Preliminary Findings of the Efficacy of Botulinum Toxin in Temporomandibular Disorders: Uncontrolled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Patient Description; Inclusion and Exclusion Criteria
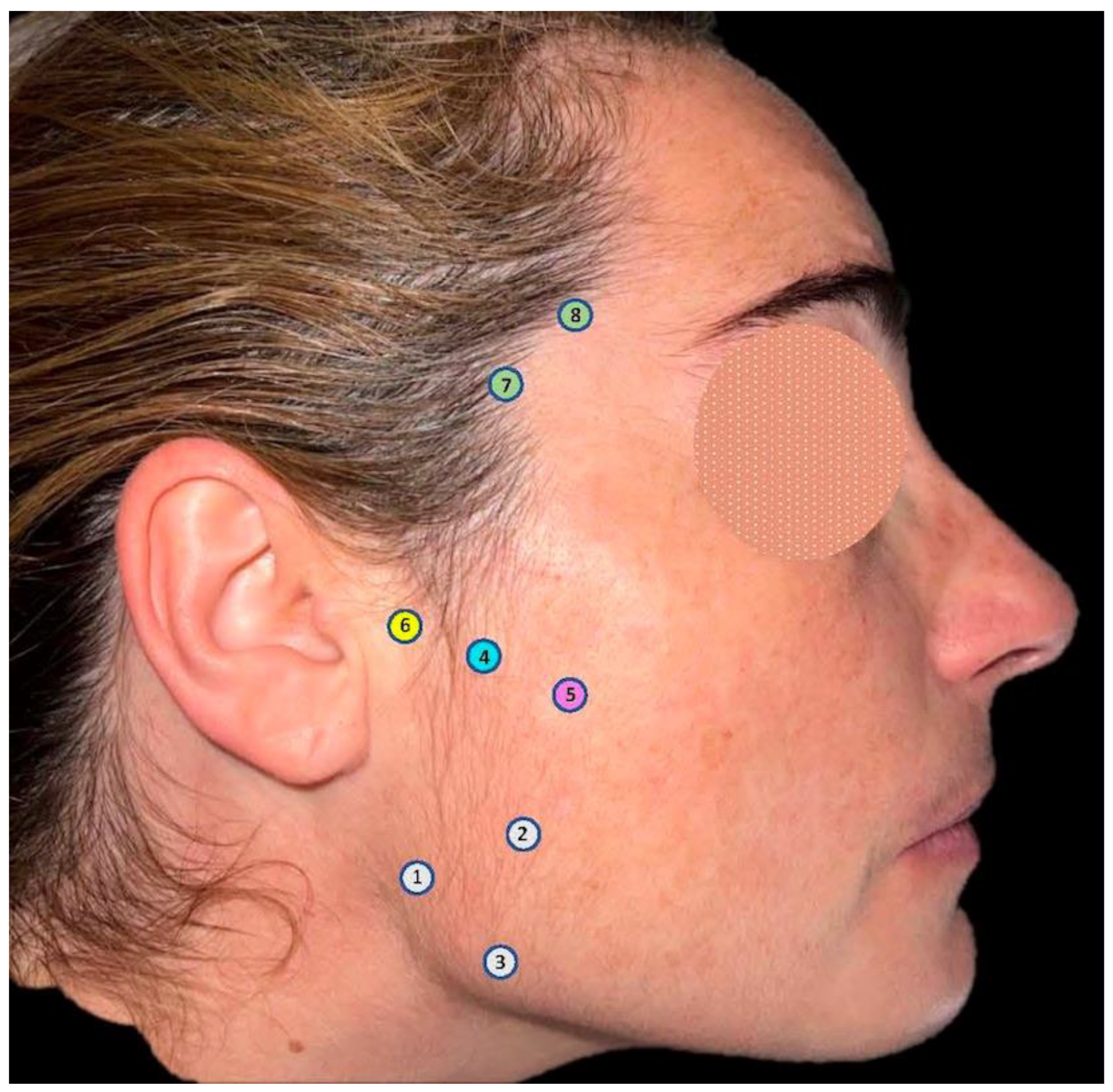
2.3. Treatments
2.4. Pain Measurement
2.5. Adverse Effect Assessment
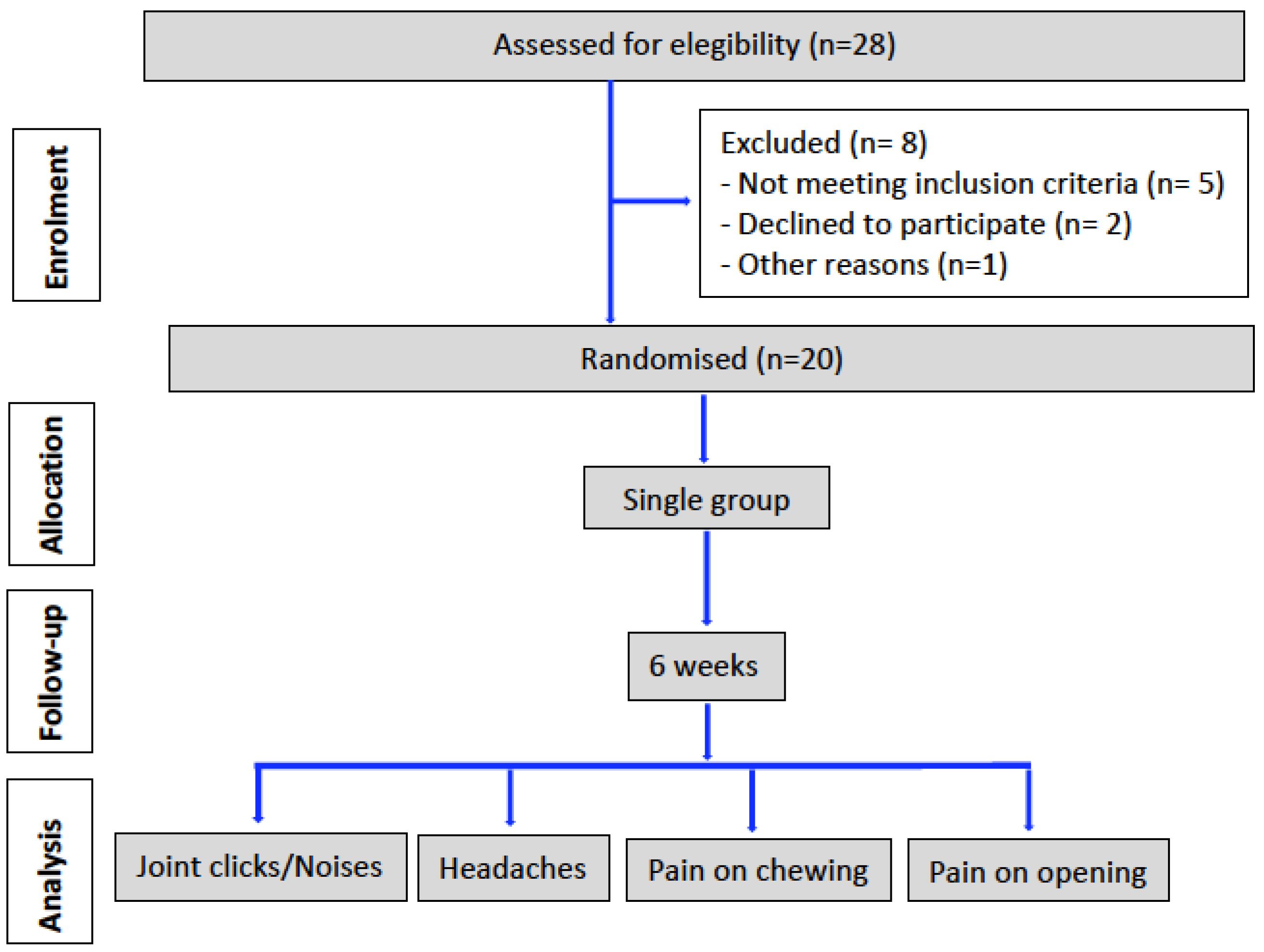
3. Results
3.1. Pain Reduction
3.2. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J.P. Current terminology and diagnostic classification schemes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 83, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Steinkeler, A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent. Clin. 2013, 57, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.Y.; Gavião, M.B.D.; Montes, A.B.M.; Marquezin, M.C.S.; Castelo, P.M. Evaluation of orofacial function in young subjects with temporomandibular disorders. J. Oral Rehabil. 2014, 41, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Tsukiyama, Y.; Yamazaki, M.; Clark, G.T. A review of temporomandibular disorder diagnostic techniques. J. Prosthet. Dent. 2001, 86, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician. 2015, 91, 378–386. [Google Scholar]
- Lomas, J.; Gurgenci, T.; Jackson, C.; Campbell, D. Temporomandibular dysfunction. Aust. J. Gen. Pract. 2018, 47, 212–215. [Google Scholar] [CrossRef]
- Shoohanizad, E.; Garajei, A.; Enamzadeh, A.; Yari, A. Nonsurgical management of temporomandibular joint autoimmune disorders. AIMS Public Health. 2019, 6, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Marotta, N.; Giudice, A.; Calafiore, D.; Curci, C.; Fortunato, L.; Ammendolia, A.; de Sire, A. Effects of Occlusal Splints on Spinal Posture in Patients with Temporomandibular Disorders: A Systematic Review. Healthcare 2022, 10, 739. [Google Scholar] [CrossRef]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: A systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022, 35, 921–936. [Google Scholar] [CrossRef]
- Ta, L.E.; Dionne, R.A. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: A randomized placebo-controlled comparison of celecoxib to naproxen. Pain 2004, 111, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.R.; Schiffman, E.L.; Look, J.O.; Rindal, D.B. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: A randomized clinical trial. J. Orofac. Pain. 2002, 16, 64–70. [Google Scholar] [PubMed]
- Hersh, E.V.; Balasubramaniam, R.; Pinto, A. Pharmacologic management of temporomandibular disorders. Oral Maxillofac Surg. Clin. N. Am. 2008, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef]
- Malgorzata, P.; Piotr, C.; Edward, K. The Mechanism of the Beneficial Effect of Botulinum Toxin Type a Used in the Treatment of Temporomandibular Joints Dysfunction. Mini. Rev. Med. Chem. 2017, 17, 445–450. [Google Scholar] [CrossRef]
