Executive Function and Working Memory Deficits in Females with Fragile X Premutation
Abstract
1. Introduction
1.1. Fragile X
1.2. Executive Functions and Phonological Working Memory in FXS
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Assessment Tools
2.2.1. Working Memory
2.2.2. Executive Functions
2.3. Procedure
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornish, K.M.; Kogan, C.S.; Li, L.; Turk, J.; Jacquemont, S.; Hagerman, R.J. Lifespan changes in working memory in Fragile X premutation males. Brain Cogn. 2009, 69, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Glineburg, M.R.; Todd, P.K.; Charlet-Berguerand, N.; Sellier, C. Repeat-associated non-AUG (RAN) translation and other molecular mechanisms in Fragile X Tremor Ataxia Syndrome. Brain Res. 2018, 1693 Pt A, 43–54. [Google Scholar] [CrossRef]
- Gabis, L.V.; Baruch, Y.K.; Jokel, A.; Raz, R. Psychiatric and autistic comorbidity in Fragile X Syndrome across ages. J. Child Neurol. 2011, 26, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Gabis, L.V.; Shefer, S.; Raas–Rothschild, A. Ethical dilemmas linked to Fragile X testing of minors- a preliminary survey among professionals. J. Mol. Neurosci. 2020, 70, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Quartier, A.; Poquet, H.; Gilbert-Dussardier, B.; Rossi, M.; Casteleyn, A.S.; Des Portes, V.; Feger, C.; Nourisson, E.; Kuentz, P.; Redin, C.; et al. Intragenic FMR1 disease-causing variants: A significant mutational mechanism leading to Fragile-X Syndrome. Eur. J. Hum. Genet. 2017, 25, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.I.; Backer, K.C.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the Fragile X mental retardation 1 gene with and without Fragile X–associated Tremor/Ataxia Syndrome (FXTAS). J. Psychiatr. Res. 2011, 45, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, L.B.; Visootsak, J.; Sherman, S.L. Cognitive aspects of Fragile X syndrome. Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 501–508. [Google Scholar] [CrossRef]
