Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila
Abstract
1. Introduction
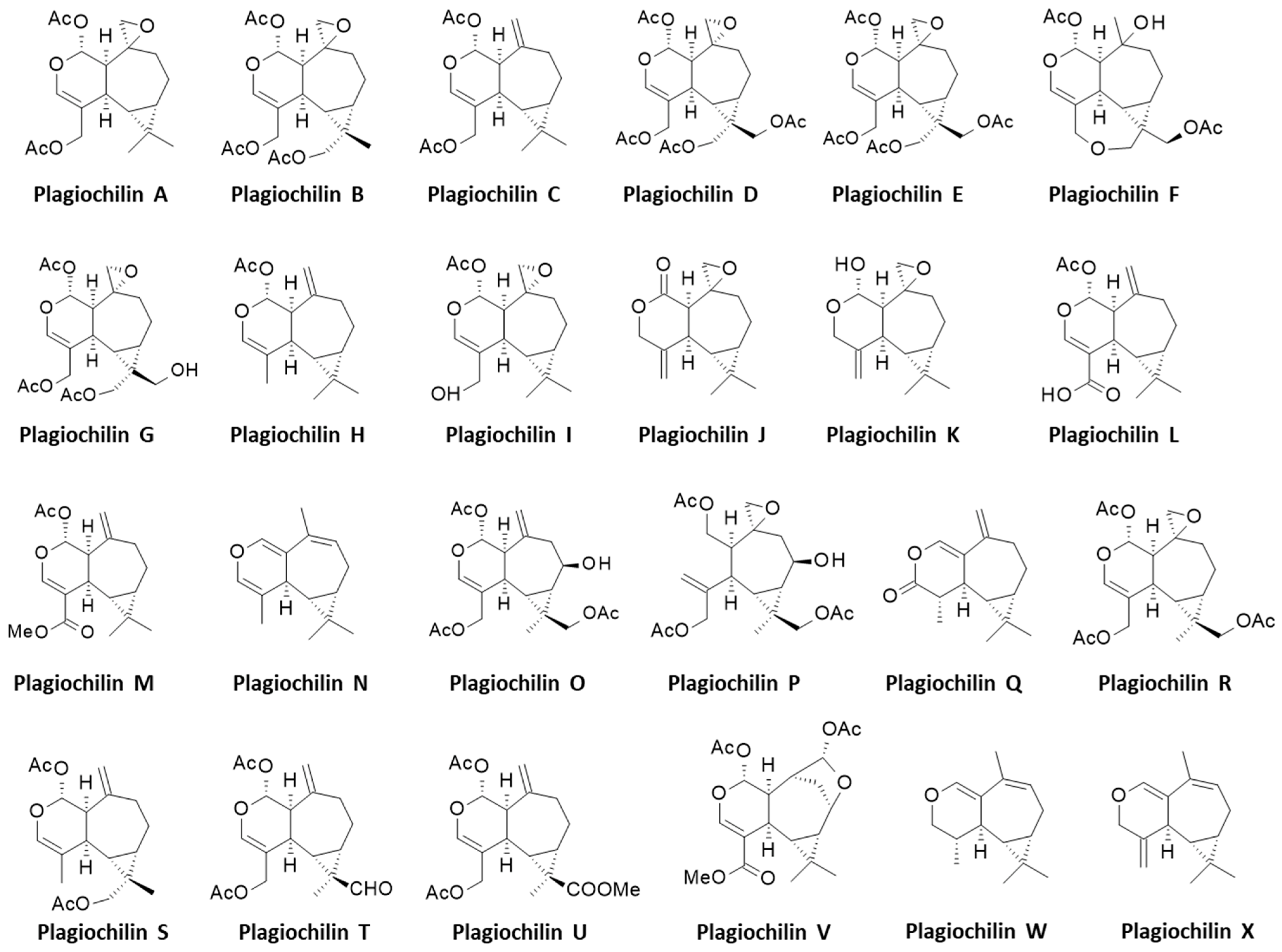
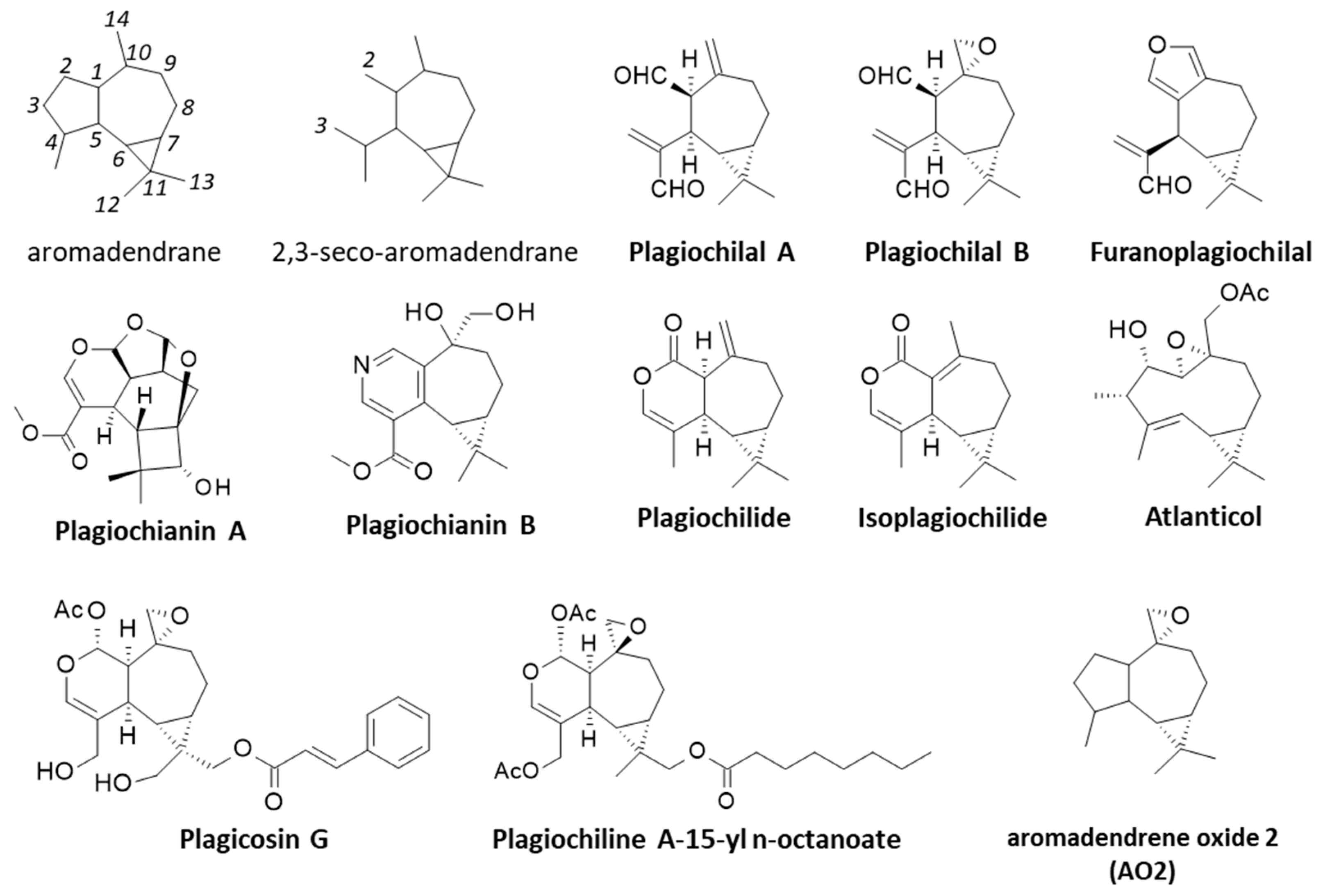
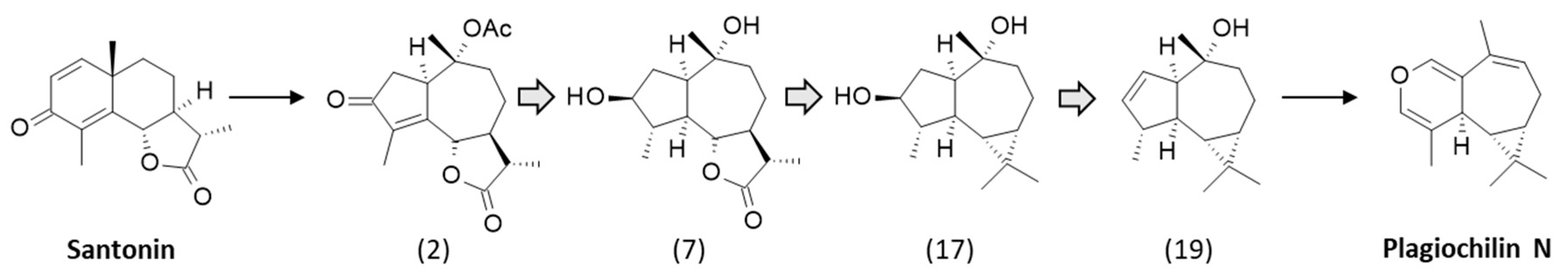
2. Discoveries of the Plagiochilins
3. Pharmacological Properties and Mechanism of Action of the Plagiochilins
3.1. Pharmacological Properties of Plagiochilins A and C
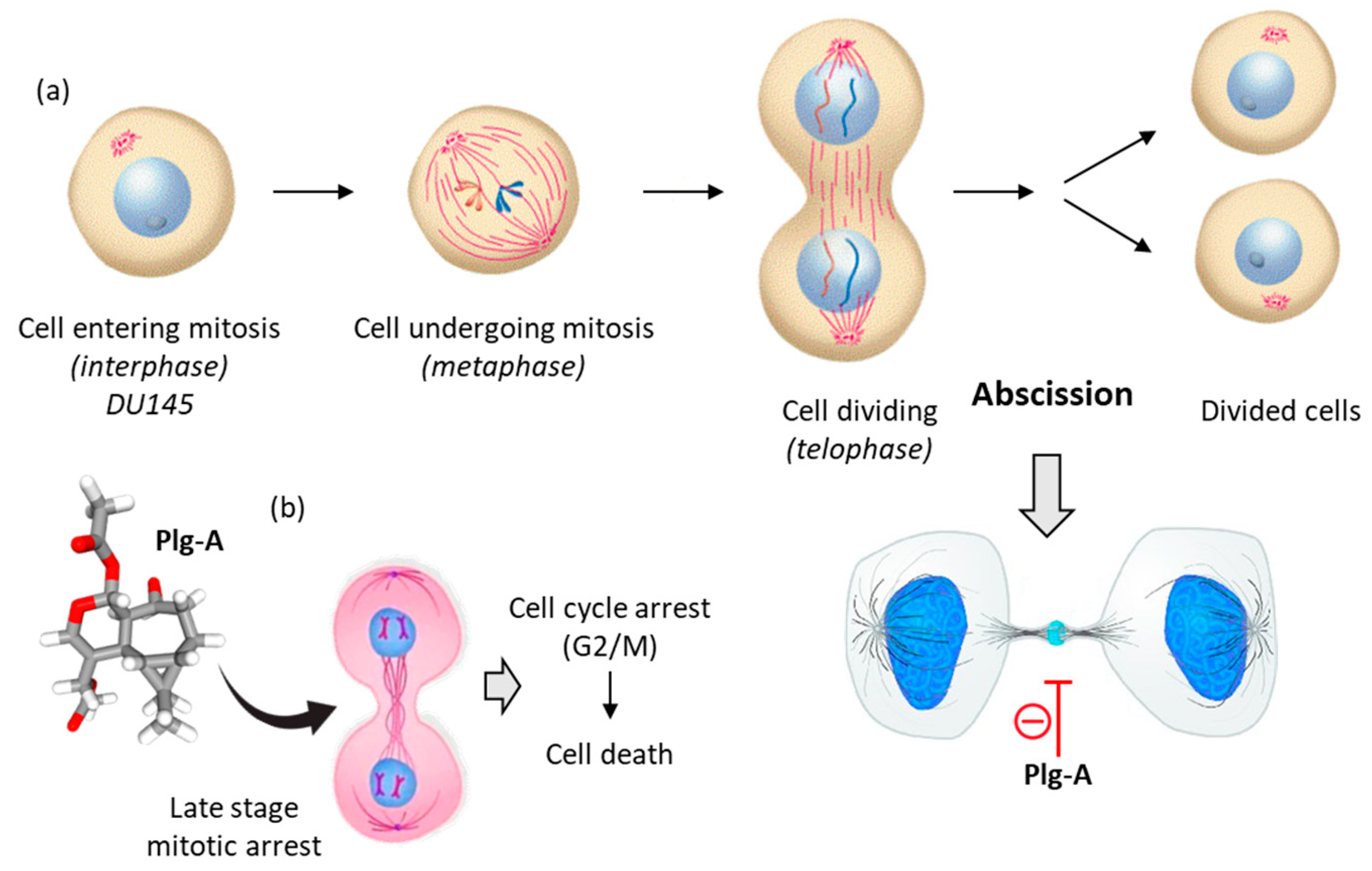
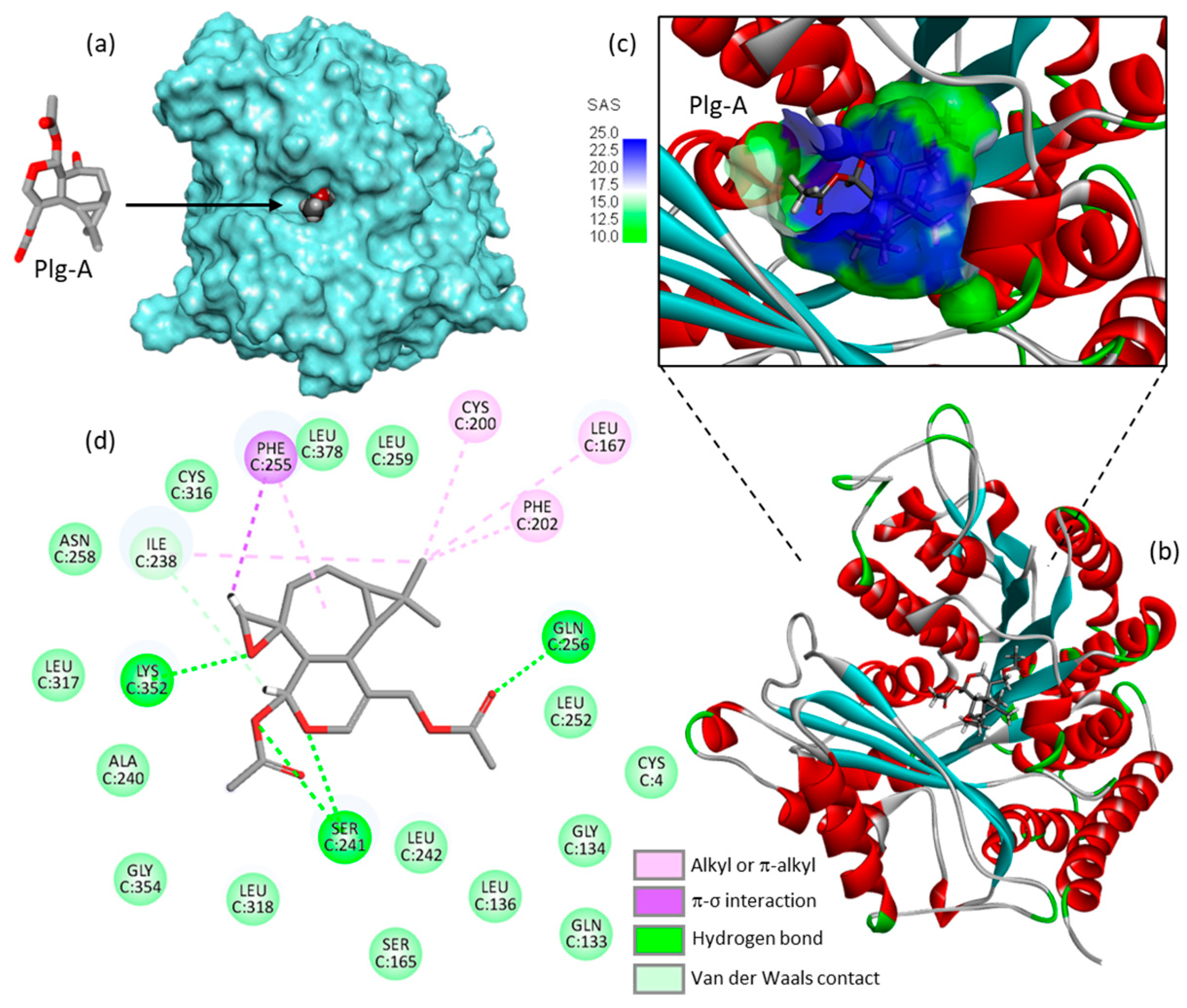
3.2. Hypothesized Mechanism of Action of Plagiochilin A
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.H.; Zhang, J.; Liu, Y.; Jia, Y.; Jiao, Y.N.; Xu, B.; Chen, Z.D. Diversity, phylogeny, and adaptation of bryophytes: Insights from genomic and transcriptomic data. J. Exp. Bot. 2022, 73, 4306–4322. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Jibran, R.; van Klink, J.W.; Zhou, Y.; Brummell, D.A.; Albert, N.W.; Schwinn, K.E.; Chagné, D.; Landi, M.; Bowman, J.L.; et al. Stress, senescence, and specialized metabolites in bryophytes. J. Exp. Bot. 2022, 73, 4396–4411. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.J.; Clark, J.W.; Schrempf, D.; Szöllősi, G.J.; Donoghue, P.C.J.; Hetherington, A.M.; Williams, T.A. Divergent evolutionary trajectories of bryophytes and tracheophytes from a complex common ancestor of land plants. Nat. Ecol. Evol. 2022, 6, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yu, J.; Zhang, L.; Goffinet, B.; Liu, Y. Phylotranscriptomics of liverworts: Revisiting the backbone phylogeny and ancestral gene duplications. Ann. Bot. 2022, 130, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L. A Brief History of Marchantia from Greece to Genomics. Plant Cell Physiol. 2016, 57, 210–229. [Google Scholar] [CrossRef]
- Söderström, L.; Hagborg, A.; von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Brown, E.; Cargill, D.C.; Costa, D.P.; Crandall-Stotler, B.J.; et al. World checklist of hornworts and liverworts. PhytoKeys 2016, 59, 1–828. [Google Scholar] [CrossRef]
- Heinrichs, J.; Hentschel, J.; Feldberg, K.; Bombosch, A.; Schneider, H. Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. J. Syst. Evol. 2009, 47, 497–508. [Google Scholar] [CrossRef]
- The World Flora Online (WFO). Available online: http://www.worldfloraonline.org (accessed on 13 January 2023).
- Renner, M.A.M. The typification of Australasian Plagiochila species (Plagiochilaceae: Jungermanniidae): A review with Recommendations. N. Z. J. Bot. 2021, 59, 323–375. [Google Scholar] [CrossRef]
- Drobnik, J.; Stebel, A. Four Centuries of Medicinal Mosses and Liverworts in European Ethnopharmacy and Scientific Pharmacy: A Review. Plants 2021, 10, 1296. [Google Scholar] [CrossRef]
- Manoj, G.S.; Murugan, K. Wound healing activity of methanolic and aqueous extracts of Plagiochila beddomei Steph. thallus in rat model. Indian J. Exp. Biol. 2012, 50, 551–558. [Google Scholar]
