Bovine Grafting: An Effective Alternative after Curettage of Benign Bone Tumors
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Galia, C.R.; de Luca, G.; Ávila, L.M.; Rosito, R.; Macedo, C.A.S. Bovine lyophilized graft (BLG): Histological analysis on behavior in humans after 49 months. Rev. Bras. Ortop. 2015, 47, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Galia, C.R.; Pagnussato, F.; Ribeiro, T.A.; Moreira, L. Biology of bone graft and the use of bovine bone for revision of total hip arthroplasty with acetabular reconstruction. In Bone Grafting; Kummoona, R., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/62749 (accessed on 1 March 2022).
- Galia, C.R.; Macedo, C.A.; Rosito, R.; de Mello, T.M.; Camargo, L.M.A.Q.; Moreira, L.F. In vitro and in vivo evaluation of lyophilized bovine bone biocompatibility. Clinics 2008, 63, 801–806. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Henning, C.; Poglia, G.; Leie, M.A.; Galia, C.R. Comparative study of subtalar arthrodesis after calcaneal frature malunion with autologous bone graft or freeze-dried xenograft. J. Exp. Orthop. 2015, 2, 10. [Google Scholar] [CrossRef]
- Rosito, R.; Galia, C.R.; Macedo, C.A.S.; Quaresma, L.M.A.C.; Moreira, L.F. Mid-term follow-up of acetabular reconstruction using bovine freeze-dried bone graft and reinforcement device. Rev. Col. Bras. Cir. 2009, 36, 230–235. [Google Scholar] [CrossRef]
- De Souza Macedo, C.A.; Galia, C.R.; Valin, M.R.; Rosito, R.; Timm, H.; Muller, L.M. Use of acetabular reinforcement in total hip arthroplasty. Rev. Bras. Ortop. 1998, 33, 307–314. [Google Scholar]
- Rosito, R.; Galia, C.R.; Macedo, C.A.S.; Moreira, L.F.; Quaresma, L.M.A.C.; Palma, H.M. Acetabular reconstruction with human and bovine freeze- dried bone grafts and a reinforcement device. Clinics 2008, 63, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Galia, C.R.; de Souza Macedo, C.A.; Rosito, R.; Camargo, L.M.A.Q.; Marinho, D.R.; Moreira, L.F. Femoral and acetabular revision using impacted nondemineralized freeze-dried bone allografts. J. Orthop. Sci. 2009, 14, 259–265. [Google Scholar] [CrossRef] [PubMed]
- De Souza Macedo, C.A.; Galia, C.R.; da Silva, A.L.B.; Sanches, P.C.; César, P.C.; Sanches, P.R.S.; Duarte, L.S. Compressive resistance of deep frozen and lyophilized bovine bone: Comparative study. Rev. Bras. Ortop. 1999, 34, 529–534. [Google Scholar]
- Wu, P.K.; Chen, C.F.; Chen, C.M.; Tsai, S.W.; Cheng, Y.C.; Chang, M.C.; Chen, W.-M. Grafting for bone defects after curettage of benign bone tumor—Analysis of factors influencing the bone healing. J. Chin. Med. Assoc. 2018, 81, 643–648. [Google Scholar] [CrossRef]
- Gava, N.F.; Engel, E.E. Treatment alternatives and clinical outcomes of bone filling after benign tumour curettage. A systematic review. Orthop. Traumatol. Surg. Res. 2022, 108, 102966. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Galia, C.R.; Lourenço, A.L.; Rosito, R.; Macedo, C.A.S.; Camargo, L.M.A. Caracterização físico-química do enxerto de osso bovino liofilizado. Rev. Bras. Ortop. 2011, 46, 444–451. [Google Scholar] [CrossRef]
- Bracey, D.; Cignetti, N.E.; Jinnah, A.H.; Stone, A.V.; Gyr, B.M.; Whitlock, P.W.; Scott, A.T. Bone xenotransplantation: A review of the history, orthopedic clinical literature, and a single-center case-series. Xenotransplantation 2020, 27, e12600. [Google Scholar] [CrossRef]
- Charalambides, C.; Beer, M.; Cobb, A.G. Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: A report of 27 cases. Acta Orthop. 2005, 76, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Newswire, P.R. Dental Bone Graft Substitutes and Other Biomaterials Market (Natural, Ceramic, Composite And Polymer). Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2014–2020. Available online: https://www.transparencymarketresearch.com/dental-bone-graft-substitutes-biomaterials.html (accessed on 21 October 2022).