- Haify, S.N.; Botta-Orfila, T.; Hukema, R.K.; Tartaglia, G.G. In silico, in vitro, and in vivo approaches to identify molecular players in Fragile X Tremor and Ataxia Syndrome. Front. Mol. Biosci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Jiraanont, P.; Sweha, S.R.; AlOlaby, R.R.; Silva, M.; Tang, H.-T.; Durbin-Johnson, B.; Schneider, A.; Espinal, G.M.; Hagerman, P.J.; Rivera, S.M.; et al. Clinical and molecular correlates in Fragile X premutation females. eNeurologicalSci 2017, 7, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Famula, J.; Ferrer, E.; Hagerman, R.J.; Tassone, F.; Schneider, A.; Rivera, S.M.; Hessl, D. Neuropsychological changes in FMR1 premutation carriers and onset of Fragile X-Associated Tremor/Ataxia Syndrome. J. Neurodev. Disord. 2022, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, P. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathology and mechanisms. Acta Neuropathol. 2013, 126, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.M.; Li, L.; Kogan, C.S.; Jacquemont, S.; Turk, J.; Dalton, A.; Hagerman, R.J.; Hagerman, P.J. Age–dependent cognitive changes in carriers of the Fragile X Syndrome. Cortex 2008, 44, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Cole, V.; Longhi, E.; Karmiloff-Smith, A.; Scerif, G. Mapping developmental trajectories of attention and working memory in Fragile X Syndrome: Developmental freeze or developmental change? Dev. Psycho-Pathol. 2013, 25, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hippolyte, L.; Battistella, G.; Perrin, A.G.; Fornari, E.; Cornish, K.M.; Beckmann, J.S.; Niederhauser, J.; Vingerhoets, F.J.G.; Draganski, B.; Maeder, P.; et al. Investigation of memory, executive functions, and anatomic correlates in asymptomatic FMR1 premutation carriers. Neurobiol. Aging 2014, 35, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Sterling, A.M.; Mailick, M.; Greenberg, J.; Warren, S.F.; Brady, N. Language dysfluencies in women with the FMR1 premutation. Brain Cogn. 2013, 82, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Kogan, C.; Turk, J.; Manly, T.; James, N.; Mills, A.; Dalton, A. The emerging Fragile X premutation phenotype: Evidence from the domain of social cognition. Brain Cogn. 2005, 57, 53–60. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Jacquemont, S.; Loesch, D.Z.; Leehey, M.A.; Goodrich, G.K.; Hagerman, R.J.; Epstein, J.; Wilson, R.; Cogswell, J.B.; et al. Impairment in the cognitive functioning of males with Fragile X–Associated Tremor/Ataxia Syndrome (FXTAS). J. Neurol. Sci. 2006, 248, 227–233. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Hagerman, P.J. The Fragile X premutation: Into the phenotypic fold. Curr. Opin. Genet. Dev. 2002, 12, 278–283. [Google Scholar] [CrossRef]
