The Prognostic Role of Spot Urinary Sodium and Chloride in a Cohort of Hospitalized Advanced Heart Failure Patients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
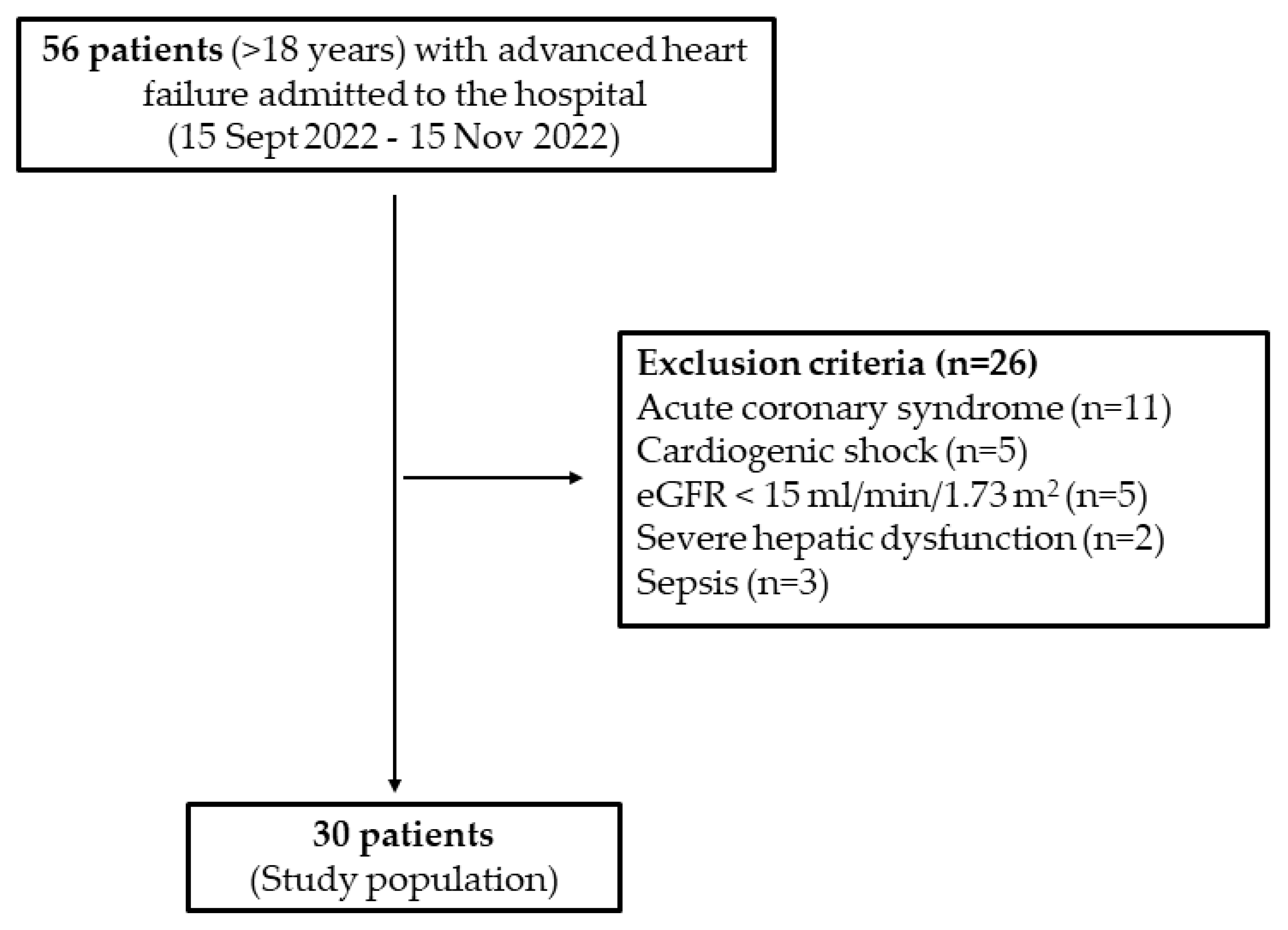
2.1. Study Population
2.2. Definitions
- Severe and persistent symptoms of HF [NYHA class III (advanced) or IV] within the last 6 months;
- LVEF ≤ 30%;
- Persistently high (or increasing) BNP or NT-proBNP values and severe left ventricular diastolic dysfunction or structural abnormalities;
- Episodes of pulmonary or systemic congestion requiring high-dose i.v. diuretics (or diuretic combinations) or episodes of low output requiring inotropes or vasoactive drugs or malignant arrhythmias causing >1 unplanned visit or hospitalization in the last 12 months.