- Manoj, G.S.; Murugan, K. Phenolic profiles, antimicrobial and antioxidant potentiality of methanolic extract of a liverwort, Plagiochila beddomei Steph. Indian J. Nat. Prod. Resour. 2012, 3, 173–183. [Google Scholar]
- Aponte, J.C.; Yang, H.; Vaisberg, A.J.; Castillo, D.; Málaga, E.; Verástegui, M.; Casson, L.K.; Stivers, N.; Bates, P.J.; Rojas, R.; et al. Cytotoxic and anti-infective sesquiterpenes present in Plagiochila disticha (Plagiochilaceae) and Ambrosia peruviana (Asteraceae). Planta Med. 2010, 76, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Tomizawa, Y.; Tsuchiya, T.; Hirasawa, Y.; Hashimoto, T.; Asakawa, Y. Antimitotic activity of two macrocyclic bis(bibenzyls), isoplagiochins A and B from the Liverwort Plagiochila fruticosa. Bioorg. Med. Chem. Lett. 2009, 19, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, S.D.; Perry, N.B.; Tangney, R.S. An antifungal bibenzyl from the New Zealand liverwort, Plagiochila stephensoniana. Bioactivity-directed isolation, synthesis, and analysis. J. Nat. Prod. 1993, 56, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, S.D.; Perry, N.B. Antifungal hydroxy-acetophenones from the New Zealand liverwort, Plagiochila fasciculata. Planta Med. 1994, 60, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Kamiya, N.; Popich, S.; Asakawa, Y.; Bardón, A. Insecticidal constituents from the argentine liverwort Plagiochila bursata. Chem. Biodivers. 2010, 7, 1855–1861. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Jin, X.Y.; Zhou, J.C.; Zhang, J.Z.; Chang, W.Q.; Li, Y.; Chen, W.; Ren, Z.J.; Zhang, C.Y.; Yuan, S.Z.; et al. Terpenoids from the Liverwort Plagiochila fruticosa and Their Antivirulence Activity against Candida albicans. J. Nat. Prod. 2020, 83, 1766–1777. [Google Scholar] [CrossRef]
- Han, J.J.; Zhang, J.Z.; Zhu, R.X.; Li, Y.; Qiao, Y.N.; Gao, Y.; Jin, X.Y.; Chen, W.; Zhou, J.C.; Lou, H.X. Plagiochianins A and B, Two ent-2,3-seco-Aromadendrane Derivatives from the Liverwort Plagiochila duthiana. Org. Lett. 2018, 20, 6550–6553. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Takemoto, T. Plagiochilide et plagiochilin a, secoaromadendrane-type sesquiterpenes de la mousse, plagiochila yokogurensis (plagiochilaceae). Tetrahedron Lett. 1978, 19, 1553–1556. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Takemoto, T. La plagiochilin a et la plagiochilin b, les sesquiterpenes du type secoaromadendrane de la mousse, Plagiochila hattoriana. Phytochemistry 1978, 17, 1794. [Google Scholar] [CrossRef]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Biotransformation of aristolane- and 2,3-secoaromadendrane-type sesquiterpenoids having a 1,1-dimethylcyclopropane ring by Chlorella fusca var. vacuolata, mucor species, and Aspergillus niger. Chem. Pharm. Bull. 2006, 54, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Naz, T.; Packer, J.; Yin, P.; Brophy, J.J.; Wohlmuth, H.; Renshaw, D.E.; Smith, J.; Elders, Y.C.; Vemulpad, S.R.; Jamie, J.F. Bioactivity