- Lightbody, A.A.; Reiss, A.L. Gene, brain, and behavior relationships in Fragile X syndrome: Evidence from neuroimaging studies. Dev. Disabil. Res. Rev. 2009, 15, 343–352. [Google Scholar] [CrossRef]
- Moore, C.J.; Daly, E.M.; Schmitz, N.; Tassone, F.; Tysoe, C.; Hagerman, R.J.; Hagerman, P.J.; Morris, R.G.; Murphy, K.C.; Murphy, D.G.M. A neuropsychological investigation of male premutation carriers of Fragile X Syndrome. Neuropsychologia 2004, 42, 1934–1947. [Google Scholar] [CrossRef]
- Toledano–Alhadef, H.; Basel–Vanagaite, L.; Magal, N.; Davidov, B.; Ehrlich, S.; Drasinover, V.; Taub, E.; Halpern, G.J.; Ginott, N.; Shohat, M. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am. J. Hum. Genet. 2001, 69, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gabis, L.V.; Gruber, N.; Berkenstadt, M.; Shefer, S.; Attia, O.L.; Mula, D.; Cohen, Y.; Elizur, S.E. Fragile X premutation carrier epidemiology and symptomatology in Israel-results from a tertiary child developmental center. Cerebellum 2016, 15, 595–598. [Google Scholar] [CrossRef]
- Zlotogora, J. The Israeli national population program of genetic carrier screening for reproductive purposes. How should it be continued? Isr. J. Health Policy Res. 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Zlotogora, J.; Grotto, I.; Kaliner, E.; Gamzu, R. The Israeli national population program of genetic carrier screening for reproductive purposes. Genet. Med. 2016, 18, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Huggins, R.M.; Bui, Q.M.; Taylor, A.K.; Pratt, C.; Epstein, J.; Hagerman, R.J. Effect of Fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. Am. J. Med. Genet. Part A 2003, 122, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Greco, C.M.; Hunsaker, M.R.; Seritan, A.L.; Berman, R.F.; Gane, L.W.; Jacquemont, S.; Basuta, K.; Jin, L.W.; Hagerman, P.J.; et al. Neuropathological, clinical and molecular pathology in female Fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012, 11, 577–585. [Google Scholar] [CrossRef]
- Goodrich–Hunsaker, N.J.; Wong, L.M.; McLennan, Y.; Srivastava, S.; Tassone, F.; Harvey, D.; Rivera, S.M.; Simon, T.J. Young adult female Fragile X premutation carriers show age–and genetically–modulated cognitive impairments. Brain Cogn. 2011, 75, 255–260. [Google Scholar] [CrossRef]
