Characteristics, Roles and Applications of Proteinaceous Elicitors from Pathogens in Plant Immunity
Abstract
1. Introduction
2. Harpins
2.1. Architectures and Functions of Harpins
2.2. Action Mechanisms Triggered by Harpins in Plants
2.3. Plant Response to Harpins Promotes Biotic and Abiotic Resistance and Growth
| Groups | Elicitors/Microbes | Plants | Treatments | Phytohormones | HR | Increase Resistance to Pathogens | Growth | Abiotic Resistance | References |
|---|---|---|---|---|---|---|---|---|---|
| Harpin | Harpin/Erwinia amylovora | N. benthamiana | infiltration | - | yes | - | - | - | [20] |
| HarpinPsph/Pseudomonas syringae pv. phaseolicola | N. benthamiana | infiltration | - | yes | - | - | - | [35] | |
| PopW/Ralstonia solanacearum | Medicago sativa L. | - | ABA, GA, JA, SA, IAA | - | - | - | drought | [52] | |
| Hpa1/Xanthomonas oryzae pv. oryzae | N. benthamiana | agrobacterium-mediated transformation | - | yes (infiltration) | yes | yes | drought | [31] | |
| PopW/Ralstonia solanacearum | Solanum lycopersicum L. | foliar application | ABA | - | - | - | drought | [15] | |
| RipX(PopA)/Ralstonia solanacearum | N. benthamiana | infiltration | - | yes | - | - | - | [29] | |
| HpaXpm/Xanthomonas phaseoli pv. manihotis | N. benthamiana | infiltration | - | yes | yes (spraying) | - | - | [19] | |
| HpaXpm/Xanthomonas phaseoli pv. manihotis | A. thaliana | soak | - | - | - | yes | - | [19] | |
| SSBXoc/X. oryzae pv. oryzicola | N. benthamiana | agrobacterium-mediated transformation | - | - | yes | yes | salt | [49] | |
| HrpZpsta/P. syringae pv. tabaci | Glycine max | agrobacterium-mediated transformation | - | - | yes | - | - | [47] | |
| NLPs | CgNLP1/Colletotrichum gloeosporioides | A. thaliana | agrobacterium-mediated transformation | -- | - | yes | - | - | [58] |
| BsNep1/Botrytis squamosa | N. benthamiana | infiltration | -- | yes | - | - | - | [59] | |
| BcNep1/Botrytis cinerea | Allium cepa | infiltration | -- | yes | - | - | - | [59] | |
| VmNLP2/Valsa mali | N. benthamiana | agroinfiltration | -- | yes | - | - | - | [11] | |
| VmNLP2/Valsa mali | apple | infiltration | -- | yes | - | - | - | [11] | |
| PiNPP1.1/ Phytophthora infestans | N. benthamiana | agroinfiltration and infiltration | -- | yes | - | - | - | [60] | |
| DserNEP1 and DserNEP2/Diplodia seriata | Vitis vinifera | dip and infiltration | -- | yes | - | - | - | [61] | |
| PeNLP1 and PeNLP2/ Penicillium expansum | N. benthamiana | agroinfiltration | -- | yes | - | - | - | [62] | |
| CoNLP1/Colletotrichum orbiculare | Several Cucurbitaceae cultivars | infiltration ** | -- | - | yes | - | - | [63] | |
| MoNLP1, MoNLP2 and MoNLP4/Magnaporthe oryzae | N. benthamiana | agroinfiltration | -- | yes | - | - | - | [64] | |
| NLPPya/Pythium aphanidermatum | N. benthamiana | infiltration | -- | yes | - | - | - | [65] | |
| NLPPya/Pythium aphanidermatum | A. thaliana | infiltration | -- | yes | - | - | - | [65] | |
| NLPPya/Pythium aphanidermatum | Phalaenopsis amabilis | infiltration | -- | yes | - | - | - | [65] | |
| NLPPp/Phytophthora parasitica | A. thaliana | infiltration | -- | yes | - | - | - | [65] | |
| Elicitin | INF1/Phytophthora infestans | N. benthamiana | infiltration | - | yes | - | - | - | [66] |
| β-CRY/Phytophthora cryptogea | Three Solanum spp. genotypes | soak | ET, JA and JA–Ile | - | yes | - | - | [67] | |
| INF1/Phytophthora infestans | N. benthamiana | agroinfiltration | - | yes | - | - | - | [68] | |
| INF1/Phytophthora infestans | N. benthamiana | agroinfiltration | - | yes | - | - | - | [69] | |
| INF1/Phytophthora infestans | Solanum microdontum | agroinfection | - | yes | - | - | - | [70] | |
| Quercinin/Phytophthora quercina | N. benthamiana | cells soak | ET | yes | - | - | - | [71] | |
| Cryptogein/Phytophthora cryptogea | N. benthamiana | place onto the fresh wound | - | yes | yes | - | - | [72] | |
| Capsicein/Phytophthora capsici | N. benthamiana | place onto the fresh wound | - | yes | yes | - | - | [72] | |
| PoEli8/Pythium oligandrum | N. benthamiana, tomato, and pepper | infiltration | - | yes | yes | - | - | [73] |
3. NLPs
3.1. Taxonomy of NLPs
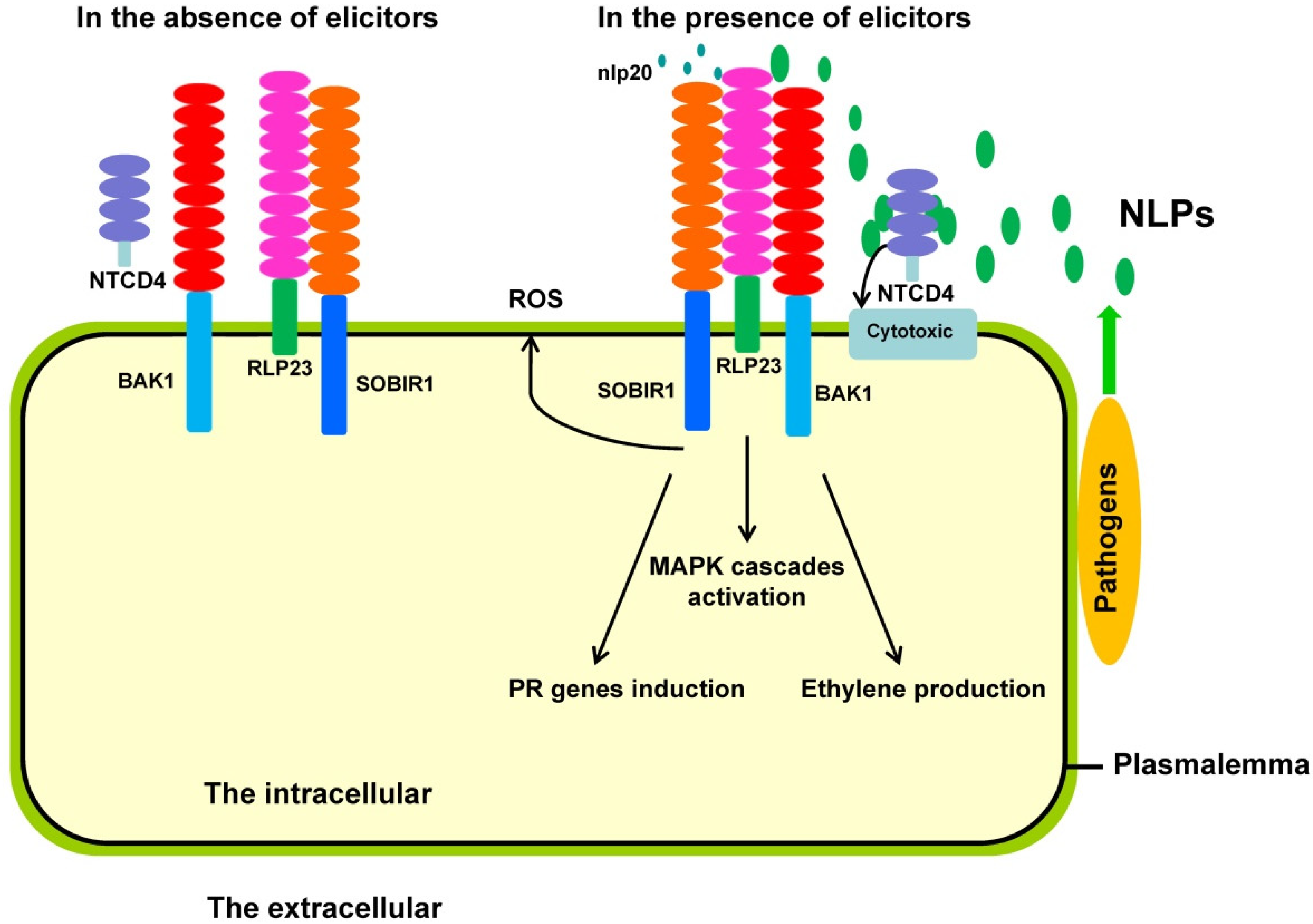
