Impact of Comorbidity of Bronchial Asthma and Type 2 Diabetes Mellitus on the Expression and Functional Activity of TLR2 and TLR4 Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of TLR2 and TLR4 Expression
2.2. Whole Blood Promotion to Cytokine Synthesis
2.3. Determination of Cytokine Content
2.4. Statistical Data Analysis
3. Results
3.1. Characterization of Patient Groups
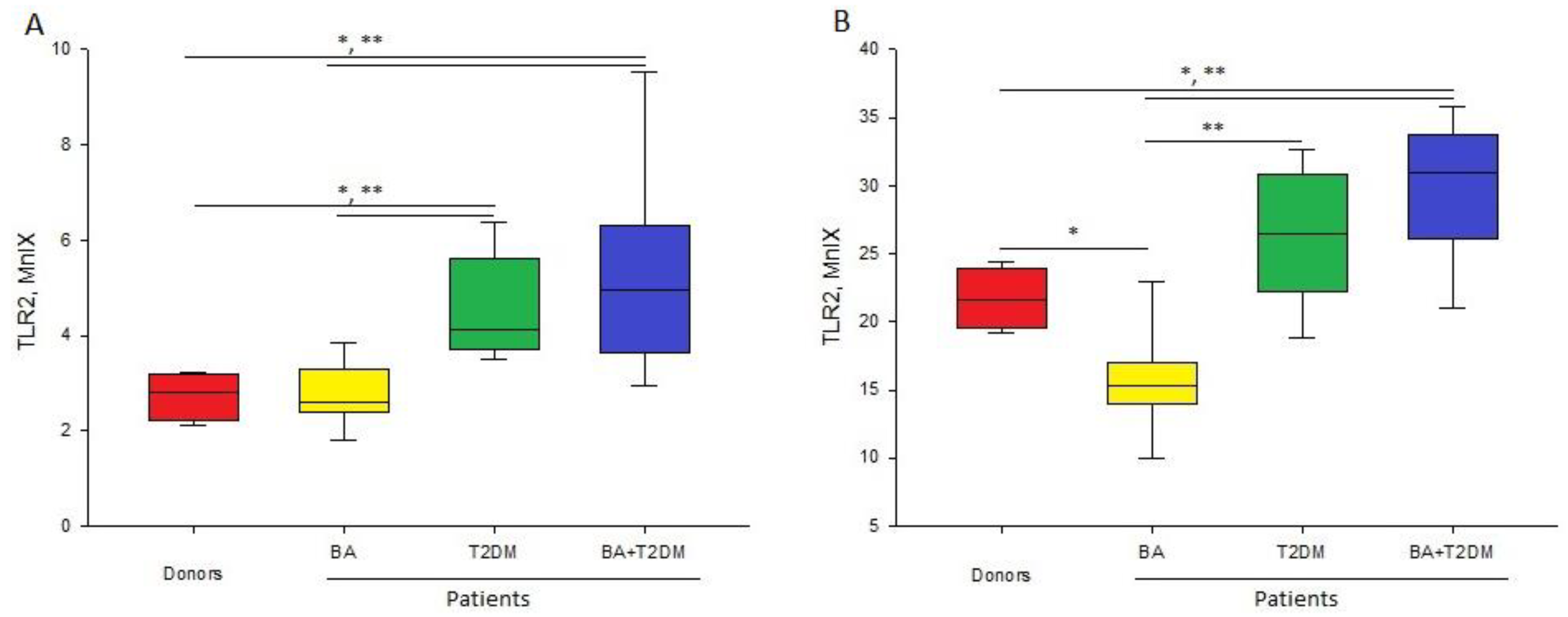
3.2. Expression of TLR2 Receptor
3.3. Expression of TLR4 Receptor
3.4. Influence of Endotoxin and Lipoteichoic Acid on the Synthesis of Cytokines by Blood Cells
- TNF-α
- IL-6
- IL-8
- IL-1β
3.5. Role of the Expression Level of TLR2 and TLR4 Receptors in Cytokine Synthesis
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Russo, M. Dual role of toll-like receptors in human and experimental asthma models. Front. Immunol. 2018, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.M.; Souza, M.D.S.; Coelho, A.C.C.; de Mello, L.M.; Souza-Machado, C. Association between Asthma and Type 2 Diabetes Mellitus: Mechanisms and Impact on Asthma Control—A Literature Review. Can. Respir. J. 2021, 2021, 8830439. [Google Scholar] [CrossRef]
- Kosmalski, M.; Różycka-Kosmalska, M.; Witusik, A.; Pietras, T. The coincidence of diabetes mellitus and asthma, their probable causal relationships and therapeutic opportunities. Adv. Respir. Med. 2020, 88, 590–598. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, T. Prevalence and influential factors for asthma among adults in Chinese. Zhong Nan Da Xue Xue Bao. Yi Xue Ban= J. Cent. South Univ. Med. Sci. 2017, 42, 1086–1093. [Google Scholar]
- Adeyeye, O.O.; Ogbera, A.O.; Ogunleye, O.O.; Brodie-Mens, A.T.; Abolarinwa, F.F.; Bamisile, R.T.; Onadeko, B.O. Understanding asthma and the metabolic syndrome-a Nigerian report. Int. Arch. Med. 2012, 5, 1. [Google Scholar] [CrossRef]
- Mueller, N.T.; Koh, W.P.; Odegaard, A.O.; Gross, M.D.; Yuan, J.M.; Pereira, M.A. Asthma and the risk of type 2 diabetes in the Singapore Chinese Health Study. Diabetes Res. Clin. Pract. 2013, 99, 192–199. [Google Scholar] [CrossRef]
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol. Res. 2017, 119, 195–207. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Worldwide asthma epidemiology: Insights from the Global Health Data Exchange database. Int. Forum Allergy Rhinol. 2020, 10, 75–80. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Bettoncelli, G.; Novelli, L.; Cricelli, C.; Rogliani, P. Asthma and comorbid medical illness. Eur. Respir. J. 2011, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Leto, G.; Mastrolorenzo, E.; Para, O.; Giordano, M. Influence of gender in diabetes mellitus and its complication. Int. J. Mol. Sci. 2022, 23, 8850. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.R.; Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Singh, D. Personalized treatment of asthma: The importance of sex and gender differences. J. Allergy Clin. Immunol. Pract. 2022, 10, 963–971.e3. [Google Scholar] [CrossRef]
- Jialal, I.; Huet, B.A.; Kaur, H.; Chien, A.; Devaraj, S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care 2012, 35, 900–904. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Wifi, M.N.A.; Assem, M.; Elsherif, R.H.; El-Azab, H.A.F.; Saif, A. Toll-like receptors-2 and-9 (TLR2 and TLR9) gene polymorphism in patients with type 2 diabetes and diabetic foot. Medicine 2017, 96, e6760. [Google Scholar] [CrossRef]
- Khoreva, M.V.; Latisheva, T.V.; Ogurtsova, A.D.; Gracheva, L.A.; Zakharov, M.V.; Switich, O.A.; Gankovskaya, L.V. Expression and functional activity of tlr2 and tlr4 in patients with allergic bronchial asthma. Russ. J. Immunol. 2019, 22, 614–616. [Google Scholar]
- Gankovskaya, L.V.; Namazova-Baranova, L.S.; Khoreva, M.V.; Bragvadze, B.G.; Ogurtsova, A.D.; Alekseeva, A.A.; Gankovskii, V.A.; Svitich, O.A. Expression features of Toll-like receptor 2 and Toll-like receptor 4 in children with asthma. Meditsinskaya Immunol. 2017, 19, 431. [Google Scholar] [CrossRef]
- Hasannejad, H.; Takahashi, R.; Kimishima, M.; Hayakawa, K.; Shiohara, T. Selective impairment of Toll-like receptor 2–mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J. Allergy Clin. Immunol. 2007, 120, 69–75. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Gupta, S.; Maratha, A.; Siednienko, J.; Natarajan, A.; Gajanayake, T.; Hoashi, S.; Miggin, S. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Sci. Rep. 2017, 7, 7623. [Google Scholar] [CrossRef]
- Su, S.C.; Hua, K.F.; Lee, H.; Chao, L.K.; Tan, S.K.; Yang, S.F.; Hsu, H.Y. LTA and LPS mediated activation of protein kinases in the regulation of inflammatory cytokines expression in macrophages. Clin. Chim. Acta 2006, 374, 106–115. [Google Scholar] [CrossRef]
- Koch, L.; Frommhold, D.; Buschmann, K.; Kuss, N.; Poeschl, J.; Ruef, P. LPS-and LTA-induced expression of IL-6 and TNF-α in neonatal and adult blood: Role of MAPKs and NF-κB. Mediat. Inflamm. 2014, 2014, 283126. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Mouratis, M.A.; Lepper, P.M.; Haston, R.M.; Baldwin, F.; Lowes, S.; Ahmed, M.A.E.; Schumann, C.; Boyd, O.; Triantafilou, K. Serum proteins modulate lipopolysaccharide and lipoteichoic acid-induced activation and contribute to the clinical outcome of sepsis. Virulence 2012, 3, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Madalina, M.; Deleanu, M.; Manduteanu, I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of g. Int. J. Nanomed. 2018, 13, 63–76. [Google Scholar]
