.R.; Lima, J.V.d.N.; de Albuquerque, I.F.; et al. Sulfated polysaccharide extracted from seaweed Gracilaria caudata attenuates acetic acid-induced ulcerative colitis. Food Hydrocoll. 2021, 111, 106221. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.-S.; Jeon, Y.-J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.V.; Barros, F.C.N.; Silva, R.O.; Dias Júnior, G.J.; Júnior, J.S.C.; Franco, Á.X.; Soares, P.M.G.; Chaves, L.S.; Abreu, C.M.W.S.; de Paula, R.C.M.; et al. Sulfated polysaccharide from the marine algae Hypnea musciformis inhibits TNBS-induced intestinal damage in rats. Carbohydr. Polym. 2016, 151, 957–964. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.-L. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 2022, 13, 100197. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Zhang, X.; Wang, Z. Potent anti-inflammatory activity of polysaccharides extracted from Blidingia minima and their effect in a mouse model of inflammatory bowel disease. J. Funct. Foods 2019, 61, 103494. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Wang, T.; Wang, P.; Liu, R.; Li, Y.; Tian, Y.; Zhang, J. Characterization of Low Molecular Weight Sulfate Ulva Polysaccharide and its Protective Effect against IBD in Mice. Mar. Drugs 2020, 18, 499. [Google Scholar] [CrossRef]
- Misra, A.; Shahiwala, A. Novel Drug Delivery Technologies; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, T.M.; Fong, P.M.; Goyal, A.; Saltzman, W.M. Targeted for drug delivery. Mater. Today 2005, 8 (Suppl. 8), 18–26. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Huang, Y.-C. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol. 2019, 131, 949–958. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.C.R.P.; Todaro, V.; do Carmo, F.A.; Frattani, F.S.; de Sousa, V.P.; Rodrigues, C.R.; Sathler, P.C.; Cabral, L.M. A promising oral fucoidan-based antithrombotic nanosystem: Development, activity and safety. Nanotechnology 2018, 29, 165102. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).