and chemical characterisation of Lophostemon suaveolens—An endemic Australian Aboriginal traditional medicinal plant. Nat. Prod. Res. 2016, 30, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Paguet, A.S.; Siah, A.; Lefèvre, G.; Moureu, S.; Cadalen, T.; Samaillie, J.; Michels, F.; Deracinois, B.; Flahaut, C.; Alves Dos Santos, H.; et al. Multivariate analysis of chemical and genetic diversity of wild Humulus lupulus L. (hop) collected in situ in northern France. Phytochemistry 2023, 205, 113508. [Google Scholar] [CrossRef] [PubMed]
- de Melo, N.I.; de Carvalho, C.E.; Fracarolli, L.; Cunha, W.R.; Veneziani, R.C.; Martins, C.H.; Crotti, A.E. Antimicrobial activity of the essential oil of Tetradenia riparia (Hochst.) Codd. (Lamiaceae) against cariogenic bacteria. Braz. J. Microbiol. 2015, 46, 519–525. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Toyota, M.; Takemoto, T.; Suire, C. Plagiochilins C, D, E and F, four novel secoaromadendrane-type sesquiterpene hemiacetals from Plagiochila asplenioides and Plagiochila semidecurrens. Phytochemistry 1979, 18, 1355–1357. [Google Scholar] [CrossRef]
- Asakawa, Y.; Inoue, H.; Toyota, M.; Takemoto, T. Sesquiterpenoids of fourteen Plagiochila species. Phytochemistry 1980, 19, 623–2626. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Toyota, M.; Asakawa, Y. Ent-kaurene diterpene from the liverwort Plagiochila pulcherrima. Phytochemistry 1988, 27, 1425–1427. [Google Scholar] [CrossRef]
- Matsuo, A.; Atsumi, K.; Nakayama, M. Structures of ent-2,3-Secoalloaromadendrane Sesquiterpenoids, which have plant growth inhibitory Activity, from Plagiochila semidecurrens (Liverwort). J. Chem. Soc. Perkin Trans. 1981, 1, 2816–2824. [Google Scholar] [CrossRef]
- Ramírez, M.; Kamiya, N.; Popich, S.; Asakawa, Y.; Bardón, A. Constituents of the Argentine Liverwort Plagiochila diversifolia and Their Insecticidal Activities. Chem. Biodivers. 2017, 14, e1700229. [Google Scholar] [CrossRef]
- Lin, S.J.; Wu, C.L. Isoplagiochilide from the liverwort Plagiochila elegans. Phytochemistry 1996, 41, 1439–1440. [Google Scholar]
- Toyota, M.; Nakamura, I.; Huneck, S.; Asakawa, Y. Sesquiterpene esters from the liverwort Plagiochila porelloides. Phytochemistry 1994, 37, 1091–1093. [Google Scholar] [CrossRef]
- Asakawa, Y. Chemosystematics of the hepaticae. Phytochemistry 2004, 65, 623–669. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Toyota, M.; Takemoto, T.; Kubo, I.; Nakanishi, K. Insect antifeedant secoaromadendrane-type sesquiterpenes from Plagiochila species. Phytochemistry 1980, 19, 2147–2154. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tanaka, H.; Asakawa, Y. Stereostructure of plagiochilin A and conversion of plagiochilin A and stearoylvelutinal into hot-tasting compounds by human saliva. Chem. Pharm. Bull. 1994, 42, 1542–1544. [Google Scholar] [CrossRef]