3.2. Involvement of NLPs in Immune Responses in Plants
3.3. Action Mechanisms of NLPs in Plant Immune Responses
4. Elicitins
4.1. Taxonomy of Elicitins
4.2. Involvement and Action Mechanisms of Elicitins in Plant Immune Responses
5. Other Elicitors
6. Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, Y. Advances in Fungal Elicitor-Triggered Plant Immunity. Int. J. Mol. Sci. 2022, 23, 12003. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Chen, X.; Zhou, J.M. From plant immunity to crop disease resistance. J. Genet. Genomics 2022, 49, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nurnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, Y.; Yue, Y.; Sui, Y.; Hao, M.; Kang, X.; Wang, Q.; Chen, D.; Liu, B.; Yin, Z.; et al. Glutathione and neodiosmin feedback sustain plant immunity. J. Exp. Bot. 2022, erac442. [Google Scholar] [CrossRef]
- Liu, J.; Nie, J.; Chang, Y.; Huang, L. Nep1-like Proteins from Valsa mali Differentially Regulate Pathogen Virulence and Response to Abiotic Stresses. J. Fungi. 2021, 7, 830. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zhang, L.; Zhuang, H.; Yang, X.; Qiu, D.; Zeng, H. Secreted protein MoHrip2 is required for full virulence of Magnaporthe oryzae and modulation of rice immunity. Appl. Microbiol. Biotechnol. 2019, 103, 6153–6167. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Han, H.; Chen, X.; Chen, Q.; Zhao, J.; Li, C. Stemphylium lycopersici Nep1-like Protein (NLP) Is a Key Virulence Factor in Tomato Gray Leaf Spot Disease. J. Fungi. 2022, 8, 518. [Google Scholar] [CrossRef]
- Sands, L.B.; Cheek, T.; Reynolds, J.; Ma, Y.; Berkowitz, G.A. Effects of Harpin and Flg22 on Growth Enhancement and Pathogen Defense in Cannabis sativa Seedlings. Plants 2022, 11, 1178. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Zhao, Y.; Gao, F.; Liu, H. The application of exogenous PopW increases the tolerance of Solanum lycopersicum L. to drought stress through multiple mechanisms. Physiol. Mol. Biol. Plants 2020, 26, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Kim, W.; Lee, C.; Oh, C.S. Harpins, multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol. Plant Microbe Interact. 2013, 26, 1115–1122. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Ji, H.; Mo, X.; Li, P.; Wang, J.; Dong, H. Hpa1 is a type III translocator in Xanthomonas oryzae pv. oryzae. BMC Microbiol. 2018, 18, 105. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Zhao, Y.; You, Z.; Dong, H.; Zhang, C. Hpa1 harpin needs nitroxyl terminus to promote vegetative growth and leaf photosynthesis in Arabidopsis. J. Biosci. 2014, 39, 127–137. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Liu, W.; Huang, J.; Liu, Q.; Sun, J.; Cai, X.; Miao, W. HpaXpm, a novel harpin of Xanthomonas phaseoli pv. manihotis, acts as an elicitor with high thermal stability, reduces disease, and promotes plant growth. BMC Microbiol. 2020, 20, 4. [Google Scholar] [CrossRef]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef]
- Tarafdar, P.K.; Vedantam, L.V.; Podile, A.R.; Swamy, M.J. Thermally stable harpin, HrpZPss is sensitive to chemical denaturants: Probing tryptophan environment, chemical and thermal unfolding by fluorescence spectroscopy. Biochimie 2013, 95, 2437–2444. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Liu, W.; Xiong, X.; Lv, C.; Zhou, X.; Miao, W. Functional regions of HpaXm as elicitors with specific heat tolerance induce the hypersensitive response or plant growth promotion in nonhost plants. PLoS ONE 2018, 13, e0188788. [Google Scholar] [CrossRef]
- Charkowski, A.O.; Alfano, J.R.; Preston, G.; Yuan, J.; He, S.Y.; Collmer, A. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 1998, 180, 5211–5217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.F.; Beer, S.V. HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectate lyases of a distinct class. J. Bacteriol. 1998, 180, 5203–5210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Liu, H.X.; Cao, J.; Chen, L.F.; Gu, C.; Allen, C.; Guo, J.H. PopW of Ralstonia solanacearum, a new two-domain harpin targeting the plant cell wall. Mol. Plant Pathol. 2010, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Gago, J.; Cui, H.; Qian, Z.; Kodama, N.; Ji, H.; Tian, S.; Shen, D.; Chen, Y.; et al. Harpin Hpa1 Interacts with Aquaporin PIP1;4 to Promote the Substrate Transport and Photosynthesis in Arabidopsis. Sci. Rep. 2015, 5, 17207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, B.; Xu, M.; Han, L.; Zhao, Y.; Liu, Z.; Dong, H.; Zhang, C. Plant growth enhancement and associated physiological responses are coregulated by ethylene and gibberellin in response to harpin protein Hpa1. Planta 2014, 239, 831–846. [Google Scholar] [CrossRef]