- Cornish, K.M.; Hocking, D.R.; Moss, S.A.; Kogan, C.S. Selective executive markers of at-risk profiles associated with the Fragile X premutation. Neurology 2011, 77, 618–622. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Engle, K.; Leehey, M.A.; Hagerman, R.J.; Tassone, F.; Hessl, D.; Hagerman, P.J.; Cogswell, J.B.; Bennett, R.E.; et al. Cognitive profile of Fragile X premutation carriers with and without Fragile X–Associated Tremor/Ataxia Syndrome. Neuropsychology 2008, 22, 48–60. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.; Kraan, C.; Georgiou–Karistianis, N.; Metcalfe, S.A.; Bradshaw, J.L.; Hocking, D.R.; Archibald, A.D.; Cohen, J.; Trollor, J.N.; et al. Exploring inhibitory deficits in female premutation carriers of Fragile X Syndrome: Through eye movements. Brain Cogn. 2014, 85, 201–208. [Google Scholar] [CrossRef]
- Johnston, C.; Eliez, S.; Dyer-Friedman, J.; Hessl, D.; Glaser, B.; Blasey, C.; Taylor, A.; Reiss, A. Neurobehavioral phenotype in carriers of the Fragile X premutation. Am. J. Med. Genet. 2001, 103, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Kogan, C.S.; Cornish, K.M. Mapping self–reports of working memory deficits to executive dysfunction in Fragile X malestal retardation 1 (FMR1) gene premutation carriers asymptomatic for FXTAS. Brain Cogn. 2010, 73, 236–243. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.; Iwahashi, C.; Cheung, K.; Schneider, A.; Hagerman, R.J.; Hagerman, P.J.; Rivera, S.M. Influence of the Fragile X mental retardation (FMR1) gene on the brain and working memory in males with normal FMR1 alleles. Neuroimage 2013, 65, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.R.; Hatton, D.; Sideris, J.; Sullivan, K.; Hammer, J.; Schaaf, J.; Mirrett, P.; Ornstein, P.A.; Bailey, D.B., Jr. Executive functions in young males with Fragile X syndrome in comparison to malestal age–matched controls: Baseline findings from a longitudinal study. Neuropsychology 2008, 22, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.W.; Mazzocco, M.M.; Kover, S.T. Assessing executive dysfunction in girls with Fragile X or Turner syndrome using the Contingency Naming Test (CNT). Dev. Neuropsychol. 2005, 28, 755–777. [Google Scholar] [CrossRef]
