Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. GWAS SNPs Selection and Detection
2.3. Statistical Analysis
2.4. SNPs/Genes Predict Functions
3. Results
3.1. Functional Genomics Data for KOA-Involved SNPs
3.1.1. SNPs Correlations with Amino Acid Replacements and Epigenetic Changes
3.1.2. KOA-Associated SNPs as Gene Quantitative Traits (eQTL and sQTL) Potential Predictors
3.1.3. Potential Interactions and Biological Pathways of KOA Putative Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Xiao, L.; Wang, Z.; Zhi, L.; Li, Q. Common variants in GNL3 gene contributed the susceptibility of hand osteoarthritis in Han Chinese population. Sci. Rep. 2022, 12, 16110. [Google Scholar] [CrossRef]
- Madry, H.; Luyten, F.P.; Facchini, A. Biological aspects of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 407–422. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Available online: https://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause (accessed on 22 November 2022).
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Michael, J.W.; Schlüter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Arztebl. Int. 2010, 107, 152–162, Erratum in Dtsch. Arztebl. Int. 2010, 107, 294. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Ortolan, A.; Lorenzin, M.; Oliviero, F.; Doria, A.; Scanzello, C.R.; Ramonda, R. Erosive hand osteoarthritis: Latest findings and outlook. Nat. Rev. Rheumatol. 2022, 18, 171–183. [Google Scholar] [CrossRef]
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344, Erratum in Lancet 2017, 390, e38.. [Google Scholar] [CrossRef]
- Losina, E.; Paltiel, A.D.; Weinstein, A.M.; Yelin, E.; Hunter, D.J.; Chen, S.P.; Klara, K.; Suter, L.G.; Solomon, D.H.; Burbine, S.A.; et al. Lifetime medical costs of knee osteoarthritis management in the United States: Impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015, 67, 203–215. [Google Scholar] [CrossRef]
- Gunaratne, R.; Pratt, D.N.; Banda, J.; Fick, D.P.; Khan, R.J.K.; Robertson, B.W. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J. Arthroplasty 2017, 32, 3854–3860. [Google Scholar] [CrossRef]
- Barbour, K.E.; Helmick, C.G.; Boring, M.; Brady, T.J. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation—United States, 2013-2015. MMWR Morb. Mortal. Wkly Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef]
- Tan, J.S.; Tikoft, E.; O’Sullivan, P.; Smith, A.; Campbell, A.; Caneiro, J.P.; Kent, P. The Relationship Between Changes in Movement and Activity Limitation or Pain in People with Knee Osteoarthritis: A Systematic Review. J. Orthop. Sports Phys. Ther. 2021, 51, 492–502. [Google Scholar] [CrossRef]
- Gaudreault, N.; Maillette, P.; Coutu, M.F.; Durand, M.J.; Hagemeister, N.; Hébert, L.J. Work disability among workers with osteoarthritis of the knee: Risks factors, assessment scales, and interventions. Int. J. Rehabil. Res. 2014, 37, 290–296. [Google Scholar] [CrossRef]
- Zengini, E.; Finan, C.; Wilkinson, J.M. The Genetic Epidemiological Landscape of Hip and Knee Osteoarthritis: Where Are We Now and Where Are We Going? J. Rheumatol. 2016, 43, 260–266. [Google Scholar] [CrossRef]
- Klein, J.C.; Keith, A.; Rice, S.J.; Shepherd, C.; Agarwal, V.; Loughlin, J.; Shendure, J. Functional testing of thousands of osteoarthritis-associated variants for regulatory activity. Nat. Commun. 2019, 10, 2434. [Google Scholar] [CrossRef]
- Aubourg, G.; Rice, S.J.; Bruce-Wootton, P.; Loughlin, J. Genetics of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 636–649. [Google Scholar] [CrossRef]
- GWAS Catalog. Available online: https://www.ebi.ac.uk/gwas/search?query=osteoarthritis,%20knee (accessed on 22 November 2022).