- Lad, N.; Murphy, A.M.; Parenti, C.; Nelson, C.P.; Williams, N.C.; Sharpe, G.R.; McTernan, P.G. Asthma and obesity: Endotoxin another insult to add to injury? Clin. Sci. 2021, 135, 2729–2748. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; von Känel, R.; Rohleder, N.; Fischer, J.E. Monocyte proinflammatory cytokine release is higher and glucocorticoid sensitivity is lower in middle aged men than in women independent of cardiovascular risk factors. Heart 2004, 90, 853–858. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, S.E.; Han, K.; Koh, E.H. Association between diabetes and asthma: Evidence from a nationwide Korean study. Ann. Allergy Asthma Immunol. 2018, 121, 699–703. [Google Scholar] [CrossRef]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell. Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Chun, E.; Lee, S.H.; Lee, S.Y.; Shim, E.J.; Cho, S.H.; Min, K.U.; Kim, Y.Y.; Park, H.W. Toll-like receptor expression on peripheral blood mononuclear cells in asthmatics; implications for asthma management. J. Clin. Immunol. 2010, 30, 459–464. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Taha, I.M.; Allah AM, A.; Abd El Gayed, E.M. Expression of toll-like receptor 4 and its connection with type 2 diabetes mellitus. Cell. Mol. Biol. 2018, 64, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Meng, Q.; Ji, J.; Lou, X.; Zhang, L. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci. Lett. 2015, 585, 28–32. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Patients with BA | Patients with T2DM | Patients with BA + T2DM | Ref. Value |
|---|---|---|---|---|
| Age, years | 48 ± 1.7 | 64 ± 2 | 64.9 ± 3 | - |
| Weight, kg | 69.8 ± 4.8 | 82.4 ± 4.1 | 80.7 ± 2 | - |
| Height, cm | 165.7 ± 2.6 | 160.5 ± 2 | 162 ± 2.2 | - |
| BMI | 25.3 ± 1.5 | 31.9 ± 1.6 | 30.3 ± 0.5 | 18.5–25 |
| Glucose, mM | 5 ± 0.2 | 8.25 ± 0.96 | 6.9 ± 0.5 | 3.9–6.1 |
| Glycated HB, mM | - | 7.9 ± 0.5 | 7 ± 0.1 | 6.5–7.5 |
| Cholesterol, mM | 6 ± 0.4 | 5.1 ± 0.3 | 5.56 ± 0.5 | 3.3–5.5 |
| Triglycerides, mM | 1.6 ± 0.15 | 1.4 ± 0.1 | 2.9 ± 1.3 | 0.44–2.29 |
| HDL, mM | 1.7 ± 0.07 | 1.3 ± 0.1 | 1.5 ± 0.2 | 0.9–3 |
| Cholesterol LDL, mM | 3.8 ± 0.2 | 3.23 ± 0.33 | 3.4 ± 0.5 | 1.6–3.5 |
| CRP, mg/dL | 0 ± 0.01 | 0.36 ± 0.17 | 0.27 ± 0.06 | 0–0.5 |
| FEV1, % | 78.8 ± 4.3 | 102.5 ± 2.2 | 81.1 ± 3.9 | >80 |
