In Vitro Pharmacological Activity, and Comparison GC-ToF-MS Profiling of Extracts from Cissus cornifolia (Baker) Planch
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection, Authentication, and Extraction
2.2. Antimicrobial Activity
2.2.1. Selected Microorganisms
2.2.2. In Vitro Microdilution Assay
2.3. Anti-Inflammatory Activity
2.3.1. Soybean Lipoxygenase (15-LOX) Assay
2.3.2. Cyclooxygenase Assay
2.4. Anticancer Activity of Extracts against Selected Cell Lines
2.5. GC-ToF-MS Analysis of Extracts from Cissus Cornifolia Bulbs
3. Statistical Analysis
4. Results
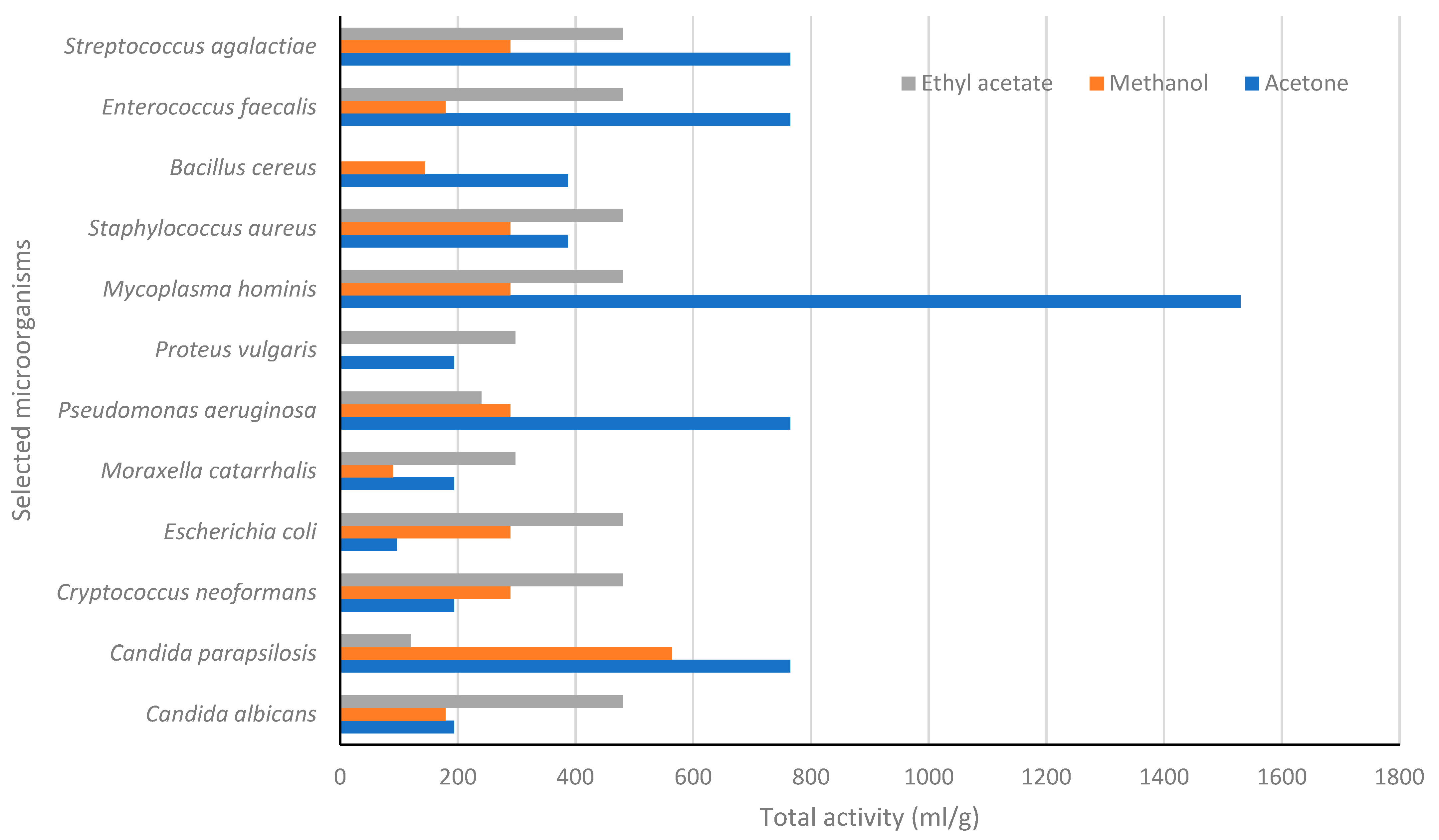
4.1. In Vitro Antimicrobial Activity
4.2. Antiproliferative and Anti-Inflammatory Activity of Extracts from Cissus Cornifolia
4.3. Comparison GC-ToF-MS Analysis of Extracts from Cissus Cornifolia
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raimondo, D.; Von Staden, L.; Foden, W.; Victor, J.E.; Helme, N.A.; Turner, R.C.; Kamundi, D.A.; Manyama, P.A. Red List of South African Plants, Strelitzia 25; South African National Biodiversity Institute: Pretoria, South Africa, 2009. [Google Scholar]
- Perovic, O.; Chetty, V.; Iyaloo, S. Antimicrobial resistance surveillance from sentinel public hospitals, South Africa. Comm. Dis. Surv. Bull. 2014, 13, 1–12. [Google Scholar]
- Padmapriyadarsini, C.; Narendran, G.; Soumya Swaminathan, S. Diagnosis, and treatment of tuberculosis in HIV co-infected patients. Ind. J. Med. Res. 2011, 134, 850–865. [Google Scholar]
- Kichu, M.; Malewska, T.; Akter, K.; Imchen, I.; Harrington, D.; Kohen, J.; Vemulpad, S.R.; Jamie, J.F. An ethnobotanical study of medicinal plants of Chungtia village, Nagaland, India. J. Ethnopharmacol. 2015, 166, 5–17. [Google Scholar] [CrossRef]
- Jayachitra, C.; Jamuna, S.; Ali, M.A.; Paulsamy, S.; Al-Hemaid, F.M.A. Evaluation of traditional medicinal plant, Cissus setosa Roxb. (Vitaceae) for the antiulcer property. Saudi J. Biol. Sci. 2018, 25, 293–297. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mohammed, A.; Isah, M.B.; Aliyu, A.B. Anti-trypanosomal activity of African medicinal plants: A review update. J. Ethnopharmacol. 2014, 154, 26–54. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Makhafola, T.J. Ethnobotanical knowledge of the lay people of Blouberg area (Pedi tribe), Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2018, 14, 46. [Google Scholar] [CrossRef]
- Rahmawati, L.; Aziz, N.; Oh, J.; Hong, Y.H.; Woo, B.Y.; Hong, Y.D.; Manilack, P.; Souladeth, P.; Jung, J.H.; Lee, W.S.; et al. Cissus subtetragona Planch. Ameliorates Inflammatory Responses in LPS-induced Macrophages, HCl/EtOH-induced Gastritis, and LPS-induced Lung Injury via Attenuation of Src and TAK1. Molecules 2021, 26, 6073. [Google Scholar] [CrossRef]
- Kokilavani, R.; Suriyakalaa, U.; Elumalai, P.; Aburami, B.; Ramachandran, R.; Sankarganesh, R.; Achiraman, S. Antioxidant mediated ameliorative steroidogenesis by Commelina benghalensis L. and Cissus quadrangularis L. against quinalphos induced male reproductive toxicity. Pestic. Biochem. Physiol. 2014, 109, 18–33. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, Y.K.; Kumar, Y.; Singh, S.; Arunraja, S. Cissus quadrangularis attenuates adjuvant-induced arthritis by down-regulating pro-inflammatory cytokine and inhibiting angiogenesis. J. Ethnopharmacol. 2015, 175, 346–355. [Google Scholar] [CrossRef]
