Abstract
Chronic wounds represent nowadays a major challenge for both clinicians and researchers in the regenerative setting. Obesity represents one of the major comorbidities in patients affected by chronic ulcers and therefore diverse studies aimed at assessing possible links between these two morbid conditions are currently ongoing. In particular, adipose tissue has recently been described as having metabolic and endocrine functions rather than serving as a mere fat storage deposit. In this setting, adipose-derived stem cells, a peculiar subset of mesenchymal stromal/stem cells (MSCs) located in adipose tissue, have been demonstrated to possess regenerative and immunological functions with a key role in regulating both adipocyte function and skin regeneration. The aim of the present review is to give an overview of the most recent findings on wound healing, with a special focus on adipose tissue biology and obesity.
Keywords:
obesity; MSC; wound healing; ulcer; stem cell; adipose tissue; leptin; cytokine; diabetes; skin 1. Introduction
At present, obesity affects 1.7 billion people worldwide [1]. Notably, obesity often leads to a wide range of possible comorbidities, including cardiovascular disorders and diabetes (type 2 diabetes mellitus, T2DM, in particular) [2]. Most of these obesity-related diseases carry an intrinsic risk of developing chronic ulcers and/or causing significant impairment in physiological wound healing. For example, venous insufficiency is the commonest cause of chronic ulcers and is often associated to—and worsened by—obesity. Venous stasis may also determine delayed wound healing because of altered capillary flow due to impaired hydrostatic pressure [3,4,5]. Another frequent cause of chronic ulcers is peripheral artery disease (PAD), which is notably caused by atherosclerosis and therefore is, in turn, associated with obesity and/or T2DM. PAD determines a reduction in oxygen flow and nutrient supply, which are essential for tissue repair, and sometimes leads to the development of arterial ulcers through tissue ischemia. Neuropathy is another relatively common cause of cutaneous ulcers and represent a key driver for the development of chronic wounds in diabetic patients [6].
Not only do obesity and chronic ulcers represent significant health-related issues from a clinical and economic point of view, but they also lead to social and psychological consequences related to body image [7,8].
Obesity is by definition associated with an excess of adipose tissue. Adipose tissue is present in the human body in the form of brown and white adipose tissue (BAT and WAT), which are mainly responsible for thermogenesis and fat storage, respectively. However, brown adipocytes have been shown to possibly appear in WAT in response to specific thermogenic stimuli, therefore suggesting a more dynamic division between BAT and WAT and undermining previously consolidated notions of a static classification of adipose tissue subtypes [9]. Moreover, current research is pointing to adipose tissue as a more complex system, involved in several other physiological and/or pathological processes, including hormone metabolism, inflammation and wound healing [10,11].
Adipocyte dysfunction seems to play a role in obesity and its comorbidities, including chronic ulcers. For example, reduced adiponectin production by adipocytes has been described in obese patients and current evidence suggests a possible correlation with impaired wound healing [12]. At the same time, adipocytes represent a possible source of leukotriene B4, which prevents macrophage M2 polarization, therefore affecting the remodeling phase of wound healing [13]. Adipocyte lipolysis is also essential in wound repair [14], but it is not clear whether significant impairment is present in obese patients. Furthermore, inflammatory mediators and insulin-related signaling proteins, such as leptin and resistin, have been postulated to contribute to delayed wound healing in the setting of obesity [15].
Beyond adipocytes, other cell types are also present in the fibrous septa of the subcutaneous fat (e.g., endothelial cells, fibroblasts, inflammatory cells, etc.). Among these, ADSCs (adipose-derived mesenchymal stromal/stem cells) are of central importance for their role in wound healing. ADSCs are, in fact, adipose-tissue specific mesenchymal stromal/stem cells (MSCs) and are currently widely studied for their regenerative properties [11,16]. ADSCs have recently been demonstrated to be able to replace the dermal compartment and to promote wound re-epithelization [16,17,18,19]. ADSCs also seem to play a key role in the orchestration of the various phases of wound healing [14]. ADSC function is regulated by the tissue microenvironment: obesity, hypoxia and inflammation affect ADSC cellular plasticity and alter their immunophenotypic profile and regenerative functions [20]. In addition, obesity enhances their migratory potential and leads to their accumulation in visceral adipose tissue (VAT) [21].
The aim of the present study is to provide a complete overview of the most recent findings in the setting of wound healing and obesity, with a special focus on adipose tissue biology (see Figure 1).
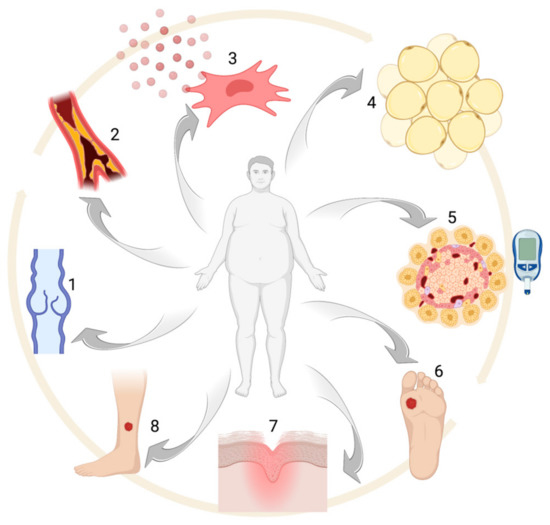
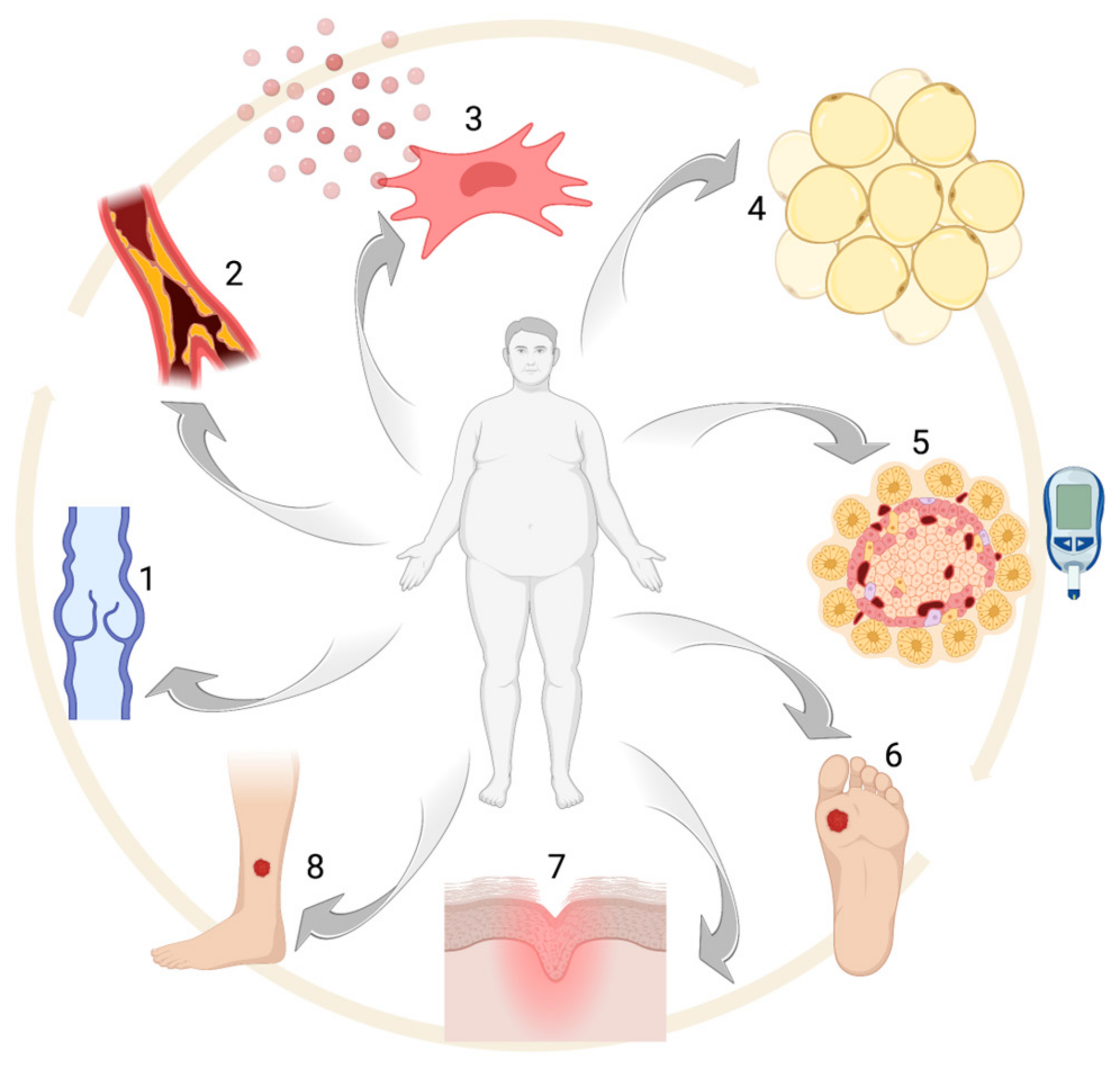
Figure 1.
Obesity-associated alterations: (1) venous insufficiency, (2) atherosclerosis, (3) pro-inflammatory MSC phenotype, (4) adipocyte hypertrophy, (5) glucose intolerance and/or diabetes, (6–8) chronic ulcers. Created with BioRender.com.
