Sex-Determined Alteration of Frontal Electroencephalographic (EEG) Activity in Social Presence
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. Data Collection
2.4. Data Analysis
3. Results
EEG Results
4. Discussion
4.1. Social Presence, Ambiguous Stimuli, and the Present Results
4.2. Sex Differences in the Area of the ACC at Cellular, Structural, and Network Level
4.3. Interpretation of the Present Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kret, M.E.; De Gelder, B. A review on sex differences in processing emotional signals. Neuropsychologia 2012, 50, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.D.; Phan, K.L.; Liberzon, I.; Taylor, S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage 2003, 19, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Placentino, A.; Carletti, F.; Landi, P.; Allen, P.; Surguladze, S.; Benedetti, F.; Abbamonte, M.; Gasparotti, R.; Barale, F.; et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009, 34, 418–432. [Google Scholar]
- Lee, T.M.; Liu, H.L.; Chan, C.C.; Fang, S.Y.; Gao, J.H. Neural activities associated with emotion recognition observed in men and women. Mol. Psychiatry 2005, 10, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rahko, J.; Paakki, J.J.; Starck, T.; Nikkinen, J.; Remes, J.; Hurtig, T.; Kuusikko-Gauffin, S.; Mattila, M.-L.; Jussila, K.; Jansson-Verkasalo, E.; et al. Functional mapping of dynamic happy and fearful facial expression processing in adolescents. Brain Imaging Behav. 2010, 4, 164–176. [Google Scholar] [CrossRef]
- Wrase, J.; Klein, S.; Gruesser, S.M.; Hermann, D.; Flor, H.; Mann, K.; Braus, D.F.; Heinz, A. Gender differences in the processing of standardized emotional visual stimuli in humans: A functional magnetic resonance imaging study. Neurosci. Lett. 2003, 348, 41–45. [Google Scholar] [CrossRef]
- Fine, J.G.; Semrud-Clikeman, M.; Zhu, D.C. Gender differences in BOLD activation to face photographs and video vignettes. Behav. Brain Res. 2009, 201, 137–146. [Google Scholar] [CrossRef]
- Stevens, J.S.; Hamann, S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia 2012, 50, 1578–1593. [Google Scholar] [CrossRef]
- Duregger, C.; Bauer, H.; Cunnington, R.; Lindinger, G.; Deecke, L.; Lang, W.; Walla, P.; Dirnberger, G. EEG evidence of gender differences in a motor related CNV study. J. Neural. Transm. 2007, 114, 359–366. [Google Scholar] [CrossRef]
- Walla, P.; Hufnagl, B.; Lindinger, G.; Deecke, L. Physiological evidence of gender differences in word recognition: A magnetoencephalographic (MEG) study. Cogn. Brain Res. 2001, 12, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gardener, E.K.T.; Carr, A.R.; MacGregor, A.; Felmingham, K.L. Sex differences and emotion regulation: An event-related potential study. PLoS ONE 2020, 8, e73475. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.; Ehlers, C.L. Gender differences in electrophysiological responses to facial stimuli. Biol. Psychiatry 1998, 44, 281–372. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M.; Zani, A.; Adorni, R. Neural markers of greater female responsiveness to social stimuli. BMC Neurosci. 2008, 30, 56. [Google Scholar] [CrossRef]
- Proverbio, A.M. Sex differences in social cognition: The case of face processing. J. Neurosci. Res. 2017, 95, 222–234. [Google Scholar] [CrossRef]
- Klein, S.; Smolka, M.N.; Wrase, J.; Gruesser, S.M.; Mann, K.; Braus, D.F.; Heinz, A.; Gruesser, S.M. The influence of gender and emotional valence of visual cues on fMRI activation in humans. Pharmacopsychiatry 2003, 36, 5191–5194. [Google Scholar]
- Gard, M.G.; Kring, A.M. Sex differences in the time course of emotion. Emotion 2007, 7, 429–437. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Adorni, R.; Zani, A.; Trestianu, L. Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia 2009, 47, 2374–2388. [Google Scholar] [CrossRef]
- Mather, M.; Lighthall, N.R.; Nga, L.; Gorlick, M.A. Sex differences in how stress affects brain activity during face viewing. Neuroreport 2010, 21, 933–937. [Google Scholar] [CrossRef]
- Soiné, A.; Flöck, A.N.; Walla, P. Electroencephalography (Eeg) reveals increased frontal activity in social presence. Brain Sci. 2021, 11, 731. [Google Scholar] [CrossRef]
- Gächter, S.; Starmer, C.; Tufano, F. Measuring the closeness of relationships: A comprehensive evaluation of the “inclusion of the other in the self” scale. PLoS ONE 2015, 10, e0129478. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, B.; Lozano, S.; Banaji, M.R. OASIS_database_2016. 2016. Available online: https://www.dropbox.com/sh/4qaoqs77c9e5muh/AABBw07ozE__2Y0LVQHVL-8ca?dl=0 (accessed on 31 May 2021).