- Meulenbelt, I.; Chapman, K.; Dieguez-Gonzalez, R.; Shi, D.; Tsezou, A.; Dai, J.; Malizos, K.N.; Kloppenburg, M.; Carr, A.; Nakajima, M.; et al. Large replication study and meta-analyses of DVWA as an osteoarthritis susceptibility locus in European and Asian populations. Hum. Mol. Genet. 2009, 18, 1518–1523. [Google Scholar] [CrossRef]
- Shi, D.; Zheng, Q.; Chen, D.; Zhu, L.; Qin, A.; Fan, J.; Liao, J.; Xu, Z.; Lin, Z.; Norman, P.; et al. Association of single-nucleotide polymorphisms in HLA class II/III region with knee osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1454–1457. [Google Scholar] [CrossRef]
- Valdes, A.M.; Styrkarsdottir, U.; Doherty, M.; Morris, D.L.; Mangino, M.; Tamm, A.; Doherty, S.A.; Kisand, K.; Kerna, I.; Tamm, A.; et al. Large scale replication study of the association between HLA class II/BTNL2 variants and osteoarthritis of the knee in European-descent populations. PLoS ONE 2011, 6, e23371. [Google Scholar] [CrossRef]
- Nakajima, M.; Shi, D.; Dai, J.; Tsezou, A.; Zheng, M.; Norman, P.E.; Chou, C.H.; Lee, M.T.; Hwang, J.Y.; Kim, D.H.; et al. A large-scale replication study for the association of rs17039192 in HIF-2α with knee osteoarthritis. J. Orthop. Res. 2012, 30, 1244–1248. [Google Scholar] [CrossRef]
- Dai, J.; Ying, P.; Shi, D.; Hou, H.; Sun, Y.; Xu, Z.; Chen, D.; Zhang, G.; Ni, M.; Teng, H.; et al. FTO variant is not associated with osteoarthritis in the Chinese Han population: Replication study for a genome-wide association study identified risk loci. J. Orthop. Surg. Res. 2018, 13, 65. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, J.; Ma, C.; Wei, J.; Wei, B.; Liu, J. Evaluation of Relationship Between Common Variants in FGF18 Gene and Knee Osteoarthritis Susceptibility. Arch. Med. Res. 2020, 51, 76–81. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Xu, X.; Zhang, H.; Lu, M.; Gao, W.; Yin, L.; Yin, Z. A novel variant near LSP1P3 is associated with knee osteoarthritis in the Chinese population. Clin. Rheumatol. 2020, 39, 2393–2398. [Google Scholar] [CrossRef]
- Litovkina, O.; Nekipelova, E.; Dvornyk, V.; Polonikov, A.; Efremova, O.; Zhernakova, N.; Reshetnikov, E.; Churnosov, M. Genes involved in the regulation of vascular homeostasis determine renal survival rate in patients with chronic glomerulo-nephritis. Gene 2014, 546, 112–116. [Google Scholar] [CrossRef]
- Reshetnikov, E.A.; Akulova, L.Y.; Dobrodomova, I.S.; Dvornyk, V.Y.; Polonikov, A.V.; Churnosov, M.I. The insertion-deletion polymorphism of the ACE gene is associated with increased blood pressure in women at the end of pregnancy. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 623–632. [Google Scholar] [CrossRef]
- Novakov, V.; Novakova, O.; Churnosova, M.; Aristova, I.; Sorokina, I.; Polonikov, A.; Reshetnikov, E.; Churnosov, M. The pronounced effect of obesity on the association of the rs143384 GDF5 with knee osteoarthritis. Life 2022, in press. [Google Scholar]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D. Criteria for classification of clinical osteoarthritis. J. Rheumatol. Suppl. 1991, 27, 10–12. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef]
- arcOGEN Consortium; arcOGEN Collaborators; Zeggini, E.; Panoutsopoulou, K.; Southam, L.; Rayner, N.W.; Day-Williams, A.G.; Lopes, M.C.; Boraska, V.; Esko, T.; et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): A genome-wide association study. Lancet 2012, 380, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Lund, S.H.; Thorleifsson, G.; Zink, F.; Stefansson, O.A.; Sigurdsson, J.K.; Juliusson, K.; Bjarnadottir, K.; Sigurbjornsdottir, S.; Jonsson, S.; et al. Meta-analysis of Icelandic and UK data sets identifies missense variants in SMO, IL11, COL11A1 and 13 more new loci associated with osteoarthritis. Nat. Genet. 2018, 50, 1681–1687. [Google Scholar] [CrossRef]
- Zengini, E.; Hatzikotoulas, K.; Tachmazidou, I.; Steinberg, J.; Hartwig, F.P.; Southam, L.; Hackinger, S.; Boer, C.G.; Styrkarsdottir, U.; Gilly, A.; et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat. Genet. 2018, 50, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Stefansson, O.A.; Gunnarsdottir, K.; Thorleifsson, G.; Lund, S.H.; Stefansdottir, L.; Juliusson, K.; Agustsdottir, A.B.; Zink, F.; Halldorsson, G.H.; et al. GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nat. Commun. 2019, 10, 2054, Erratum in Nat. Commun. 2019, 10, 2358. [Google Scholar] [CrossRef] [PubMed]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.G.; Hatzikotoulas, K.; Southam, L.; Stefánsdóttir, L.; Zhang, Y.; Coutinho de Almeida, R.; Wu, T.T.; Zheng, J.; Hartley, A.; Teder-Laving, M.; et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell 2021, 184, 4784–4818.e17, Erratum in Cell 2021, 184, 6003–6005. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Bushueva, O.Y.; Bulgakova, I.V.; Freidin, M.B.; Churnosov, M.I.; Solodilova, M.A.; Shvetsov, Y.D.; Ivanov, V.P. A comprehensive contribution of genes for aryl hydrocarbon receptor signaling pathway to hypertension susceptibility. Pharm. Genom. 2017, 27, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Verzilina, I.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with uterine leiomyoma. Front. Genet. 2021, 11, 512940. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef]