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Study Outcomes
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Mullens, W. Current Approach to Decongestive Therapy in Acute Heart Failure. Curr. Heart Fail Rep. 2015, 12, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Tersalvi, G.; Dauw, J.; Gasperetti, A.; Winterton, D.; Cioffi, G.M.; Scopigni, F.; Pedrazzini, G.; Mullens, W. The value of urinary sodium assessment in acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Tang, W.H.W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Brater, D.C. Pharmacokinetics of loop diuretics in congestive heart failure. Br. Heart J. 1994, 72 (Suppl. 2), S40–S43. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kjeldsen, K.; Rossi, G.P.; Voors, A.A.; Cleland, J.G.; Anker, S.D.; Gheorghiade, M.; Fiuzat, M.; Rossignol, P.; Zannad, F.; et al. Decongestion in acute heart failure. Eur. J. Heart Fail. 2014, 16, 471–482. [Google Scholar] [CrossRef]
- Biegus, J.; Zymlinski, R.; Sokolski, M.; Todd, J.; Cotter, G.; Metra, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur. J. Heart Fail. 2019, 21, 624–633. [Google Scholar] [CrossRef]
- Matsue, Y.; Damman, K.; Voors, A.A.; Kagiyama, N.; Yamaguchi, T.; Kuroda, S.; Okumura, T.; Kida, K.; Mizuno, A.; Oishi, S.; et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 3042–3051. [Google Scholar] [CrossRef]
- Biegus, J.; Zymlinski, R.; Testani, J.; Marciniak, D.; Zdanowicz, A.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur. J. Heart Fail. 2021, 23, 729–739. [Google Scholar] [CrossRef]
- Collins, S.P.; Jenkins, C.A.; Baughman, A.; Miller, K.F.; Storrow, A.B.; Han, J.H.; Brown, N.J.; Liu, D.; Luther, J.M.; McNaughton, C.D.; et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Heart Fail. 2019, 6, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef]
