1. Introduction
The economic production of broiler chickens is critical for sustained meat supply, and feed is a crucial factor that influences the economic and productive performance of broiler chickens [
1,
2]. Antibiotic supplements are used in animal diets to boost their growth, feed efficiency, and disease resistance. Irrational use of antibiotics in animal feed has resulted in an increase in antibiotic-resistant bacteria, thereby endangering human health and influencing the natural growth of livestock production. Since the prohibition of antibiotic use as a feed additive in 2006 by the European Union, there is an urgent need to investigate and broaden the range of antibiotic alternatives [
3]. Several potential immune modulators may act as substitutes for antibiotics used in animal production to promote growth and disease resistance [
4,
5,
6]. Specific feed ingredients in poultry diets improve digestion by creating a favorable gut environment [
2]. Different feed additives can now be used as growth promoters instead of antibiotics [
4,
7].
β-glucans (GLC) are glucan polymers derived from the cell walls of yeast, fungi, and bacteria as well as cereal grains such as barley and oat [
8]. 1,3 β-glucans are chains of glucose molecules linked together by β-glycosidic bonds between carbons #1 and #3. Significant structural variations in GLC from these sources lead to differences in their physiological mechanisms [
9]. Because of their highly branched structure, GLC derived from yeast and fungal sources are the most efficient for boosting immunity against pathogenic organisms [
10,
11]. These GLC can reduce coccidia infection in growing pullets and laying hens [
12]. GLC can boost both innate and adaptive immune responses [
8].
The addition of GLC to feed enhanced gut health and disease resistance in birds challenged with necrotic enteritis by stimulating the gene expression of antimicrobial peptides [
13]. By enhancing the populations of cells secreting IgA and goblet cells, yeast-based GLC can serve as growth inducers and prospective antibiotic alternatives against specific microorganisms in birds [
14]. Moreover, macrophages obtained from chickens fed a diet supplemented with GLC showed upregulated expression of IL-1 [
15], IL-2, interferon (IFN)-γ [
16], IL-4, and IL-18 [
17]. Additionally, yeast GLC boosted humoral and cell-mediated immune responses in broilers [
8,
18]. Other studies have found that yeast GLC are absorbed through the intestinal mucosa and promote the innate immune system, thereby causing a higher production of macrophages, monocytes, and cytokines and a lower susceptibility to diseases [
8,
19]. GLC supplementation increased lysozyme and complement levels, increased CD3 and CD20 expression, altered tight junction protein expression, and improved phagocytic activity [
20,
21].
In the present study, we used algae-based GLC produced from a unique species of microalgae (Euglena gracilis) with more than 50% content of 1,3 β-glucan, unlike yeast-based GLC that contain 5–15% 1,3 β-glucan. The high digestibility of algal cells makes GLC more bioavailable without extraction. Therefore, the aim of the present study was to assess the potential effects of the dietary addition of algae-based GLC as antibiotic alternative growth promoters on some production performance and health aspects of broiler chickens such as growth, carcass traits, economic efficiency, chemical composition of breast muscles, blood biochemical parameters, liver histopathology, antioxidant activity, and proinflammatory responses in broiler chickens.
4. Discussion
GLC have important biological activities and play an important function in disease prevention and animal production performance [
40]. In the present study, birds fed GLC-enriched diets grew at the same rate as the GLC0 group. In contrast, the GLC50 and GLC100 groups showed an increase in their total feed intake. This is supported by the fact that the energy contribution of GLC is used for immunity and other physiological functions, such as antioxidant activity, rather than bird growth. Similarly, Chae et al. [
41] reported that GLC-fed birds consumed more feed than nonsupplemented birds. Zhang et al. [
42] noted no significant effect of 1,000 mg kg
−1 GLC (derived from
Agrobacterium sp.) on birds’ growth. Cox et al. [
8] showed no significant differences in the growth of broilers fed diets supplemented with 200 or 1000 mg glucans kg
−1 diet with or without an
Eimeria challenge. Zhang et al. [
16] demonstrated an increased average daily gain and improved FCR in broiler chickens fed β1,3/1,6-glucan-supplemented diets. Zhang et al. [
43] reported enhanced BWG and FCR of heat-stressed chicks by increasing energy digestibility. Chae et al. [
41] found increased BWG of chickens fed finisher diets containing 200 and 400 mg kg
−1 GLC (derived from
Saccharomyces cerevisiae). In contrast, Kazempour et al. [
44] reported that GLC supplementation (30 g kg
−1) reduced feed intake and daily average gain and improved FCR in broiler chickens during the starter, grower, and finisher periods.