- Boffano, M.; Ratto, N.; Conti, A.; Pellegrino, P.; Rossi, L.; Perale, G.; Piana, R. A preliminary study on the mechanical reliability and regeneration capability of artificial bone grafts in oncologic cases, with and without osteosynthesis. J. Clin. Med. 2020, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
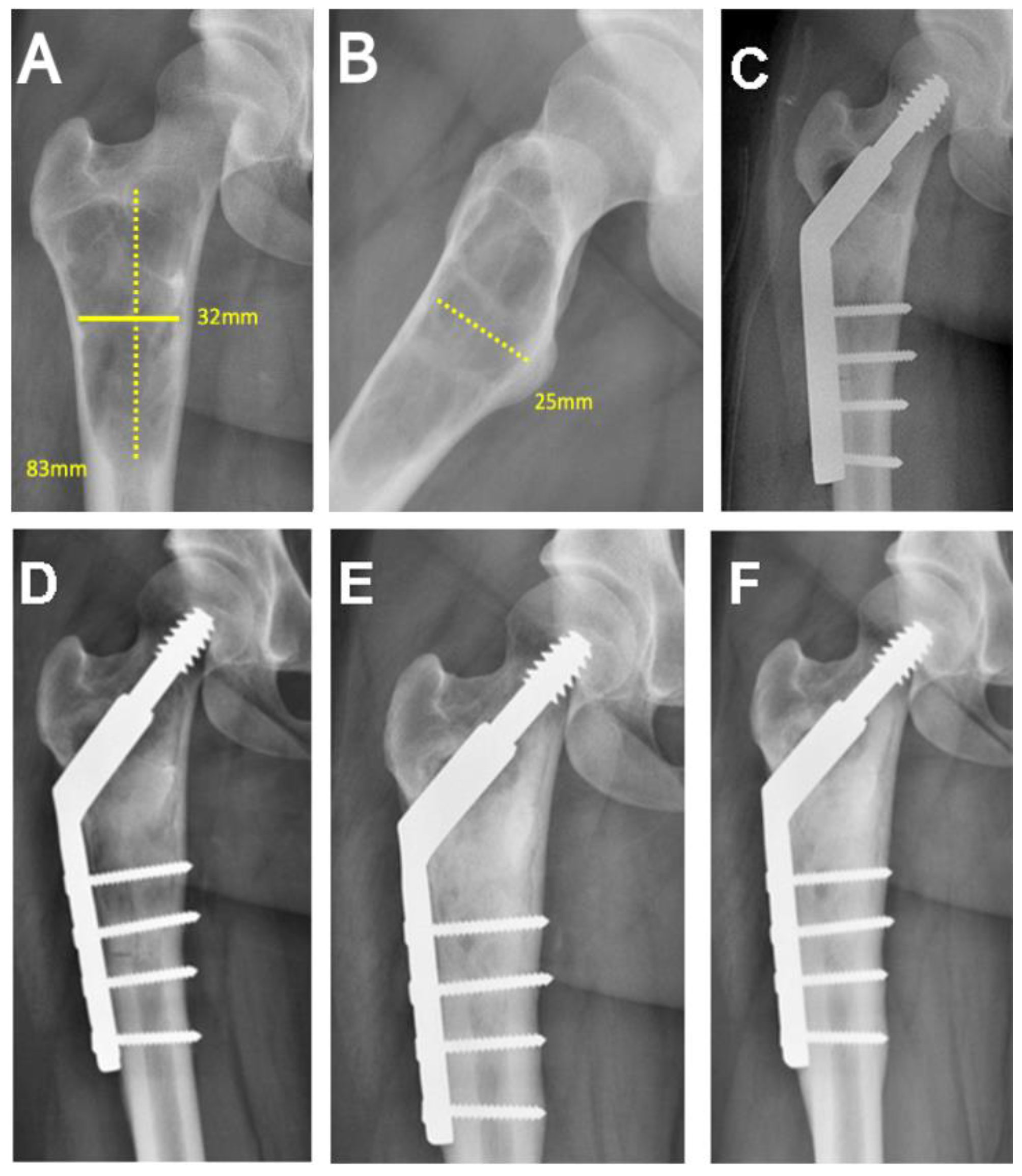
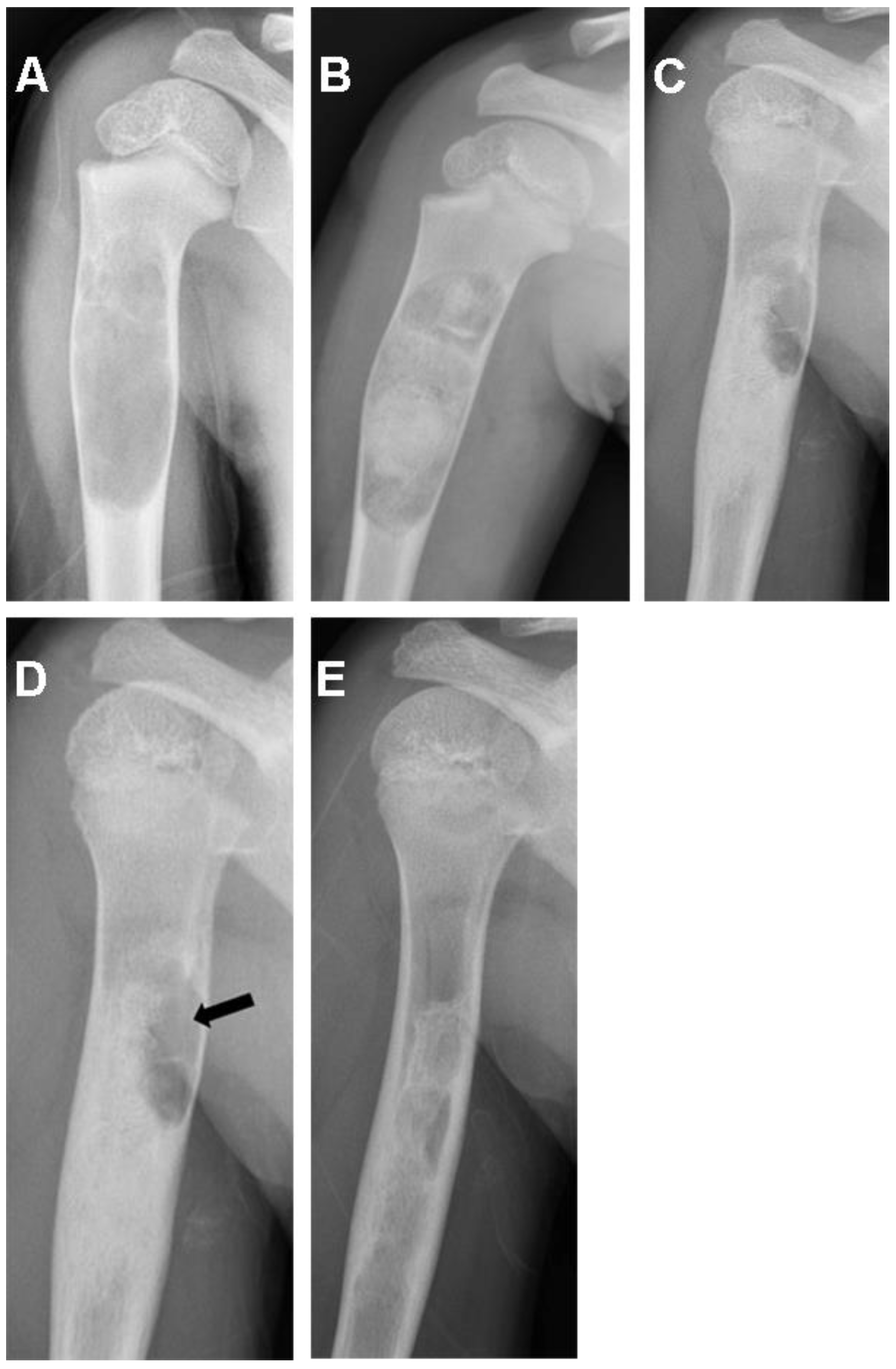
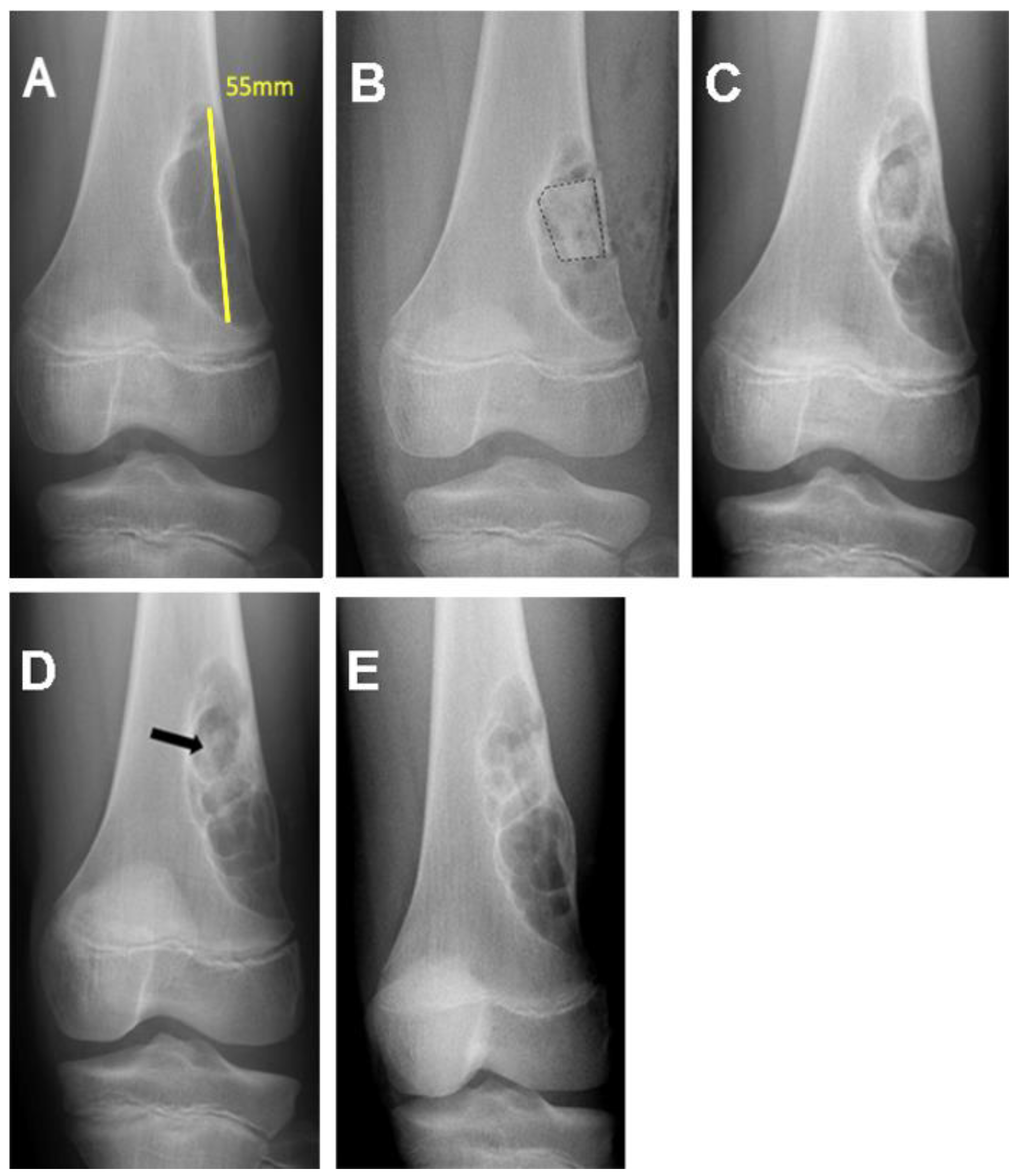
| Patient Registry/Sex/Age (Years) | Tumor Location | Pathological Diagnosis | Tumor Volume (cm3) |
|---|---|---|---|
| 1/F/14 | Calcaneus | Simple bone cyst | 5.82 |
| 2/F/8 | Humerus | Simple bone cyst | 4.12 |
| 3/M/5 | Proximal femur | Simple bone cyst | 7.05 |
| 4/F/28 | Calcaneus | Simple bone cyst | 8.53 |
| 5/M/75 | Proximal tibia | Ganglion cyst | 21.84 |
| 6/M/7 | Distal femur | Simple bone cyst | 62.96 |
| 7/M/45 | Phalanx (foot) | Gouty tophi | 8.24 |