- Al-Bukhaiti, W.Q.; Noman, A.; Mahdi, A.A.; Ali, A.H.; Abed, S.M.; Rashed, M.M.A.; Wang, H. Profiling of phenolic compounds and antioxidant activities of Cissus rotundifolia (Forssk.) as influenced by untrasonic-assisted extraction conditions. J. Food Meas. Character. 2019, 13, 634–647. [Google Scholar] [CrossRef]
- Dutta, T.; Paul, A.; Majumder, M.; Sultan, R.A.; Emran, T.B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophy Rep. 2020, 21, 100715. [Google Scholar] [CrossRef]
- Coates Palgrave, M. Keith Coates Palgrave Trees of Southern Africa, 3rd ed.; Third Impression; Struik Publishers: Cape Town, South Africa, 2002; p. 680. [Google Scholar]
- Rasingam, L. Ethnobotanical studies on the wild edible plants of Irula tribes of Pillur Valley, Coimbatore District, Tamil Nadu, India. Asian Pac. J. Trop. Biomed. 2012, 2, S1493–S1497. [Google Scholar] [CrossRef]
- Mbakazi, Y.; Kappo, A.P.; Soyingbe, O.S.; Nety, N.S.; Makhafola, T.J.; Chukwuma, C.I.; Dikhoba, M.P.; Mariri, N.G.; Mongalo, N.I. GC/TOF-MS-based phytochemical analysis, in vitro antiproliferative effects, antioxidant, and antibacterial activity of Sarcophyte sanguinea subsp. piriei (Hutch.) B. Hansen. S. Afr. J. Bot. 2022, 150, 752–758. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Mangal, M.; Sagar, P.; Singh, H.; Raghava, G.P.S.; Agarwal, S.M. NPACT: Naturally occurring plant-based anti-cancer compound-activity-target database. Nuc. Acids Res. 2013, 41, D1124–D1129. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant-derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Samarghandian, S.; Boskabady, M.H.; Davoodi, S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharm. Mag. 2010, 6, 309–314. [Google Scholar] [CrossRef]
- Steger-Hartmann, T.; Raschke, M. Translating in vitro to in vivo and animal to human. Curr. Opin. Toxicol. 2020, 23, 6–10. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation, and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Schultz, F.; Osuji, O.F.; Wack, B.; Anywar, G.; Garbe, L.A. Anti-inflammatory medicinal plants from the Ugandan Greater Mpigi region act as potent inhibitors in the COX-2/PGH2 pathway. Plants 2021, 10, 351. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Masoko, P.; Picard, P.; Eloff, J.N. Antifungal activities of six South African Terminalia species (Combretaceae). J. Ethnopharmacol. 2005, 99, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.D.C.; Tejeda, A.; Duque, A.L.; Macias, P. Determination of lipoxygenase activity in plant extracts using a modified ferrous oxidation-xylenol orange assay. J. Agric. Food Chem. 2007, 55, 5956. [Google Scholar] [CrossRef]
- Fadipe, V.O.; Mongalo, N.I.; Opoku, A.R. In vitro evaluation of the comprehensive antimicrobial and antioxidant properties of Curtisia dentata (Burm.F) C.A. SM: Toxicological effect on the human embryonic kidney (HEK 293) and human hepatocellular carcinoma (HepG2) cell lines. EXCLI J. 2015, 14, 971–983. [Google Scholar]