- Schmitt, L.; Shaffer, R.; Hessl, D.; Erickson, C. Executive function in Fragile X Syndrome: A systematic review. Brain Sci. 2019, 9, 15. [Google Scholar] [CrossRef]
- Anderson, P.J. Towards a developmental model of executive function. In Executive Functions and the Frontal Lobes: A Lifespan Perspective; Anderson, V., Jacobs, R., Anderson, P.J., Eds.; Taylor & Francis: New York, NY, USA, 2008; pp. 154–196. [Google Scholar]
- Ando, J.; Ono, Y.; Wright, M.J. Genetic structure of spatial and verbal working memory. Behav. Genet. 2001, 31, 615–624. [Google Scholar] [CrossRef]
- Karlsgodt, K.H.; Bachman, P.; Winkler, A.M.; Bearden, C.E.; Glahn, D.C. Genetic influence on the working memory circuitry: Behavior, structure, function and extensions to illness. Behav. Brain Res. 2011, 225, 610–622. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Hitch, G. Working memory. Psychol. Learn. Motiv. 1974, 8, 47–89. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory and language: An overview. J. Commun. Disord. 2003, 36, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Brega, A.G.; Goodrich, G.; Bennett, R.E.; Hessl, D.; Engle, K.; Leehey, M.A.; Bounds, L.S.; Paulich, M.J.; Hagerman, R.J.; Hagerman, P.J.; et al. The primary cognitive deficit among males with Fragile X–Associated Tremor/Ataxia Syndrome (FXTAS) is a dysexecutive syndrome. J. Clin. Exp. Neuropsychol. 2008, 30, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory. Curr. Biol. 2010, 20, R136–R140. [Google Scholar] [CrossRef]
- Baddeley, A.; Gathercole, S.; Papagno, C. The phonological loop as a language learning device. Psychol. Rev. 1998, 105, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Lahey, M. Nonword repetitions of children with specific language impairment: Exploration of some explanations for their inaccuracies. Appl. Psycholinguist. 1998, 19, 279–309. [Google Scholar] [CrossRef]
- Gathercole, S.E. Nonword repetition and word learning: The nature of the relationship. Appl. Psycholinguist. 2006, 27, 513–543. [Google Scholar] [CrossRef]
- Estes, K.G.; Evans, J.L.; Else–Quest, N.M. Differences in the nonword repetition performance of children with and without specific language impairment: A meta–analysis. J. Speech Lang. Hear. Res. 2007, 50, 177–195. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Lohmeier, H.L.; Campbell, T.F.; Dollaghan, C.A.; Green, J.R.; Moore, C.A. A nonword repetition task for speakers with misarticulations: The Syllable Repetition Task (SRT). J. Speech Lang. Hear. Res. 2009, 52, 1189–1212. [Google Scholar] [CrossRef]