- Fallah, H.M.; Currimbhoy, S. Use of botulinum toxin A for treatment of myofascial pain and dysfunction. J. Oral Maxillofac Surg. 2012, 70, 1243–1245. [Google Scholar] [CrossRef]
- Patel, J.; Cardoso, J.A.; Mehta, S. A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism. Br. Dent. J. 2019, 226, 667–672. [Google Scholar] [CrossRef]
- Serrera-Figallo, M.A.; Ruiz-de-León-Hernández, G.; Torres-Lagares, D.; Castro-Araya, A.; Torres-Ferrerosa, O.; Hernández-Pacheco, E.; Gutierrez-Perez, J.L. Use of Botulinum Toxin in Orofacial Clinical Practice. Toxins 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- von Lindern, J.J.; Niederhagen, B.; Bergé, S.; Appel, T. Type A botulinum toxin in the treatment of chronic facial pain associated with masticatory hyperactivity. J. Oral Maxillofac. Surg. 2003, 61, 774–778. [Google Scholar] [CrossRef]
- Muñoz Lora, V.R.M.; Del Bel Cury, A.A.; Jabbari, B.; Lacković, Z. Botulinum Toxin Type A in Dental Medicine. J. Dent. Res. 2019, 98, 1450–1457. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, R.H.; Park, J.B. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast. Reconstr. Surg. 2010, 125, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Tan, K.H. Botulinum toxin A for myofascial trigger point injection: A qualitative systematic review. Eur. J. Pain. 2007, 11, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Jiménez, J.A.; Sánchez-Gutiérrez, J.; de-la-Hoz-Aizpurua, J.L.; Fernández-de-las-Peñas, C. Cadaveric validation of dry needle placement in the lateral pterygoid muscle. J. Manip. Physiol. Ther. 2015, 38, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, L.M.; Infante-Cossio, P.; Granados-Nuñez, M.; Urresti-Lopez, F.J. Treatment of temporomandibular myofascial pain with deep dry needling. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e781-5. [Google Scholar] [CrossRef] [PubMed]
- Kucukguven, A.; Demiryurek, M.D.; Kucukguven, M.B.; Vargel, I. A Novel Injection Technique to the Lateral Pterygoid Muscle for Temporomandibular Disorders: A Cadaveric Study. Plast. Reconstr. Surg. 2021, 148, 785e–790e. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef]
- Sidebottom, A.J.; Patel, A.A.; Amin, J. Botulinum injection for the management of myofascial pain in the masticatory muscles. A prospective outcome study. Br. J. Oral Maxillofac. Surg. 2013, 51, 199–205. [Google Scholar] [CrossRef]
- Laskin, D.M. The Use of Botulinum Toxin for the Treatment of Myofascial Pain in the Masticatory Muscles. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 287–289. [Google Scholar] [CrossRef]
- Soares, A.; Andriolo, R.B.; Atallah, A.N.; da Silva, E.M. Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst. Rev. 2014, 2014, CD007533. [Google Scholar]