- Oh, C.S.; Beer, S.V. AtHIPM, an ortholog of the apple HrpN-interacting protein, is a negative regulator of plant growth and mediates the growth-enhancing effect of HrpN in Arabidopsis. Plant Physiol. 2007, 145, 426–436. [Google Scholar] [CrossRef]
- Sun, T.; Wu, W.; Wu, H.; Rou, W.; Zhou, Y.; Zhuo, T.; Fan, X.; Hu, X.; Zou, H. Ralstonia solanacearum elicitor RipX Induces Defense Reaction by Suppressing the Mitochondrial atpA Gene in Host Plant. Int. J. Mol. Sci. 2020, 21, 2000. [Google Scholar] [CrossRef]
- Niu, L.; Yang, J.; Zhang, J.; He, H.; Xing, G.; Zhao, Q.; Guo, D.; Sui, L.; Zhong, X.; Yang, X. Introduction of the harpinXooc-encoding gene hrf2 in soybean enhances resistance against the oomycete pathogen Phytophthora sojae. Transgenic Res. 2019, 28, 257–266. [Google Scholar] [CrossRef]
- Ji, Z.L.; Yu, M.H.; Ding, Y.Y.; Li, J.; Zhu, F.; He, J.X.; Yang, L.N. Coiled-Coil N21 of Hpa1 in Xanthomonas oryzae pv. oryzae Promotes Plant Growth, Disease Resistance and Drought Tolerance in Non-Hosts via Eliciting HR and Regulation of Multiple Defense Response Genes. Int. J. Mol. Sci. 2020, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.L.; Bao, Z.L.; Ren, H.Y.; Wang, J.S.; Dong, H.S. Expression of harpin(xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 2004, 94, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Ma, W.X.; Che, Y.Z.; Zou, L.F.; Zakria, M.; Zou, H.S.; Chen, G.Y. A highly-conserved single-stranded DNA-binding protein in Xanthomonas functions as a harpin-like protein to trigger plant immunity. PLoS ONE 2013, 8, e56240. [Google Scholar] [CrossRef]
- Wang, D.; Wang, B.; Wang, J.; Wang, S.; Wang, W.; Niu, Y. Exogenous Application of Harpin Protein Hpa1 onto Pinellia ternata Induces Systemic Resistance Against Tobacco Mosaic Virus. Phytopathology 2020, 110, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Klessig, D.F.; Nurnberger, T. A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell 2001, 13, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Racape, J.; Belbahri, L.; Engelhardt, S.; Lacombe, B.; Lee, J.; Lochman, J.; Marais, A.; Nicole, M.; Nurnberger, T.; Parlange, F.; et al. Ca2+-dependent lipid binding and membrane integration of PopA, a harpin-like elicitor of the hypersensitive response in tobacco. Mol. Microbiol. 2005, 58, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Klusener, B.; Tsiamis, G.; Stevens, C.; Neyt, C.; Tampakaki, A.P.; Panopoulos, N.J.; Noller, J.; Weiler, E.W.; Cornelis, G.R.; et al. HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc. Natl. Acad Sci. USA 2001, 98, 289–294. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, Z. Harpin-induced hypersensitive cell death is associated with altered mitochondrial functions in tobacco cells. Mol. Plant Microbe Interact. 2000, 13, 183–190. [Google Scholar] [CrossRef]
- Krause, M.; Durner, J. Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol. Plant Microbe Interact. 2004, 17, 131–139. [Google Scholar] [CrossRef]
- Samuel, M.A.; Hall, H.; Krzymowska, M.; Drzewiecka, K.; Hennig, J.; Ellis, B.E. SIPK signaling controls multiple components of harpin-induced cell death in tobacco. Plant J. 2005, 42, 406–416. [Google Scholar] [CrossRef]