- Goodrich–Hunsaker, N.J.; Wong, L.M.; McLennan, Y.; Tassone, F.; Harvey, D.; Rivera, S.M.; Simon, T.J. Adult female Fragile X premutation carriers exhibit age– and CGG repeat length–related impairments on an attentionally-based enumeration task. Front. Hum. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef]
- Hunter, J.E.; Allen, E.G.; Abramowitz, A.; Rusin, M.; Leslie, M.; Novak, G.; Hamilton, D.; Shubeck, L.; Charen, K.; Sherman, S.L. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am. J. Hum. Genet. 2008, 83, 692–702. [Google Scholar] [CrossRef]
- Yang, J.C.; Simon, C.; Niu, Y.Q.; Bogost, M.; Schneider, A.; Tassone, F.; Seritan, A.; Grigsby, J.; Hagerman, P.J.; Hagerman, R.J.; et al. Phenotypes of hypofrontality in older female Fragile X premutation carriers. Ann. Neurol. 2013, 74, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, M.J.W.; Huizinga, M.; Huizenga, H.M.; Ridderinkhof, K.R.; Van der Molen, M.W.; Hamel, B.J.C.; Curfs, L.M.G.; Ramakers, G.J.A. Profiling Fragile X Syndrome in males: Strengths and weaknesses in cognitive abilities. Res. Dev. Disabil. 2010, 31, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Memory Scale Memory, 3rd ed.; Psychological Press: London, UK, 1997. [Google Scholar]
- Gvion, A.; Friedmann, N. FriGvi: A battery for the diagnosis of phonological working memory. Lang. Brain 2008, 7, 161–180. (In Hebrew) [Google Scholar]
- Gvion, A.; Friedmann, N. Phonological short–term memory in conduction aphasia. Aphasiology 2012, 26, 579–614. [Google Scholar] [CrossRef]
- Gaudino, E.A.; Geisler, M.W.; Squires, N.K. Construct validity in the Trail Making Test: What makes part B harder? J. Clin. Exp. Neuropsychol. 1995, 17, 529–535. [Google Scholar] [CrossRef]
- Sánchez–Cubillo, I.; Perianez, J.A.; Adrover–Roig, D.; Rodriguez–Sanchez, J.M.; Rios–Lago, M.; Tirapu, J.E.E.A.; Barcelo, F. Construct validity of the Trail Making Test: Role of task–switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. BRIEF–A: Behavior Rating Inventory of Executive Function—Adult Version; Psychological Assessment Resources: Lutz, FL, USA, 2005. [Google Scholar]