- Eliseeva, N.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. LOXL1 gene polymorphism candidates for exfoliation glaucoma are also associated with a risk for primary open-angle glaucoma in a Caucasian population from central Russia. Mol. Vis. 2021, 27, 262–269. [Google Scholar]
- Ponomarenko, I.; Reshetnikov, E.; Altuchova, O.; Polonikov, A.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Golovchenko, O.; Churnosov, M. Association of genetic polymorphisms with age at menarche in Russian women. Gene 2019, 686, 228–236. [Google Scholar] [CrossRef]
- Reshetnikov, E.; Zarudskaya, O.; Polonikov, A.; Bushueva, O.; Orlova, V.; Krikun, E.; Dvornyk, V.; Churnosov, M. Genetic markers for inherited thrombophilia are associated with fetal growth retardation in the population of Central Russia. J. Obstet. Gynaecol. Res. 2017, 43, 1139–1144. [Google Scholar] [CrossRef]
- Moskalenko, M.I.; Milanova, S.N.; Ponomarenko, I.V.; Polonikov, A.V.; Churnosov, M.I. Study of associations of polymorphism of matrix metalloproteinases genes with the development of arterial hypertension in men. Kardiologiia 2019, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, O.; Abramova, M.; Ponomarenko, I.; Reshetnikov, E.; Aristova, I.; Polonikov, A.; Dvornyk, V.; Churnosov, M. Functionally significant polymorphisms of ESR1and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Starikova, D.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Novel data about association of the functionally significant polymorphisms of the MMP9 gene with exfoliation glaucoma in the caucasian population of Central Russia. Ophthalmic Res. 2021, 64, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Bushueva, O.; Solodilova, M.; Churnosov, M.; Ivanov, V.; Polonikov, A. The Flavin-Containing Monooxygenase 3 Gene and Essential Hypertension: The Joint Effect of Polymorphism E158K and Cigarette Smoking on Disease Susceptibility. Int. J. Hypertens. 2014, 2014, 712169. [Google Scholar] [CrossRef]
- Tikunova, E.; Ovtcharova, V.; Reshetnikov, E.; Dvornyk, V.; Polonikov, A.; Bushueva, O.; Churnosov, M. Genes of tumor necrosis factors and their receptors and the primary open angle glaucoma in the population of Central Russia. Int. J. Ophthalmol. 2017, 10, 1490–1494. [Google Scholar] [CrossRef]
- Pavlova, N.; Demin, S.; Churnosov, M.; Reshetnikov, E.; Aristova, I.; Churnosova, M.; Ponomarenko, I. The Modifying Effect of Obesity on the Association of Matrix Metalloproteinase Gene Polymorphisms with Breast Cancer Risk. Biomedicines 2022, 10, 2617. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with endometriosis. Reprod. Biomed. Online 2020, 41, 943–956. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Calle, M.L.; Urrea, V.; Malats, N.; Van Steen, K. Mbmdr: An R package for exploring gene-gene interactions associated with binary or quantitative traits. Bioinformatics 2010, 26, 2198–2199. [Google Scholar] [CrossRef]
- Ponomarenko, I.V. Using the method of Multifactor Dimensionality Reduction (MDR) and its modifications for analysis of gene-gene and gene-environment interactions in genetic-epidemiological studies (review). Res. Results Biomed. 2019, 5, 4–21. [Google Scholar] [CrossRef]
- Reshetnikov, E.; Ponomarenko, I.; Golovchenko, O.; Sorokina, I.; Batlutskaya, I.; Yakunchenko, T.; Dvornyk, V.; Polonikov, A.; Churnosov, M. The VNTR polymorphism of the endothelial nitric oxide synthase gene and blood pressure in women at the end of pregnancy. Taiwan J. Obstet. Gynecol. 2019, 58, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Minyaylo, O.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Functionally significant polymorphisms of the MMP-9 gene are associated with peptic ulcer disease in the Caucasian population of Central Russia. Sci. Rep. 2021, 11, 13515. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Jack, J.R.; Motsinger-Reif, A.A.; Brown, C.C. An adaptive permutation approach for genome-wide association study: Evaluation and recommendations for use. BioData Min. 2014, 7, 9. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with endometrial hyperplasia. Gene 2020, 757, 144933. [Google Scholar] [CrossRef] [PubMed]
- Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci. Rep. 2021, 11, 5224. [Google Scholar] [CrossRef]
- Pavlova, N.; Demin, S.; Churnosov, M.; Reshetnikov, E.; Aristova, I.; Churnosova, M.; Ponomarenko, I. Matrix Metalloproteinase Gene Polymorphisms Are Associated with Breast Cancer in the Caucasian Women of Russia. Int. J. Mol. Sci. 2022, 23, 12638. [Google Scholar] [CrossRef] [PubMed]
- SOURCEFORGE. Available online: http://sourceforge.net/projects/mdr (accessed on 22 November 2022).