- Honda, S.; Nagai, T.; Nishimura, K.; Nakai, M.; Honda, Y.; Nakano, H.; Iwakami, N.; Sugano, Y.; Asaumi, Y.; Aiba, T.; et al. Long-term prognostic significance of urinary sodium concentration in patients with acute heart failure. Int. J. Cardiol. 2018, 254, 189–194. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Damman, K.; Hanberg, J.S.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; Ponikowski, P.; Cotter, G.; Davison, B.; et al. Hypochloremia, Diuretic Resistance, and Outcome in Patients With Acute Heart Failure. Circ. Heart Fail. 2016, 9, e003109. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Martens, P.; Boonen, L.; Nijst, P.; Verhaert, D.; Noyens, P.; De Vusser, P.; Dupont, M.; Tang, W.H.W.; Mullens, W. Loop diuretic down-titration in stable chronic heart failure is often achievable, especially when urinary chloride concentration is low. Acta Cardiol. 2018, 73, 335–341. [Google Scholar] [CrossRef]
- Brinkley, D.M.; Burpee, L.J., Jr.; Chaudhry, S.P.; Smallwood, J.A.; Lindenfeld, J.; Lakdawala, N.K.; Desai, A.S.; Stevenson, L.W. Spot Urine Sodium as Triage for Effective Diuretic Infusion in an Ambulatory Heart Failure Unit. J. Card Fail. 2018, 24, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, A.; Frea, S.; Boretto, P.; Pidello, S.; Volpe, A.; Canavosio, F.G.; Golzio, P.G.; Bergerone, S.; De Ferrari, G.M. Spot urinary sodium in acute decompensation of advanced heart failure and dilutional hyponatremia: Insights from DRAIN trial. Clin. Res. Cardiol. 2020, 109, 1251–1259. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Garcia, A.A.; Anker, S.D.; Armstrong, P.W.; Calvo, G.; Cleland, J.G.; Cohn, J.N.; Dickstein, K.; Domanski, M.J.; Ekman, I.; et al. Clinical outcome endpoints in heart failure trials: A European Society of Cardiology Heart Failure Association consensus document. Eur. J. Heart Fail. 2013, 15, 1082–1094. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Biegus, J.; Zymlinski, R.; Fudim, M.; Testani, J.; Sokolski, M.; Marciniak, D.; Ponikowska, B.; Guzik, M.; Garus, M.; Urban, S.; et al. Spot urine sodium in acute heart failure: Differences in prognostic value on admission and discharge. ESC Heart Fail. 2021, 8, 2597–2602. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients With Heart Failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Dupont, M.; Verbrugge, F.H.; Damman, K.; Degryse, N.; Nijst, P.; Reynders, C.; Penders, J.; Tang, W.H.W.; Testani, J.; et al. Urinary Sodium Profiling in Chronic Heart Failure to Detect Development of Acute Decompensated Heart Failure. JACC Heart Fail. 2019, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Nijst, P.; Dupont, M.; Reynders, C.; Penders, J.; Tang, W.H.; Mullens, W. Prognostic value of glomerular filtration changes versus natriuretic response in decompensated heart failure with reduced ejection. J. Card Fail. 2014, 20, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Proposal for heart failure progression based on the ‘chloride theory’: Worsening heart failure with increased vs. non-increased serum chloride concentration. ESC Heart Fail. 2017, 4, 623–631. [Google Scholar] [CrossRef]
- Zandijk, A.J.L.; van Norel, M.R.; Julius, F.E.C.; Sepehrvand, N.; Pannu, N.; McAlister, F.A.; Voors, A.A.; Ezekowitz, J.A. Chloride in Heart Failure: The Neglected Electrolyte. JACC Heart Fail. 2021, 9, 904–915. [Google Scholar] [CrossRef]
- Rivera, F.B.; Alfonso, P.; Golbin, J.M.; Lo, K.; Lerma, E.; Volgman, A.S.; Kazory, A. The Role of Serum Chloride in Acute and Chronic Heart Failure: A Narrative Review. Cardiorenal. Med. 2021, 11, 87–98. [Google Scholar] [CrossRef]
- Kataoka, H. Estimation of Plasma Renin Activity on the Basis of Serum and Urinary Chloride Concentrations versus Sodium Concentrations. Cardiorena.l Med. 2022, 12, 205–213. [Google Scholar] [CrossRef]
- Patoulias, D.; Fragakis, N.; Rizzo, M. The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life 2022, 12, 2062. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided therapy in acute heart failure: Rationale and design of the Pragmatic Urinary Sodium-based treatment algoritHm in Acute Heart Failure (PUSH-AHF) trial. Eur. J. Heart Fail. 2022, 24, 385–392. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Mills, R.M.; Wynne, J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am. Heart J. 2008, 155, 339–347. [Google Scholar] [CrossRef]