- Cao, H.; Yu, R.; Choi, Y. Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol. Res. 2010, 61, 519–524. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Soyingbe, S.O.; Makhafola, T.J. Antimicrobial, cytotoxicity anticancer and antioxidant activities of Jatropha zeyheri Sond. roots (Euphorbiaceae). Asian Pac. J. Trop. Biomed. 2019, 9, 307–314. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Systematic review in South Africa reveals antibiotic resistance genes shared between clinical and environmental settings. Infect. Drug Resist. 2018, 11, 1907–1920. [Google Scholar] [CrossRef]
- Chetty, S.; Reddy, M.; Ramsamy, Y.; Naidoo, A.; Essack, S. Antimicrobial stewardship in South Africa: A scoping review of the published literature. JAC-Ant. Res. 2019, 1, dlz060. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Masur, H. Recurring and emerging questions related to management of HIV-related opportunistic infections. Top Antivir Med. 2018, 26, 79–84. [Google Scholar] [PubMed]
- Tumbarello, M.; Ventura, G.; Caldarola, G.; Morace, G.; Cauda, R.; Ortona, L. An emerging opportunistic infection in HIV patients: A retrospective analysis of 11 cases of pulmonary aspergillosis. Eur. J. Epidemiol. 1993, 9, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Chandra, H.; Nautiyal, A.R.; Kalra, S.J. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDAms) as an alternative drug line to control infections. 3 Biotech. 2014, 4, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) C.A.Sm-medicinal plants used in South Africa. BMC Complement Altern Med. 2018, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Komape, N.P.M.; Bagla, V.P.; Kabongo-Kayoka, P.; Masoko, P. Antimycobacterial potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province, South Africa. BMC Complement. Alt. Med. 2017, 17, 128. [Google Scholar]
- Musa, A.M.; Tajuddeen, N.; Idris, A.Y.; Rafindadi, A.Y.; Abdullahi, M.I.; Aliyu, A.B.; Abdullahi, M.S.; Ibrahim, M.A. A New antimicrobial prenylated benzo-lactone from the rhizome of Cissus cornifolia. Pharmacogn. Res. 2014, 7, 363–366. [Google Scholar]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Pharmacological properties of extracts from six South African medicinal plants used to treat sexually transmitted infections (STIs) and related infections. S. Afr. J. Bot. 2017, 112, 290–295. [Google Scholar] [CrossRef]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 4, 606–625. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef]
- Bitew, A.; Abebaw, Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Women’s Health 2018, 18, 94. [Google Scholar] [CrossRef]
- Nurain, A.M.; Bilal, N.E.; Ibrahim, M.E. The frequency and antimicrobial resistance patterns of nosocomial pathogens recovered from cancer patients and hospital environments. Asian Pac. J. Trop Biomed. 2015, 5, 1055–1059. [Google Scholar] [CrossRef]