| 8/F/40 | Phalanx (foot) | Enchondroma | 4.71 |
| 9/M/17 | Humerus | Fibrous dysplasia | 16.28 |
| 10/F/32 | Phalanx (hand) | Epithelial bone cyst | 8.24 |
| 11/F/10 | Tibia | Nonossifying fibroma | 23.55 |
| 12/F/11 | Tibia | Chondromyxoid fibroma | 14.87 |
| 13/F/29 | Proximal femur | Simple bone cyst | 7.63 |
| 14/M/9 | Tibia | Aneurysmal bone cyst | 26.82 |
| 15/F/9 | Humerus | Simple bone cyst | 70.2 |
| 16/M/5 | Humerus | Aneurysmal bone cyst | 3.6 |
| 17/M/11 | Distal femur | Nonossifying fibroma | 7.11 |
| 18/M/16 | Fibula | Chondromyxoid fibroma | 27.66 |
| 19/F/15 | Proximal femur | Simple bone cyst | 52.25 |
| 20/M/4 | Humerus | Simple bone cyst | 14.49 |
| 21/F/4 | Tibia | Aneurysmal bone cyst | 12.35 |
| 22/F/12 | Humerus | Simple bone cyst | 8.22 |
| 23/F/8 | Tibia | Nonossifying fibroma | 9.36 |
| 24/M/38 | Proximal femur | Simple bone cyst | 101.42 |