- Rotenberg–Shpigelman, S.; Rapaport, R.; Stern, A.; Hartman–Maeir, A. Content validity and internal consistency reliability of the Behavior rating inventory of executive function–adult version (BRIEF–A) in Israeli adults with attention–deficit/hyperactivity disorder. Isr. J. Occup. Ther. 2008, 17, H77–H96. (In Hebrew) [Google Scholar]
- Kavé, G. Phonemic fluency, semantic fluency, and difference scores: Normative data for adult Hebrew speakers. J. Clin. Exp. Neuropsychol. 2005, 27, 690–699. [Google Scholar] [CrossRef]
- Munir, F.; Cornish, K.M.; Wilding, J. Nature of the working memory deficit in Fragile–X Syndrome. Brain Cogn. 2000, 44, 387–401. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.M.; Kraan, C.M.; Lozano, R.; Bui, M.; Fielding, J. Executive dysfunction in female FMR1 premutation carriers. Cerebellum 2016, 15, 565–569. [Google Scholar] [CrossRef]
- Kremales, W.S.; Jacobsen, K.C.; Xian, H.; Eisen, S.A.; Eaves, L.J.; Tsuang, M.T.; Lyons, M.J. Genetics of verbal working memory processes: A twin study of middle–aged males. Neuropsychology 2007, 21, 569–580. [Google Scholar] [CrossRef]
- Newbury, D.F.; Bishop, D.V.; Monaco, A.P. Genetic influences on language impairment and phonological short–term memory. Trends Cogn. Sci. 2005, 9, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Tost, H.; Meyer-Lindenberg, A. Working memory genetics in schizophrenia and related disorders: An RDoC perspective. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 121–131. [Google Scholar] [CrossRef]
- Bishop, D.V.; North, T.; Donlan, C. Nonword repetition as a behavioral marker for inherited language impairment: Evidence from a twin study. J. Child Psychol. Psychiatry 1996, 37, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Karmiloff-Smith, A.; Scerif, G.; Thomas, M. Different approaches to relating genotype to phenotype in develop-mental disorders. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2002, 40, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Devanna, P.; Dediu, D.; Vernes, S.C. The genetics of language: From complex genes to complex communication. In The Oxford Handbook of Psycholinguistics, 2nd ed.; Rueschemeyer, S., Gasskell, M.G., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 865–898. [Google Scholar]
- Peterson, R.L.; McGrath, L.M.; Smith, S.D.; Pennington, B.F. Neuropsychology and genetics of speech, language, and literacy disorders. Pediatr. Clin. N. Am. 2007, 54, 543–561. [Google Scholar] [CrossRef] [PubMed]