2. Materials and Methods
A search was conducted in the PubMed/MedLine databases from their inception to the present. The following search terms were used: obesity, wound healing, MSCs, chronic ulcers, ADSCs. All the major journals were indexed. Articles without the full text electronically available and/or English translation were also excluded. After removal of papers not focusing on the link between MSCs, obesity and wound healing, we considered the articles referenced in the present review.
3. Obesity: Epidemiology and Comorbidities
Obesity is defined by the presence of a body mass index (BMI) ≥ 30 kg/m2, with normal range varying from 18.5 to 24.9 kg/m2. A BMI between 25.0 to 29.9 kg/m2 is considered overweight [22]. Obesity and being overweight are almost invariably caused by excessive caloric intake compared to the necessary amount, which in turns determines fat storage increase and adipocyte hypertrophy [23]. An unhealthy lifestyle is often the leading cause of obesity, but also genetic and epigenetic factors seem to play a very important role [24]. About 30% of the adult population in the world is overweight or obese, with western countries showing the highest prevalence. The Organization for Economic Cooperation and Development (OECD) estimated a prevalence for obesity ranging from 3.7% in Japan up to 38.2% in the US [25]. In the last two decades, the number of obese patients has tripled in Europe, where obesity is estimated to account for 7% of total healthcare costs [26]. However, the prevalence of obesity is nowadays constantly rising, even in developing countries [27,28], and rising childhood obesity rates portend worsening statistics [29].
According to the UK National Audit Office, obesity-related disorders cause significant loss in terms of both working days and deaths, with subsequent direct and indirect costs being estimated at approximately £480,000,000 and £2,150,000,000 per year, respectively [30]. In the U.S, the economic burden for obesity and its comorbidities was estimated to be around $147 billion in 2008 and $126 billion in 2016 [31,32].
Beyond these numbers, a large number of people is currently at risk of becoming obese, including children with familial history of obesity and/or metabolic syndrome, former smokers, lower social classes and older people [33,34,35,36]. As just mentioned, obesity is usually framed in the broader context of metabolic syndrome (or syndrome X), where it is associated with hypertriglyceridemia, atherosclerosis, reduced HDL, hypertension and impaired glucose tolerance [37]. In particular, metabolic syndrome is defined by the presence of three or more of the following criteria: (1) abdominal obesity (waist circumference ≥ 102 cm for men and ≥ 88 cm for women); (2) triglycerides ≥ 150 mg/dL; (3) high-density lipoprotein (HDL) cholesterol < 40 mg/dL for men and < 50 mg/dL for women; (4) systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg and (5) fasting serum glucose ≥ 100 mg/dL [38].
One of the main issues in obese subjects is T2D, which is notably associated with insulin resistance [39,40,41]. Obese subjects also have an increased cardiovascular risk which, like other obesity-related comorbidities, can be explained by lipid accumulation in internal organs. Atherosclerosis, due to lipid accumulation in arterial walls, has got a pivotal role in coronary and cerebrovascular disease [42]. However, obesity also leads to increased platelet activation, which is responsible for thrombosis and subsequent further inflammation, increasing the likelihood of developing ischemic complications [40]. Triglyceride accumulation in the liver causes non-alcoholic fatty liver disease [43]. Moreover, obesity is a well-established risk factor for cholelithiasis due to cholesterol gallstones [44].
Obesity is also associated with a large variety of other possible comorbid conditions, including endocrine, oncological neurological, dermatological, respiratory and psychological disorders. Alterations in the hypothalamic–pituitary–gonadal (HPG) axis are often present in obese subjects [44]. In particular, polycystic ovary syndrome (PCOS) is strictly connected with metabolic syndrome, and weight loss is often part of the therapeutic regimen [45]. Obesity also carries a higher risk of developing several types of malignancies, such as colorectal, gastric, liver and gallbladder, endometrial and esophageal cancer [46].
As for the neurological complications, obese patients are more likely to develop small fiber sensory neuropathy (SFSN) [47] and recent studies suggest a higher risk of developing a form of cortical atrophy similar to Alzheimer’s disease (AD) [48].
Ulcers, lymphedema, intertrigo, hidradenitis suppurativa, striae distensae, skin tags, acanthosis nigricans, psoriasis, acne, hirsutism and androgenetic alopecia are the main skin condition connected to obesity and metabolic syndrome [49].
Biomechanical stress caused by a high body mass is responsible for numerous comorbidities of the musculoskeletal, respiratory, gastrointestinal and skin systems [44].
From a respiratory point of view, obesity finally increases the risk of obstructive sleep apnea syndrome (OSAS), chronic obstructive pulmonary disease (COPD) and asthma [50]. Even if not universally accepted, obesity is also associated with psychiatric/psychological conditions, including anxiety and depression [51].
Most of the aforementioned comorbidities are associated with the low-grade inflammation which nearly invariably comes with obesity. In fact, adipocytes can produce proinflammatory cytokines, such as IL-6, therefore maintaining the pro-inflammatory state typical of obese subjects, as also confirmed by elevated levels of c-reactive protein (CRP) in these patients [52,53]. This chronic inflammatory state is associated with hemodynamic and cardiac changes due to excessive body weight and contributes to the increased likelihood of having heart failure for obese subjects [54].
4. Wound Healing
A wound is defined as a disruption in the normal continuity of the skin. When the skin is injured, a series of events takes place in order to close and heal the area where the barrier is compromised [55]. Wound healing is an evolutionary-conserved process comprised of four sequential yet overlapping phases: hemostasis, inflammation, proliferation and remodeling (see Figure 2) [56]. These phases are strictly regulated through specific molecules that are expressed at different levels at each time interval [57].
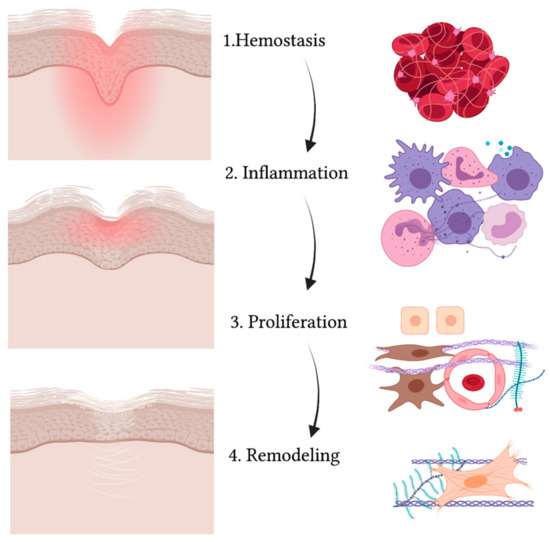
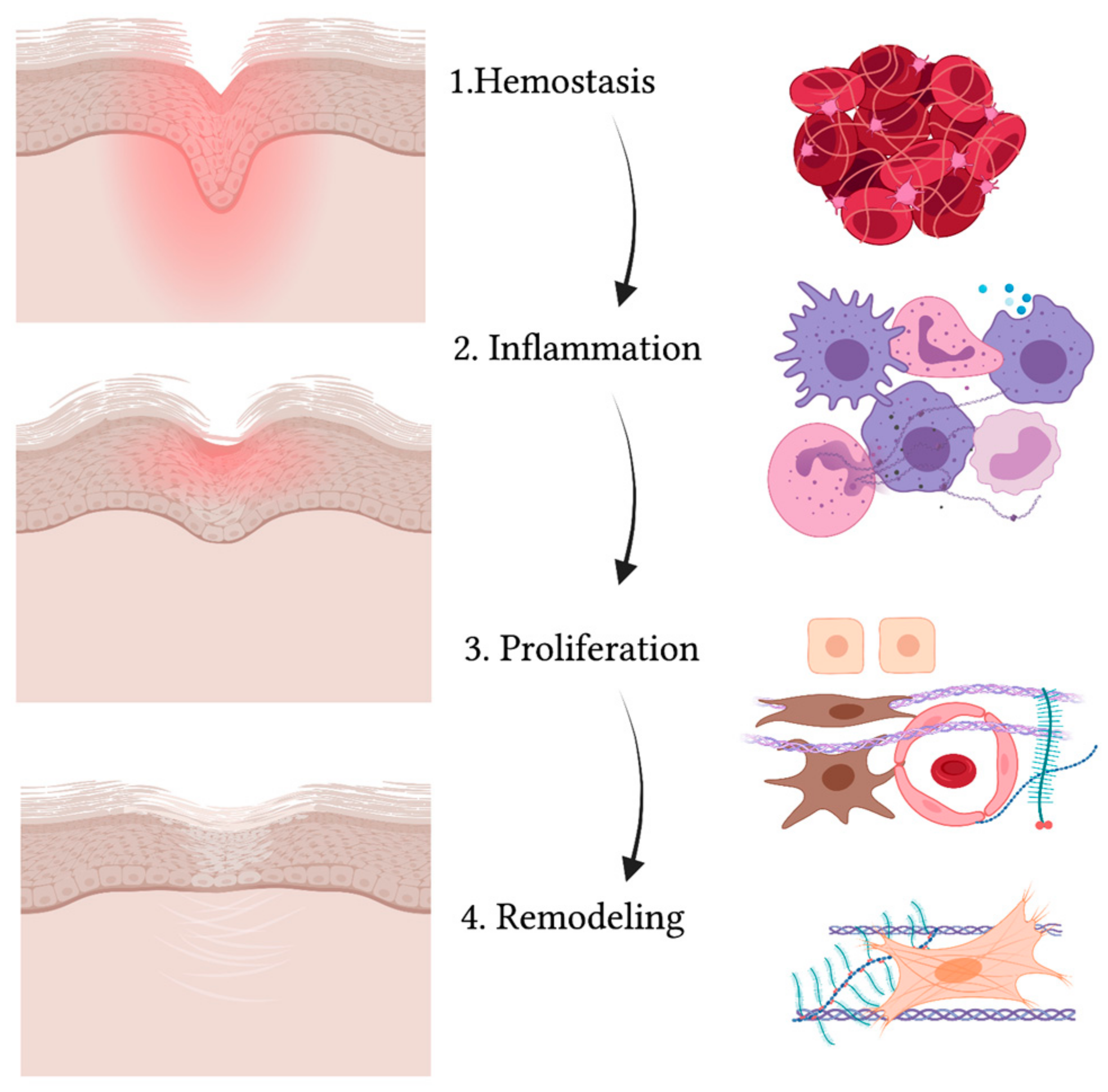
Figure 2.