In the current study, dietary GLC supplementation did not influence the carcass traits; dressing percentage; and the weights of the spleen, liver, intestine, and gizzard. These results indicate that GLC supplementation had no negative effects on the internal organs of birds. The bursal weight was decreased at the highest GLC concentration of 150 mg kg
−1; this may be due to increased inflammatory response. Similarly, Tang et al. [
45] reported no effect of GLC on the carcass features of Peking ducks. In the study of Kazempour et al. [
44], GLC at 30 g kg
−1 concentration had no effect on the internal organs, namely the liver, spleen, and pancreas of broiler chickens; however, it decreased the abdominal fat and increased the weight of the small intestine and gizzards.
Many factors affect meat quality, such as diet composition, genetic structure, sex, housing management, slaughter method, and the type of muscle fibers [
46]. The recent trend in poultry consumption demonstrates the significance of meat quality assurance in poultry establishments [
47]. The chemistry of chicken meat is important because it affects storage and processing. Scarce information is available on the effect of 1,3-β-glucans on the fatty acid content of broiler muscles. Hence, it was difficult to compare the present results with previous reports. In the present study, dietary GLC changed the fatty acid profile of breast muscles. In the breast muscles of chickens fed GLC-supplemented diets, the percentages of α-linolenic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid, linoleic acid, arachidonic acid, total n-3, and total n-6 PUFA continued to increase in a concentration-dependent manner. Our results suggested that dietary GLC may affect lipid metabolism. The n-3 and n-6 PUFA play critical roles in human nutrition. They are precursors to various biological compounds, including prostaglandins, eicosanoids, and thromboxanes, which regulate the activity of the immune and cardiovascular systems [
48]. Salah et al. [
49] found increased n-3 PUFA content in the breast muscles of birds fed a synbiotic-supplemented diet. In the current study, however, GLC addition had no effect on breast muscle composition as measured by the content of dry matter, crude protein, fat, and ash. These findings support the growth performance results and demonstrate that the energy contribution of GLC was not used for the bird’s growth or muscle formation. Tang et al. [