- Desikan, R.; Clarke, A.; Atherfold, P.; Hancock, J.T.; Neill, S.J. Harpin induces mitogen-activated protein kinase activity during defence responses in Arabidopsis thaliana suspension cultures. Planta 1999, 210, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Hancock, J.T.; Ichimura, K.; Shinozaki, K.; Neill, S.J. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 2001, 126, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Campa, M.; Piazza, S.; Righetti, L.; Oh, C.S.; Conterno, L.; Borejsza-Wysocka, E.; Nagamangala, K.C.; Beer, S.V.; Aldwinckle, H.S.; Malnoy, M. HIPM Is a Susceptibility Gene of Malus spp.: Reduced Expression Reduces Susceptibility to Erwinia amylovora. Mol. Plant Microbe Interact. 2019, 32, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, X.B.; Li, P.; Wang, H.; Ji, H.T.; Xie, J.Y.; Qiu, Q.L.; Shen, D.; Dong, H.S. Plant Aquaporin AtPIP1;4 Links Apoplastic H2O2 Induction to Disease Immunity Pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef]
- Fontanilla, J.M.; Montes, M.; De Prado, R. Induction of resistance to the pathogenic agent Botrytis cinerea in the cultivation of the tomato by means of the application of the protein “Harpin”(Messenger). Commun. Agric. Appl. Biol. Sci. 2005, 70, 35–40. [Google Scholar] [PubMed]
- Fontanilla, M.; Montes, M.; De Prado, R. Effects of the foliar-applied protein “Harpin(Ea)” (messenger) on tomatoes infected with Phytophthora infestans. Commun. Agric. Appl. Biol. Sci. 2005, 70, 41–45. [Google Scholar]
- Du, Q.; Yang, X.; Zhang, J.; Zhong, X.; Kim, K.S.; Yang, J.; Xing, G.; Li, X.; Jiang, Z.; Li, Q.; et al. Over-expression of the Pseudomonas syringae harpin-encoding gene hrpZm confers enhanced tolerance to Phytophthora root and stem rot in transgenic soybean. Transgenic Res. 2018, 27, 277–288. [Google Scholar] [CrossRef]
- Miao, W.; Wang, X.; Song, C.; Wang, Y.; Ren, Y.; Wang, J. Transcriptome analysis of Hpa1Xoo transformed cotton revealed constitutive expression of genes in multiple signalling pathways related to disease resistance. J. Exp. Bot. 2010, 61, 4263–4275. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, M.; Ma, W.; Sun, Y.; Chen, G. Overexpression of SSBXoc, a Single-Stranded DNA-Binding Protein From Xanthomonas oryzae pv. oryzicola, Enhances Plant Growth and Disease and Salt Stress Tolerance in Transgenic Nicotiana benthamiana. Front. Plant Sci. 2018, 9, 953. [Google Scholar] [CrossRef]
- Chen, L.; Qian, J.; Qu, S.; Long, J.; Yin, Q.; Zhang, C.; Wu, X.; Sun, F.; Wu, T.; Hayes, M.; et al. Identification of specific fragments of HpaG Xooc, a harpin from Xanthomonas oryzae pv. oryzicola, that induce disease resistance and enhance growth in plants. Phytopathology 2008, 98, 781–791. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.J.; Zhang, S.S.; Qu, S.; Ren, X.; Long, J.; Yin, Q.; Qian, J.; Sun, F.; Zhang, C.; et al. A fragment of the Xanthomonas oryzae pv. oryzicola harpin HpaG Xooc reduces disease and increases yield of rice in extensive grower plantings. Phytopathology 2008, 98, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, G. PopW enhances drought stress tolerance of alfalfa via activating antioxidative enzymes, endogenous hormones, drought related genes and inhibiting senescence genes. Plant Physiol. Biochem. 2021, 166, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Peng, J.; Bao, Z.; Meng, X.; Bonasera, J.M.; Chen, G.; Beer, S.V.; Dong, H. Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol. 2004, 136, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, P.; Zhang, C. Harpin Hpa1 promotes flower development in Impatiens and Parochetus plants. Bot. Stud. 2016, 57, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Jeon, E.; Oh, J.; Moon, J.S.; Hwang, I. Mutational analysis of Xanthomonas harpin HpaG identifies a key functional region that elicits the hypersensitive response in nonhost plants. J. Bacteriol. 2004, 186, 6239–6247. [Google Scholar] [CrossRef]
- Haapalainen, M.; Engelhardt, S.; Kufner, I.; Li, C.M.; Nurnberger, T.; Lee, J.; Romantschuk, M.; Taira, S. Functional mapping of harpin HrpZ of Pseudomonas syringae reveals the sites responsible for protein oligomerization, lipid interactions and plant defence induction. Mol. Plant Pathol. 2011, 12, 151–166. [Google Scholar] [CrossRef]
- Reboutier, D.; Bouteau, F. Harpins and ion channels modulations: Many ways to die. Plant Signal. Behav. 2008, 3, 314–316. [Google Scholar] [CrossRef]