- Kurdi, B.; Lozano, S.; Banaji, M.R. Introducing the open affective standardized image set (OASIS). Behav. Res. Methods 2017, 49, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.; Stein, M.B.; Matthews, S.C.; Feinstein, J.S.; Paulus, M.P. Affective ambiguity for a group recruits ventromedial prefrontal cortex. Neuroimage 2006, 29, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Braver, T.S.; Barch, D.M.; Botvinick, M.M.; Noll, D.; Cohen, J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998, 280, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Kolling, N.; Behrens, T.E.J.; Wittmann, M.K.; Rushworth, M.F.S. Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol. 2016, 37, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Nystrom, L.E.; Fissell, K.; Carter, C.S.; Cohen, J.D. Conflict monitoring versus selection for-action in anterior cingulate cortex. Nature 1999, 402, 179–181. [Google Scholar] [CrossRef]
- Fritz, J.; Dreisbach, G. Conflicts as aversive signals: Conflict priming increases negative judgments for neutral stimuli. Cogn. Affect. Behav. Neurosci. 2013, 13, 311–317. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Yeung, N. An Integrative Theory of Anterior Cingulate Cortex Function: Option Selection in Hierarchical Reinforcement Learning. Neural Basis Motiv. Cogn. Control 2013, 16, 332–349. [Google Scholar] [CrossRef]
- Dalgleish, T.; Walsh, N.D.; Mobbs, D.; Schweizer, S.; Van Harmelen, A.L.; Dunn, B.; Dunn, V.; Goodyer, I.; Stretton, J. Social pain and social gain in the adolescent brain: A common neural circuitry underlying both positive and negative social evaluation. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Li, P.; Peng, W.; Li, H.; Holroyd, C.B. Electrophysiological measures reveal the role of anterior cingulate cortex in learning from unreliable feedback. Cogn. Affect. Behav. Neurosci. 2018, 18, 949–963. [Google Scholar] [CrossRef]
- Van Veen, V.; Carter, C.S. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and Emotional Influence in Anterior Cingulate Cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Lavin, C.; Melis, C.; Mikulan, E.; Gelormini, C.; Huepe, D.; Ibañez, A. The anterior cingulate cortex: An integrative hub for human socially-driven interactions. Front. Neurosci. 2013, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does rejection hurt? An fMRI study of social exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Onoda, K.; Okamoto, Y.; Nakashima, K.; Nittono, H.; Ura, M.; Yamawaki, S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc. Neurosci. 2009, 4, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Roelofs, A.; Van Turennout, M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J. Neurosci. 2008, 28, 4671–4678. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Schmidt, P.J. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 2019, 44, 111–128. [Google Scholar] [CrossRef]