- Zhou, J.Y.; Wang, D. An update on botulinum toxin A injections of trigger points for myofascial pain. Curr. Pain Headache Rep. 2014, 18, 386. [Google Scholar] [CrossRef]
- Freund, B.; Schwartz, M.; Symington, J.M. The use of botulinum toxin for the treatment of temporomandibular disorders: Preliminary findings. J. Oral Maxillofac. Surg. 1999, 57, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Freund, B.; Schwartz, M.; Symington, J.M. Botulinum toxin: New treatment for temporomandibular disorders. Br. J. Oral Maxillofac. Surg. 2000, 38, 466–471. [Google Scholar] [CrossRef]
- Nayyar; Kumar, P.; Nayyar, P.V.; Singh, A. BOTOX: Broadening the Horizon of Dentistry. J. Clin. Diagn. Res. 2014, 8, ZE25–ZE29. [Google Scholar] [PubMed]
- Hoque, A.; McAndrew, M. Use of botulinum toxin in dentistry. N. Y. State Dent. J. 2009, 75, 52–55. [Google Scholar] [PubMed]
- Al-Wayli, H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J. Clin. Exp. Dent. 2017, 9, e112–e117. [Google Scholar] [CrossRef]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef]
- Srivastava, S.; Kharbanda, S.; Pal, U.S.; Shah, V. Applications of botulinum toxin in dentistry: A comprehensive review. Natl. J. Maxillofac. Surg. 2015, 6, 152–159. [Google Scholar]
- Laskin, D.M. Botulinum toxin A in the treatment of myofascial pain and dysfunction: The case against its use. J. Oral Maxillofac. Surg. 2012, 70, 1240–1242. [Google Scholar] [CrossRef]
- Pihut, M.; Ferendiuk, E.; Szewczyk, M.; Kasprzyk, K.; Wieckiewicz, M. The efficiency of botulinum toxin type A for the treatment of masseter muscle pain in patients with temporomandibular joint dysfunction and tension-type headache. J. Headache Pain 2016, 17, 29. [Google Scholar] [CrossRef]
- Chikhani, L.; Dichamp, J. Bruxisme, syndrome algodysfonctionnel des articulations temporo-mandibulaires et toxine botulique [Bruxism, temporo-mandibular dysfunction and botulinum toxin]. Ann. Readapt. Med. Phys. 2003, 46, 333–337. [Google Scholar] [CrossRef]
- Hosgor, H.; Altindis, S. Efficacy of botulinum toxin in the management of temporomandibular myofascial pain and sleep bruxism. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Lerner, M.Z.; Blitzer, A. IncobotulinumtoxinA Injection for Temporomandibular Joint Disorder. Ann. Otol. Rhinol. Laryngol. 2017, 126, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Aronovich, S. Does Adjunctive Botulinum Toxin a Reduce Pain Scores When Combined with Temporomandibular Joint Arthroscopy for the Treatment of Concomitant Temporomandibular Joint Arthralgia and Myofascial Pain? J. Oral Maxillofac. Surg. 2017, 75, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Freund, B. Treatment of temporomandibular disorders with botulinum toxin. Clin. J. Pain 2002, 18 (Suppl. S6), S198–S203. [Google Scholar] [CrossRef]