| SaO2, % | 97.1 ± 0.3 | 96.9 ± 0.2 | 95.9 ± 0.4 | >96 |
| Cell Type | Conditionally Healthy Donors | Patients with BA | Patients with T2DM | Patients with BA + T2DM |
|---|---|---|---|---|
| Lymphocytes | 1.2 | 1.3 | 1.3 | 1.3 |
| [1.2; 1.3] | [1.2; 1.4] | [1.1; 1.6] | [1.2; 1.4] | |
| Neutrophils | 2.8 | 2.6 | 4.1 *,** | 4.9 *,** |
| [2.3; 3.1] | [2.4; 2.9] | [3.7; 5.6] | [3.6; 6.3] | |
| Monocytes | 21.6 | 15.2 * | 26.4 ** | 30.9 *,** |
| [19.9; 23.4] | [14.2; 16.3] | [22.2; 30.1] | [27.8; 33] |
| Cell Type | Conditionally Healthy Donors | Patients with BA | Patients with T2DM | Patients with BA + T2DM |
|---|---|---|---|---|
| Lymphocytes | 1.2 | 1.2 | 1.1 | 1.1 |
| [1.1; 1.3] | [1.2; 1.4] | [1; 1.3] | [1.1; 1.2] | |
| Neutrophils | 1.3 | 1.8 | 1.4 | 1.7 |
| [1.2; 2.3] | [1.3; 2] | [1; 2] | [1.3; 2.2] | |
| Monocytes | 1.8 | 2.3 * | 2.4 * | 2.9 * |
| [1.8; 1.9] | [2.2; 3.8] | [2.9; 10.2] | [1.9; 2.9] |
| Parameters | TLR2(M)—TNF-α | TLR2(M)—IL-6 | TLR2(M)—IL-1β | TLR2(N)—IL-8 |
|---|---|---|---|---|
| Donors | 0.4 | 0.8 | −0.6 | 0.4 |
| Patients with BA | 0.29 | 0.07 | 0.31 | 0.43 |
| Patients with T2DM | −0.2 | −0.1 | 0.19 | −0.05 |
| Patients with BA + T2DM | −0.26 | −0.09 | −0.66 | −0.8 |
| Parameters | TLR4(M)—TNF-α | TLR4(M)—IL-6 | TLR4(M)—IL-1β | TLR4(N)—IL-8 |
| Donors | 0.1 | 0.8 | −0.2 | 0.5 |
| Patients with BA | 0.29 | 0.38 | 0.38 | 0.86 * |
| Patients with T2DM | 0.1 | 0.06 | −0.19 | 0.36 |
| Patients with BA + T2DM | 0.26 | −0.66 | −0.49 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyukevich, Y.V.; Kosyakova, N.I.; Prokhorenko, I.R. Impact of Comorbidity of Bronchial Asthma and Type 2 Diabetes Mellitus on the Expression and Functional Activity of TLR2 and TLR4 Receptors. Life 2023, 13, 550. https://doi.org/10.3390/life13020550
Radzyukevich YV, Kosyakova NI, Prokhorenko IR. Impact of Comorbidity of Bronchial Asthma and Type 2 Diabetes Mellitus on the Expression and Functional Activity of TLR2 and TLR4 Receptors. Life. 2023; 13(2):550. https://doi.org/10.3390/life13020550
Chicago/Turabian StyleRadzyukevich, Yaroslav V., Ninel I. Kosyakova, and Isabella R. Prokhorenko. 2023. "Impact of Comorbidity of Bronchial Asthma and Type 2 Diabetes Mellitus on the Expression and Functional Activity of TLR2 and TLR4 Receptors" Life 13, no. 2: 550. https://doi.org/10.3390/life13020550
APA StyleRadzyukevich, Y. V., Kosyakova, N. I., & Prokhorenko, I. R. (2023). Impact of Comorbidity of Bronchial Asthma and Type 2 Diabetes Mellitus on the Expression and Functional Activity of TLR2 and TLR4 Receptors. Life, 13(2), 550. https://doi.org/10.3390/life13020550