- Churnosov, M.; Abramova, M.; Reshetnikov, E.; Lyashenko, I.V.; Efremova, O.; Churnosova, M.; Ponomarenko, I. Polymorphisms of hypertension susceptibility genes as a risk factors of preeclampsia in the Caucasian population of central Russia. Placenta 2022, 129, 51–61. [Google Scholar] [CrossRef]
- Golovchenko, I.; Aizikovich, B.; Golovchenko, O.; Reshetnikov, E.; Churnosova, M.; Aristova, I.; Ponomarenko, I.; Churnosov, M. Sex Hormone Candidate Gene Polymorphisms Are Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 13691. [Google Scholar] [CrossRef]
- Abramova, M.; Churnosova, M.; Efremova, O.; Aristova, I.; Reshetnikov, E.; Polonikov, A.; Churnosov, M.; Ponomarenko, I. Effects of pre-pregnancy over-weight/obesity on the pattern of association of hypertension susceptibility genes with preeclampsia. Life 2022, 12, 2018. [Google Scholar] [CrossRef]
- Zhabin, S.N. Association of the rs10508336 polymorphism of the TAF3 transcription factor gene with the risk of lower limb arterial disease in the Russian population. Res. Results Biomed. 2022, 8, 439–447. [Google Scholar] [CrossRef]
- Polonikov, A.; Kharchenko, A.; Bykanova, M.; Sirotina, S.; Ponomarenko, I.; Bocharova, A.; Vagaytseva, K.; Stepanov, V.; Bushueva, O.; Churnosov, M.; et al. Polymorphisms of CYP2C8, CYP2C9 and CYP2C19 and risk of coronary heart disease in Russian population. Gene 2017, 627, 451–459. [Google Scholar] [CrossRef]
- Polonikov, A.; Rymarova, L.; Klyosova, E.; Volkova, A.; Azarova, I.; Bushueva, O.; Bykanova, M.; Bocharova, I.; Zhabin, S.; Churnosov, M.; et al. Matrix metalloproteinases as target genes for gene regulatory networks driving molecular and cellular pathways related to a multistep pathogenesis of cerebrovascular disease. J. Cell Biochem. 2019, 120, 16467–16482. [Google Scholar] [CrossRef]
- Sirotina, S.; Ponomarenko, I.; Kharchenko, A.; Bykanova, M.; Bocharova, A.; Vagaytseva, K.; Stepanov, V.; Churnosov, M.; Solodilova, M.; Polonikov, A. A Novel Polymorphism in the Promoter of the CYP4A11 Gene Is Associated with Susceptibility to Coronary Artery Disease. Dis. Markers. 2018, 2018, 5812802. [Google Scholar] [CrossRef]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef]
- Westra, H.J.; Peters, M.J.; Esko, T.; Yaghootkar, H.; Schurmann, C.; Kettunen, J.; Christiansen, M.W.; Fairfax, B.P.; Schramm, K.; Powell, J.E.; et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 2013, 45, 1238–1243. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 7, 1073–1081. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef]
- Rice, S.J.; Cheung, K.; Reynard, L.N.; Loughlin, J. Discovery and analysis of methylation quantitative trait loci (mQTLs) mapping to novel osteoarthritis genetic risk signals. Osteoarthr. Cartil. 2019, 27, 1545–1556. [Google Scholar] [CrossRef]
- Rice, S.J.; Beier, F.; Young, D.A.; Loughlin, J. Interplay between genetics and epigenetics in osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 268–281. [Google Scholar] [CrossRef]
- Lopez-Rodríguez, C.; Aramburu, J.; Rakeman, A.S.; Rao, A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. USA 1999, 96, 7214–7219. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.; Han, E.J.; Hong, B.K.; Choi, S.Y.; Kwon, H.M.; Hwang, S.Y.; Cho, C.S.; Kim, W.U. The xanthine oxidase-NFAT5 pathway regulates macrophage activation and TLR-induced inflammatory arthritis. Eur. J. Immunol. 2014, 44, 2721–2736. [Google Scholar] [CrossRef]