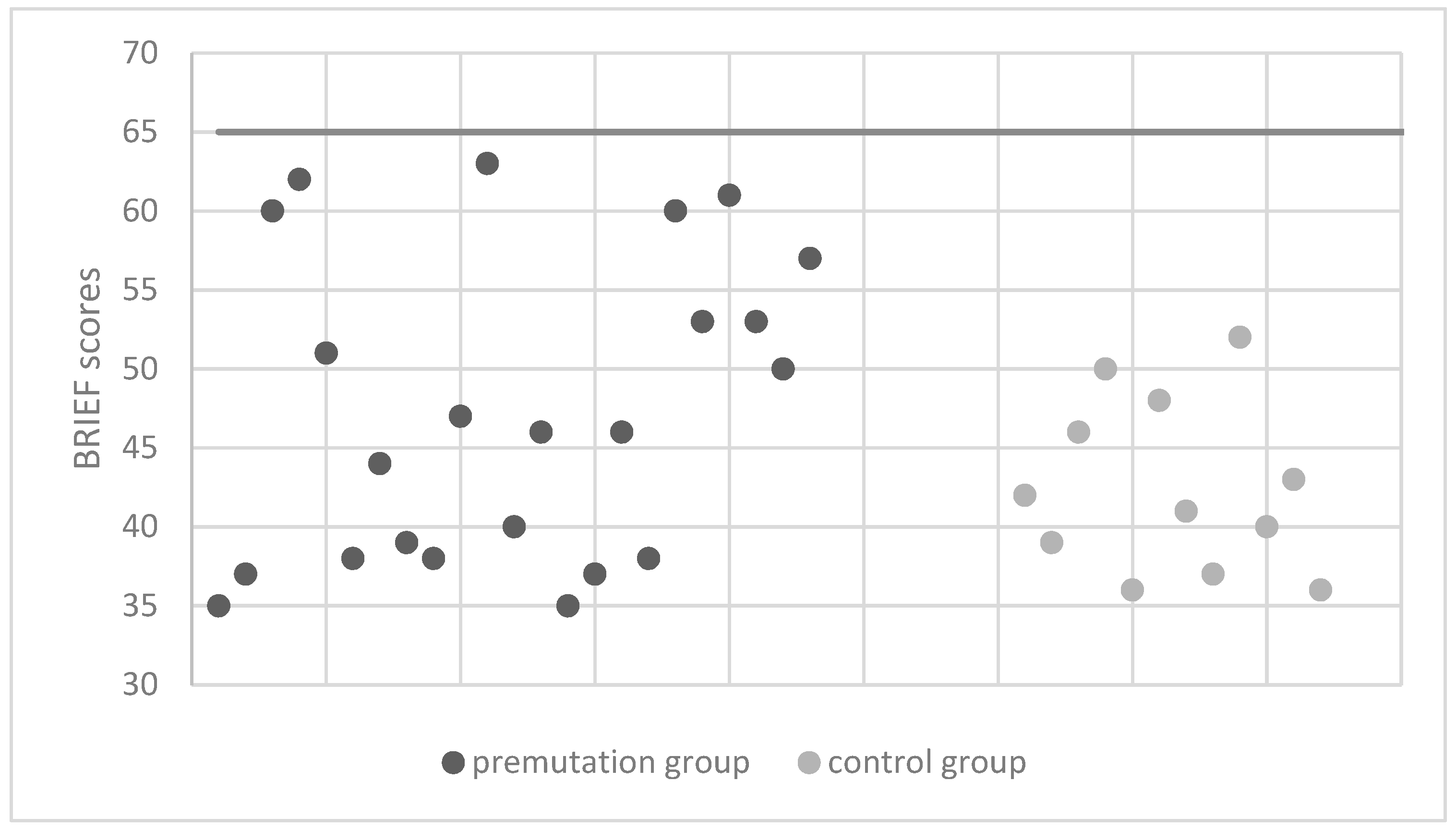
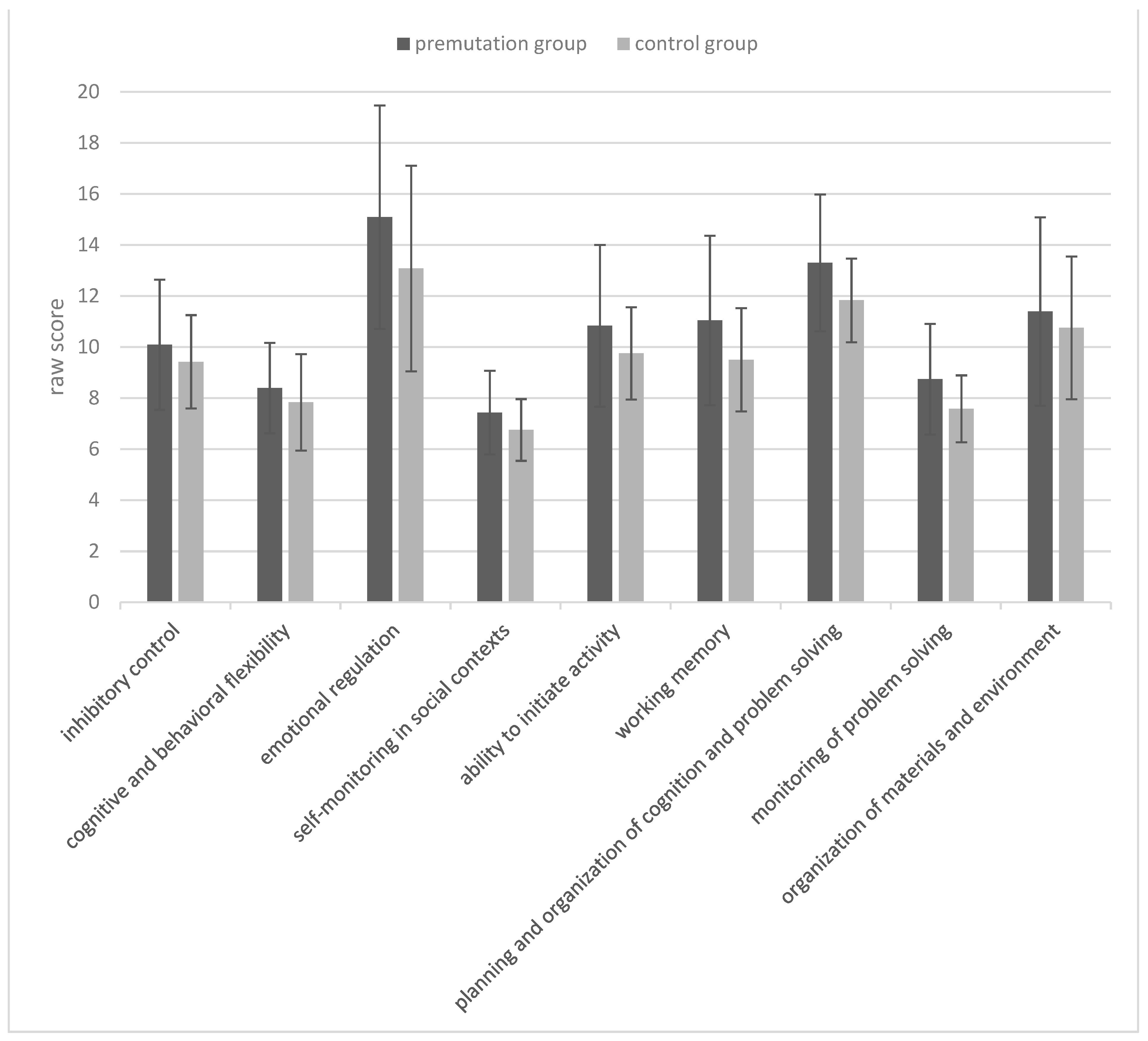
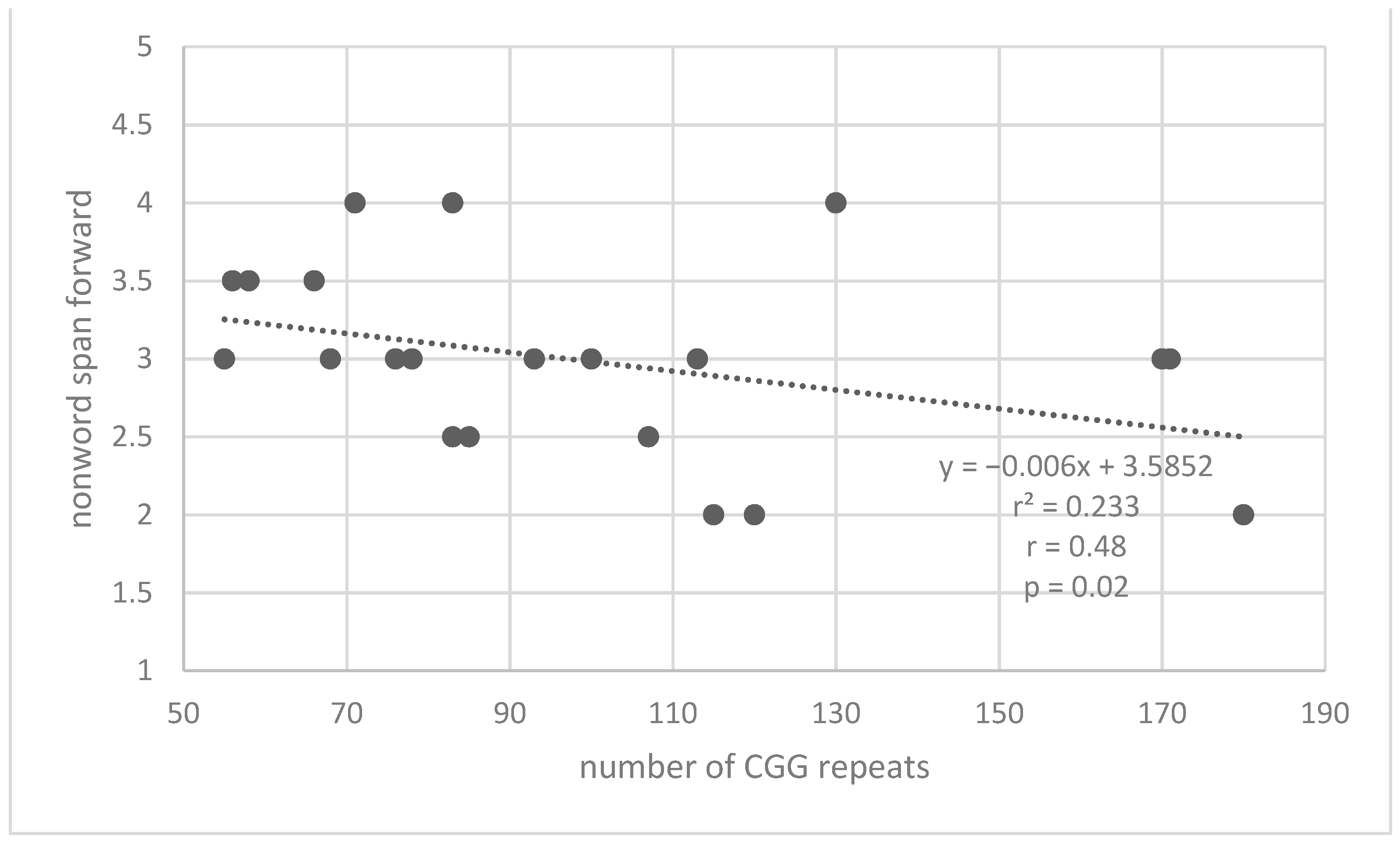
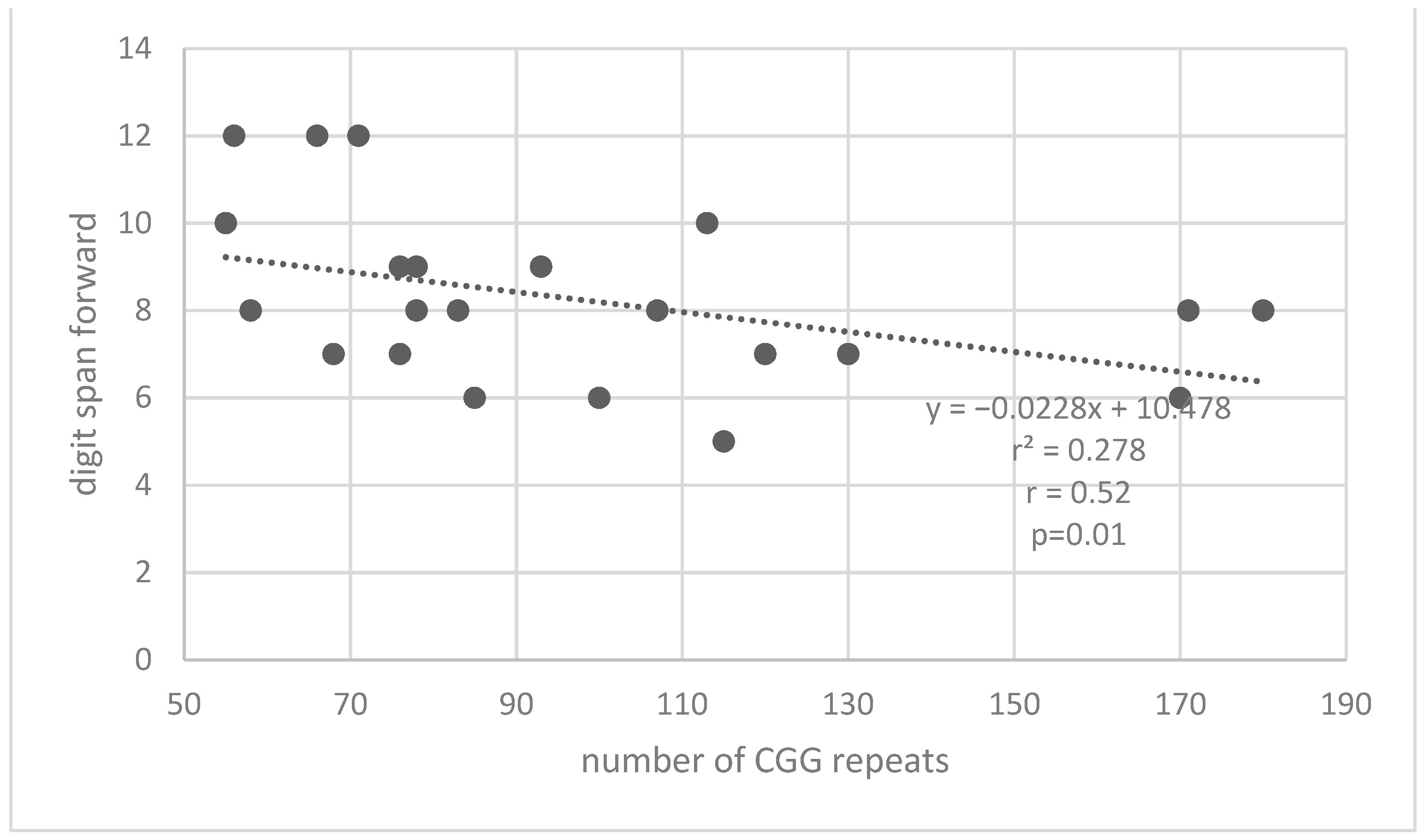
| Measures | Premutation Group | Control Group | |
|---|---|---|---|
| BRIEF-A | raw score | 96.3 (19.78) | (10.95) 86.5 |
| standard score | 47.39 (9.68) | 42.5 (5.43) | |
| TMT (seconds) | Part A | 22.28 (10.7) | 20.48 (7.58) |
| Part B | 55.419 (29.71) | 41.212 (10.59) | |
| Digit span (number of items) | forward | 8.19 (1.91) | 8.42 (1.5) |
| backward | 6.27 (2.57) | 6.17 (2.16) | |
| total raw score | 14.9 (3.84) | 14.58 (3.2) | |
| total standard score | 8.59 (2.57) | 8.41 (2.15) | |
| FriGvi (memory span) | basic word span | 4.81 (0.69) | 5.13 (0.64) |
| phonological similarity span | 3.85 (0.77) | 4.04 (0.33) | |
| long word span | 3.96 (0.37) | 4.04 (0.25) | |
| nonword span | 2.92 (0.61) | 2.96 (0.33) | |
| FriGvi (effect size) | phonological similarity effect | 0.96 (9.67) | 1.08 (0.66) |
| length effect | 0.84 (0.62) | 1.08 (0.59) | |
| lexical effect | 1.88 (0.76) | 2.16 (0.49) | |
| Phonemic fluency (number of items) | Bet (/b/) | 13.65 (4.12) | 14 (4.88) |
| Gimel (/g/) | 12.92 (4.0) | 13.16 (2.2) | |
| Shin (/S/) | 13.80 (3.74) | 15.58 (5.43) | |
| Semantic fluency (number of items) | animals | 22.34 (4.67) | 26.66 (3.47) |
| fruits and vegetables | 24.11 (4.72) | 27.41 (4.23) | |
| vehicles | 15.15 (3.69) | 18.58 (5.41) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segal, O.; Kowal, T.; Banet-Levi, Y.; Gabis, L.V. Executive Function and Working Memory Deficits in Females with Fragile X Premutation. Life 2023, 13, 813. https://doi.org/10.3390/life13030813
Segal O, Kowal T, Banet-Levi Y, Gabis LV. Executive Function and Working Memory Deficits in Females with Fragile X Premutation. Life. 2023; 13(3):813. https://doi.org/10.3390/life13030813
Chicago/Turabian StyleSegal, Osnat, Tamar Kowal, Yonit Banet-Levi, and Lidia V. Gabis. 2023. "Executive Function and Working Memory Deficits in Females with Fragile X Premutation" Life 13, no. 3: 813. https://doi.org/10.3390/life13030813
APA StyleSegal, O., Kowal, T., Banet-Levi, Y., & Gabis, L. V. (2023). Executive Function and Working Memory Deficits in Females with Fragile X Premutation. Life, 13(3), 813. https://doi.org/10.3390/life13030813