- Asakawa, Y.; Toyota, M.; Takemoto, T. Three ent-secoaromadendrane-type sesquiterpene hemiacetals and a bicyclogermacrene from Plagiochila ovalifolia and Plagiochila yokogurensis. Phytochemistry 1980, 19, 2141–2145. [Google Scholar] [CrossRef]
- Toyota, M.; Tanimura, K.; Asakawa, Y. Cytotoxic 2,3-secoaromadendrane-type sesquiterpenoids from the liverwort Plagiochila ovalifolia. Planta Med. 1998, 64, 462–464. [Google Scholar] [CrossRef]
- Nagashima, F.; Tanaka, H.; Toyota, M.; Hashimoto, T. Sesqui- and diterpenoids from Plagiochila species. Phytochemistry 1994, 36, 1425–1430. [Google Scholar] [CrossRef]
- Nagashima, F.; Tanaka, H.; Toyota, M.; Hashimoto, T.; Okamoto, Y.; Tori, M.; Asakawa, Y. 2,3-Secoaromadendrane-, Aromadendrane and Maaliane-Type Sesquiterpenoids from Liverwort. J. Essent. Oil Res. 1995, 7, 343–345. [Google Scholar] [CrossRef]
- Birladeanu, L. The stories of santonin and santonic acid. Angew. Chem. Int. Ed. Engl. 2003, 42, 1202–1208. [Google Scholar] [CrossRef]
- Blay, G.; Cardona, L.; García, B.; Lahoz, L.; Pedro, J.R. Synthesis of plagiochilin N from santonin. J. Org. Chem. 2001, 66, 7700–7705. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Asakawa, Y. Neurotrophic secoaromadendrane-type sesquiterpenes from the liverwort Plagiochila fruticosa. Phytochemistry 1991, 30, 4061–4065. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakamura, I.; Tori, M.; Takaoka, S.; Asakawa, Y. Epi-neoverrucosane- and ent-clerodane-type diterpenoids and ent-2,3-secoaromadendrane- and calamenene-type sesquiterpenoids from the liverwort heteroscyphus planus. Phytochemistry 1995, 38, 119–127. [Google Scholar] [CrossRef]
- Heinrichs, J.; Anton, H.; Holz, I.; Grolle, R. The andine Plagiochila tabinensis Steph. and the identity of Acrobolbus laceratus R.M. Schust. (Hepaticae). Nova Hedwig. 2001, 73, 445–452. [Google Scholar] [CrossRef]
- Valcic, S.; Zapp, J.; Becker, H. Plagiochilins and other sesquiterpenoids from Plagiochila (Hepaticae). Phytochemistry 1997, 44, 89–99. [Google Scholar] [CrossRef]
- Kraut, L.; Mues, R. The First Biflavone Found in Liverworts and Other Phenolics and Terpenoids from Chandonanthus hirtellus ssp. giganteus and Plagiochila asplenioides. Z. Naturforsch. 1999, 54, 6–10. [Google Scholar] [CrossRef]
- Rycroft, D.S.; Cole, W.J.; Lamont, Y.M. Plagiochilins T and U, 2,3-secoaromadendranes from the liverwort Plagiochila carringtonii from Scotland. Phytochemistry 1999, 51, 663–667. [Google Scholar] [CrossRef]
- Rycroft, D.S. Plagiochila carringtonii: Carrington’s Featherwort. In Mosses and Liverworts of Britain and Ireland: A Field Guide; Atherton, I., Bosanquet, S.D.S., Lawley, M., Eds.; British Bryological Society: Middlewich, UK, 2010; p. 197. ISBN 9780956131010. [Google Scholar]
- Rycroft, D.S.; Cole, W.J. Atlanticol, an epoxybicyclogermacrenol from the liverwort Plagiochila atlanticaF. Rose. Phytochemistry 1998, 49, 1641–1644. [Google Scholar] [CrossRef]