- Cen, L.; Xing, F.; Xu, L.; Cao, Y. Potential Role of Gene Regulator NFAT5 in the Pathogenesis of Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 6927429. [Google Scholar] [CrossRef]
- Gwon, D.H.; Kim, S.I.; Lee, S.H.; Noh, C.; Kim, Y.; Yun, S.; Lee, W.H.; Oh, J.Y.; Kim, D.W.; Hong, J.; et al. NFAT5 Deficiency Alleviates Formalin-Induced Inflammatory Pain Through mTOR. Int. J. Mol. Sci. 2021, 22, 2587. [Google Scholar] [CrossRef]
- Buxadé, M.; Lunazzi, G.; Minguillón, J.; Iborra, S.; Berga-Bolaños, R.; Del Val, M.; Aramburu, J.; López-Rodríguez, C. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J. Exp. Med. 2012, 209, 379–393. [Google Scholar] [CrossRef]
- Lee, N.; Kim, D.; Kim, W.U. Role of NFAT5 in the Immune System and Pathogenesis of Autoimmune Diseases. Front. Immunol. 2019, 10, 270. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Pope, R.M. The role of toll-like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 357–364. [Google Scholar] [CrossRef]
- Yoon, H.J.; You, S.; Yoo, S.A.; Kim, N.H.; Kwon, H.M.; Yoon, C.H.; Cho, C.S.; Hwang, D.; Kim, W.U. NF-AT5 is a critical regulator of inflammatory arthritis. Arthritis Rheum. 2011, 63, 1843–1852. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Mills, S.T.; Jones, K.A.; Ho, S.N.; Pavlath, G.K. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J. Cell Sci. 2007, 120, 149–159. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Ritter, S.Y.; Wright, J.; Tsang, K.; Hu, D.; Glimcher, L.H.; Aliprantis, A.O. NFATc1 and NFATc2 repress spontaneous osteoarthritis. Proc. Natl. Acad. Sci. USA 2013, 110, 19914–19919. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, B.; Chen, L.; Sun, J. Role of nuclear factor of activated T cells 1 in the pathogenesis of osteoarthritis. Exp. Ther. Med. 2014, 7, 195–198. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, Q.; Budden, T.; Wang, J. NFAT1 protects articular cartilage against osteoarthritic degradation by directly regulating transcription of specific anabolic and catabolic genes. Bone Joint Res. 2019, 8, 90–100. [Google Scholar] [CrossRef]
- Liang, X.Q.; Du, Y.P.; Wang, D.L.; Yang, Y. The biological functions of lysine methyltransferase PR-SET7. Yi Chuan 2013, 35, 241–254. (In Chinese) [Google Scholar] [CrossRef]
- Kumarasinghe, D.D.; Hopwood, B.; Kuliwaba, J.S.; Atkins, G.J.; Fazzalari, N.L. An update on primary hip osteoarthritis including altered Wnt and TGF-β associated gene expression from the bony component of the disease. Rheumatology 2011, 50, 2166–2175. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, S.; Lin, W.; Wang, L.; Ying, J.; Ding, Y.; Chen, X. Roles of Cell Cyle Regulators Cyclin D1, CDK4, and p53 in Knee Osteoarthritis. Genet. Test. Mol. Biomark. 2016, 20, 529–534. [Google Scholar] [CrossRef]
- Madzuki, I.N.; Lau, S.F.; Mohamad Shalan, N.A.A.; Mohd Ishak, N.I.; Mohamed, S. Does cartilage ERα overexpression correlate with osteoarthritic chondrosenescence? Indications from Labisia pumila OA mitigation. J. Biosci. 2019, 44, 100. [Google Scholar] [CrossRef]