- Yancy, C.W.; Saltzberg, M.T.; Berkowitz, R.L.; Bertolet, B.; Vijayaraghavan, K.; Burnham, K.; Oren, R.M.; Walker, K.; Horton, D.P.; Silver, M.A. Safety and feasibility of using serial infusions of nesiritide for heart failure in an outpatient setting (from the FUSION I trial). Am. J. Cardiol. 2004, 94, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.C.; Gelijns, A.C.; Miller, L.; Wang, C.; Nickens, P.; Arons, R.; Aaronson, K.; Richenbacher, W.; van Meter, C.; Nelson, K.; et al. Left ventricular assist devices as permanent heart failure therapy: The price of progress. Ann. Surg. 2003, 238, 577–583; discussion 583–575. [Google Scholar] [CrossRef] [PubMed]
- De Rango, P. Prospective Cohort Studies. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 151. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Iwanaga, Y.; Kanaoka, K.; Sumita, Y.; Nishioka, Y.; Myojin, T.; Kubo, S.; Okada, K.; Soeda, T.; Noda, T.; et al. Contemporary use of SGLT2 inhibitors in heart failure patients with diabetes mellitus: A comparison of DPP4 inhibitors in a nationwide electric health database of the superaged society. Cardiovasc. Diabetol. 2022, 21, 157. [Google Scholar] [CrossRef]
- Hofer, F.; Kazem, N.; Richter, B.; Sulzgruber, P.; Schweitzer, R.; Pailer, U.; Hammer, A.; Koller, L.; Hengstenberg, C.; Niessner, A. Prescription Patterns of Sodium-Glucose Cotransporter 2 Inhibitors and Cardiovascular Outcomes in Patients with Diabetes Mellitus and Heart Failure. Cardiovasc. Drugs Ther. 2022, 36, 497–504. [Google Scholar] [CrossRef]
- Sangha, V.; Lipska, K.; Lin, Z.; Inzucchi, S.E.; McGuire, D.K.; Krumholz, H.M.; Khera, R. Patterns of Prescribing Sodium-Glucose Cotransporter-2 Inhibitors for Medicare Beneficiaries in the United States. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e008381. [Google Scholar] [CrossRef]
| Demographics | Number of Patients, n = 30 |
|---|---|
| Female, n (%) | 15 (50) |
| Mean age, years (SD) | 73 (13) |
| Sodium Consumption, n (%) | 28 (100) |
| Systolic arterial pressure, mmHg (SD) | 126 (25) |
| Diastolic arterial pressure, mmHg (SD) | 77 (17) |
| Heart rate | 88 (21) |
| NYHA class, n (%) | |
| I | 0 (0) |
| II | 0 (0) |
| III | 21 (70) |
| IV | 9 (30) |