Schematic representation of the wound healing process. While the wound heals and the injured area (in red) reduces, gradually leading to a scar, the four phases take place (from upper to lower panel): hemostasis, inflammation, proliferation and remodeling. The main cell types are indicated on the right and include: red blood cells and platelets (1), leucocytes and professional phagocytes (2), keratinocytes (3), endothelial cells (3), MSCs (3) and fibroblasts (3,4). Created with BioRender.com.
The first process that takes place in the unwinding of wound healing consists of coagulation and hemostasis, aimed at stopping the bleeding while creating a temporary matrix for cells to infiltrate the site of injury [58]. In fact, not only does vascular smooth muscle contraction reduce the diameter of injured vessels as a mechanism of reflex, but also activation of the coagulation cascade allows platelets to form a clot with fibronectin, fibrin, vitronectin and thrombospondin [59]. Platelets also release several growth factors and cytokines when degranulating [60]. Growth factors and cytokines such as PDGF (platelet-derived growth factor), TGFβ (transforming growth factor β) and EGF (epidermal growth factor) activate neutrophils, macrophages, endothelial cells, fibroblasts and keratinocytes [61]. Then comes the inflammatory phase, where neutrophils, monocytes and macrophages flood to the site of injury [62]. Neutrophils help in removing cell debris and microorganisms that may have slipped into the wound via phagocytosis [63]. Macrophages also enter the site not only as professional phagocytes but also as regulatory cells secreting TGFα and TGFβ, HB-EGF (heparin-binding epidermal growth factor), FGFs (fibroblast growth factor) and collagenases [64,65]. Partially overlapping with the inflammatory phase, the proliferation stage is characterized by the activation, expansion and migration of fibroblasts, keratinocytes and endothelial cells [66]. Proliferation also involves the production of collagen, proteoglycans, hyaluronic acid and other ECM structural proteins that, along with fibroblast recruitment, give rise to granulation tissue [67]. Moreover, endothelial cells aid in forming new vessels and thus epithelization takes place [68]. Cytokines and growth factors activate keratinocytes so that they can migrate from the edges of the wound over the dermal matrix in order to close up the wound [69]. The expression of specific keratins, such as K6 and K16, is observed in migrating keratinocytes [70]. Lastly, collagenases, matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) are secreted by fibroblasts in the remodeling phase [65]. At this stage, fibroblasts differentiate towards a myofibroblast phenotype [71], and type III collagen is replaced by type I [72]. The remodeling phase is essential in order to restore skin elasticity as much as possible [66]. However, skin can only reach up to 80% of its original strength in a normal wound healing process and unavoidably heals with scarring, which represents the main difference between adult tissue repair and embryonic tissue regeneration [73,74].
Wounds can be either acute or chronic [75]. Acute wounds, such as surgical wounds, burns and skin tears, generally progress through the main phases of tissue repair and tend to heal quite easily within 6 weeks [76]. On the contrary, a prolonged inflammatory phase often accompanies chronic wounds due to a reduced nutrient supply, local hypoxic conditions and excess of exudates [62]. The presence of chronic ulcers and wounds can be associated with malnutrition, vitamin C deficiency, dehydration, autoimmune disorders, diabetes, arterial obstruction and/or venous congestion [77]. The vast majority of chronic wounds can be classified into a few major categories: venous and arterial ulcers, diabetic ulcers, pressure ulcers and post-surgical ulcers [78,79]. All these cases are associated by the need for the specific treatment of underlying causes and proper wound care in order to prevent wounds from remaining unhealed [80]. Healing may also be impaired and become exaggerated in keloid and hypertrophic scar formation, where excessive type III collagen formation in the proliferative phase causes an overgrowth of scar tissue [81]. To date, a lot of studies have been focusing on wound healing biology [82], and cell-based strategies are currently under investigation as possible therapies for skin regeneration [83,84].
5. Chronic Ulcers
During the last two decades, scientific interest for the wound healing setting has been constantly rising. Possible explanations for this reside in an increasing incidence of chronic ulcers, a larger population of elderly people and intensive medical care for chronic disorders [85]. Both older people and those who live with chronic diseases are at increased risk of developing chronic wounds [86]. Reduced mobility, age, diabetes, malnutrition and obesity are some of the clinical factors that can determine a delay in the wound healing process [87]. Chronic wounds are very debilitating morbid conditions, not only for the clinical and social consequences for patients but also for the related healthcare costs: nearly 2% of the US population is nowadays affected by chronic ulcers, accounting for a total cost of 20–25 billion USD per year [88,89]. It is estimated that the cost of wound care may account for 2–3% of the total healthcare budget in European countries. Moreover, from 27 to 50% of acute hospital beds are likely to be occupied on any day by patients with a wound [90]. Moreover, the psychological burden of living with chronic ulcers often implies reduced self-esteem, altered body image and, as a consequence, a reduced quality of life [91,92]. As already stated, chronic wounds can be due to a wide variety of possible causes, the most common being immobilization (pressure ulcers), arterial or venous insufficiency (arterial or venous ulcers), neuropathy (diabetic ulcers), ulcerated skin and/or soft-tissue neoplasms, with sometimes more than one cause being implicated in skin barrier disruption [93].
6. Obesity and Wound Healing
Wound healing seems to be impaired in obese patients compared to subjects with a normal BMI (body mass index), despite the mechanism underlying such a difference not being totally understood [94]. A multidisciplinary team is thus often essential for optimal wound care management in obese patients [89]. Decreased vascularization can partially explain wound healing delay [95]. In fact, the increase in the size of the adipocytes typical of obesity is generally not accompanied by an adequate rise in the number of vessels [96]. This eventually leads to a fibrotic environment [97], characterized by reduced elastin and increased collagen V and VI levels [98].
Wound healing disorders seem to have a huge impact in obese patients, both form a clinical and a social point of view. Venous insufficiency, caused or worsened by an elevated intra-abdominal pressure due to fat accumulation in the abdominal area [99], can cause leakage of proteinaceous-like material in the interstitial space, which can eventually occlude smaller vessels [100]. The resulting reduction in oxygen tension not only affects the proliferative and remodeling phases but also increases the risk of wound infection through the impairment of leukocyte phagocytic properties [12,101]. Arterial ulcers are associated to PAD, with atherosclerosis [89] being a well-known comorbidity in obese patients affected by metabolic syndrome [102] and/or T2D. Pressure ulcers are demonstrated to be common among obese patients staying in nursing homes [103]. However, contrasting data regarding the effects of obesity on the risk of development of pressure ulcers have been published so far in other patient subpopulations [104,105].
Peripheral neuropathy is another factor that can cause, aggravate or delay wound healing, and often represent the leading cause of cutaneous ulcers in diabetic patients [106,107]. Microangiopathy often represents the principal cause of impaired wound healing in T2D patients, leading to reduced nerve vascularization, endothelial dysfunction and impaired microcirculation [108]. On the other hand, macroangiopathy causes the production of prothrombotic factors and prevents the formation of an efficient network of collateral vessels, thus contributing to the pathogenesis of chronic wounds [109]. Finally, Advanced Glycation End-products (AGEs) appear to play a role in delaying wound healing in diabetic patients, affecting both angiogenesis and [110] extracellular matrix (ECM) production and remodeling [111]. Recent studies have demonstrated insulin to promote cell migration, and insulin-based therapies have therefore been suggested to surmount the impact of insulin resistance on wound repairing [111,112]. In addition, leptin, an anti-obesity hormone, improves wound repairing by accelerating angiogenesis and promoting the proliferation, differentiation and migration of keratinocytes [113]. Leptin is physiologically produced by adipocytes and leads to a reduction in the caloric intake, regulating body weight. Nevertheless, a high-fat diet leads to a leptin-resistant condition over time, thus limiting the effects of this hormone [114]. In obese patients, high leptin plasma levels are associated with peripheral receptor resistance [115].