- Rodrigues e Rocha, M.; da Cunha, C.P.; Filho, R.B.; Vieira, I.J. A Novel Alkaloid Isolated from Spiranthera atlantica (Rutaceae). Nat. Prod. Commun. 2016, 11, 393–395. [Google Scholar]
- Söderström, L.; Rycroft, D.S.; Cole, W.J.; Wei, S. Plagiochila porelloides (Plagiochilaceae, Hepaticae) from Changbai Moutain, new to China, with chemical characterization and chromosome measurements. Bryothera 1999, 5, 195–201. [Google Scholar]
- Silva-E-Costa, J.D.C.; Luizi-Ponzo, A.P.; McLetchie, D.N. Sex Differences in Desiccation Tolerance Varies by Colony in the Mesic Liverwort Plagiochila porelloides. Plants 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Adio, A.M.; König, W.A. Sesquiterpene constituents from the essential oil of the liverwort Plagiochila asplenioides. Phytochemistry 2005, 66, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Phytochemistry of Bryophytes. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Springer: Boston, MA, USA, 1999; Volume 33. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Asakawa, Y. Bryophytes as a source of bioactive volatile terpenoids—A review. Food Chem. Toxicol. 2019, 132, 110649. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.S.; Lin, Z.M.; Li, R.J.; Wang, X.N.; Zhou, J.C.; Lou, H.X. Terpenoids from the Chinese liverwort Plagiochila pulcherrima and their cytotoxic effects. J. Asian Nat. Prod. Res. 2013, 15, 473–481. [Google Scholar] [CrossRef]
- Stivers, N.S.; Islam, A.; Reyes-Reyes, E.M.; Casson, L.K.; Aponte, J.C.; Vaisberg, A.J.; Hammond, G.B.; Bates, P.J. Plagiochilin A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules 2018, 23, 1418. [Google Scholar] [CrossRef]
- Andrade, V.; Echard, A. Mechanics and regulation of cytokinetic abscission. Front. Cell Dev. Biol. 2022, 10, 1046617. [Google Scholar] [CrossRef]
- Sechi, S.; Piergentili, R.; Giansanti, M.G. Minor Kinases with Major Roles in Cytokinesis Regulation. Cells 2022, 11, 3639. [Google Scholar] [CrossRef]
- Wenzel, D.M.; Mackay, D.R.; Skalicky, J.J.; Paine, E.L.; Miller, M.S.; Ullman, K.S.; Sundquist, W.I. Comprehensive analysis of the human ESCRT-III-MIT domain interactome reveals new cofactors for cytokinetic abscission. Elife 2022, 11, e77779. [Google Scholar] [CrossRef]
- Chircop, M.; Perera, S.; Mariana, A.; Lau, H.; Ma, M.P.; Gilbert, J.; Jones, N.C.; Gordon, C.P.; Young, K.A.; Morokoff, A.; et al. Inhibition of dynamin by dynole 34-2 induces cell death following cytokinesis failure in cancer cells. Mol. Cancer Ther. 2011, 10, 1553–1562. [Google Scholar] [CrossRef]