- Yang, G.; Yang, J.; Zhang, Q.; Wang, W.; Feng, L.; Zhao, L.; An, B.; Wang, Q.; He, C.; Luo, H. The Effector Protein CgNLP1 of Colletotrichum gloeosporioides Affects Invasion and Disrupts Nuclear Localization of Necrosis-Induced Transcription Factor HbMYB8-Like to Suppress Plant Defense Signaling. Front. Microbiol. 2022, 13, 911479. [Google Scholar] [CrossRef] [PubMed]
- Steentjes, M.B.F.; Herrera Valderrama, A.L.; Fouillen, L.; Bahammou, D.; Leisen, T.; Albert, I.; Nurnberger, T.; Hahn, M.; Mongrand, S.; Scholten, O.E.; et al. Cytotoxic activity of Nep1-like proteins on monocots. New Phytol. 2022, 235, 690–700. [Google Scholar] [CrossRef]
- Schumacher, S.; Grosser, K.; Voegele, R.T.; Kassemeyer, H.H.; Fuchs, R. Identification and Characterization of Nep1-Like Proteins From the Grapevine Downy Mildew Pathogen Plasmopara viticola. Front. Plant Sci. 2020, 11, 65. [Google Scholar] [CrossRef]
- Cobos, R.; Calvo-Pena, C.; Alvarez-Perez, J.M.; Ibanez, A.; Diez-Galan, A.; Gonzalez-Garcia, S.; Garcia-Angulo, P.; Acebes, J.L.; Coque, J.J.R. Necrotic and Cytolytic Activity on Grapevine Leaves Produced by Nep1-Like Proteins of Diplodia seriata. Front. Plant Sci. 2019, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.; Raphael, G.; Ma, J.; Ballester, A.R.; Feygenberg, O.; Norelli, J.; Aly, R.; Gonzalez-Candelas, L.; Wisniewski, M.; Droby, S. Identification and Functional Analysis of NLP-Encoding Genes from the Postharvest Pathogen Penicillium expansum. Microorganisms 2019, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.S.A.; Singkaravanit-Ogawa, S.; Ikeda, K.; Kitakura, S.; Inoue, Y.; Narusaka, Y.; Shirasu, K.; Kaido, M.; Mise, K.; Takano, Y. Inappropriate Expression of an NLP Effector in Colletotrichum orbiculare Impairs Infection on Cucurbitaceae cultivars via Plant Recognition of the C-Terminal Region. Mol. Plant Microbe Interact. 2018, 31, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Peng, Y.L.; Fan, J. The Nep1-like protein family of Magnaporthe oryzae is dispensable for the infection of rice plants. Sci. Rep. 2017, 7, 4372. [Google Scholar] [CrossRef] [PubMed]
- Lenarcic, T.; Albert, I.; Bohm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Zagar, E.; Pruitt, R.; et al. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef]
- Imano, S.; Fushimi, M.; Camagna, M.; Tsuyama-Koike, A.; Mori, H.; Ashida, A.; Tanaka, A.; Sato, I.; Chiba, S.; Kawakita, K.; et al. AP2/ERF Transcription Factor NbERF-IX-33 Is Involved in the Regulation of Phytoalexin Production for the Resistance of Nicotiana benthamiana to Phytophthora infestans. Front. Plant Sci. 2021, 12, 821574. [Google Scholar] [CrossRef]
- Stary, T.; Satkova, P.; Piterkova, J.; Mieslerova, B.; Luhova, L.; Mikulik, J.; Kasparovsky, T.; Petrivalsky, M.; Lochman, J. The elicitin β-cryptogein’s activity in tomato is mediated by jasmonic acid and ethylene signalling pathways independently of elicitin-sterol interactions. Planta 2019, 249, 739–749. [Google Scholar] [CrossRef]
- Turnbull, D.; Wang, H.; Breen, S.; Malec, M.; Naqvi, S.; Yang, L.; Welsh, L.; Hemsley, P.; Zhendong, T.; Brunner, F.; et al. AVR2 Targets BSL Family Members, Which Act as Susceptibility Factors to Suppress Host Immunity. Plant Physiol. 2019, 180, 571–581. [Google Scholar] [CrossRef]
- Domazakis, E.; Wouters, D.; Visser, R.G.F.; Kamoun, S.; Joosten, M.; Vleeshouwers, V. The ELR-SOBIR1 Complex Functions as a Two-Component Receptor-Like Kinase to Mount Defense Against Phytophthora infestans. Mol. Plant Microbe Interact. 2018, 31, 795–802. [Google Scholar] [CrossRef]
- Du, J.; Verzaux, E.; Chaparro-Garcia, A.; Bijsterbosch, G.; Keizer, L.C.; Zhou, J.; Liebrand, T.W.; Xie, C.; Govers, F.; Robatzek, S.; et al. Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat. Plants 2015, 1, 15034. [Google Scholar] [CrossRef]