- Che, Y.M.; Mao, S.H.; Jiao, W.L.; Fu, Z.Y. Susceptibilities of Mycoplasma hominis to herbs. Am. J. Chin. Med. 2005, 33, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Friis, H.; Plesner, A.; Scheibel, J.; Justesen, T.; Lind, K. Mycoplasma hominis septicaemia. Br. Med. J. Clin. Res. Ed. 1983, 286, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Jaweed, A.; Jyoti, R.; Neha, D.; Neena, K.; Benu, D. Mycoplasma hominis: An under-recognized pathogen. Ind. J. Med. Microbiol. 2021, 39, 88–97. [Google Scholar]
- Matyanga, C.M.J.; Morse, G.D.; Gundidza, M. African potato (Hypoxis hemerocallidea): A systematic review of its chemistry, pharmacology and ethnomedicinal properties. BMC Complement Med. Ther. 2020, 20, 182. [Google Scholar] [CrossRef]
- Mwinga, J.L.; Asong, J.A.; Amoo, S.O.; Nkadimeng, S.M.; McGaw, L.J.; Aremu, A.O.; Otang-Mbeng, W. In vitro antimicrobial effects of Hypoxis hemerocallidea against six pathogens with dermatological relevance and its phytochemical characterization and cytotoxicity evaluation. J. Ethnopharmacol. 2019, 242, 112048. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Mbeng, W.O. Are plants used in the Eastern Cape province for cosmetics fully commercialized? Indian J. Pharmacol. 2019, 51, 140–149. [Google Scholar] [CrossRef]
- Kaczorowski, Ł.; Powierska-Czarny, J.; Wolko, Ł.; Piotrowska-Cyplik, A.; Cyplik, P.; Czarny, J. the influence of bacteria causing subclinical mastitis on the structure of the cow’s milk microbiome. Molecules 2022, 27, 1829. [Google Scholar] [CrossRef]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural agents against bovine mastitis pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments-A review. Asian-Australas J. Anim Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and methicillin resistance pattern of potential mastitis-inducing Staphylococcus aureus and coagulase-negative Staphylococci isolates from the mammary secretion of dairy goats. Biology 2022, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Pangprasit, N.; Srithanasuwan, A.; Suriyasathaporn, W.; Pikulkaew, S.; Bernard, J.K.; Chaisri, W. Antibacterial activities of acetic acid against major and minor pathogens isolated from mastitis in dairy cows. Pathogens 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Haxhiaj, K.; Wishart, D.S.; Ametaj, B.N. Mastitis: What it is, current diagnostics, and the potential of metabolomics to identify new predictive biomarkers. Dairy 2022, 3, 722–746. [Google Scholar] [CrossRef]
- Mdee, L.K.; Masoko, P.; Eloff, J.N. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S. Afr. J. Bot. 2009, 75, 375–379. [Google Scholar] [CrossRef]
- Eloff, J.N. On expressing the antibacterial activity of plant extractsa small first step in applying scientific knowledge to rural primary health care. S. Afr. J. Sci. 2000, 96, 116–118. [Google Scholar]
- Eloff, J.N. Quantification the bioactivity of plant extracts during screening and bioassay-guided fractionation. Phytomed 2004, 11, 370–371. [Google Scholar] [CrossRef]
- Meshram, M.A.; Bhise, U.O.; Makhal, P.N.; Kaki, V.R. Synthetically tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. Eur. J. Med. Chem. 2021, 225, 113804. [Google Scholar] [CrossRef]