- Tremblay, C.S.; Chiu, S.K.; Saw, J.; McCalmont, H.; Litalien, V.; Boyle, J.; Sonderegger, S.E.; Chau, N.; Evans, K.; Cerruti, L.; et al. Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells. Nat. Commun. 2020, 11, 6211. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.S.; Wong, H.L.; Georg, G.I. Synthesis and Cytotoxicity Evaluation of C4- and C5-Modified Analogues of the α,β-Unsaturated Lactone of Pironetin. ChemMedChem 2017, 12, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Coulup, S.K.; Georg, G.I. Revisiting microtubule targeting agents: α-Tubulin and the pironetin binding site as unexplored targets for cancer therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat. Prod. Rep. 2015, 32, 1236–1248. [Google Scholar] [CrossRef]
- Sabovljević, M.S.; Sabovljević, A.D.; Ikram, N.K.K.; Peramuna, A.; Bae, H.; Simonsen, H.T. Bryophytes—An emerging source for herbal remedies and chemical production. (Special Issue 4: Evolving trends in plant-based drug discovery). Plant Genet. Resour. Charact. Util. 2016, 14, 314–327. [Google Scholar] [CrossRef]
- Marques, R.V.; Sestito, S.E.; Bourgaud, F.; Miguel, S.; Cailotto, F.; Reboul, P.; Jouzeau, J.Y.; Rahuel-Clermont, S.; Boschi-Muller, S.; Simonsen, H.T.; et al. Anti-Inflammatory Activity of Bryophytes Extracts in LPS-Stimulated RAW264.7 Murine Macrophages. Molecules 2022, 27, 1940. [Google Scholar] [CrossRef]
- Vollár, M.; Gyovai, A.; Szűcs, P.; Zupkó, I.; Marschall, M.; Csupor-Löffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, antimicrobial and antioxidant activities of mosses obtained from open habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Manoj, G.S.; Murugan, K. Wound healing potential of aqueous and methnolic extracts of Plagiochila beddomei Steph.—A briophyte. Int. J. Pharm. Pharm. Sci. 2012, 4, 222–227. [Google Scholar]
- Nandy, S.; Dey, A. Bibenzyls and bisbybenzyls of bryophytic origin as promising source of novel therapeutics: Pharmacology, synthesis and structure-activity. Daru 2020, 28, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Métoyer, B.; Benatrehina, A.; Rakotondraibe, L.H.; Thouvenot, L.; Asakawa, Y.; Nour, M.; Raharivelomanana, P. Dimeric and esterified sesquiterpenes from the liverwort Chiastocaulon caledonicum. Phytochemistry 2020, 179, 112495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qian, L.; Qiao, Y.; Jin, X.; Zhou, J.; Yuan, S.; Zhang, J.; Zhang, C.; Lou, H. Cembrane-type diterpenoids from the Chinese liverwort Chandonanthus birmensis. Phytochemistry 2022, 203, 113376. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Nagashima, F.; Ludwiczuk, A. Distribution of Bibenzyls, Prenyl Bibenzyls, Bis-bibenzyls, and Terpenoids in the Liverwort Genus Radula. J. Nat. Prod. 2020, 83, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Novakovic, M.; Bukvicki, D.; Anchang, K.Y. Bis-bibenzyls, Bibenzyls, and Terpenoids in 33 Genera of the Marchantiophyta (Liverworts): Structures, Synthesis, and Bioactivity. J. Nat. Prod. 2022, 85, 729–762. [Google Scholar] [CrossRef]