- An, J.; Zhang, J.; Wang, Z.; Wang, K.; Liang, W.; Liu, X.; Zhou, H.; Zhang, H.; Wang, S. KMT5A Knockdown Suppresses Osteosarcoma Cell Proliferation and Metastasis Through Ꞵ-Catenin Signalling. Clin. Investig. Med. 2022, 45, E23–E31. [Google Scholar] [CrossRef]
- Liu, Y.; Yau, M.S.; Yerges-Armstrong, L.M.; Duggan, D.J.; Renner, J.B.; Hochberg, M.C.; Mitchell, B.D.; Jackson, R.D.; Jordan, J.M. Genetic Determinants of Radiographic Knee Osteoarthritis in African Americans. J. Rheumatol. 2017, 44, 1652–1658. [Google Scholar] [CrossRef]
- Yau, M.S.; Yerges-Armstrong, L.M.; Liu, Y.; Lewis, C.E.; Duggan, D.J.; Renner, J.B.; Torner, J.; Felson, D.T.; McCulloch, C.E.; Kwoh, C.K.; et al. Genome-Wide Association Study of Radiographic Knee Osteoarthritis in North American Caucasians. Arthritis Rheumatol. 2017, 69, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fontenla, C.; Calaza, M.; Evangelou, E.; Valdes, A.M.; Arden, N.; Blanco, F.J.; Carr, A.; Chapman, K.; Deloukas, P.; Doherty, M.; et al. Assessment of osteoarthritis candidate genes in a meta-analysis of nine genome-wide association studies. Arthritis Rheumatol. 2014, 66, 940–949. [Google Scholar] [CrossRef]
- Lindner, C.; Thiagarajah, S.; Wilkinson, J.M.; Panoutsopoulou, K.; Day-Williams, A.G.; arcOGEN Consortium; Cootes, T.F.; Wallis, G.A. Investigation of association between hip osteoarthritis susceptibility loci and radiographic proximal femur shape. Arthritis Rheumatol. 2015, 67, 2076–2084. [Google Scholar] [CrossRef]
- Barr, R.J.; Gregory, J.S.; Reid, D.M.; Aspden, R.M.; Yoshida, K.; Hosie, G.; Silman, A.J.; Alesci, S.; Macfarlane, G.J. Predicting OA progression to total hip replacement: Can we do better than risk factors alone using active shape modelling as an imaging biomarker? Rheumatology 2012, 51, 562–570. [Google Scholar] [CrossRef]
- Agricola, R.; Reijman, M.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; Weinans, H.; Waarsing, J.H. Total hip replacement but not clinical osteoarthritis can be predicted by the shape of the hip: A prospective cohort study (CHECK). Osteoarthr. Cartil. 2013, 21, 559–564. [Google Scholar] [CrossRef]
- Gee, F.; Clubbs, C.F.; Raine, E.V.; Reynard, L.N.; Loughlin, J. Allelic expression analysis of the osteoarthritis susceptibility locus that maps to chromosome 3p21 reveals cis-acting eQTLs at GNL3 and SPCS1. BMC Med. Genet. 2014, 15, 53. [Google Scholar] [CrossRef]
- Rushton, M.D.; Reynard, L.N.; Young, D.A.; Shepherd, C.; Aubourg, G.; Gee, F.; Darlay, R.; Deehan, D.; Cordell, H.J.; Loughlin, J. Methylation quantitative trait locus analysis of osteoarthritis links epigenetics with genetic risk. Hum. Mol. Genet. 2015, 24, 7432–7444. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, H.; Ma, W.; Gong, F.; Wang, X.; Duan, N.; Dang, X. Common variants in the GNL3 contribute to the increasing risk of knee osteoarthritis in Han Chinese population. Sci. Rep. 2018, 8, 9610. [Google Scholar] [CrossRef]
- Moll, T.; Shaw, P.J.; Cooper-Knock, J. Disrupted glycosylation of lipids and proteins is a cause of neurodegeneration. Brain 2020, 143, 1332–1340. [Google Scholar] [CrossRef]
- Baddoo, M.; Hill, K.; Wilkinson, R.; Gaupp, D.; Hughes, C.; Kopen, G.C.; Phinney, D.G. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell Biochem. 2003, 89, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Romanova, L.; Kellner, S.; Katoku-Kikyo, N.; Kikyo, N. Novel role of nucleostemin in the maintenance of nucleolar architecture and integrity of small nucleolar ribonucleoproteins and the telomerase complex. J. Biol. Chem. 2009, 284, 26685–26694. [Google Scholar] [CrossRef] [PubMed]