- Bisi-Johnson, M.A.; Obi, C.L.; Hattori, T.; Oshima, Y.; Li, S.; Kambizi, L.; Vasaikar, S.D. Evaluation of the antibacterial and anticancer activities of some South African medicinal plants. BMC Compl. Alt. Med. 2011, 11, 14. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement Altern Med. 2017, 17, 60. [Google Scholar] [CrossRef]
- Minorics, R.; Bózsity, N.; Wölfling, J.; Mernyák, E.; Schneider, G.; Márki, A.; Falkay, G.; Ocsovszki, I.; Zupkó, I. Antiproliferative effect of normal and 13-epi-D-homoestrone and their 3-methyl ethers on human reproductive cancer cell lines. J. Steroid Biochem. Mol. Biol. 2012, 132, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro experiments to in vivo and clinical studies; Pros and Cons. Curr Drug Discov Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Vijayarathna, S.; Sasidharan, S. Cytotoxicity of methanol extracts of Elaeis guineensison against MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Vu, T.Y.; Chandi, V. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep 2020, 10, 15965. [Google Scholar] [CrossRef]
- Orafaie, A.; Matin, M.M.; Sadeghian, H. The importance of 15-lipoxygenase inhibitors in cancer treatment. Cancer Metastasis Rev. 2018, 37, 397–408. [Google Scholar] [CrossRef]
- Eleftheriadis, N.; Thee, S.; Biesebeek, J.; van der Wouden, P.; Baas, B.J.; Dekker, F.J. Identification of 6-benzyloxysalicylates as a novel class of inhibitors of 15-lipoxygenase-1. Eur. J. Med. Chem. 2015, 13, 265–275. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Deepak, H.B.; Thiyagarajan, P.; Kathiresan, S.; Sangli, G.K.; Deepak, M.; Agarwal, A. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine 2011, 18, 278–284. [Google Scholar] [CrossRef]
- Patil, A.; Jadhav, V. GC-MS analysis of bioactive components from methanol leaf extract of Toddalia asiatica (L.). Int. J. Pharm. Sci. Rev. Res. 2014, 29, 18–20. [Google Scholar]
- Abushaheen, M.A.; Fatani, M.A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; Vellappally, S.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Disease-a-Month 2020, 66, 100971. [Google Scholar] [CrossRef]
- Parente, L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: Is two better than one? J. Rheumatol. 2001, 28, 2375–2382. [Google Scholar]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of their structure-activity relationships. Iran J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Aklilu, A.; Seid, M.; Merdikios, B.; Tsegaye, B. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: Efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Cho, K.; Bae, D.R.; Joo, N.E.; Kim, H.H.; Mabasa, L.; Fowler, A.W. Methyl-donor nutrients inhibit breast cancer cell growth. In Vitro Cell Dev. Biol. Anim. 2008, 44, 268–272. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mahomoodally, M.F.; Picot-Allain, C.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Ak, G.; Polat, R.; Urusan, Z.; et al. A comparative study on biological properties and chemical profiles of different solvent extracts from Centaurea bingoelensis, an endemic plant of Turkey. Process Biochem. 2021, 102, 315–324. [Google Scholar] [CrossRef]