- Shanmugan, S.; Loughead, J.; Cao, W.; Sammel, M.D.; Satterthwaite, T.D.; Ruparel, K.; Epperson, C.N.; Gur, R.C. Impact of trypotophan depletion on executive system function during menopause is moderated by childhood adversity. Neuropsychopharmacology 2017, 42, 2398–2406. [Google Scholar] [CrossRef]
- Berent-Spillson, A.; Persad, C.C.; Love, T.; Tkaczyk, A.; Wang, H.; Reame, N.K.; Smith, Y.R.; Zubieta, J.-K.; Frey, K.A. Early menopaus hormone use influences brain regions used for visual working memory. Menopause 2010, 17, 692–699. [Google Scholar] [CrossRef]
- Berman, K.F.; Schmidt, P.J.; Rubinow, D.R.; Danaceau, M.A.; Van Horn, J.D.; Esposito, G.; Weinberger, D.R.; Ostrem, J.L. Modulation of cognition-specific cortical acivity by gondadal steroids: A position-emission tomotgraphy study in women. Proc. Natl. Acad. Sci. USA 1997, 94, 8836–8841. [Google Scholar] [CrossRef]
- Macoveanu, J.; Henningsson, S.; Pinborg, A.; Jensen, P.; Knudsen, G.M.; Frokjaer, V.G.; Siebner, H.R. Sex-steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology 2016, 41, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bayer, J.; Bandurski, P.; Sommer, T. Differential modulatio of acitivity related to the anticipation of monetary gains and losses across the menstrual cycle. Eur. J. Neurosicence 2013, 38, 3519–3526. [Google Scholar] [CrossRef]
- Dreher, J.; Schmidt, P.J.; Kohn, P.; Furman, D.; Rubinow, D.R.; Berman, K.F. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci. USA 2007, 104, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Rupp, H.A.; James, T.W.; Ketterson, E.D.; Sengelaub, D.R.; Janssen, E.; Heiman, J.R. Neural activation in women in response to masculinized male faces: Mediation by hormones and psychosexual factors. Evol. Hum. Behav. 2009, 30, 1–10. [Google Scholar] [CrossRef]
- Joffe, H.; Deckersbach, T.; Lin, N.U.; Makris, N.; Skaar, T.C.; Rauch, S.L.; Dougherty, D.D.; Hall, J.E. Metabolic activity in the insular cortex and hypothalamus predicts hot flashes: An FDG-PET study. J. Clin. Endocrinol. Metab. 2012, 97, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Arelin, K.; Mueller, K.; Barth, C.; Rekkas, P.V.; Kratzsch, J.; Burmann, I.; Sacher, J.; Villringer, A. Progesterone mediates brain functional connectivity changes during the menstrucal cycle-a pilot resting state MRI study. Front. Neurosci. 2015, 9, 44. [Google Scholar] [PubMed]