45] demonstrated no significant changes in the chemical composition (crude protein, fat, and ash) and fatty acid content in the meat of Peking ducks fed Sophy β-glucans-supplemented diet in comparison with the control and the bacitracin zinc groups.
Regarding the liver and kidney function tests, the current study showed that the GLC150 group had higher serum ALT and uric acid levels, while their levels did not stray from the normal levels [
50]. Moreover, no differences in serum AST and creatinine levels were noted between the groups. These results may be attributed to the mild inflammatory responses caused by the highest level of GLC (150 mg kg
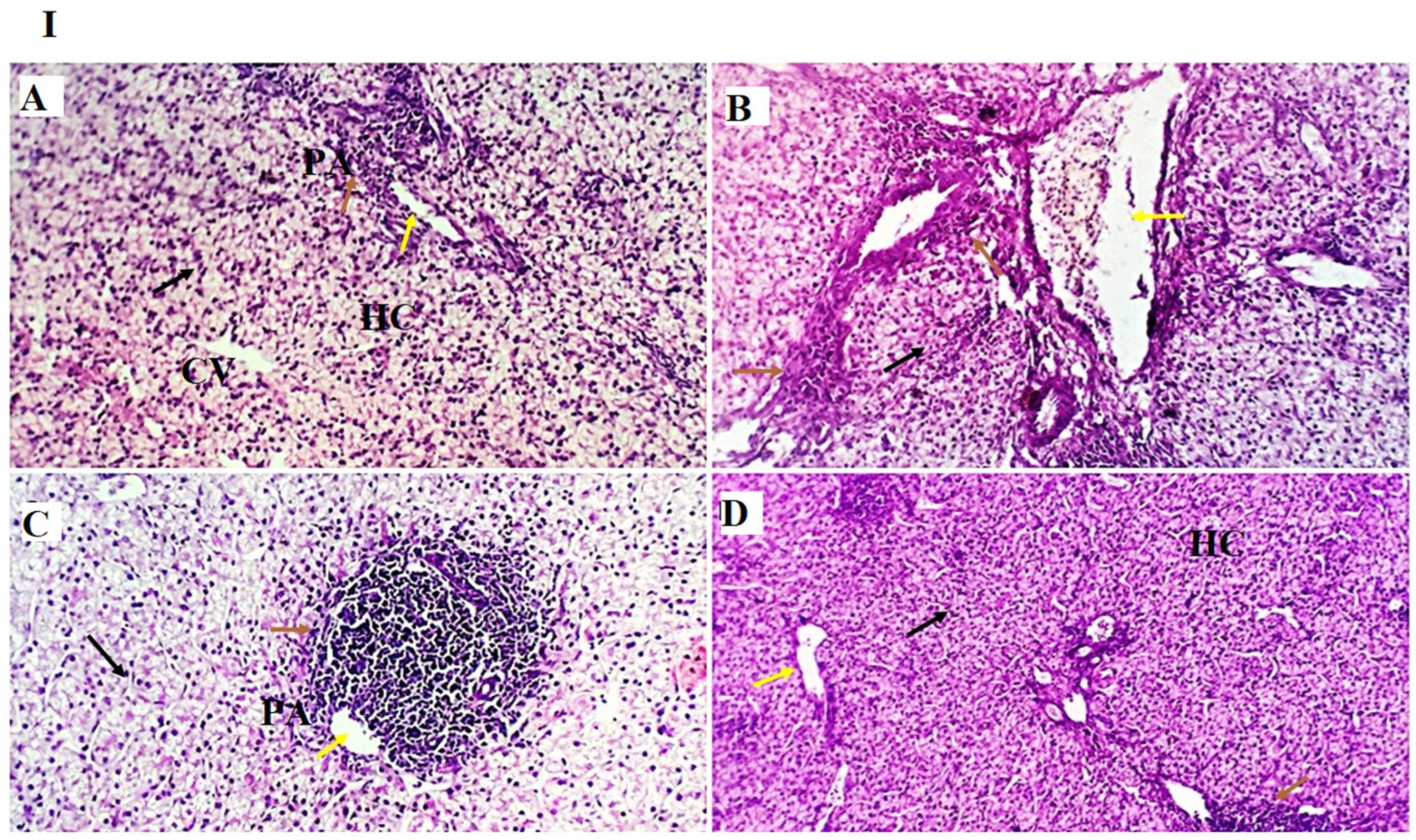
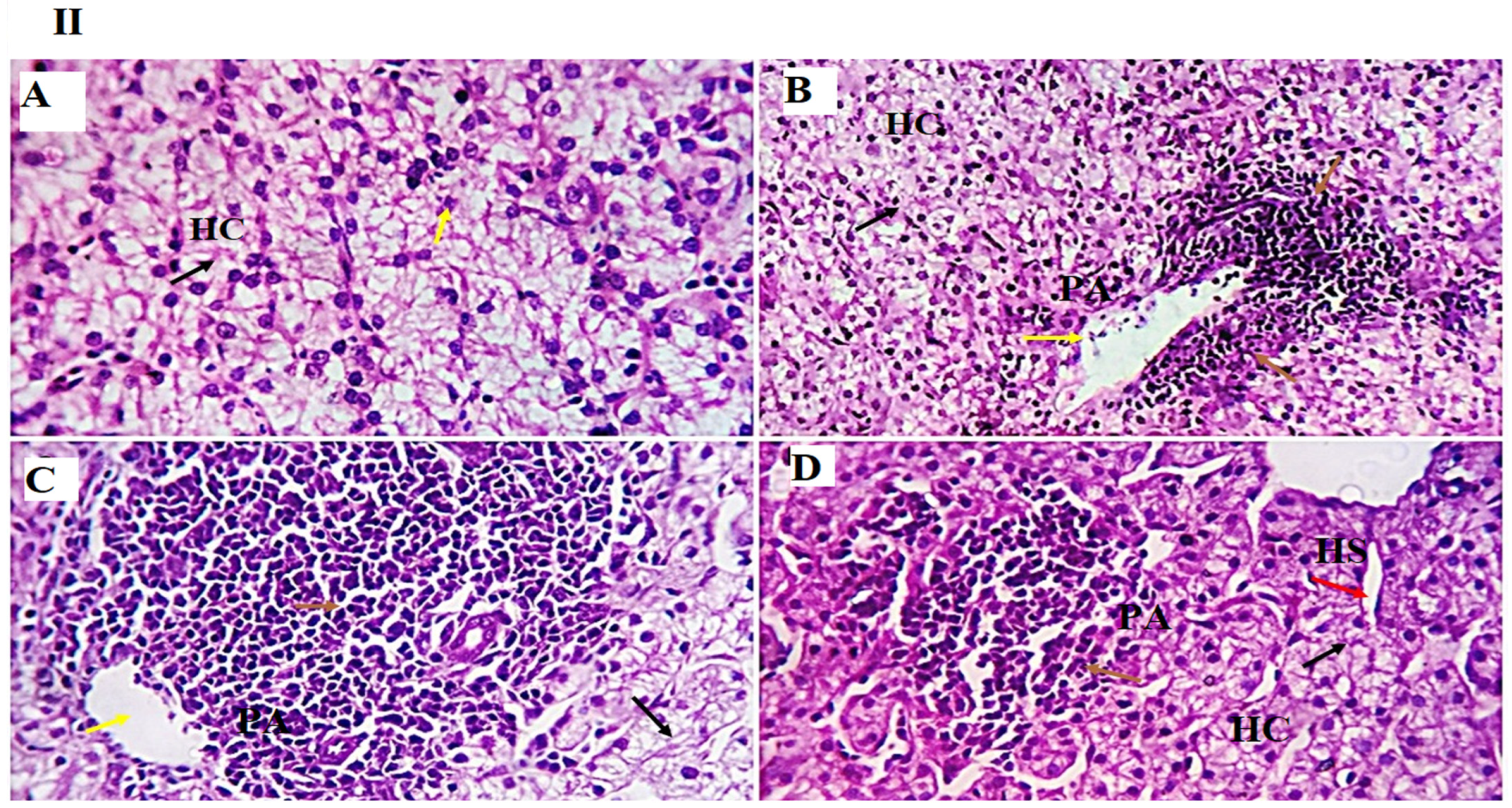
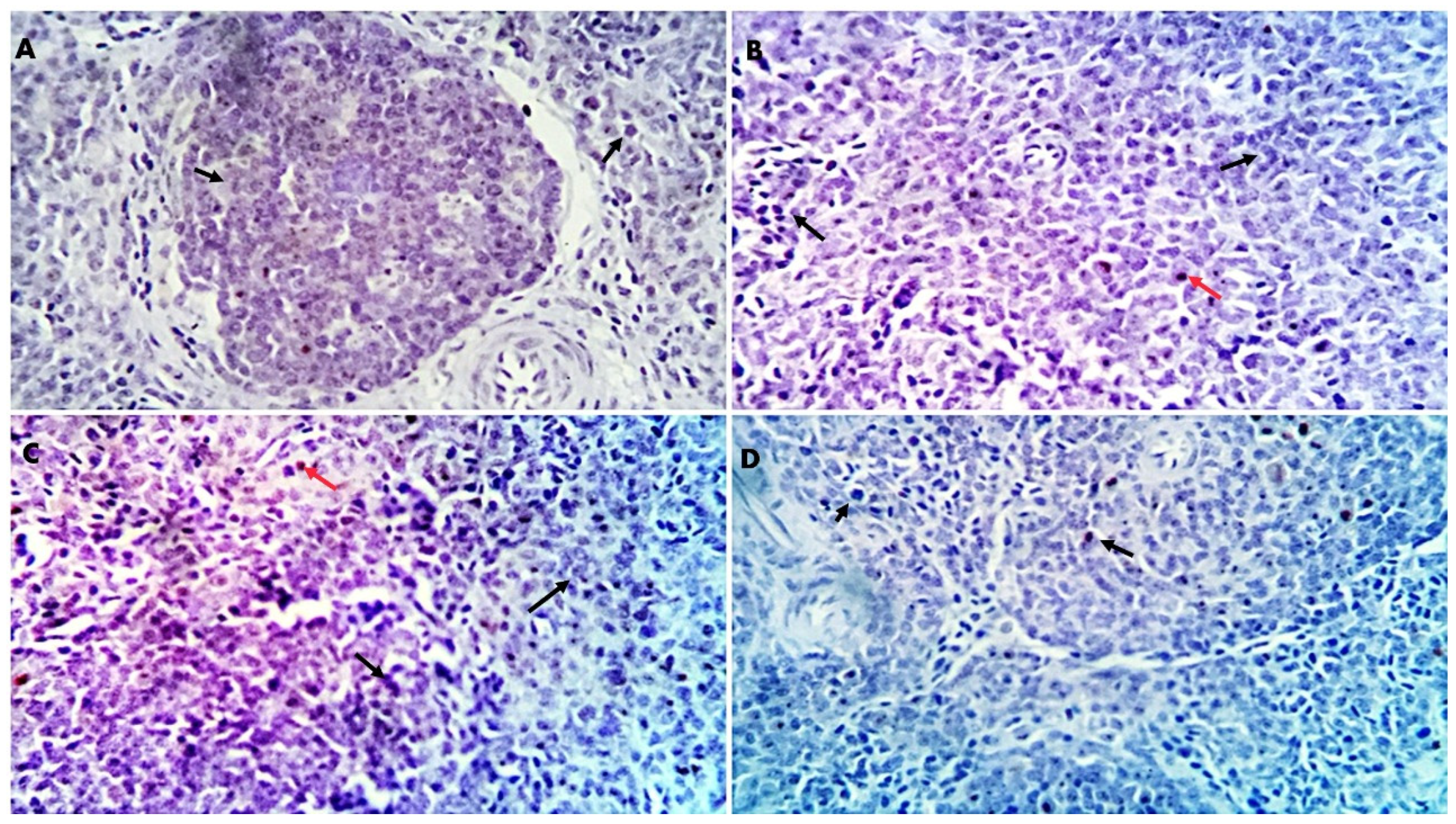
−1), as observed in the results of the liver histopathological examination, where normal liver histoarchitecture was found in all experimental groups with mild portal vascular dilatation and lymphoplasmacytic aggregations in the GLC100 group. A minimal number of inflammatory cells were recorded in the GLC0 and GLC50 groups. The GLC150 group showed moderate peri-portal lymphocytic aggregation.
Regarding the antioxidant status of broiler chickens in response to dietary GLC supplementation, the results showed increased serum levels of TAC, CAT, and SOD. The MDA levels in GLC-fed groups were decreased in a concentration-dependent manner. GLC can boost the antioxidant capacity of animals by enhancing the activity of the antioxidant enzymes CAT, SOD, and glutathione peroxidase, as well as by enhancing the scavenging activity of the superoxide anion and the hydroxyl radical and by preventing lipid peroxidation [
40]. The combination of the hydroxyl groups of GLC and the metal ion inhibits the generation of hydroxyl radicals and prevents lipid peroxidation [
51,
52]. Guo et al. [
53] reported that GLC protects the cells of the porcine jejunum epithelial cell line IPEC-J2 cells from oxidative stress induced by deoxysqualenol by reducing the levels of reactive oxygen species and MDA and by upregulating the glutathione level. Our results proposed that GLC can provide a protective effect against oxidative stress by stimulating the antioxidant system. Liu et al. [
54] discovered that the addition of yeast cell wall components to the broiler chicken diet increased the levels of intestinal reduced glutathione and glutathione reductase.