- Koehl, J.; Djulic, A.; Kirner, V.; Nguyen, T.T.; Heiser, I. Ethylene is required for elicitin-induced oxidative burst but not for cell death induction in tobacco cell suspension cultures. J. Plant Physiol. 2007, 164, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Ricci, P.; Bonnet, P.; Huet, J.C.; Sallantin, M.; Beauvais-Cante, F.; Bruneteau, M.; Billard, V.; Michel, G.; Pernollet, J.C. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur. J. Biochem. 1989, 183, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, Y.; Zhao, H.; Shen, D.; Dou, D.; Jing, M. Novel EIicitin from Pythium oligandrum Confers Disease Resistance against Phytophthora capsici in Solanaceae Plants. J. Agric. Food Chem. 2022, 70, 16135–16145. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Huang, X.; Tang, X.; Qin, L.; Liu, Y.; Xia, Y.; Peng, Z.; Xia, S. SsNEP2 Contributes to the Virulence of Sclerotinia sclerotiorum. Pathogens 2022, 11, 446. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Kirungu, J.N.; Lu, P.; Cai, X.; Zhou, Z.; Xu, Y.; Hou, Y.; Agong, S.G.; Wang, K.; Liu, F. Map-Based Functional Analysis of the GhNLP Genes Reveals Their Roles in Enhancing Tolerance to N-Deficiency in Cotton. Int. J. Mol. Sci. 2019, 20, 4953. [Google Scholar] [CrossRef]
- Seidl, M.F.; Van den Ackerveken, G. Activity and Phylogenetics of the Broadly Occurring Family of Microbial Nep1-Like Proteins. Annu. Rev. Phytopathol. 2019, 57, 367–386. [Google Scholar] [CrossRef]
- Pirc, K.; Clifton, L.A.; Yilmaz, N.; Saltalamacchia, A.; Mally, M.; Snoj, T.; Znidarsic, N.; Srnko, M.; Borisek, J.; Parkkila, P.; et al. An oomycete NLP cytolysin forms transient small pores in lipid membranes. Sci. Adv. 2022, 8, eabj9406. [Google Scholar] [CrossRef]
- Irieda, H.; Maeda, H.; Akiyama, K.; Hagiwara, A.; Saitoh, H.; Uemura, A.; Terauchi, R.; Takano, Y. Colletotrichum orbiculare Secretes Virulence Effectors to a Biotrophic Interface at the Primary Hyphal Neck via Exocytosis Coupled with SEC22-Mediated Traffic. Plant Cell 2014, 26, 2265–2281. [Google Scholar] [CrossRef]
- Bailey, B.A. Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 1995, 6, 1250–1255. [Google Scholar] [CrossRef]
- Fellbrich, G.; Romanski, A.; Varet, A.; Blume, B.; Brunner, F.; Engelhardt, S.; Felix, G.; Kemmerling, B.; Krzymowska, M.; Nurnberger, T. NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J. 2002, 32, 375–390. [Google Scholar] [CrossRef]
- Oome, S.; Van den Ackerveken, G. Comparative and functional analysis of the widely occurring family of Nep1-like proteins. Mol. Plant Microbe Interact. 2014, 27, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, C.; Luberacki, B.; Kufner, I.; Koch, W.; Brunner, F.; Weyand, M.; Mattinen, L.; Pirhonen, M.; Anderluh, G.; Seitz, H.U.; et al. A common toxin fold mediates microbial attack and plant defense. Proc. Natl. Acad Sci. USA 2009, 106, 10359–10364. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, C.; Wang, Y.; Li, J.; Dong, X.; Cheng, Y.; Zhang, H.; Zhai, Y.; Ai, G.; Song, Q.; et al. Nep1-Like Proteins From the Biocontrol Agent Pythium oligandrum Enhance Plant Disease Resistance Independent of Cell Death and Reactive Oxygen Species. Front. Plant Sci. 2022, 13, 830636. [Google Scholar] [CrossRef] [PubMed]
- Dallal Bashi, Z.; Hegedus, D.D.; Buchwaldt, L.; Rimmer, S.R.; Borhan, M.H. Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene-inducing peptides (NEPs). Mol. Plant Pathol. 2010, 11, 43–53. [Google Scholar] [CrossRef]
- Tian, H.; Wu, Z.; Chen, S.; Ao, K.; Huang, W.; Yaghmaiean, H.; Sun, T.; Xu, F.; Zhang, Y.; Wang, S.; et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature 2021, 598, 500–503. [Google Scholar] [CrossRef]
- Lenarcic, T.; Pirc, K.; Hodnik, V.; Albert, I.; Borisek, J.; Magistrato, A.; Nurnberger, T.; Podobnik, M.; Anderluh, G. Molecular basis for functional diversity among microbial Nep1-like proteins. PLoS Pathog. 2019, 15, e1007951. [Google Scholar] [CrossRef]
- Pirc, K.; Albert, I.; Nurnberger, T.; Anderluh, G. Disruption of plant plasma membrane by Nep1-like proteins in pathogen-plant interactions. New Phytol. 2022, 237, 746–750. [Google Scholar] [CrossRef]
- Zaparoli, G.; Barsottini, M.R.; de Oliveira, J.F.; Dyszy, F.; Teixeira, P.J.; Barau, J.G.; Garcia, O.; Costa-Filho, A.J.; Ambrosio, A.L.; Pereira, G.A.; et al. The crystal structure of necrosis- and ethylene-inducing protein 2 from the causal agent of cacao’s Witches’ Broom disease reveals key elements for its activity. Biochemistry 2011, 50, 9901–9910. [Google Scholar] [CrossRef]