7. MSCs
MSCs are defined as a subset of multipotent cells present in tissues of mesenchymal origin responsible for their regeneration [116,117]. MSCs were initially identified as spindle-shaped cells in the bone marrow (bone marrow stromal stem cells, BMSCs) [118,119,120]. Afterwards, other MSC reservoirs have been identified, including skeletal muscle, dental tissue, tendons, dermis, subcutaneous fat, liver, lungs, placenta, synovium, umbilical-cord, amniotic-fluid and breast milk [116,121,122,123,124,125,126]. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy identified three minimal defining criteria for MSCs: adherence to plastic, multipotency (ability to differentiate into chondrocytes, osteoblasts and adipocytes in vitro) and specific surface markers, such as CD105, CD73 and CD90 [127]. The absence of hematopoietic markers (e.g., CD34, CD45, CD14, CD19) is also crucial for identifying MSCs [127]. More recently, STRO-1, CD106 and CD146 have also been identified as MSC-defining markers [128,129]. Various authors already identified MSCs and their secretomes as key therapeutic tools in regenerative medicine, with possible applications in different medical settings, including—among others—neurology, orthopedics, dentistry and dermatology [130,131,132,133]. However, MSCs are currently studied not only for their regenerative properties, but also for their immunomodulating action [134]. Several mechanisms and molecules have been hypothesized so far for MSC-mediated immunoregulatory activity, involving both cell-to-cell contact and the secretion of soluble mediators [135,136]. For this reason, MSCs are currently under investigation for their potential therapeutic role in a wide range of autoimmune and/or autoinflammatory diseases [137,138,139]. Interestingly, aging seems to affect MSC function and to reduce their immunosuppressive activity [140]. Finally, a growing body of evidence also points at a possible pathogenetic role for MSCs in cancer, due to their anti-apoptotic, pro-angiogenic and immunosuppressive properties [141].
8. MSCs and Wound Healing
As already discussed, effective therapies for chronic wounds are urgently needed, not only to improve patient quality of life but to also reduce the burden of healthcare-related costs [142]. Despite advances in the context of wound care and the increasing employment of tissue-engineered skin substitutes in this setting, the results achieved with conventional techniques are still not completely satisfactory, and cell-based strategies are currently under investigation [143]. Adipose tissue and wound healing are closely related and a key role in this interplay is certainly played by ADSCs, fat-tissue resident MSCs. ADSCs are characterized by multipotency, plastic adherence and by the presence of specific surface molecules (CD73, CD90, CD105, CD106, CD146, STRO-1) in the absence of other lineage-specific markers [127,129,144]. ADSCs are particularly interesting for their relative abundance and the ease of the isolation procedure compared to other MSC subtypes [19,145,146]. Regenerative medicine and immune-mediated disorders certainly represent the principal applications for ADSC-based strategies [16,147,148,149,150,151,152]. As well as for other MSC sources, ADSC therapeutic functions are mostly mediated by the production of extracellular vesicles and the secretion of specific soluble mediators [153,154]. Several studies already demonstrated ADSC efficacy in the context of wound healing [155,156], therefore giving rise to a dedicated field of research.
Our group previously described the ability of ADSCs to secrete the ECM components and to produce an ADSC-based scaffolding material for the treatment of skin wounds [19,157]. Moreover, we demonstrated ADSC’s ability to promote re-epithelization in organotypic models through a direct action on basal stem keratinocytes [16]. Several authors also postulated that ADSCs themselves could differentiate into keratinocytes [158,159]. However, whether this is likely to happen in real-life remains to be determined, since basal and hair-follicle stem cells are considered to be physiologically more prone to contributing to re-epithelization [160] in the wound healing process. As well as for their immunomodulatory action, the regenerative and pro-epithelizing properties of MSCs seem to be mainly mediated by the secretion of soluble factors [161]. In order to prevent the potential oncogenic and immunogenic risks of direct cell transplantation [141], an increasing [67,130,162] trend in using ADSC-derived extracellular vesicles has been observed in the last few years, with exosomes being the most studied ones [163,164].
However, the use of ADSCs for the treatment of obesity-related disorders could not be limited to chronic wounds. In fact, ADSC-derived exosomes have been shown to inhibit both adipogenic differentiation and lipogenesis in ADSCs through activation of the Hedgehog (Hh) pathway [165,166]. Despite the promising results achieved with MSCs in the treatment of diabetes and its associated comorbidities, clinical data on the efficacy of autologous ADSCs are still lacking [167,168].
9. MSC Function and Obesity
MSCs’ regenerative properties strictly rely on their multipotency in terms of differentiation potential. MSC differentiation varies upon the environment where they are found and the specific stimuli they receive [122]. In particular, adipose differentiation is of central interest for a deeper understanding of obesity and its pathogenetic mechanisms. Despite MSC biology currently being widely studied, the intermediate stages between MSCs and terminally differentiated adipocytes are not well known. The chronic inflammatory milieu that characterizes obesity possibly affects MSC differentiation potential and, at a more local level, determines an increase in infiltrating macrophages (especially the M1 subtype) together with a significant decrease in Treg lymphocytes in WAT (See Figure 3) [11].
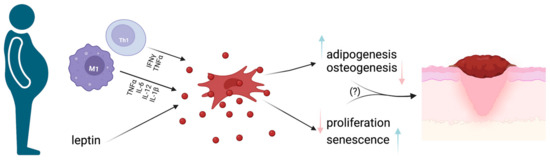
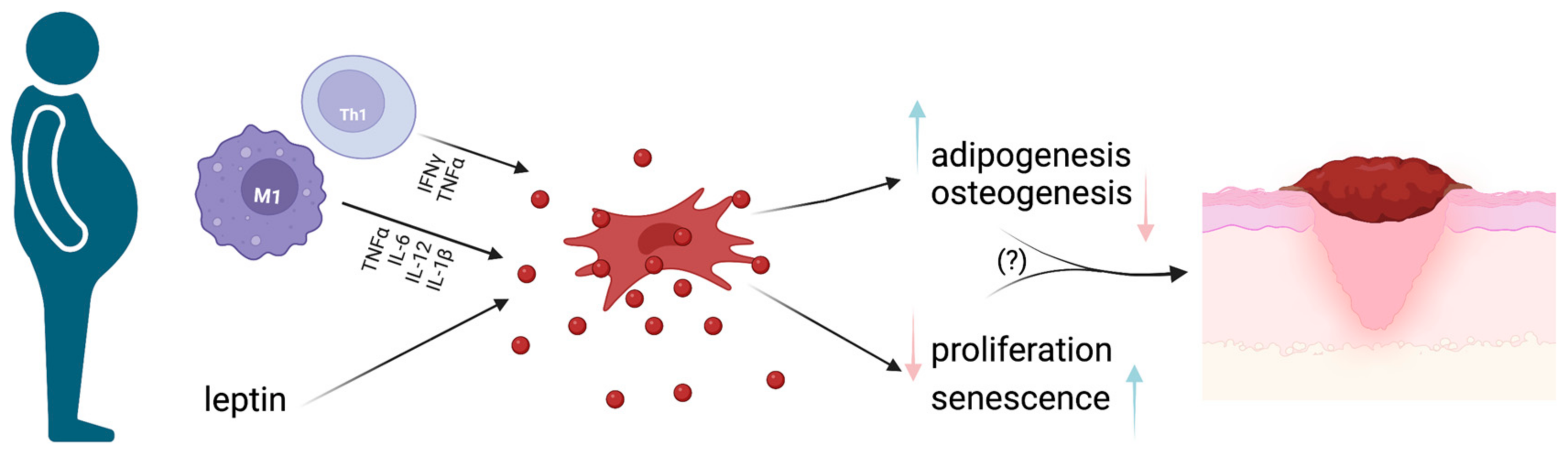
Figure 3.
MSCs and obesity. Schematic representation of how the pro-inflammatory milieu found in obese subjects determines MSC impairment, with subsequent pro-inflammatory cytokine production, cell senescence and reduced proliferative ability. Such changes could be at least partially responsible for impaired wound healing and, subsequently, the occurrence of chronic wounds in obese subjects. Image created with BioRender.com.
ADSC functions seem to be particularly affected by obesity, which has been demonstrated to lead to cell senescence [169]. As highlighted by a very recent review, obese subjects have increased ADSC content, but are characterized by lower proliferative ability, cell senescence and increased production of inflammatory cytokines [170]. A recent publication suggests that the presence of senescent ADSC in visceral WAT secreting pro-inflammatory and pro-aging factors could drive the typical increase in size and number of adipocytes observed in obese subjects [171]. Very recent studies also suggest a possible role for endocrine-disrupting chemicals (EDCs), such as bisphenol A (BPA), in inducing adipogenesis in ADSCs, therefore possibly contributing to the pathogenesis of obesity and its associated comorbidities [172,173]. An interesting study from Alessio [169] et al. clarified the molecular mechanisms implicated in MSC senescence, demonstrating the RB and p53 pathways to be activated in obese mice but not in controls. Moreover, the authors observed reduced proliferation rates in bone marrow and visceral fat MSCs but not in subcutaneous fat ADSCs, therefore suggesting substantial functional differences among the various WAT compartments of the body [169]. Other authors demonstrated obesity to be associated with BMSCs’ increased migratory activities. A Brazilian group demonstrated that [174] inflammatory cytokines—TNF alpha in particular—are able to induce the expression of CXCR4 on MSCs, which gives a plausible explanation of their enhanced migration in animal models of obesity. Moreover, leptin and interleukin-10 (IL-10) were found to be increased in visceral but not in subcutaneous WAT from obese mice, confirming site-dependent variations in the MSC microenvironment [21]. A high-fat diet is also demonstrated to promote adipogenesis and inhibit osteogenesis through leptin receptor signaling in bone marrow MSCs [175]. Consistent with these findings, an exercise-induced switch in MSC differentiation from adipogenesis to osteogenesis has been proposed as a potential biomarker for monitoring the effects of a healthier lifestyle on diabetes [176]. The fat content in umbilical cord-derived MSCs has been demonstrated to be an independent predictor of the offspring metabolic phenotype in terms of adiposity and fasting glucose levels [177]; such results confirm MSC alterations to be possibly involved in the development of obesity-related disorders.