- Petersen, N.; Kilpatrick, L.A.; Goharzad, A.; Cahill, L. Oral contraceptives pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroim 2014, 90, 24–32. [Google Scholar] [CrossRef]
- Syan, S.K.; Minuzzi, L.; Costesu, D.; Smith, M.; Allega, O.R.; Coote, M.; Hall, G.B.; Frey, B.N. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil. Steril. 2017, 107, 1246–1255. [Google Scholar] [CrossRef]
- Hjelmervik, H.; Hausmann, M.; Osnes, B.; Westerhausen, R.; Specht, K. Resting states are resting traits-an FMRI study on sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS ONE 2014, 9, e103492. [Google Scholar] [CrossRef]
- Andreano, J.M.; Touroutoglou, A.; Dickerson, B.; Barrett, L.F. Hormonal Cycles, Brain Network Connectivity, and Windows of Vulnerability to Affective Disorder. Trends Neurosci. 2018, 41, 660–676. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Jerram, M.; Poldrack, R.; Ahern, T.; Kennedy, D.N.; Seidman, L.J.; Makris, N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Handb. Clin. Neurol. 2005, 25, 9309–9316. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, S.; Madsen, K.H.; Pinborg, A.; Heede, M.; Knudsen, G.M.; Siebner, H.R.; Al, E. Role of emotional processing in depressive responses to sex-hormone manipulation: A pharmacological fMRI study. Transl. Psychiatry 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Protopopescu, X.; Hong, P.; Margaret, A.; Oliver, T.; Margaret, P.; Bruce, M.; David, S.; Emily, S.; Raichle, E.M. Orbitofrontal Cortex Activity Related to Emotional Processing Changes across the Menstrual Cycle. Proc. Natl. Acad. Sci. USA 2005, 102, 16060–16065. [Google Scholar] [CrossRef] [PubMed]
- Shafir, T.; Love, T.; Berent-Spillson, A.; Persad, C.; Wang, H.; Reame, N.; Frey, K.; Zubieta, J.; Smith, Y. Postmenopausal hormone use impact on emotion processing circuitry. Behav. Brain Res. 2012, 226, 147–153. [Google Scholar] [CrossRef]
- Toffoletto, S.; Lanzenberger, R.; Gingnell, M.; Sundstrom-Poromaa, I.; Comasco, E. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. Psychoneuroendocrinology 2014, 50, 28–52. [Google Scholar] [CrossRef]
- Valentino, R.J.; Reyes, B.; Van Bockstaele, E.; Bangassser, D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology 2011, 62, 13–20. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. Cerebral asymmetry and the effects of sex and handedness on brainstructure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001, 14, 658–700. [Google Scholar]