- Ayipo, Y.O.; Osunniran, W.A.; Babamale, H.F.; Ayinde, M.O.; Mordi, M.N. Metalloenzyme mimicry and modulation strategies to conquer antimicrobial resistance: Metal-ligand coordination perspectives. Coord. Chem. Rev. 2022, 453, 214317. [Google Scholar] [CrossRef]
- Haq, I.; Kalamdhad, A.S.; Pandey, A. Genotoxicity evaluation of paper industry wastewater prior and post-treatment with laccase producing Pseudomonas putida MTCC 7525. J. Cleaner Prod. 2022, 342, 130981. [Google Scholar] [CrossRef]
- Leng, T.C.; Ping, N.S.; Lim, B.P.; Keng, C.L. Detection of bioactive compounds from Spilanthes acmella (L.) plants and its various in vitro culture products. J. Med. Plants Res. 2011, 5, 371–378. [Google Scholar]
- Tayade, A.B.; Dhar, P.; Sharma, M.; Chauhan, R.S.; Chaurasia, O.P.; Srivastava, R.B. Antioxidant capacities, phenolic contents, and GC/MS analysis of Rhodiola imbricata Edgew. Root extracts from Trans-Himalaya. J. Food Sci. 2013, 78, C402–C410. [Google Scholar] [CrossRef]
- Boğa, M.; Ertaş, A.; Yılmaz, M.A.; Kızıl, M.; Çeken, B.; Haşimi, N.; Deveci, Ö. UHPLC-ESI-MS/MS and GC-MS analyses on phenolic, fatty acid and essential oil of Verbascum pinetorum with antioxidant, anticholinesterase, antimicrobial and DNA damage protection effects. IJPR 2016, 15, 393. [Google Scholar]
| Microorganisms | Extracts and Control Drugs | |||||
|---|---|---|---|---|---|---|
| Acetone | Methanol | Dichloromethane | Ethyl Acetate | Hexane | Control Drugs | |
| Yeasts | Amphotericin B | |||||
| Candida albicans | 0.78 | 0.63 | 0.16 | 0.39 | 0.31 | 0.001 |
| Candida parapsilosis | 0.20 | 0.20 | ≥12.50 | 1.56 | 1.56 | 0.004 |
| Cryptococcus neoformans | 0.78 | 0.39 | 1.25 | 0.39 | 0.39 | 0.001 |
| Gram-negative bacteria | Neomycin | |||||
| Escherichia coli | 1.56 | 0.39 | 0.39 | 0.39 | ≥12.50 | 0.001 |
| Moraxella catarrhalis | 0.78 | 1.25 | 1.25 | 0.63 | ≥12.50 | 0.013 |
| Proteus vulgaris | 0.78 | ≥12.50 | ≥12.50 | 0.63 | ≥12.50 | 0.013 |
| Pseudomonas aeruginosa | 0.20 | 0.39 | ≥12.50 | 0.78 | ≥12.50 | 0.008 |
| Mycoplasma hominis | 0.10 | 0.39 | ≥12.50 | 0.39 | ≥12.50 | 0.008 |
| Gram-positive bacteria | ||||||
| Staphylococcus aureus | 0.39 | 0.39 | 0.39 | 0.39 | ≥12.50 | 0.003 |
| Bacillus cereus | 0.39 | 0.78 | ≥12.50 | ≥12.50 | ≥12.50 | 0.004 |
| Enterococcus faecalis | 0.20 | 0.63 | 0.63 | 0.31 | 0.625 | 0.008 |
| Streptococcus agalactiae | 0.20 | 0.39 | ≥12.50 | 0.39 | 1.56 | 0.004 |
| Extracts | HeLa | MCF7-21 | HepG2 | A547 |
|---|---|---|---|---|
| Hexane | 119.67 ± 0.09 b | 314.81 ± 0.13 c | 138.43 ± 0.18 d | 104.33 ± 1.11 c |
| Acetone | >1000 | 161.85 ± 0.09 b | >1000 | 421.22 ± 1.89 d |
| Ethyl acetate | 123.83 ± 0.98 bc | 10.82 ± 0.04 a | 113.21 ± 0.98 bc | 88.84 ± 0.11 c |
| Methanol | 103.89 ± 0.11 c | 24.06 ± 0.01 a | 96.45 ± 0.16 d | >1000 |
| Dichloromethane | >1000 | 162.55 ± 0.12 c | >1000 | >1000 |
| Doxorubicin | 1.43 ± 0.04 a | 1.26 ± 0.03 a | 1.11 ± 0.02 a | 2.10 ± 0.01 a |
| Extracts | 15-LOX | COX-1 | COX-2 |
|---|---|---|---|
| Hexane | 188.13 ± 1.22 e | 5.87 ± 0.09 b | >200 |