MSC-based therapeutic protocols are widely studied in a large variety of diseases and have recently been explored as possible strategies for treating obesity-related disorders in pre-clinical models. For example, intraperitoneal injection of ADSCs was demonstrated to be effective in improving body weight and composition, glycemic control and lipid metabolism in obese mice [178]. The MSC therapeutic potential in this setting was also confirmed by another recent study using a murine model of obesity and T2D [179]. However, due to the significant functional impairment observed in obese subjects, the clinical efficacy of autologous MSCs in human diseases remains to be determined in this setting.
10. Conclusions
Obesity is currently a severe public health issue which raises the likelihood of suffering from concomitant morbid conditions including diabetes, cardiovascular diseases and, among others, chronic ulcers. Taken together, obesity-related disorders and chronic wounds represent major issues in human health, with significant burdens both in terms of healthcare costs and patient quality of life. The typical increase in terms of the visceral adipose tissue observed in obese subjects partially explains most of such possible comorbidities, due to its peculiar endocrine and immunological functions. In fact, WAT is nowadays recognized as being implicated in many different physiological processes beyond fat storage, including wound healing. However, adipose tissue also represents a strategic source for MSCs, which, in turn, play a pivotal role in both inflammation and tissue repair. Nevertheless, further studies are needed on the clinical efficacy of autologous ADSC-based strategies for the treatment of chronic wounds in obese subjects, due to the potentially detrimental effects of the subcutaneous pro-inflammatory milieu on MSC function.
Author Contributions
Conceptualization, A.P.; methodology, A.A., C.M., E.R.; investigation, A.P., A.A.; data curation, A.P.; writing—original draft preparation, A.A.; writing—review and editing, A.P., G.D.M.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from the authors upon reasonable request.
Acknowledgments
The author thanks the support of Marina Bondi (Department of Linguistic and Cultural Studies) for English editing and revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deitel, M. Overweight and Obesity Worldwide Now Estimated to Involve 1.7 Billion People. Obes. Surg. 2003, 13, 329–330. [Google Scholar] [CrossRef]
- Baghbani-Naghadehi, F.; Armijo-Olivo, S.; Prado, C.M.; Woodhouse, L.J. Obesity, Comorbidities, and the Associated Risk among Patients Who Underwent Total Knee Arthroplasty in Alberta. J. Knee Surg. 2022, s-0042-1742646. [Google Scholar] [CrossRef]
- Ligi, D.; Croce, L.; Mosti, G.; Raffetto, J.D.; Mannello, F. Chronic Venous Insufficiency: Transforming Growth Factor-β Isoforms and Soluble Endoglin Concentration in Different States of Wound Healing. Int. J. Mol. Sci. 2017, 18, 2206. [Google Scholar] [CrossRef]
- Moss, J.-L.; Pugliese, M.; Richards, T. Ultrasound Patterns of Venous Disease in Patients with Venous Leg Ulcers and Morbid Obesity. Phlebology 2022, 37, 732–738. [Google Scholar] [CrossRef]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928. [Google Scholar] [CrossRef]
- Dayya, D.; O’Neill, O.J.; Huedo-Medina, T.B.; Habib, N.; Moore, J.; Iyer, K. Debridement of Diabetic Foot Ulcers. Adv. Wound Care 2022, 11, 666–686. [Google Scholar] [CrossRef]
- Luo, R.; Ji, Y.; Liu, Y.-H.; Sun, H.; Tang, S.; Li, X. Relationships among Social Support, Coping Style, Self-Stigma, and Quality of Life in Patients with Diabetic Foot Ulcer: A Multicentre, Cross-Sectional Study. Int. Wound J. 2023, 20, 716–724. [Google Scholar] [CrossRef]
- Jung, F.U.C.E.; Riedel-Heller, S.G.; Luck-Sikorski, C. The Relationship between Weight History and Psychological Health-Differences Related to Gender and Weight Loss Patterns. PLoS ONE 2023, 18, e0281776. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Li, J.; Xu, R. Obesity-Associated ECM Remodeling in Cancer Progression. Cancers 2022, 14, 5684. [Google Scholar] [CrossRef]
- Esteve Ràfols, M. Adipose Tissue: Cell Heterogeneity and Functional Diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and Surgical Wound Healing: A Current Review. ISRN Obes. 2014, 2014, 638936. [Google Scholar] [CrossRef]
- Friedman, A.; Siewe, N. Mathematical Model of Chronic Dermal Wounds in Diabetes and Obesity. Bull. Math. Biol. 2020, 82, 137. [Google Scholar] [CrossRef] [PubMed]
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; López-Giráldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e6. [Google Scholar] [CrossRef]
- Rawal, K.; Patel, T.P.; Purohit, K.M.; Israni, K.; Kataria, V.; Bhatt, H.; Gupta, S. Influence of Obese Phenotype on Metabolic Profile, Inflammatory Mediators and Stemness of HADSC in Adipose Tissue. Clin. Nutr. 2020, 39, 3829–3835. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Benassi, L.; Rossi, E.; Tarentini, E.; Magnoni, C. Mesenchymal Stromal Cells Promote the Proliferation of Basal Stem Cells and Efficient Epithelization in Organotypic Models of Wound Healing. Microsc. Res. Tech. 2022, 85, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, I.; Elvira, G.; Zapata, A.G.; Lamana, M.L.; Ramírez, M.; Castro, J.G.; Arranz, M.G.; Vicente, A.; Bueren, J.; García-Olmo, D. Mesenchymal Stem Cells: Biological Properties and Clinical Applications. Expert Opin. Biol. Ther. 2010, 10, 1453–1468. [Google Scholar] [CrossRef]
- Villagrasa, A.; Posada-González, M.; García-Arranz, M.; Zapata, A.G.; Vorwald, P.; Olmedillas-López, S.; Vega-Clemente, L.; García-Olmo, D. Implicación de las células madre derivadas del tejido adiposo en la cicatrización de heridas de pacientes obesos y pacientes oncológicos. CIRU 2022, 90, 6528. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Pastar, I.; Pellegrini, M.; Azzoni, P.; Vaschieri, C.; Pisciotta, A.; Carnevale, G.; Pellacani, G.; Magnoni, C. In Vitro Engineering of a Skin Substitute Based on Adipose-Derived Stem Cells. Cells Tissues Organs 2019, 207, 46–57. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- De Assis-Ferreira, A.; Saldanha-Gama, R.; de Brito, N.M.; Renovato-Martins, M.; Simões, R.L.; Barja-Fidalgo, C.; Vargas da Silva, S. Obesity Enhances the Recruitment of Mesenchymal Stem Cells to Visceral Adipose Tissue. J. Mol. Endocrinol. 2021, 67, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic Obesity in People with Normal Body Weight (MONW)-Review of Diagnostic Criteria. Int. J. Environ. Res. Public Health 2022, 19, 624. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Nam, G.E.; Park, H.S. Perspective on Diagnostic Criteria for Obesity and Abdominal Obesity in Korean Adults. JOMES 2018, 27, 134–142. [Google Scholar] [CrossRef]
- Hyde, R. Europe Battles with Obesity. Lancet 2008, 371, 2160–2161. [Google Scholar] [CrossRef]
- Żukiewicz-Sobczak, W.; Wróblewska, P.; Zwoliński, J.; Chmielewska-Badora, J.; Adamczuk, P.; Krasowska, E.; Zagórski, J.; Oniszczuk, A.; Piątek, J.; Silny, W. Obesity and Poverty Paradox in Developed Countries. Ann. Agric. Environ. Med. 2014, 21, 590–594. [Google Scholar] [CrossRef]
- Héraïef, E. The contribution of epidemiology to the definition of obesity and its risk factors. Ther. Umsch. 1989, 46, 275–280. [Google Scholar]
- Levine, J.A. Poverty and Obesity in the U.S. Diabetes 2011, 60, 2667–2668. [Google Scholar] [CrossRef]
- Avenell, A.; Broom, J.; Brown, T.J.; Poobalan, A.; Aucott, L.; Stearns, S.C.; Smith, W.C.S.; Jung, R.T.; Campbell, M.K.; Grant, A.M. Systematic Review of the Long-Term Effects and Economic Consequences of Treatments for Obesity and Implications for Health Improvement. Health Technol. Assess. 2004, 8, iii–iv, 1–182. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual Medical Spending Attributable to Obesity: Payer-and Service-Specific Estimates. Health Aff. 2009, 28, w822–w831. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek-Altenburg, E.; Atherly, A.; Holladay, E. Changes in Healthcare Spending Attributable to Obesity and Overweight: Payer- and Service-Specific Estimates. BMC Public Health 2022, 22, 962. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Lapidus, L.; Beckers, F.; Lambert, A.; Björntorp, P. Familial Trends of Obesity through Three Generations: The Belgian-Luxembourg Child Study. Int. J. Obes. Relat. Metab. Disord. 1995, 19, S5–S9. [Google Scholar] [PubMed]
- Grio, R.; Porpiglia, M. Obesity: Internal Medicine, Obstetric and Gynecological Problems Related to Overweight. Panminerva Med. 1994, 36, 138–141. [Google Scholar] [PubMed]