- Batifol, D. Les différents types d’injection pour traiter les dysfonctions de l’articulation temporomandibulaire [Different types of injection in temporomandibular disorders (TMD) treatment]. Rev. Stomatol. Chir. Maxillo-Faciale Chir. Orale 2016, 117, 256–258. [Google Scholar] [CrossRef]
- Gadhia, K.; Walmsley, D. The therapeutic use of botulinum toxin in cervical and maxillofacial conditions. Evid. Based Dent. 2009, 10, 53. [Google Scholar] [CrossRef]
- Blumenfeld, A.M.; Stark, R.J.; Freeman, M.C.; Orejudos, A.; Manack Adams, A. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J. Headache Pain 2018, 19, 13. [Google Scholar] [CrossRef]
- Binder, W.J.; Brin, M.F.; Blitzer, A.; Schoenrock, L.D.; Pogoda, J.M. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open-label study. Otolaryngol. Head Neck Surg. 2000, 123, 669–676. [Google Scholar] [CrossRef]
- Freund, B.; Schwartz, M. Treatment of chronic cervical-associated headache with botulinum toxin A: A pilot study. Headache J. Head Face Pain 2000, 40, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Freund, B.; Schwartz, M. Treatment of whiplash associated with neck pain with botulinum toxin-A: A pilot study. J. Rheumatol. 2000, 27, 481–484. [Google Scholar]
- Ihde, S.K.; Konstantinovic, V.S. The therapeutic use of botulinum toxin in cervical and maxillofacial conditions: An evidence-based review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.; Sulica, L. Botulinum toxin: Basic science and clinical uses in otolaryngology. Laryngoscope 2000, 111, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Nowak, Z.; Cheęciński, M.; Nitecka-Buchta, A.; Bulanda, S.; Ilczuk-Rypuła, D.; Postek-Stefańska, L.; Baron, S. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain. Systematic Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 9552. [Google Scholar] [CrossRef] [PubMed]
- Sari, B.C.; Develi, T. The effect of intraarticular botulinum toxin-A injection on symptoms of temporomandibular joint disorder. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e316–e320. [Google Scholar] [CrossRef] [PubMed]
- Lora, V.R.; Clemente-Napimoga, J.T.; Abdalla, H.B.; Macedo, C.G.; Canales, G.T.; Barbosa, C.M. Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomadibular joint of rats. Toxicon 2017, 129, 52–57. [Google Scholar] [CrossRef]
- Drinovac Vlah, V.; Filipović, B.; Bach-Rojecky, L.; Lacković, Z. Role of central versus peripheral opioid system in antinociceptive and anti-inflammatory effect of botulinum toxin type A in trigeminal region. Eur. J. Pain. 2018, 22, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Møller, E.; Werdelin, L.M.; Dalager, T.; Kitai, N.; Kreiborg, S. Treatment of severe temporomandibular joint clicking with botulinum toxin in the lateral pterygoid muscle in two cases of anterior disc displacement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 693–700. [Google Scholar] [CrossRef]