| Acetone | 125.88 ± 0.01 a | >200 | 120.22 ± 0.01 a |
| Dichloromethane | 44.12 ± 0.16 d | 8.08 ± 0.04 a | 58.77 ± 0.04 a |
| Ethyl acetate | 164.36 ± 0.15 d | >200 | 15.59 ± 0.02 b |
| Methanol | 68.88 ± 0.11 bc | >200 | 15.78 ± 0.01 a |
| Quercetin (µg/mL) | 31.55 ± 0.01 a | - | - |
| Celecoxib (µM) | - | 12.88 ± 0.01 a | 4.33 ± 0.01 a |
| Extracts | RT min:s | Compound Detected | Similarity | Area % |
|---|---|---|---|---|
| Acetone | 09:86,1 | 5,8,11-Heptadecatriynoic acid | 664 | 9.454 |
| 11:39,5 | 2-(2’,4’,4’,6’,6’,8’,8’-Heptamethyltetrasiloxan-2’-yloxy)-2,4,4,6,6,8,8,10,10-nonamethylcyclopentasiloxane | 936 | 15.714 | |
| 13:09,0 | 3-Butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 756 | 12.281 | |
| 15:33,50 | 2-Pyrrolidinone, 1-methyl- | 955 | 6.344 | |
| 16:16,0 | Hexadecane | 961 | 4.496 | |
| 17:17,9 | Octanedioic acid | 594 | 2.207 | |
| 17:36,4 | 2,6-Nonadienoic acid | 627 | 2.227 | |
| 19:15,9 | Spiro[2.4]hept-5-ene,5-trimethylsilylmethyl-1-trimethylsilyl- | 779 | 1.806 | |
| Ethyl acetate | 09:50,5 | 9-Borabicyclo[3.3.2]decan-10-ol, 9-(1-oxopropoxy)-, propanoate | 591 | 6.127 |
| 12:26,7 | 2-(2’,4’,4’,6’,6’,8’,8’-Heptamethyltetrasiloxan-2’-yloxy)-2,4,4,6,6,8,8,10,10-decamethylcyclopentasiloxane | 935 | 39.225 | |
| 12:44,8 | 3-Butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | 757 | 10.275 | |
| 15:01,6 | Butylated Hydroxytoluene | 932 | 6.127 | |
| 16:38,1 | 5,8,11-Heptadecatriynoic acid | 674 | 5.999 | |
| 16:55,5 | 2,6-Nonadienoic acid | 620 | 2.084 | |
| 17:22,1 | Quinoline | 778 | 1.88 | |
| Methanol | 09:39,3 | Spiro[2.4]hept-5-ene, 5-trimethylsilylmethyl-1-trimethylsilyl- | 701 | 1.36 |
| 11:50,5 | 2-(2’,4’,4’,6’,6’,8’,8’-Heptamethyltetrasiloxan-2’-yloxy)-2,4,4,6,6,8,8,10,10-nonamethylcyclopentasiloxane | 806 | 19.478 | |
| 12:44,7 | Trisiloxane, 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]- | 887 | 17.705 | |
| 15:18,5 | Butylated Hydroxytoluene | 934 | 3.769 | |
| 15:39,0 | 1,6-Heptadiene | 654 | 4.442 | |
| 16:55,9 | 1,3-Dioxolane | 883 | 2.224 | |
| 17:13,2 | Octanedioic acid | 551 | 1.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongalo, N.I.; Raletsena, M.V.; Munyai, R. In Vitro Pharmacological Activity, and Comparison GC-ToF-MS Profiling of Extracts from Cissus cornifolia (Baker) Planch. Life 2023, 13, 728. https://doi.org/10.3390/life13030728
Mongalo NI, Raletsena MV, Munyai R. In Vitro Pharmacological Activity, and Comparison GC-ToF-MS Profiling of Extracts from Cissus cornifolia (Baker) Planch. Life. 2023; 13(3):728. https://doi.org/10.3390/life13030728
Chicago/Turabian StyleMongalo, Nkoana I., Maropeng Vellry Raletsena, and Rabelani Munyai. 2023. "In Vitro Pharmacological Activity, and Comparison GC-ToF-MS Profiling of Extracts from Cissus cornifolia (Baker) Planch" Life 13, no. 3: 728. https://doi.org/10.3390/life13030728
APA StyleMongalo, N. I., Raletsena, M. V., & Munyai, R. (2023). In Vitro Pharmacological Activity, and Comparison GC-ToF-MS Profiling of Extracts from Cissus cornifolia (Baker) Planch. Life, 13(3), 728. https://doi.org/10.3390/life13030728