- Paus, T.; Otaky, N.; Caramanos, Z.; MacDonalds, D.; Zijdenbos, A.; Davirro, D.; Gutmans, D.; Holmes, C.; Tomaiuolo, F.; Evans, A.C. In vivo morphometry of intrasulcal gray matter in human cingulate, paracingulate, and superior-rostral sulci: Hemispheric asymmetries, gender differences and probability maps. J. Comp. Neurol. 1996, 376, 664–673. [Google Scholar] [CrossRef]
- Boes, A.D.; Tranel, D.; Anderson, S.W.; Nopoulos, P. Right anterior-cingulate: A neuroanatomical correlate of aggression and definance in boys. Behav. Neurosci. 2008, 122, 677–684. [Google Scholar] [CrossRef]
- Kateri, M.; Kevin, N.O.; Iris, B.M.; John, J.D.G.; James, J.G. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Gr. Process. Intergr. Relations 2008, 11, 143–162. [Google Scholar]
- Kogler, L.; Gur, R.C.; Derntl, B. Sex differences in cognitive regulation of psychosocial achievement stress: Brain and behavior. Hum. Brain Mapp. 2015, 36, 1028–1066. [Google Scholar] [CrossRef] [PubMed]
- Spalek, K.; Fastenrath, M.; Ackermann, S.; Auschra, B.; Coynel, D.; Frey, J.; Gschwind, L.; Hartmann, F.; Van der Maarel, N.; Papassotiropoulos, A.; et al. Sex-dependent dissociation between emotional appraisal and memory: A large-scale behavioral and fMRI study. J. Neurosci. 2015, 35, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Korczykowski, M.; Rao, H.; Fan, Y.; Pluta, J.; Gur, R.C.; McEwen, B.S.; Detre, J.A. Gender difference in neural response to psychological stress. Soc. Cogn. Affect. Neurosci. 2007, 2, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Levy, I. Neuroanatomical Substrates for Risk Behavior. Neuroscientist 2017, 23, 275–286. [Google Scholar] [CrossRef]
- Botvinick, M.M. Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 2007, 7, 356–366. [Google Scholar] [CrossRef]
- Apps, M.A.J.; Rushworth, M.F.S.; Chang, S.W.C. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron 2016, 90, 692–707. [Google Scholar] [CrossRef]
- Demolliens, M.; Isbaine, F.; Takerkart, S.; Huguet, P.; Boussaoud, D. Social and asocial prefrontal cortex neurons: A new look at social facilitation and the social brain. Soc. Cogn. Affect. Neurosci. 2017, 12, 1241–1248. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Buckley, M.J.; Walton, M.E.; Rushworth, M.F.S. Reports 4. 2005, 566, 1–4. [Google Scholar]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef]
- Shenhav, A.; Botvinick, M.M.; Cohen, J.D. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 2013, 79, 217–240. [Google Scholar] [CrossRef]