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=GNL3 (accessed on 22 November 2022).
- Zhu, Z.; Xie, J.; Manandhar, U.; Yao, X.; Bian, Y.; Zhang, B. RNA binding protein GNL3 up-regulates IL24 and PTN to promote the development of osteoarthritis. Life Sci. 2021, 267, 118926. [Google Scholar] [CrossRef]
- Louka, M.L.; Zakaria, Z.M.; Nagaty, M.M.; Elsebaie, M.A.; Nabil, L.M. Expression of nucleostemin gene in primary osteoarthritis. Gene 2016, 587, 27–32. [Google Scholar] [CrossRef]

| Parameters | KOA Patients ± SD/% (n) | Controls ± SD/% (n) | p |
|---|---|---|---|
| n | 500 | 500 | - |
| Men/Women | 41.60/58.40 (208/292) | 40.40/59.60 (202/298) | 0.75 |
| Age, years (min–max) | 52.69 ± 5.67 (40–68) | 52.96 ± 6.72 (40–70) | 0.76 |
| Height, cm | 169.30 ± 7.89 | 169.90 ± 7.61 | 0.42 |
| BMI, kg/m2 | 30.50 ± 5.05 | 26.04 ± 3.41 | <1 × 10−06 |
| Obesity (BMI ≥ 30) (yes) | 51.00 (255) | 13.40 (67) | 0.0005 |
| Alcohol (yes) | 76.00 (380) | 78.80 (394) | 0.33 |
| Smoker (yes) | 19.00 (95) | 21.00 (105) | 0.48 |
| Hereditary burden (yes) | 39.00 (195) | 14.60 (73) | 0.0005 |
| Occupation-related physical workload | |||
| Low | 18.40 (92) | 39.00 (195) | 0.0005 |
| Medium | 50.20 (251) | 45.20 (226) | 0.13 |
| High | 31.40 (157) | 15.80 (79) | 0.0005 |
| Leisure time physical activity | |||
| Little | 69.60 (348) | 56.40 (282) | 0.0005 |
| Irregular | 25.00 (125) | 31.00 (155) | 0.04 |
| Regular | 5.40 (27) | 12.60 (63) | 0.0007 |
| Concomitant pathology | |||
| Digestive system | 12.00 (60) | 10.20 (51) | 0.42 |
| Cardiovascular system | 36.80 (184) | 18.60 (93) | 0.0005 |
| Genitourinary system | 5.80 (29) | 5.20 (26) | 0.78 |
| Central nervous system | 10.40 (52) | 8.40 (42) | 0.33 |
| Musculoskeletal system | 7.80 (39) | 0(0) | 0.0005 |
| Endocrine system | 10.20 (51) | 6.00 (30) | 0.02 |
| Respiratory system | 11.80 (59) | 9.6 (48) | 0.31 |
| Other | 5.80 (29) | 5.00 (25) | 0.67 |
| SNP | Gene | Minor Allele | n | Allelic Model | Additive Model | Dominant Model | Recessive Model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | ||||||||
| L95 | U95 | L95 | U95 | L95 | U95 | L95 | U95 | ||||||||||||
| rs2820436 | LYPLAL1 | A | 998 | 0.82 | 0.68 | 0.99 | 0.042 | 0.77 | 0.60 | 0.99 | 0.038 | 0.76 | 0.56 | 1.06 | 0.115 | 0.58 | 0.33 | 1.00 | 0.052 |
| rs2820443 | LYPLAL1 | C | 984 | 1.04 | 0.85 | 1.27 | 0.700 | 0.94 | 0.73 | 1.21 | 0.617 | 0.95 | 0.69 | 1.31 | 0.749 | 0.83 | 0.46 | 1.52 | 0.552 |
| rs3771501 | TGFA | A | 997 | 1.06 | 0.88 | 1.26 | 0.552 | 1.00 | 0.80 | 1.24 | 0.968 | 1.01 | 0.72 | 1.40 | 0.965 | 0.96 | 0.66 | 1.44 | 0.902 |
| rs11177 | GNL3 | A | 1000 | 0.86 | 0.71 | 1.02 | 0.073 | 0.78 | 0.62 | 0.98 | 0.031 | 0.71 | 0.50 | 0.99 | 0.046 | 0.74 | 0.50 | 1.10 | 0.135 |
| rs6976 | GLT8D1 | T | 962 | 0.84 | 0.70 | 1.00 | 0.049 | 0.77 | 0.62 | 0.97 | 0.026 | 0.70 | 0.47 | 0.95 | 0.024 | 0.76 | 0.51 | 1.13 | 0.172 |
| rs1060105 | SBNO1 | T | 1000 | 1.04 | 0.84 | 1.29 | 0.703 | 1.18 | 0.91 | 1.54 | 0.202 | 1.14 | 0.82 | 1.56 | 0.444 | 1.75 | 0.91 | 3.35 | 0.092 |