- Kirisanth, A.; Nafas, M.N.M.; Dissanayake, R.K.; Wijayabandara, J. Antimicrobial and Alpha-Amylase Inhibitory Activities of Organic Extracts of Selected Sri Lankan Bryophytes. Evid. Based Complement. Altern. Med. 2020, 2020, 3479851. [Google Scholar] [CrossRef]
- Asakawa, Y.; Nagashima, F.; Hashimoto, T.; Toyota, M.; Ludwiczuk, A.; Komala, I.; Ito, T.; Yagi, Y. Pungent and bitter, cytotoxic and antiviral terpenoids from some bryophytes and inedible fungi. Nat. Prod. Commun. 2014, 9, 409–417. [Google Scholar] [CrossRef]
- Mierzwa, B.; Gerlich, D.W. Cytokinetic abscission: Molecular mechanisms and temporal control. Dev. Cell 2014, 31, 525–538. [Google Scholar] [CrossRef]
- McNeely, K.C.; Dwyer, N.D. Cytokinetic Abscission Regulation in Neural Stem Cells and Tissue Development. Curr. Stem Cell Rep. 2021, 7, 161–173. [Google Scholar] [CrossRef]
- Halcrow, E.F.J.; Mazza, R.; Diversi, A.; Enright, A.; D’Avino, P.P. Midbody Proteins Display Distinct Dynamics during Cytokinesis. Cells 2022, 11, 3337. [Google Scholar] [CrossRef]
- Chatterjee, N.; Wang, W.L.; Conklin, T.; Chittur, S.; Tenniswood, M. Histone deacetylase inhibitors modulate miRNA and mRNA expression, block metaphase, and induce apoptosis in inflammatory breast cancer cells. Cancer Biol. Ther. 2013, 14, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Jarman, P.J.; Hearnden, V.; Fairbanks, S.D.; Bassetto, M.; Maib, H.; Palmer, J.; Ayscough, K.R.; Thomas, J.A.; Smythe, C. A Ruthenium(II) Polypyridyl Complex Disrupts Actin Cytoskeleton Assembly and Blocks Cytokinesis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117449. [Google Scholar] [CrossRef] [PubMed]
- Petsalaki, E.; Zachos, G. An ATM-Chk2-INCENP pathway activates the abscission checkpoint. J. Cell Biol. 2021, 220, e202008029. [Google Scholar] [CrossRef] [PubMed]
- Sardina, F.; Monteonofrio, L.; Ferrara, M.; Magi, F.; Soddu, S.; Rinaldo, C. HIPK2 Is Required for Midbody Remnant Removal Through Autophagy-Mediated Degradation. Front. Cell Dev. Biol. 2020, 8, 572094. [Google Scholar] [CrossRef] [PubMed]
- Boullé, M.; Davignon, L.; Nabhane Saïd Halidi, K.; Guez, S.; Giraud, E.; Hollenstein, M.; Agou, F. High-Content RNAi Phenotypic Screening Unveils the Involvement of Human Ubiquitin-Related Enzymes in Late Cytokinesis. Cells 2022, 11, 3862. [Google Scholar] [CrossRef] [PubMed]
- Sabovljević, M.S.; Ćosić, M.V.; Jadranin, B.Z.; Pantović, J.P.; Giba, Z.S.; Vujičić, M.M.; Sabovljević, A.D. The Conservation Physiology of Bryophytes. Plants 2022, 11, 1282. [Google Scholar] [CrossRef]
- Basile, D.V.; Lin, J.J.; Varner, J.E. The metabolism of exogenous hydroxyproline by gametophytes of Plagiochila arctica Bryhn et Kaal. (Hepaticae). Planta 1988, 175, 539–545. [Google Scholar] [CrossRef]
- Ugur, A.; Sarac, N.; Duru, M.E. Antimicrobial activity and chemical composition of Senecio sandrasicus on antibiotic resistant staphylococci. Nat. Prod. Commun. 2009, 4, 579–584. [Google Scholar] [CrossRef]