| Comorbidities | |
| Hypertension, n (%) | 9 (30) |
| Diabetes Mellitus, n (%) | 8 (26.7) |
| Coronary Artery Disease, n (%) | 8 (26.7) |
| Dyslipidemia, n (%) | 5 (16.7) |
| Valvular disease (at least moderate), n (%) | 6 (20) |
| Arterial blood gases | |
| pH | 7.44 (0.06) |
| pO2, mm Hg (SD) | 63 (20) |
| pCO2, mm Hg (SD) | 42 (10) |
| HCO3, mmol/L (SD) | 20 (8) |
| Lactate, mmol/L (SD) | 1.3 (1.1) |
| Blood tests | |
| Hematocrit, % (SD) | 38.5 (6.8) |
| Hemoglobin, g/dL (SD) | 12.2 (2.3) |
| Platelets, K/μL (SD) | 261 (100) |
| Prothrombin Time, sec (SD) | 19.4 (9.3) |
| International Normalized Ratio (SD) | 1.6 (0.8) |
| Activated Partial Thromboplastin Clotting Time, s (SD) | 31 (5) |
| Fibrinogen, mg/dL (SD) | 344 (81) |
| C-reactive protein, mg/dL (SD) | 2 (3) |
| Glucose, mg/dL (SD) | 137 (47) |
| Urea, mg/dL (SD) | 80 (50) |
| Creatinine, mg/dL (SD) | 1.4 (0.7) |
| K+, mmol/L (SD) | 4.6 (0.5) |
| Na+, mmol/L (SD) | 135.4 (6.9) |
| NT-proBNP, ng/L (SD) | 13319.6 (9577.2) |
| Iron, mg/dL (SD) | 106.6 (63.7) |
| Ferritin, ng/mL (SD) | 159.2 (134.4) |
| Urine collection | |
| Baseline (admission) | |
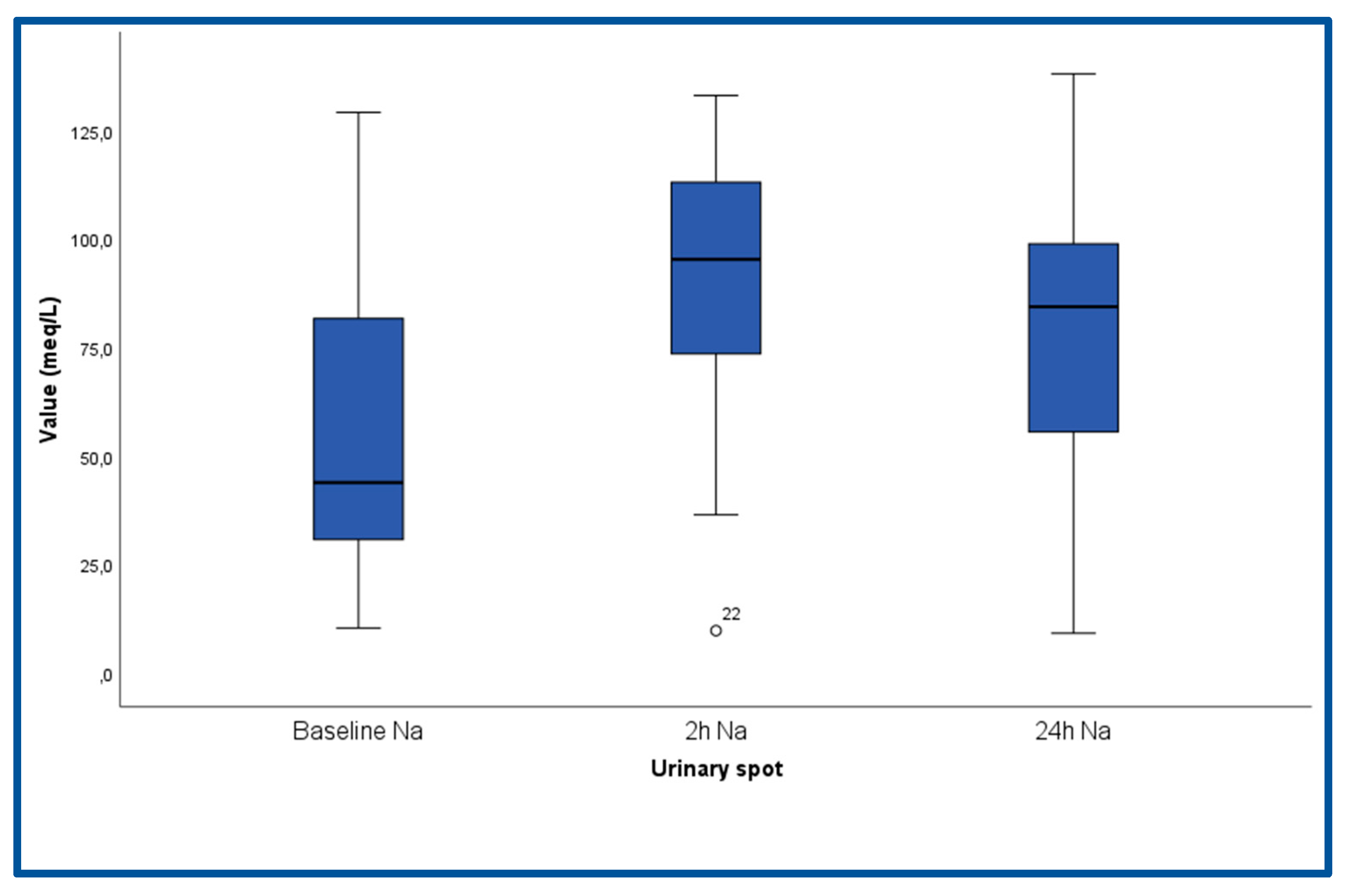
| Spot Na+, meq/L (SD) | 55.9 (34.5) |
| Spot Cre, mg/dL (SD) | 18.5 (15.3) |
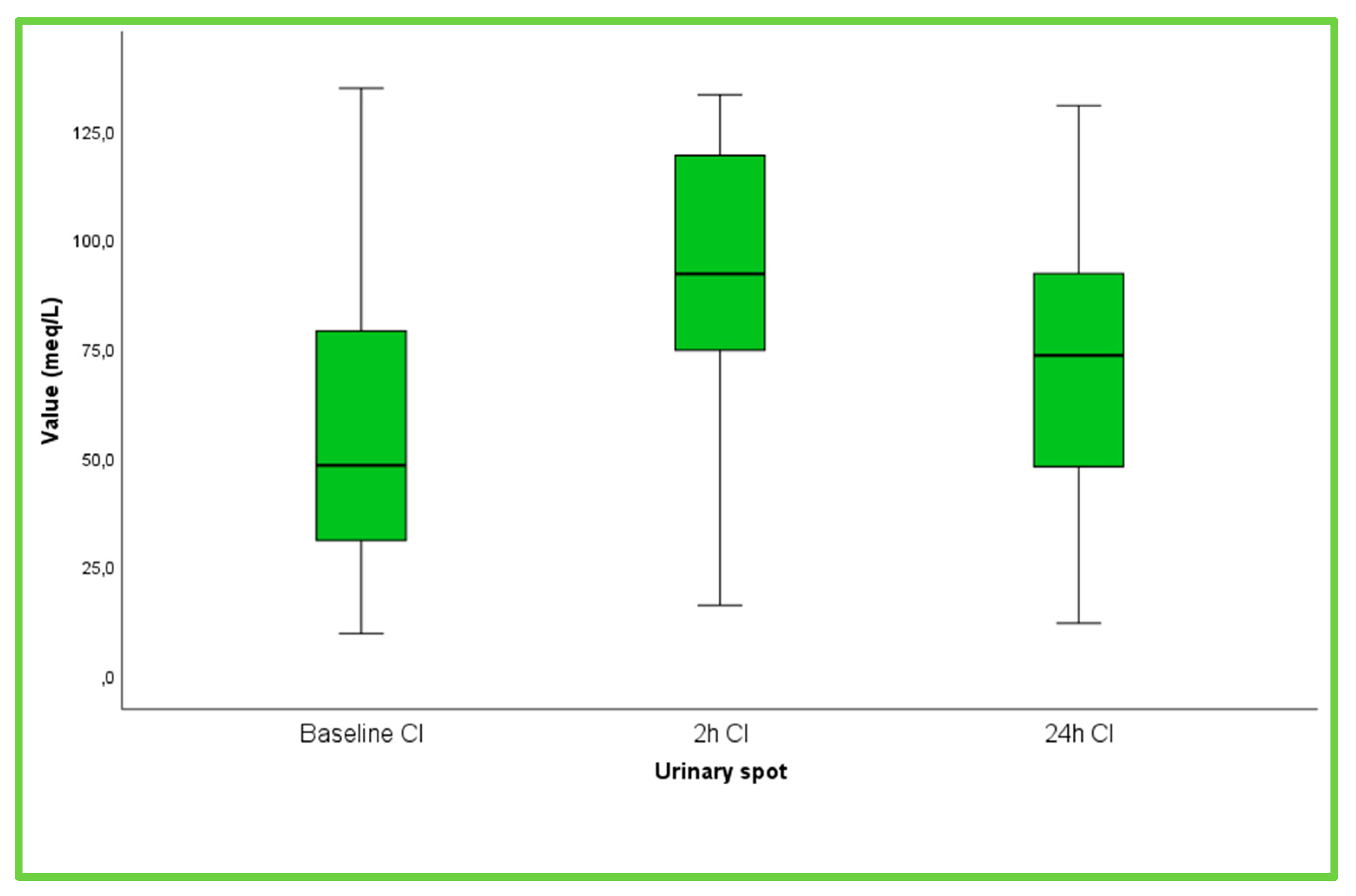
| Spot Cl−, meq/L (SD) | 90.7 (28.4) |
| At 2 h | |
| Spot Na+, meq/L (SD) | 90.2 (30.4) |
| Spot Cre, mg/dL (SD) | 19.4 (15.5) |
| Spot Cl−, meq/L (SD) | 90.6 (28.3) |
| At 24 h | |
| Spot Na+, meq/L (SD) | 78.98 (30.58) |
| Spot Cre, mg/dL (SD) | 44.73 (36.24) |
| Spot Cl−, meq/L (SD) | 71.35 (32.37) |
| Urine output at 6 h, mL (SD) | 1676.67 (616.9) |
| Urine output at 24 h, mL (SD) | 3985.42 (1384.5) |
| Cardiac echo | |
| LVEDD, mm (SD) | 54.8 (8.9) |
| LVEF %, (SD) | 37.3 (15) |
| Left Atrium, mm (SD) | 51.4 (6.6) |
| TDI RV S wave velocity, cm/sec (SD) | 9.2 (1.7) |
| RVSP, mm Hg (SD) | 51 (14) |
| IVC, mm (SD) | 24 (4.5) |
| Medical treatment | |
| b-blocker, n (%) | 24 (80) |
| ACE-inhibitor/ARB/ARNI, n (%) | 16 (53.3) |
| MRA, n (%) | 12 (40) |
| Loop Diuretic, n (%) | 23 (76.7) |