- Flegal, K.M.; Troiano, R.P.; Pamuk, E.R.; Kuczmarski, R.J.; Campbell, S.M. The Influence of Smoking Cessation on the Prevalence of Overweight in the United States. N. Engl. J. Med. 1995, 333, 1165–1170. [Google Scholar] [CrossRef]
- Parsons, T.J.; Power, C.; Logan, S.; Summerbell, C.D. Childhood Predictors of Adult Obesity: A Systematic Review. Int. J. Obes. Relat. Metab. Disord. 1999, 23, S1–S107. [Google Scholar] [PubMed]
- Mauras, N.; Delgiorno, C.; Kollman, C.; Bird, K.; Morgan, M.; Sweeten, S.; Balagopal, P.; Damaso, L. Obesity without Established Comorbidities of the Metabolic Syndrome Is Associated with a Proinflammatory and Prothrombotic State, Even before the Onset of Puberty in Children. J. Clin. Endocrinol. Metab. 2010, 95, 1060–1068. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Zapsalis, K.; Giannis, D.; Tsoulfas, G. Metabolic Syndrome and Liver Disease in the Era of Bariatric Surgery: What You Need to Know! World J. Hepatol. 2020, 12, 709–721. [Google Scholar] [CrossRef]
- Schienkiewitz, A.; Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Body Mass Index History and Risk of Type 2 Diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am. J. Clin. Nutr. 2006, 84, 427–433. [Google Scholar] [CrossRef]
- Samad, F.; Ruf, W. Inflammation, Obesity, and Thrombosis. Blood 2013, 122, 3415–3422. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin Resistance: The Link Between Obesity and Cardiovascular Disease. Med. Clin. N. Am. 2011, 95, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, Inflammation, and Atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zaman, C.F.; Sultana, J.; Dey, P.; Dutta, J.; Mustarin, S.; Tamanna, N.; Roy, A.; Bhowmick, N.; Khanam, M.; Sultana, S.; et al. A Multidisciplinary Approach and Current Perspective of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e29657. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Hofland, J., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Yue, W.; Huang, X.; Zhang, W.; Li, S.; Liu, X.; Zhao, Y.; Shu, J.; Liu, T.; Li, W.; Liu, S. Metabolic Surgery on Patients With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 848947. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Ontaneda, D.; Sperling, J. Metabolic Syndrome in Small Fiber Sensory Neuropathy. J. Clin. Neuromuscul. Dis. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- For the Alzheimer’s Disease Neuroimaging Initiative; Morys, F.; Potvin, O.; Zeighami, Y.; Vogel, J.; Lamontagne-Caron, R.; Duchesne, S.; Dagher, A. Obesity-Associated Neurodegeneration Pattern Mimics Alzheimer’s Disease in an Observational Cohort Study. J. Alzheimers Dis. 2023, 91, 1059–1071. [Google Scholar] [CrossRef]
- Tobin, A.-M.; Ahern, T.; Rogers, S.; Collins, P.; O’Shea, D.; Kirby, B. The Dermatological Consequences of Obesity: Dermatological Consequences of Obesity. Int. J. Dermatol. 2013, 52, 927–932. [Google Scholar] [CrossRef]
- Sebastian, J.C. Respiratory Physiology and Pulmonary Complications in Obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 157–161. [Google Scholar] [CrossRef]
- Kivimäki, M.; Batty, G.D.; Singh-Manoux, A.; Nabi, H.; Sabia, S.; Tabak, A.G.; Akbaraly, T.N.; Vahtera, J.; Marmot, M.G.; Jokela, M. Association between Common Mental Disorder and Obesity over the Adult Life Course. Br. J. Psychiatry 2009, 195, 149–155. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-Alpha, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Bastard, J.P.; Jardel, C.; Delattre, J.; Hainque, B.; Bruckert, E.; Oberlin, F. Evidence for a Link between Adipose Tissue Interleukin-6 Content and Serum C-Reactive Protein Concentrations in Obese Subjects. Circulation 1999, 99, 2221–2222. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Broszczak, D.A.; Sydes, E.R.; Wallace, D.; Parker, T.J. Molecular Aspects of Wound Healing and the Rise of Venous Leg Ulceration: Omics Approaches to Enhance Knowledge and Aid Diagnostic Discovery. Clin. Biochem. Rev. 2017, 38, 35–55. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Żurawska-Płaksej, E.; Kuliczkowski, W.; Karolko, B.; Cielecka-Prynda, M.; Dębski, J.; Kaaz, K.; Mysiak, A.; Wróbel, T.; Podolak-Dawidziak, M.; Usnarska-Zubkiewicz, L. Platelet Polyphosphate Level Is Elevated in Patients with Chronic Primary Thrombocytopenia: A Preliminary Study. Adv. Clin. Exp. Med. 2020, 29, 1051–1056. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Yang, S.; Cui, Y.-H.; He, Y.-Y. Keratinocyte Autophagy Enables the Activation of Keratinocytes and Fibroblastsand Facilitates Wound Healing. Autophagy 2021, 17, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Stricklin, G.P.; Li, L.; Jancic, V.; Wenczak, B.A.; Nanney, L.B. Localization of MRNAs Representing Collagenase and TIMP in Sections of Healing Human Burn Wounds. Am. J. Pathol. 1993, 143, 1657–1666. [Google Scholar]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, Medicine and the Future: Healing Chronic Wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, P.; Pan, C.; Wang, Y.; Liu, Z.; Chen, Y.; Chen, C.; Fu, S.; Xue, K.; Zhou, Q.; et al. Production and Biological Effects of Extracellular Vesicles from Adipose-Derived Stem Cells Were Markedly Increased by Low-Intensity Ultrasound Stimulation for Promoting Diabetic Wound Healing. Stem Cell Rev. Rep. 2022, 1–23. [Google Scholar] [CrossRef]
- Zwaginga, J.J.; Doevendans, P. Stem Cell-Derived Angiogenic/Vasculogenic Cells: Possible Therapies for Tissue Repair and Tissue Engineering. Clin. Exp. Pharmacol. Physiol. 2003, 30, 900–908. [Google Scholar] [CrossRef]
- Pilcher, B.K.; Wang, M.; Qin, X.-J.; Parks, W.C.; Senior, R.M.; Welgus, H.G. Role of Matrix Metalloproteinases and Their Inhibition in Cutaneous Wound Healing and Allergic Contact Hypersensitivity. Ann. N. Y. Acad. Sci. 1999, 878, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular Pathogenesis of Chronic Wounds: The Role of Beta-Catenin and c-Myc in the Inhibition of Epithelialization and Wound Healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts Differentiate from Fibroblasts When Plated at Low Density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef]
- Campelo, M.B.D.; de Alencar Fonseca Santos, J.; Maia Filho, A.L.M.; Ferreira, D.C.L.; Sant’Anna, L.B.; de Oliveira, R.A.; Maia, L.F.; Arisawa, E.Â.L. Effects of the Application of the Amniotic Membrane in the Healing Process of Skin Wounds in Rats. Acta Cir. Bras. 2018, 33, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ireton, J.E.; Unger, J.G.; Rohrich, R.J. The Role of Wound Healing and Its Everyday Application in Plastic Surgery: A Practical Perspective and Systematic Review. Plast. Reconstr. Surg. Glob. Open 2013, 1, e10–e19. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, H.; Yi, J.; Chen, Z.; Chen, J.; Zhang, J.; Gao, K.; He, S.; Wang, A.; Jin, P.; et al. Identification of Differentially Expressed Proteins Involved in Fetal Scarless Wound Healing Using a Rat Model of Cleft Lip. Mol. Med. Rep. 2021, 24, 596. [Google Scholar] [CrossRef]
- Situm, M.; Kolić, M. Atypical wounds: Definition and classification. Acta Med. Croat. 2012, 66, 5–11. [Google Scholar]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Haughey, L.; Barbul, A. Nutrition and Lower Extremity Ulcers: Causality and/or Treatment. Int. J. Low Extrem. Wounds 2017, 16, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and Guidelines for Assessment of Wounds and Evaluation of Healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef]
- Dean, S.M. Cutaneous Manifestations of Chronic Vascular Disease. Prog. Cardiovasc. Dis. 2018, 60, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Cerbone, A.M.; Tufano, A.; Coppola, A.; Cimino, E.; Di Minno, M.N.; Di Minno, G. Pharmacological Treatment and Prevention of Chronic Venous Ulcers: A Review. Minerva Cardioangiol. 2015, 63, 231–238. [Google Scholar] [PubMed]
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloids: The Paradigm of Skin Fibrosis-Pathomechanisms and Treatment. Matrix Biol. 2016, 51, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic Wound Pathogenesis and Current Treatment Strategies: A Unifying Hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of Adipose-Derived Stem Cells in Wound Healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with Autologous Adipose-Derived Regenerative Cells for the Care of Chronic Ulcer of Lower Limbs in Patients with Peripheral Arterial Disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Mezera, V.; Bureš, I. Chronic Non-Healing Wounds in Geriatrics. Vnitr. Lek. 2018, 64, 1098–1104. [Google Scholar] [CrossRef]