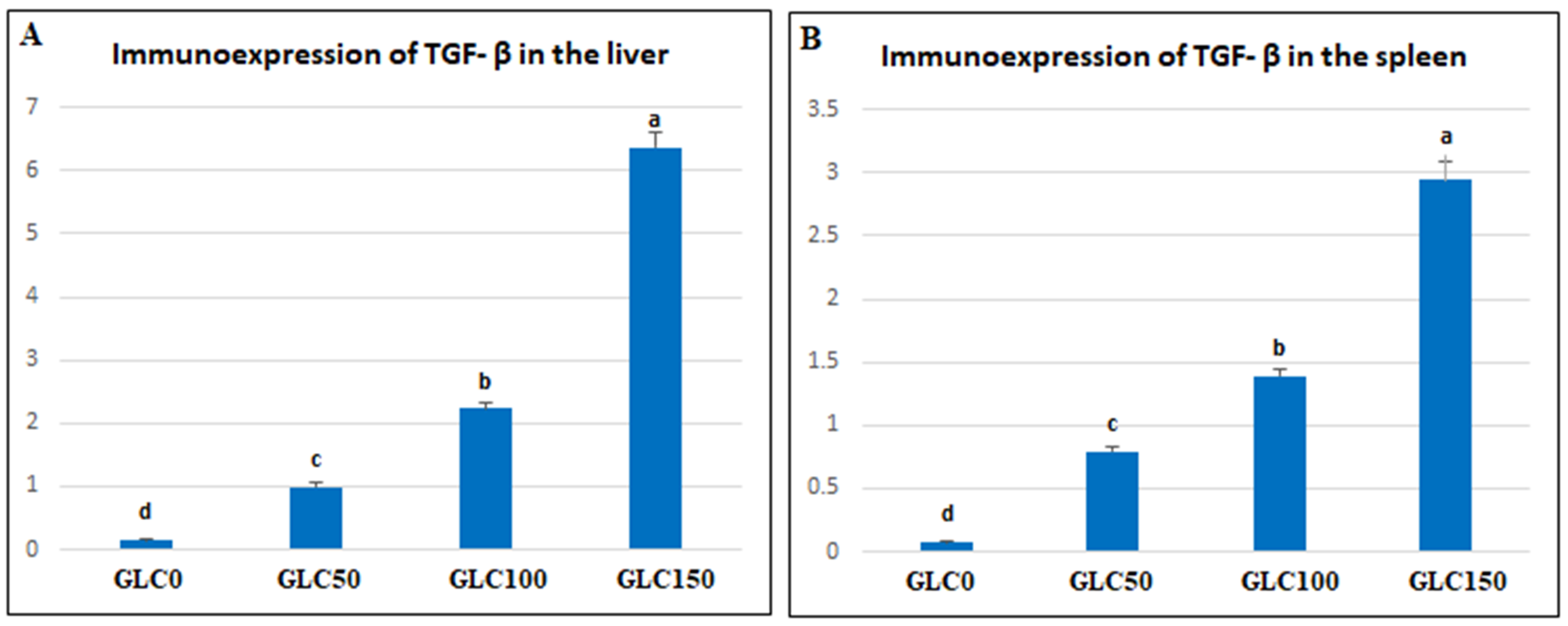
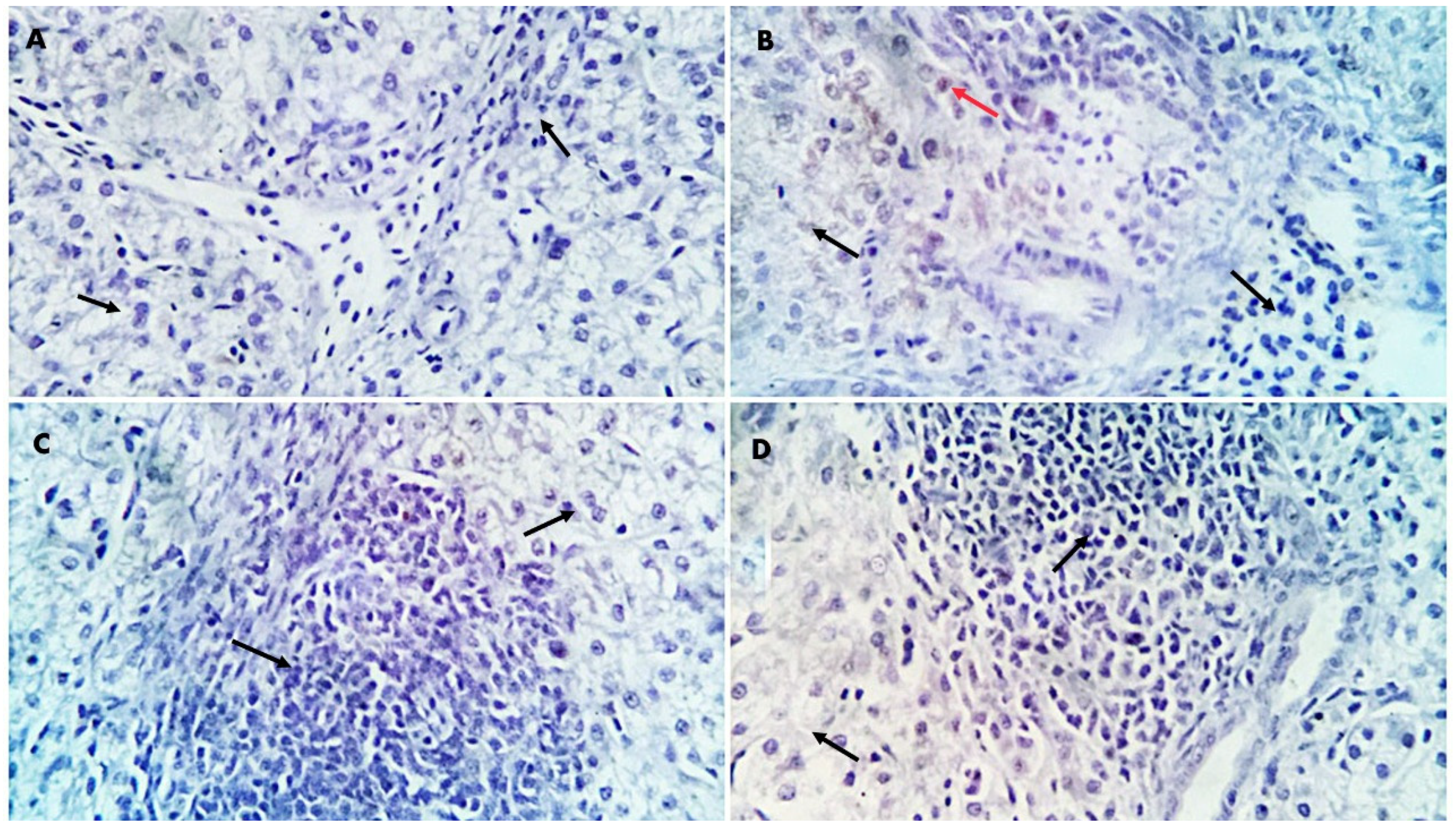
Regarding the inflammatory responses in broiler chickens fed with a GLC-supplemented diet, the results showed increased serum levels of IL-1β and IFN-γ in the GLC-fed groups in a concentration-dependent manner. Moreover, dietary GLC supplementation increased TGF-β immunoexpression in chicken liver and spleen tissues in a concentration-dependent manner. Proinflammatory cytokines (interferon-γ and IL-1β), which are essential immune proteins, are endogenous signaling molecules that facilitate cellular defense against inflammatory response [
55]. Broiler chickens fed diets supplemented with GLC showed improved cell-mediated [
41,
56] and humoral immune responses [
15,
16]. The ability of GLC to enhance the immune response establishes their possible use as an antibiotic alternative by preventing numerous economically critical pathogens, for instance,
Escherichia coli and
Salmonella enterica [
19,
57]. GLC may activate macrophages or monocytes by inducing the major histocompatibility complex (MHC) compound [
16]. Immune potentiation occurs because of this activity, which includes the activation of T-helper and natural killer cells, cytotoxic macrophages, T-cell development and differentiation, and the initiation of the alternative complement pathway [
58]. The other signaling messengers, such as IL-1, IL-2, IFN-γ, and TNF-α, may also be included in the immuno-regulating system. The results of liver histopathology confirmed these findings, wherein moderate-to-high peri-portal lymphocytic aggregation was observed in the GLC100 and GLC150 groups. The accumulation of portal inflammatory cells appears to be a highly resistant and defensive mechanism rather than a destructive inflammatory process.
From an economic point of view, dietary GLC supplementation did not affect the TR, NP, performance index, and EE of the treated groups. This effect was due to the increase in the amount of feed intake by birds in the GLC50 and GLC100 groups with no change in the growth rate of birds. However, birds fed GLC-enriched diets have the advantages of transferring energy to the development of immune response and antioxidant capability, which improves the health and performance of birds. These results have more economic value than weight gain in maintaining adequate health of chicken flocks because clinical and subclinical diseases result in reduced feed intake, weight, and severe morbidity and mortality, which consequently harm animal welfare and cause substantial economic problems to poultry producers [
13,
59].