| Dose of loop diuretic (furosemide) at admission (mg) | 118.0 (80.7) |
| SGLT-2 inhibitor, n (%) | 5 (16.7) |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.04 | 0.98, 1.10 | 0.21 |
| Systolic arterial pressure | 0.99 | 0.97, 1.03 | 0.95 |
| Diastolic arterial pressure | 0.97 | 0.92, 1.02 | 0.24 |
| NYHA III | 0.37 | 0.07, 1.92 | 0.23 |
| Heart rate | 0.96 | 0.91, 1.00 | 0.05 |
| Hypertension | 1.37 | 0.28, 6.60 | 0.69 |
| Diabetes Mellitus | 4.33 | 0.70, 26.53 | 0.11 |
| Coronary Artery Disease | 1.00 | 0.19, 5.04 | 1.00 |
| Dyslipidemia | 5.09 | 0.49, 52.28 | 0.17 |
| Valvular disease (at least moderate) | 1.00 | 0.16, 5.98 | 1.00 |
| pH | 0.39 | 0.30, 0.55 | 0.89 |
| pO2 | 1.00 | 0.96, 1.04 | 0.98 |
| pCO2 | 1.06 | 0.97, 1.19 | 0.24 |
| HCO3 | 1.09 | 0.96, 1.32 | 0.31 |
| Lactate | 1.20 | 0.53, 3.76 | 0.68 |
| Hct | 1.04 | 0.94, 1.17 | 0.46 |
| Hgb | 1.09 | 0.78, 1.54 | 0.61 |
| Plt | 1.00 | 0.99, 1.00 | 0.61 |
| PT | 1.07 | 0.97, 1.22 | 0.23 |
| INR | 2.22 | 0.71, 11.15 | 0.23 |
| APTT | 1.07 | 0.91, 1.27 | 0.41 |
| FIB | 0.99 | 0.98, 1.00 | 0.22 |
| CRP | 0.88 | 0.63, 1.15 | 0.39 |
| Glucose | 1.00 | 0.99, 1.02 | 0.76 |
| Urea | 1.01 | 1.00, 1.04 | 0.11 |
| Creatinine | 1.55 | 0.53, 5.31 | 0.44 |
| K | 0.76 | 0.15, 3.52 | 0.73 |
| Na | 0.92 | 0.80, 1.02 | 0.16 |
| NT-proBNP | 1.00 | 1.00, 1.00 | 0.27 |
| Iron | 1.00 | 0.994, 1.01 | 0.35 |
| Ferritin | 0.99 | 0.99, 1.00 | 0.33 |
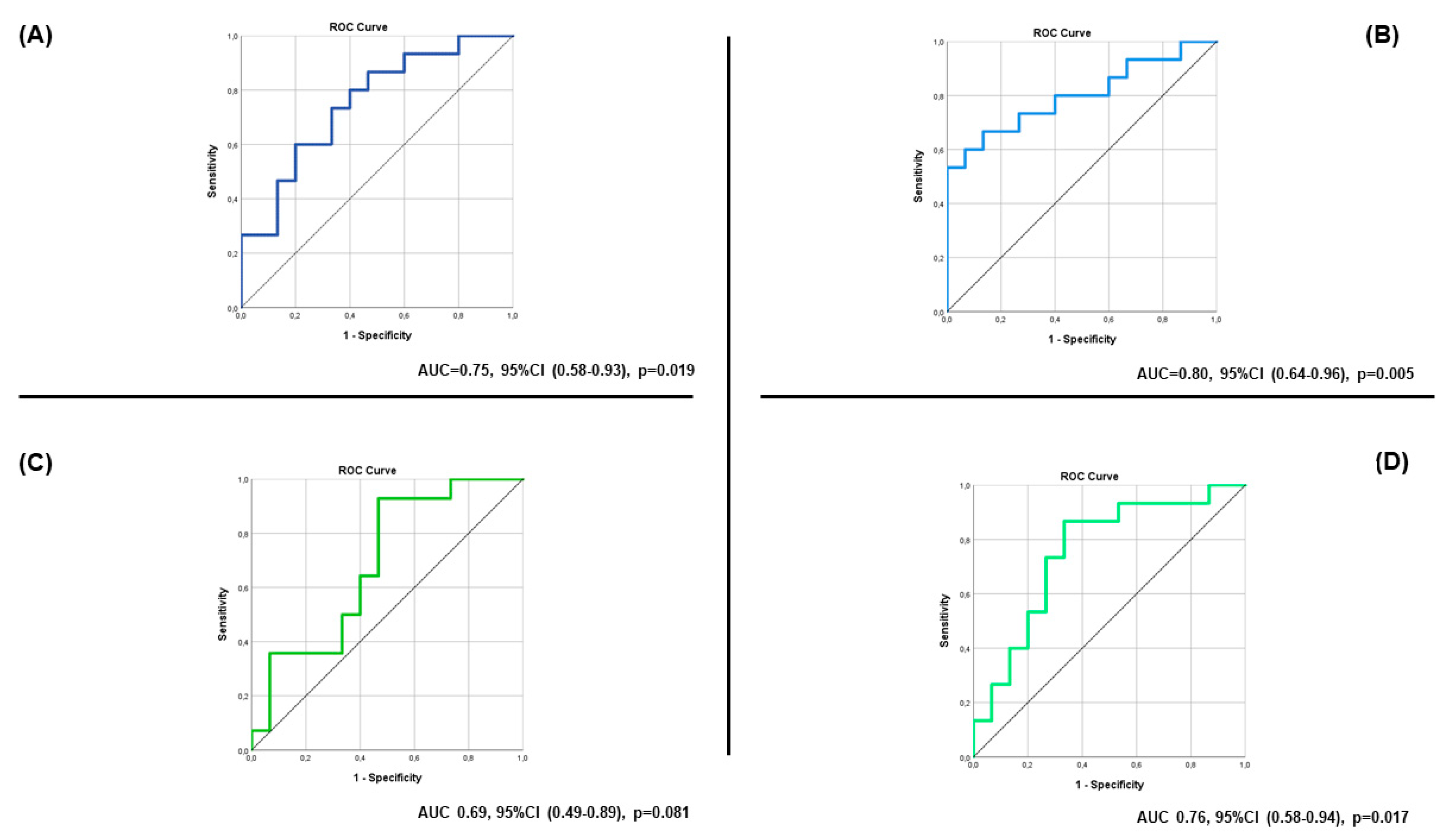
| Baseline Spot Na | 0.97 | 0.94, 0.99 | 0.02 |
| Spot Na 2 h | 0.95 | 0.91, 0.99 | 0.01 |
| Spot Na 24 h | 0.98 | 0.95, 1.01 | 0.20 |
| Baseline Spot Cre | 1.04 | 0.99, 1.12 | 0.20 |
| Spot Cre 2 h | 1.05 | 0.98, 1.13 | 0.10 |
| Spot Cre 24 h | 0.99 | 0.96, 1.01 | 0.55 |
| Baseline Spot Cl | 0.97 | 0.95, 1.0 | 0.06 |
| Spot Cl 2 h | 0.96 | 0.92, 0.99 | 0.02 |
| Spot Cl 24 h | 0.98 | 0.96, 1.01 | 0.33 |
| Urine output 6 h | 0.99 | 0.996, 0.999 | 0.01 |
| Urine output 24 h | 1.00 | 0.99, 1.00 | 0.16 |
| LVEDD | 1.02 | 0.91, 1.16 | 0.71 |
| LVEF | 1.00 | 0.95, 1.05 | 0.90 |
| Left Atrium | 1.08 | 0.93, 1.30 | 0.37 |
| TDI RV | 0.46 | 0.18, 0.88 | 0.05 |