| 25/F/24 | Pelvis | Ganglion cyst | 2.73 |
| 26/F/24 | Distal femur | Chondroblastoma | 5.61 |
| 27/M/9 | Humerus | Simple bone cyst | 37.28 |
| 28/M/46 | Distal femur | Enchondroma | 14.62 |
| Radiological Evaluation | 6 Months n (%) | 12 Months n (%) | 24 Months n (%) |
|---|---|---|---|
| (n = 28) | (n = 27) | (n = 16) | |
| Neer * I (healed cavity) | 21 (75.0) | 21 (77.8) | 12 (75.0) |
| Neer II (healed with defects) | 5 (17.9) | 5 (18.5) | 1 (6.3) |
| Neer III (persistent lesion) | 2 (7.1) | 1 (3.7) | 3 (18.8) |
| Neer IV (recurring lesion) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| (Neer I) | (Neer II, III, IV) | |
|---|---|---|
| n (%) | n (%) | |
| Percentage of cavity filled | ||
| <90% | 4 (19.0) | 4 (66.7) |
| >90% | 17 (81.0) | 2 (33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanhini, P.; Antunes, B.P.; Pestilho, J.F.C.; Galia, C.R.; Guedes, A.; Becker, R.G. Bovine Grafting: An Effective Alternative after Curettage of Benign Bone Tumors. Life 2023, 13, 789. https://doi.org/10.3390/life13030789
Montanhini P, Antunes BP, Pestilho JFC, Galia CR, Guedes A, Becker RG. Bovine Grafting: An Effective Alternative after Curettage of Benign Bone Tumors. Life. 2023; 13(3):789. https://doi.org/10.3390/life13030789
Chicago/Turabian StyleMontanhini, Priscilla, Bruno P. Antunes, Julie Francine Cerutti Pestilho, Carlos Roberto Galia, Alex Guedes, and Ricardo Gehrke Becker. 2023. "Bovine Grafting: An Effective Alternative after Curettage of Benign Bone Tumors" Life 13, no. 3: 789. https://doi.org/10.3390/life13030789
APA StyleMontanhini, P., Antunes, B. P., Pestilho, J. F. C., Galia, C. R., Guedes, A., & Becker, R. G. (2023). Bovine Grafting: An Effective Alternative after Curettage of Benign Bone Tumors. Life, 13(3), 789. https://doi.org/10.3390/life13030789