- Manley, S.; Mitchell, A. The Impact of Nutrition on Pressure Ulcer Healing. Br. J. Nurs. 2022, 31, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; DiPietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic Wound Repair and Healing in Older Adults: Current Status and Future Research. Wound Repair Regen. 2015, 23, 1–13. [Google Scholar] [CrossRef]
- Schneider, C.; Stratman, S.; Kirsner, R.S. Lower Extremity Ulcers. Med. Clin. N. Am. 2021, 105, 663–679. [Google Scholar] [CrossRef]
- Green, J.; Jester, R.; McKinley, R.; Pooler, A. The Impact of Chronic Venous Leg Ulcers: A Systematic Review. J. Wound Care 2014, 23, 601–612. [Google Scholar] [CrossRef]
- Putri, N.M.M.E.; Yasmara, D.; Yen, M.-F.; Pan, S.-C.; Fang, S.-Y. Body Image as a Mediator Between Gender and Quality of Life Among Patients With Diabetic Foot Ulcers in Indonesia. J. Transcult. Nurs. 2021, 32, 655–663. [Google Scholar] [CrossRef]
- Salomé, G.M.; de Almeida, S.A.; de Jesus Pereira, M.T.; Massahud, M.R.; de Oliveira Moreira, C.N.; de Brito, M.J.A.; Ferreira, L.M. The Impact of Venous Leg Ulcers on Body Image and Self-Esteem. Adv. Ski. Wound Care 2016, 29, 316–321. [Google Scholar] [CrossRef]
- Wilson, J.A.; Clark, J.J. Obesity: Impediment to Postsurgical Wound Healing. Adv. Ski. Wound Care 2004, 17, 426–432. [Google Scholar] [CrossRef]
- Gallagher Camden, S.; Gates, J. Obesity: Changing the Face of Geriatric Care. Ostomy Wound Manag. 2006, 52, 36–38, 40–44. [Google Scholar]
- Lazar, M.; Ershadi, S.; Bolton, L.; Phillips, T. Patient-Centered Outcomes for Individuals with a Venous Leg Ulcer: A Scoping Review. Adv. Ski. Wound Care 2023, 36, 10–17. [Google Scholar] [CrossRef]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in Adipose Tissue and Obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E.; Peterson, C.A.; Kern, P.A. Adipose Tissue Extracellular Matrix and Vascular Abnormalities in Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Impediments to Wound Healing. Am. J. Surg. 1998, 176, 39S–47S. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; DeVore, A.; Dawn, A. Obesity and the Skin: Skin Physiology and Skin Manifestations of Obesity. J. Am. Acad. Dermatol. 2007, 56, 901–916. [Google Scholar] [CrossRef]
- Robson, M.C. Wound infection. Surg. Clin. N. Am. 1997, 77, 637–650. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, S.; Wang, L.; Wu, J.; Li, X.; Yuan, J. Metabolically Healthy Obesity and Carotid Plaque among Steelworkers in North China: The Role of Inflammation. Nutrients 2022, 14, 5123. [Google Scholar] [CrossRef]
- Cai, S.; Rahman, M.; Intrator, O. Obesity and Pressure Ulcers among Nursing Home Residents. Med. Care 2013, 51, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, E.; Mehrdadi, P.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Association of Overweight and Obesity with the Prevalence and Incidence of Pressure Ulcers: A Systematic Review and Meta-Analysis. Clin. Nutr. 2021, 40, 5089–5098. [Google Scholar] [CrossRef] [PubMed]
- Großschädl, F.; Bauer, S. The Relationship between Obesity and Nursing Care Problems in Intensive Care Patients in Austria. Nurs. Crit. Care 2022, 27, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Whitehouse, R.W. The Diabetic Foot. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Andrews, K.L.; Dyck, P.J.; Kavros, S.J.; Vella, A.; Kazamel, M.; Clark, V.; Litchy, W.J.; Dyck, P.J.B.; Lodermeier, K.A.; Davies, J.L.; et al. Plantar Ulcers and Neuropathic Arthropathies: Associated Diseases, Polyneuropathy Correlates, and Risk Covariates. Adv. Ski. Wound Care 2019, 32, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Caprino, F.; Giannotta, N.; Sisinni, A.; Abramo, A.; Ssempijja, L.; Andreucci, M.; Bracale, U.M.; Serra, R. Social Aspects of Diabetic Foot: A Scoping Review. Soc. Sci. 2022, 11, 149. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Hart, D.A. Influence of Comorbidities: Neuropathy, Vasculopathy, and Diabetes on Healing Response Quality. Adv. Wound Care 2013, 2, 410–421. [Google Scholar] [CrossRef]
- Peppa, M.; Raptis, S.A. Glycoxidation and Wound Healing in Diabetes: An Interesting Relationship. Curr. Diabetes Rev. 2011, 7, 416–425. [Google Scholar] [CrossRef]
- Strollo, F.; Gentile, S.; Pipicelli, A.M.V.; Mambro, A.; Monici, M.; Magni, P. Space Flight-Promoted Insulin Resistance as a Possible Disruptor of Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 868999. [Google Scholar] [CrossRef]
- Yang, P.; Pei, Q.; Yu, T.; Chang, Q.; Wang, D.; Gao, M.; Zhang, X.; Liu, Y. Compromised Wound Healing in Ischemic Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0152068. [Google Scholar] [CrossRef]
- Tadokoro, S.; Ide, S.; Tokuyama, R.; Umeki, H.; Tatehara, S.; Kataoka, S.; Satomura, K. Leptin Promotes Wound Healing in the Skin. PLoS ONE 2015, 10, e0121242. [Google Scholar] [CrossRef]
- Fam, B.C.; Morris, M.J.; Hansen, M.J.; Kebede, M.; Andrikopoulos, S.; Proietto, J.; Thorburn, A.W. Modulation of Central Leptin Sensitivity and Energy Balance in a Rat Model of Diet-Induced Obesity. Diabetes Obes. Metab. 2007, 9, 840–852. [Google Scholar] [CrossRef]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The Role of Leptin in Selected Skin Diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Bianco, P.; Gehron Robey, P. Marrow Stromal Stem Cells. J. Clin. Investig. 2000, 105, 1663–1668. [Google Scholar] [CrossRef]
- Owen, M. Marrow Stromal Stem Cells. J. Cell Sci. Suppl. 1988, 10, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Fridenshteĭn, A.I.; Petrakova, K.V.; Kuralesova, A.I.; Frolova, G.I. Precursor cells for osteogenic and hemopoietic tissues. Analysis of heterotopic transplants of bone marrow. Tsitologiia 1968, 10, 557–567. [Google Scholar] [PubMed]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Patki, S.; Kadam, S.; Chandra, V.; Bhonde, R. Human Breast Milk Is a Rich Source of Multipotent Mesenchymal Stem Cells: Multipotent Breast Milk Stem Cells. Hum. Cell 2010, 23, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Selich, A.; Zimmermann, K.; Tenspolde, M.; Dittrich-Breiholz, O.; von Kaisenberg, C.; Schambach, A.; Rothe, M. Umbilical Cord as a Long-Term Source of Activatable Mesenchymal Stromal Cells for Immunomodulation. Stem Cell Res. Ther. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Salingcarnboriboon, R.; Yoshitake, H.; Tsuji, K.; Obinata, M.; Amagasa, T.; Nifuji, A.; Noda, M. Establishment of Tendon-Derived Cell Lines Exhibiting Pluripotent Mesenchymal Stem Cell-like Property. Exp. Cell Res. 2003, 287, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal Stem versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Long, C.; Wang, J.; Gan, W.; Qin, X.; Yang, R.; Chen, X. Therapeutic Potential of Exosomes from Adipose-Derived Stem Cells in Chronic Wound Healing. Front. Surg. 2022, 9, 1030288. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Li, Y.; Wang, L.; Zhao, Y.; Yuan, R.; Yang, M.-M.; Chen, Y.; Zhang, H.; Zhou, F.-H.; Qian, Z.-R.; et al. Mesenchymal Stem Cell-Derived Exosomes Regulate Microglia Phenotypes: A Promising Treatment for Acute Central Nervous System Injury. Neural Regen. Res. 2023, 18, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, S.; Guo, S.; Tian, W. Mechanisms and Clinical Application Potential of Mesenchymal Stem Cells-Derived Extracellular Vesicles in Periodontal Regeneration. Stem Cell Res. Ther. 2023, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.J.; Miller, J.M.; Sparks, T.H.; Georgiou, A.E.; Reid, J. Efficacy of Autologous Mesenchymal Stromal Cell Treatment for Chronic Degenerative Musculoskeletal Conditions in Dogs: A Retrospective Study. Front. Vet. Sci. 2022, 9, 1014687. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal Stem Cells: Immunomodulatory Capability and Clinical Potential in Immune Diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Soundara Rajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human Periodontal Ligament Stem Cells Secretome from Multiple Sclerosis Patients Suppresses NALP3 Inflammasome Activation in Experimental Autoimmune Encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Kaleci, S.; Benassi, L.; Pellacani, G.; Magnoni, C. Mesenchymal Stem Cells and Psoriasis: State of the Art and Future Perspectives. Dermatol. Ther. 2020, 33, e13247. [Google Scholar] [CrossRef] [PubMed]
- Rad, F.; Ghorbani, M.; Mohammadi Roushandeh, A.; Habibi Roudkenar, M. Mesenchymal Stem Cell-Based Therapy for Autoimmune Diseases: Emerging Roles of Extracellular Vesicles. Mol. Biol. Rep. 2019, 46, 1533–1549. [Google Scholar] [CrossRef]