| RVSP | 1.01 | 0.96, 1.07 | 0.65 |
| IVC | 1.04 | 0.88, 1.24 | 0.67 |
| b-blocker | 2.36 | 0.36, 15.45 | 0.36 |
| ACE-inhibitor/ARB/ARNI | 1.00 | 0.23, 4.19 | 1.00 |
| MRA | 3.14 | 0.68, 14.50 | 0.14 |
| Loop Diuretic | - | - | 0.99 |
| Dose of loop diuretic at admission | 1.01 | 1.00, 1.02 | 0.01 |
| SGLT-2 inhibitor | 5.09 | 0.49, 52.28 | 0.17 |
| Variable | B | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Spot Na 2h | −0.04 | 0.95 | 0.91, 0.99 | 0.02 |
| Loop diuretic dose at admission | 0.01 | 1.01 | 1.00, 1,02 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulos, A.; Christofidis, C.; Pantsios, C.; Magouliotis, D.; Bourazana, A.; Leventis, I.; Skopeliti, N.; Skoularigki, E.; Briasoulis, A.; Giamouzis, G.; et al. The Prognostic Role of Spot Urinary Sodium and Chloride in a Cohort of Hospitalized Advanced Heart Failure Patients: A Pilot Study. Life 2023, 13, 698. https://doi.org/10.3390/life13030698
Xanthopoulos A, Christofidis C, Pantsios C, Magouliotis D, Bourazana A, Leventis I, Skopeliti N, Skoularigki E, Briasoulis A, Giamouzis G, et al. The Prognostic Role of Spot Urinary Sodium and Chloride in a Cohort of Hospitalized Advanced Heart Failure Patients: A Pilot Study. Life. 2023; 13(3):698. https://doi.org/10.3390/life13030698
Chicago/Turabian StyleXanthopoulos, Andrew, Charalambos Christofidis, Chris Pantsios, Dimitrios Magouliotis, Angeliki Bourazana, Ioannis Leventis, Niki Skopeliti, Evangelia Skoularigki, Alexandros Briasoulis, Grigorios Giamouzis, and et al. 2023. "The Prognostic Role of Spot Urinary Sodium and Chloride in a Cohort of Hospitalized Advanced Heart Failure Patients: A Pilot Study" Life 13, no. 3: 698. https://doi.org/10.3390/life13030698
APA StyleXanthopoulos, A., Christofidis, C., Pantsios, C., Magouliotis, D., Bourazana, A., Leventis, I., Skopeliti, N., Skoularigki, E., Briasoulis, A., Giamouzis, G., Triposkiadis, F., & Skoularigis, J. (2023). The Prognostic Role of Spot Urinary Sodium and Chloride in a Cohort of Hospitalized Advanced Heart Failure Patients: A Pilot Study. Life, 13(3), 698. https://doi.org/10.3390/life13030698