- Silva, E.A.J.; Estevam, E.B.B.; Silva, T.S.; Nicolella, H.D.; Furtado, R.A.; Alves, C.C.F.; Souchie, E.L.; Martins, C.H.G.; Tavares, D.C.; Barbosa, L.C.A.; et al. Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae). Braz. J. Biol. 2019, 79, 697–702. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Aromadendrene oxide 2, induces apoptosis in skin epidermoid cancer cells through ROS mediated mitochondrial pathway. Life Sci. 2018, 197, 19–29. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Pornpakakul, S.; Suwancharoen, S.; Petsom, A.; Roengsumran, S.; Muangsin, N.; Chaichit, N.; Piapukiew, J.; Sihanonth, P.; Allen, J.W. A new sesquiterpenoid metabolite from Psilocybe samuiensis. J. Asian Nat. Prod. Res. 2009, 11, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Özerkan, D.; Erol, A.; Altuner, E.M.; Canlı, K.; Kuruca, D.S. Some Bryophytes Trigger Cytotoxicity of Stem Cell-like Population in 5-Fluorouracil Resistant Colon Cancer Cells. Nutr. Cancer 2022, 74, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chandra, D.; Barh, A.; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of remedies, an ethno-medicinal review. J. Tradit. Complement. Med. 2016, 7, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Guarino, F.; Marchi, L.; Muto, A.; Piro, A.; Degola, F. Bryo-Activities: A Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds against Fungi. Plants 2021, 10, 203. [Google Scholar] [CrossRef]

| Compounds | Bioactivities | Tests/Species | End Points | Ref. |
|---|---|---|---|---|
| Plagiochilin A | Antifeedant | African armyworm Spodoptera exempta | Activity observed at 1–10 ng/cm2 | [35] |
| Plagiochilin A | Antiparasitic | Leishmania amazonensis axenic amastigotes | IC50 = 7.1 µM | [13] |
| Plagiochilin A | Antiparasitic | Trypanosoma cruzi trypomastigotes | MIC = 14.5 µM | [13] |
| Plagiochilin A | Anti- proliferative | P-388 murine leukemia cells | IC50 = 3.0 µg/mL | [38] |
| Plagiochilin A | Anti- proliferative | A172 glioblastoma cells | IC50 = 19.4 µM. | [56] |
| Plagiochilin-A-15-yl n-octanoate | Anti- proliferative | P-388 murine leukemia cells | IC50 = 0.05 µg/mL | [38] |
| Plagiochilin C | Antiplatelet | Inhibition of arachidonate-induced rabbit platelet aggregation | 95% and 45% inhibition at 100 and 50 µg/mL, respectively. | [32] |
| Plagiochilin C | Anti- proliferative | A172 glioblastoma cells | IC50 = 4.3 µM | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila. Life 2023, 13, 758. https://doi.org/10.3390/life13030758
Bailly C. Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila. Life. 2023; 13(3):758. https://doi.org/10.3390/life13030758
Chicago/Turabian StyleBailly, Christian. 2023. "Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila" Life 13, no. 3: 758. https://doi.org/10.3390/life13030758
APA StyleBailly, C. (2023). Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila. Life, 13(3), 758. https://doi.org/10.3390/life13030758