- Antonioli, E.; Torres, N.; Ferretti, M.; de Azevedo Piccinato, C.; Sertie, A.L. Individual Response to MTOR Inhibition in Delaying Replicative Senescence of Mesenchymal Stromal Cells. PLoS ONE 2019, 14, e0204784. [Google Scholar] [CrossRef]
- Paganelli, A.; Rossi, E.; Magnoni, C. The Dark Side of Adipose-Derived Mesenchymal Stromal Cells in Cutaneous Oncology: Roles, Expectations, and Potential Pitfalls. Stem Cells Dev. 2022, 31, 593–603. [Google Scholar] [CrossRef]
- De Leon, J.; Bohn, G.A.; DiDomenico, L.; Fearmonti, R.; Gottlieb, H.D.; Lincoln, K.; Shah, J.B.; Shaw, M.; Taveau, H.S.; Thibodeaux, K.; et al. Wound Care Centers: Critical Thinking and Treatment Strategies for Wounds. Wounds 2016, 28, S1–S23. [Google Scholar]
- Brett, E.; Chung, N.; Leavitt, W.T.; Momeni, A.; Longaker, M.T.; Wan, D.C. A Review of Cell-Based Strategies for Soft Tissue Reconstruction. Tissue Eng. Part B Rev. 2017, 23, 336–346. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS Collection: Using Human Adipose-Derived Stem/Stromal Cells for the Production of New Skin Substitutes. Stem Cells 2008, 26, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Liu, M.; Gao, G.; Zhao, W.; Fu, Q.; Wang, Y. Implantation of Adipose-Derived Mesenchymal Stem Cell Sheets Promotes Axonal Regeneration and Restores Bladder Function after Spinal Cord Injury. Stem Cell Res. Ther. 2022, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Hu, Y.; Shi, Y. Current Research and Use of Mesenchymal Stem Cells in the Therapy of Autoimmune Diseases. Curr. Stem Cell Res. Ther. 2019, 14, 579–582. [Google Scholar] [CrossRef]
- Berry, C.E.; Abbas, D.B.; Lintel, H.A.; Churukian, A.A.; Griffin, M.; Guo, J.L.; Cotterell, A.C.; Parker, J.B.L.; Downer, M.A.; Longaker, M.T.; et al. Adipose-Derived Stromal Cell-Based Therapies for Radiation-Induced Fibrosis. Adv. Wound Care 2022. [Google Scholar] [CrossRef] [PubMed]
- Fode, M.; Nadler, N.; Lund, L.; Azawi, N. Feasibility of Minimally Invasive, Same-Day Injection of Autologous Adipose-Derived Stem Cells in the Treatment of Erectile Dysfunction. Scand. J. Urol. 2022, 57, 110–114. [Google Scholar] [CrossRef]
- Naeimi, A.; Zaminy, A.; Amini, N.; Balabandi, R.; Golipoor, Z. Effects of Melatonin-Pretreated Adipose-Derived Mesenchymal Stem Cells (MSC) in an Animal Model of Spinal Cord Injury. BMC Neurosci. 2022, 23, 65. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Vu, N.B.; Nguyen, H.T.; Palumbo, R.; Pellicano, R.; Fagoonee, S.; Pham, P.V. Stem Cell-Derived Exosomes for Wound Healing: Current Status and Promising Directions. Minerva Med. 2021, 112, 384–400. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef] [PubMed]
- Huayllani, M.T.; Sarabia-Estrada, R.; Restrepo, D.J.; Boczar, D.; Sisti, A.; Nguyen, J.H.; Rinker, B.D.; Moran, S.L.; Quiñones-Hinojosa, A.; Forte, A.J. Adipose-Derived Stem Cells in Wound Healing of Full-Thickness Skin Defects: A Review of the Literature. J. Plast. Surg. Hand Surg. 2020, 54, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound Healing Effect of Adipose-Derived Stem Cells: A Critical Role of Secretory Factors on Human Dermal Fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Benassi, L.; Rossi, E.; Magnoni, C. Extracellular Matrix Deposition by Adipose-Derived Stem Cells and Fibroblasts: A Comparative Study. Arch. Dermatol. Res. 2020, 312, 295–299. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Borçari, N.R.; da Silva Araújo, M.; Nunes, V.A. Mesenchymal Stem Cells Differentiate into Keratinocytes and Express Epidermal Kallikreins: Towards an in Vitro Model of Human Epidermis. J. Cell. Biochem. 2019, 120, 13141–13155. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Freitas-Marchi, B.L.; Reigado, G.R.; de Assis, S.R.; Maria Engler, S.S.; Chambergo Alcalde, F.S.; Nunes, V.A. Mesenchymal Stem Cells Express Epidermal Markers in an in Vitro Reconstructed Human Skin Model. Front. Cell Dev. Biol. 2022, 10, 1012637. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Chen, X.; Shi, Y.; Xie, J. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 2020, 9148310. [Google Scholar] [CrossRef]
- Kim, M.H.; Wu, W.H.; Choi, J.H.; Kim, J.; Jun, J.H.; Ko, Y.; Lee, J.H. Galectin-1 from Conditioned Medium of Three-Dimensional Culture of Adipose-Derived Stem Cells Accelerates Migration and Proliferation of Human Keratinocytes and Fibroblasts. Wound Repair Regen. 2018, 26, S9–S18. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Z.; Jiang, X.; Wang, C.; Tang, T.; Xu, T.; Chen, H.; Li, X.; Du, X.; Cui, W. Hypoxia-Pretreated ADSC-Derived Exosome-Embedded Hydrogels Promote Angiogenesis and Accelerate Diabetic Wound Healing. Acta Biomater. 2023, 157, 175–186. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Zhao, S.; Yuan, H.; Shi, J.; Zhao, H. Hypoxic ADSC-Derived Exosomes Enhance Wound Healing in Diabetic Mice via Delivery of Circ-Snhg11 and Induction of M2-like Macrophage Polarization. Biomed. Pharmacother. 2022, 153, 113463. [Google Scholar] [CrossRef]
- Mou, S.; Li, Y.; Sun, D.; Zhou, M.; Li, J.; Chen, L.; Liu, S.; Yang, J.; Xiao, P.; Tong, J.; et al. Delayed Supplementation Strategy of Extracellular Vesicles from Adipose-Derived Mesenchymal Stromal Cells with Improved Proregenerative Efficiency in a Fat Transplantation Model. Stem Cells Int. 2022, 2022, 2799844. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Cai, Z.; Gu, S.; He, Y.; Zhang, Z.; Li, T.; Wei, Q.; Wang, J.; Ke, C.; Li, L. Exosomes Derived From Human Adipose-Derived Stem Cells Inhibit Lipogenesis Involving Hedgehog Signaling Pathway. Front. Bioeng. Biotechnol. 2021, 9, 734810. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L.-H.; Sun, S.-Y.; Li, Y.; Ran, X.-W. Mesenchymal Stem Cell-Derived Exosomes: The Dawn of Diabetic Wound Healing. World J. Diabetes 2022, 13, 1066–1095. [Google Scholar] [CrossRef]
- Lv, J.; Hao, Y.-N.; Wang, X.-P.; Lu, W.-H.; Xie, L.-Y.; Niu, D. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MiR-30e-5p Ameliorates High-Glucose Induced Renal Proximal Tubular Cell Pyroptosis by Inhibiting ELAVL1. Ren. Fail. 2023, 45, 2177082. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Du, Z.; Wu, T.; Yang, C. Hair Follicle Mesenchymal Stem Cell Exosomal LncRNA H19 Inhibited NLRP3 Pyroptosis to Promote Diabetic Mouse Skin Wound Healing. Aging 2023, 15, 791. [Google Scholar] [CrossRef]
- Alessio, N.; Acar, M.B.; Demirsoy, I.H.; Squillaro, T.; Siniscalco, D.; Bernardo, G.D.; Peluso, G.; Özcan, S.; Galderisi, U. Obesity Is Associated with Senescence of Mesenchymal Stromal Cells Derived from Bone Marrow, Subcutaneous and Visceral Fat of Young Mice. Aging 2020, 12, 12609–12621. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Grant, A.; Durgam, S.; El-Hayek, K.; Flanigan, D.C.; Malanga, G.; Vasileff, W.K.; Baria, M.R. Adipose-Derived Stem Cells, Obesity, and Inflammation: A Systematic Review and Implications for Osteoarthritis Treatment. Am. J. Phys. Med. Rehabil. 2022, 101, 879–887. [Google Scholar] [CrossRef]
- Acar, M.B.; Ayaz-Güner, Ş.; Di Bernardo, G.; Güner, H.; Murat, A.; Peluso, G.; Özcan, S.; Galderisi, U. Obesity Induced by High-Fat Diet Is Associated with Critical Changes in Biological and Molecular Functions of Mesenchymal Stromal Cells Present in Visceral Adipose Tissue. Aging 2020, 12, 24894–24913. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef]
- Salehpour, A.; Shidfar, F.; Hedayati, M.; Farshad, A.A.; Tehrani, A.N.; Mohammadi, S. Molecular Mechanisms of Vitamin D plus Bisphenol A Effects on Adipogenesis in Human Adipose-Derived Mesenchymal Stem Cells. Diabetol. Metab. Syndr. 2021, 13, 41. [Google Scholar] [CrossRef]
- Da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity Modifies Bone Marrow Microenvironment and Directs Bone Marrow Mesenchymal Cells to Adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef]
- Sen, S. Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes. Diabetes Metab. J. 2019, 43, 744–751. [Google Scholar] [CrossRef]
- Gyllenhammer, L.E.; Duensing, A.M.; Keleher, M.R.; Kechris, K.; Dabelea, D.; Boyle, K.E. Fat Content in Infant Mesenchymal Stem Cells Prospectively Associates with Childhood Adiposity and Fasting Glucose. Obesity 2023, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.; Issa, K.; Eid, A.; Saleh, F.A. The Therapeutic Effects of Adipose-Derived Mesenchymal Stem Cells on Obesity and Its Associated Diseases in Diet-Induced Obese Mice. Sci. Rep. 2021, 11, 6291. [Google Scholar] [CrossRef] [PubMed]
- Bortin, M.M.; Rose, W.C.; Truitt, R.L.; Rimm, A.A.; Saltzstein, E.C.; Rodey, G.E. Graft versus Leukemia. VI. Adoptive Immunotherapy in Combination with Chemoradiotherapy for Spontaneous Leukemia-Lymphoma in AKR Mice. J. Natl. Cancer Inst. 1975, 55, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).