- Emara, A.S.; Faramawey, M.I.; Hassaan, M.A.; Hakam, M.M. Botulinum toxin injection for management of temporomandibular joint clicking. Int. J. Oral Maxillofac. Surg. 2013, 42, 759–764. [Google Scholar] [CrossRef]
- Karacalar, A.; Yilmaz, N.; Bilgici, A.; Baş, B.; Akan, H. Botulinum toxin for the treatment of temporomandibular joint disk disfigurement: Clinical experience. J. Craniofac. Surg. 2005, 16, 476–481. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Esnaashari, N.; Azad, A.; Emad, S. The effects of botulinum toxin A injection on the lateral pterygoid muscle in patients with a painful temporomandibular joint click: A randomized clinical trial study. BMC Oral Health 2022, 22, 217. [Google Scholar] [CrossRef]
- Truong, D.; Brodsky, M.; Lew, M.; Brashear, A.; Jankovic, J.; Molho, E.; Orlova, O.; Timerbaeva, S. Global Dysport Cervical Dystonia Study Group. Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Park. Relat. Disord. 2010, 16, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Martimbianco, A.L.C.; Bussadori, S.K.; Pacheco, R.L.; Riera, R.; Santos, E.M. Botulinum Toxin Type A for Painful Temporomandibular Disorders: Systematic Review and Meta-Analysis. J. Pain 2020, 21, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Sipahi Calis, A.; Colakoglu, Z.; Gunbay, S. The use of botulinum toxin-a in the treatment of muscular temporomandibular joint disorders. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 322–325. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Rueda, J.A.; López-Valverde, A.; Márquez-Vera, A.; Méndez-Sánchez, R.; López-García, E.; López-Valverde, N. Preliminary Findings of the Efficacy of Botulinum Toxin in Temporomandibular Disorders: Uncontrolled Pilot Study. Life 2023, 13, 345. https://doi.org/10.3390/life13020345
Blanco-Rueda JA, López-Valverde A, Márquez-Vera A, Méndez-Sánchez R, López-García E, López-Valverde N. Preliminary Findings of the Efficacy of Botulinum Toxin in Temporomandibular Disorders: Uncontrolled Pilot Study. Life. 2023; 13(2):345. https://doi.org/10.3390/life13020345
Chicago/Turabian StyleBlanco-Rueda, José A., Antonio López-Valverde, Antonio Márquez-Vera, Roberto Méndez-Sánchez, Eva López-García, and Nansi López-Valverde. 2023. "Preliminary Findings of the Efficacy of Botulinum Toxin in Temporomandibular Disorders: Uncontrolled Pilot Study" Life 13, no. 2: 345. https://doi.org/10.3390/life13020345
APA StyleBlanco-Rueda, J. A., López-Valverde, A., Márquez-Vera, A., Méndez-Sánchez, R., López-García, E., & López-Valverde, N. (2023). Preliminary Findings of the Efficacy of Botulinum Toxin in Temporomandibular Disorders: Uncontrolled Pilot Study. Life, 13(2), 345. https://doi.org/10.3390/life13020345