| rs56116847 | SBNO1 | A | 998 | 0.93 | 0.77 | 1.11 | 0.414 | 0.86 | 0.68 | 1.08 | 0.200 | 0.84 | 0.61 | 1.16 | 0.296 | 0.77 | 0.47 | 1.24 | 0.283 |
| rs6499244 | NFAT5 | A | 999 | 1.02 | 0.86 | 1.22 | 0.790 | 1.13 | 0.91 | 1.41 | 0.269 | 1.29 | 0.91 | 1.82 | 0.156 | 1.07 | 0.73 | 1.56 | 0.730 |
| rs34195470 | WWP2 | A | 996 | 1.00 | 0.84 | 1.20 | 0.997 | 1.12 | 0.89 | 1.41 | 0.340 | 1.11 | 0.78 | 1.59 | 0.559 | 1.21 | 0.82 | 1.79 | 0.326 |
| rs143384 | GDF5 | G | 998 | 1.13 | 0.95 | 1.35 | 0.165 | 1.20 | 0.96 | 1.03 | 0.101 | 1.41 | 1.00 | 1.97 | 0.049 | 1.14 | 0.77 | 1.69 | 0.524 |
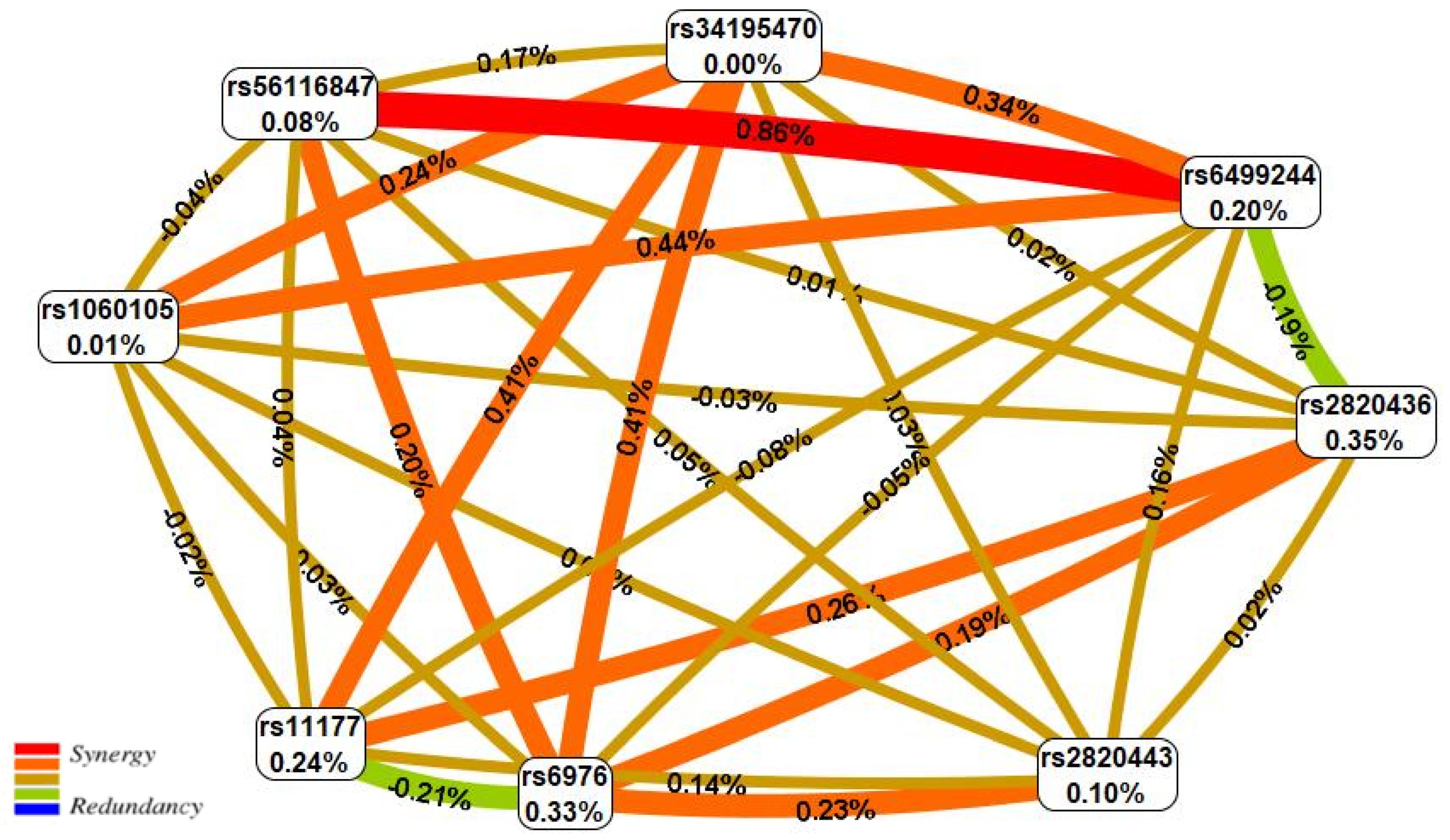
| N | SNP × SNP Interaction Models | NH | betaH | WH | NL | betaL | WL | pperm |
|---|---|---|---|---|---|---|---|---|
| Two-order interaction models (p < 9.25 × 10−04) | ||||||||
| 1 | rs11177 GNL3 × rs2820436 LYPLAL1 | 1 | 0.418 | 4.88 | 2 | −0.060 | 12.20 | 0.007 |
| 2 | rs6499244 NFAT5 × rs56116847 SBNO1 | 0 | - | - | 2 | −0.574 | 11.98 | 0.008 |
| 3 | rs34195470 WWP2 × rs6976 GLT8D1 | 3 | 0.561 | 13.33 | 1 | 0.786 | 4.98 | 0.010 |
| 4 | rs6976 GLT8D1 × rs2820443 LYPLAL1 | 3 | 0.567 | 11.50 | 1 | −0.475 | 5.18 | 0.014 |
| 5 | rs6976 GLT8D1 × rs2820436 LYPLAL1 | 1 | 0.467 | 5.85 | 2 | −0.989 | 10.97 | 0.018 |
| Three-order interaction models (p < 2.71 × 10−11) | ||||||||
| 1 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs6976 GLT8D1 | 1 | 0.558 | 4.12 | 5 | −1.279 | 44.38 | <0.001 |
| 2 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs11177 GNL3 | 2 | 0.670 | 9.04 | 5 | −1.198 | 39.69 | <0.001 |
| Four-order interaction models (p < 5.34 × 10−12) | ||||||||
| 1 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs6976 GLT8D1 × rs2820443 LYPLAL1 | 3 | 0.927 | 15.13 | 7 | −1.580 | 47.56 | <0.001 |
| 2 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs1060105 SBNO1 × rs6976 GLT8D1 | 4 | 1.187 | 16.97 | 7 | −1.377 | 43.43 | <0.001 |
| 3 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs11177 GNL3 × rs2820443 LYPLAL1 | 5 | 1.179 | 30.55 | 7 | −1.475 | 43.00 | <0.001 |
| 4 | rs6499244 NFAT5 × rs34195470 WWP2 × rs56116847 SBNO1 × rs6976 GLT8D1 | 5 | 1.289 | 24.91 | 7 | −1.518 | 42.00 | <0.001 |
| 5 | rs6499244 NFAT5 × rs56116847 SBNO1 × rs11177 GNL3 × rs6976 GLT8D1 | 2 | 0.670 | 9.04 | 5 | −1.257 | 41.00 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novakov, V.; Novakova, O.; Churnosova, M.; Sorokina, I.; Aristova, I.; Polonikov, A.; Reshetnikov, E.; Churnosov, M. Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia. Life 2023, 13, 405. https://doi.org/10.3390/life13020405
Novakov V, Novakova O, Churnosova M, Sorokina I, Aristova I, Polonikov A, Reshetnikov E, Churnosov M. Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia. Life. 2023; 13(2):405. https://doi.org/10.3390/life13020405
Chicago/Turabian StyleNovakov, Vitaly, Olga Novakova, Maria Churnosova, Inna Sorokina, Inna Aristova, Alexey Polonikov, Evgeny Reshetnikov, and Mikhail Churnosov. 2023. "Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia" Life 13, no. 2: 405. https://doi.org/10.3390/life13020405
APA StyleNovakov, V., Novakova, O., Churnosova, M., Sorokina, I., Aristova, I., Polonikov, A., Reshetnikov, E., & Churnosov, M. (2023). Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia. Life, 13(2), 405. https://doi.org/10.3390/life13020405