- Taylor, S.E.; Klein, L.C.; Lewi, B.P.; Gruenewald, T.L.; Gurung, R.A.; Updegraff, J.A. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000, 107, 411–429. [Google Scholar] [CrossRef]
- Morrison, R.L. Are Women Tending and Befriending in the Workplace? Gender Differences in the Relationship Between Workplace Friendships and Organizational Outcomes. Sex Roles 2009, 60, 1–13. [Google Scholar] [CrossRef]
- Wood, W.; Eagly, A.H. Two Traditions of Research on Gender Identity. Sex Roles 2015, 73, 461–473. [Google Scholar] [CrossRef]
- Clow, K.A.; Ricciardelli, R. Women and Men in Conflicting Social Roles: Implications from Social Psychological Research. Soc. Issues Policy Rev. 2011, 5, 191–226. [Google Scholar] [CrossRef]
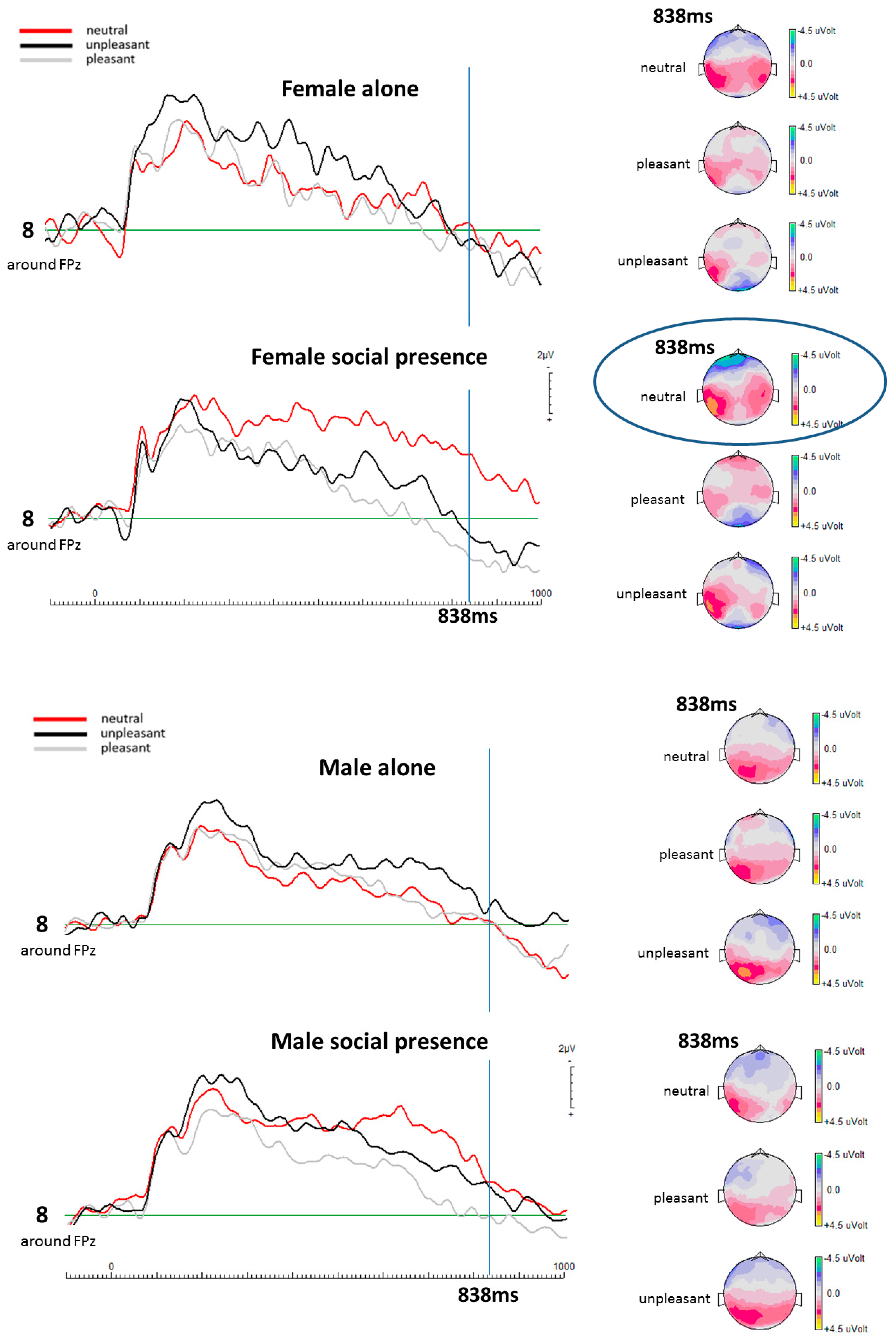
| Social Presence Condition | ||
|---|---|---|
| Female Participants | at 838 ms | |
| Valence Categories | p-Value | |
| Electrode 8 (mid-frontal) | Neutral–Pleasant | p = 0.013 (t(13) = −0.429) |
| Neutral–Unpleasant | p = 0.013 (t(13) = 0.466) | |
| Unpleasant–Pleasant | p = 0.492 (t(13) = 0.631) | |
| Male Participants | at 838 ms | |
| Valence Categories | p-Value | |
| Electrode 8 (mid-frontal) | Neutral–Pleasant | p = 0.547 (t(12) = 0.594) |
| Neutral–Unpleasant | p = 0.787 (t(12) = 0.447) | |
| Unpleasant–Pleasant | p = 0.701 (t(12) = 0.497) | |
| Alone Condition | ||
|---|---|---|
| Female Participants | at 838 ms | |
| Valence Categories | p-Value | |
| Electrode 8 (around FPz) | Neutral–Pleasant | p = 0.634 (t(13) = 0.537) |
| Neutral–Unpleasant | p = 0.977 (t(13) = 0.347) | |
| Unpleasant–Pleasant | p = 0.320 (t(13) = 0.754) | |
| Male Participants | at 838 ms | |
| Valence Categories | p-Value | |
| Electrode 8 (around FPz) | Neutral–Pleasant | p = 0.735 (t(12) = 0.722) |
| Neutral–Unpleasant | p = 0.415 (t(12) = 0.995) | |
| Unpleasant–Pleasant | p = 0.510 (t(12) = 0.775) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soiné, A.; Walla, P. Sex-Determined Alteration of Frontal Electroencephalographic (EEG) Activity in Social Presence. Life 2023, 13, 585. https://doi.org/10.3390/life13020585
Soiné A, Walla P. Sex-Determined Alteration of Frontal Electroencephalographic (EEG) Activity in Social Presence. Life. 2023; 13(2):585. https://doi.org/10.3390/life13020585
Chicago/Turabian StyleSoiné, Anna, and Peter Walla. 2023. "Sex-Determined Alteration of Frontal Electroencephalographic (EEG) Activity in Social Presence" Life 13, no. 2: 585. https://doi.org/10.3390/life13020585
APA StyleSoiné, A., & Walla, P. (2023). Sex-Determined Alteration of Frontal Electroencephalographic (EEG) Activity in Social Presence. Life, 13(2), 585. https://doi.org/10.3390/life13020585