- Albert, I.; Bohm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23-SOBIR1-BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Wan, W.L.; Zhang, L.; Pruitt, R.; Zaidem, M.; Brugman, R.; Ma, X.; Krol, E.; Perraki, A.; Kilian, J.; Grossmann, G.; et al. Comparing Arabidopsis receptor kinase and receptor protein-mediated immune signaling reveals BIK1-dependent differences. New Phytol. 2019, 221, 2080–2095. [Google Scholar] [CrossRef]
- Chen, J.B.; Bao, S.W.; Fang, Y.L.; Wei, L.Y.; Zhu, W.S.; Peng, Y.L.; Fan, J. An LRR-only protein promotes NLP-triggered cell death and disease susceptibility by facilitating oligomerization of NLP in Arabidopsis. New Phytol. 2021, 232, 1808–1822. [Google Scholar] [CrossRef] [PubMed]
- Derevnina, L.; Dagdas, Y.F.; De la Concepcion, J.C.; Bialas, A.; Kellner, R.; Petre, B.; Domazakis, E.; Du, J.; Wu, C.H.; Lin, X.; et al. Nine things to know about elicitins. New Phytol. 2016, 212, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Janku, M.; Cincalova, L.; Luhova, L.; Lochman, J.; Petrivalsky, M. Biological effects of oomycetes elicitins. Plant Prot. Sci. 2020, 56, 1–8. [Google Scholar] [CrossRef]
- Jiang, R.H.; Tyler, B.M.; Whisson, S.C.; Hardham, A.R.; Govers, F. Ancient origin of elicitin gene clusters in Phytophthora genomes. Mol. Biol. Evol. 2006, 23, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Boissy, G.; de La Fortelle, E.; Kahn, R.; Huet, J.C.; Bricogne, G.; Pernollet, J.C.; Brunie, S. Crystal structure of a fungal elicitor secreted by Phytophthora cryptogea, a member of a novel class of plant necrotic proteins. Structure 1996, 4, 1429–1439. [Google Scholar] [CrossRef]
- Janku, M.; Jedelska, T.; Cincalova, L.; Sedlar, A.; Mikulik, J.; Luhova, L.; Lochman, J.; Petrivalsky, M. Structure-activity relationships of oomycete elicitins uncover the role of reactive oxygen and nitrogen species in triggering plant defense responses. Plant Sci. 2022, 319, 111239. [Google Scholar] [CrossRef]
- O’Donohue, M.J.; Gousseau, H.; Huet, J.C.; Tepfer, D.; Pernollet, J.C. Chemical synthesis, expression and mutagenesis of a gene encoding β-cryptogein, an elicitin produced by Phytophthora cryptogea. Plant Mol. Biol. 1995, 27, 577–586. [Google Scholar] [CrossRef]
- Pleskova, V.; Kasparovsky, T.; Oboril, M.; Ptackova, N.; Chaloupkova, R.; Ladislav, D.; Damborsky, J.; Lochman, J. Elicitin-membrane interaction is driven by a positive charge on the protein surface: Role of Lys13 residue in lipids loading and resistance induction. Plant Physiol. Biochem. 2011, 49, 321–328. [Google Scholar] [CrossRef]
- Dokladal, L.; Oboril, M.; Stejskal, K.; Zdrahal, Z.; Ptackova, N.; Chaloupkova, R.; Damborsky, J.; Kasparovsky, T.; Jeandroz, S.; Zd’arska, M.; et al. Physiological and proteomic approaches to evaluate the role of sterol binding in elicitin-induced resistance. J. Exp. Bot. 2012, 63, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Ponchet, M.; Panabieres, F.; Milat, M.L.; Mikes, V.; Montillet, J.L.; Suty, L.; Triantaphylides, C.; Tirilly, Y.; Blein, J.P. Are elicitins cryptograms in plant-Oomycete communications? Cell. Mol. Life Sci. 1999, 56, 1020–1047. [Google Scholar] [CrossRef]
- Qutob, D.; Huitema, E.; Gijzen, M.; Kamoun, S. Variation in structure and activity among elicitins from Phytophthora sojae. Mol. Plant Pathol. 2003, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux-Ouaked, F.; Pugin, A.; Lebrun-Garcia, A. Phosphoproteins involved in the signal transduction of cryptogein, an elicitor of defense reactions in tobacco. Mol. Plant Microbe Interact. 2000, 13, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Wendehenne, D.; Lamotte, O.; Frachisse, J.M.; Barbier-Brygoo, H.; Pugin, A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell 2002, 14, 1937–1951. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Qiu, A.L.; Shi, L.P.; Cai, J.S.; Huang, X.Y.; Yang, S.; Wang, B.; Shen, L.; Huang, M.K.; Mou, S.L.; et al. SRC2-1 is required in PcINF1-induced pepper immunity by acting as an interacting partner of PcINF1. J. Exp. Bot. 2015, 66, 3683–3698. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Garcia, A.; Wilkinson, R.C.; Gimenez-Ibanez, S.; Findlay, K.; Coffey, M.D.; Zipfel, C.; Rathjen, J.P.; Kamoun, S.; Schornack, S. The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana. PLoS ONE 2011, 6, e16608. [Google Scholar] [CrossRef]
- Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 2008, 20, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, N.; Yamada, R.; Yoshioka, M.; Katou, S.; Yoshioka, H. Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell 2011, 23, 1153–1170. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY Transcription Factors Phosphorylated by MAPK Regulate a Plant Immune NADPH Oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef] [PubMed]
- Noirot, E.; Der, C.; Lherminier, J.; Robert, F.; Moricova, P.; Kieu, K.; Leborgne-Castel, N.; Simon-Plas, F.; Bouhidel, K. Dynamic changes in the subcellular distribution of the tobacco ROS-producing enzyme RBOHD in response to the oomycete elicitor cryptogein. J. Exp. Bot. 2014, 65, 5011–5022. [Google Scholar] [CrossRef]
- Saito, S.; Yamamoto-Katou, A.; Yoshioka, H.; Doke, N.; Kawakita, K. Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant Cell Physiol. 2006, 47, 689–697. [Google Scholar] [CrossRef]
- Yamamoto-Katou, A.; Katou, S.; Yoshioka, H.; Doke, N.; Kawakita, K. Nitrate reductase is responsible for elicitin-induced nitric oxide production in Nicotiana benthamiana. Plant Cell Physiol. 2006, 47, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.; Noirot, E.; Grandperret, V.; Bourque, S.; Fromentin, J.; Salloignon, P.; Truntzer, C.; Dobrowolska, G.; Simon-Plas, F.; Wendehenne, D. Interplays between nitric oxide and reactive oxygen species in cryptogein signalling. Plant Cell Environ. 2015, 38, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, X.; Qiu, D.; Guo, L.; Zeng, H.; Mao, J.; Gao, Q. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol. Biol. Rep. 2011, 38, 2549–2556. [Google Scholar] [CrossRef]
- Kulye, M.; Liu, H.; Zhang, Y.; Zeng, H.; Yang, X.; Qiu, D. Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence-related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 2012, 35, 2104–2120. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, H.; Qiu, D.; Guo, L.; Yang, X.; Shi, H.; Zhou, T.; Zhao, J. Purification and characterization of a novel hypersensitive response-inducing elicitor from Magnaporthe oryzae that triggers defense response in rice. PLoS ONE 2012, 7, e37654. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Zeng, H.; Qiu, D. Purification, crystallization and preliminary X-ray diffraction analysis of effector protein MoHrip2 from Magnaporthe oryzae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 463–467. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Zi, Q.; Qiu, D.; Liu, W.; Zeng, H. A novel elicitor identified from Magnaporthe oryzae triggers defense responses in tobacco and rice. Plant Cell Rep. 2014, 33, 1865–1879. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liu, Q.; Qiu, D.; Zhang, Y.; Zeng, H.; Yuan, J.; Mao, J. Purification of novel protein elicitor from Botrytis cinerea that induces disease resistance and drought tolerance in plants. Microbiol. Res. 2010, 165, 142–151. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, X.; Zhong, L.; Fan, Q.; Lan, Z.; Ye, X.; Huang, Y.; Li, Z.; Cui, Z. Functional Characterization of the Novel Laminaripentaose-Producing beta-1,3-Glucanase MoGluB and Its Biocontrol of Magnaporthe oryzae. J. Agric. Food Chem. 2021, 69, 9571–9584. [Google Scholar] [CrossRef]
- Mateos, F.V.; Rickauer, M.; Esquerre-Tugaye, M.T. Cloning and characterization of a cDNA encoding an elicitor of Phytophthora parasitica var. nicotianae that shows cellulose-binding and lectin-like activities. Mol. Plant Microbe Interact. 1997, 10, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, T.; Yao, M.; Feng, Z.; Miriam, K.; Wu, J.; Zhou, X.; Tao, X. The 2a protein of Cucumber mosaic virus induces a hypersensitive response in cowpea independently of its replicase activity. Virus Res. 2012, 170, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, P.; Yoshioka, K.; Shah, J.; Dooner, H.K.; Klessig, D.F. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 2000, 12, 677–690. [Google Scholar] [CrossRef]
- Garcia, J.A.; Pallas, V. Viral factors involved in plant pathogenesis. Curr. Opin. Virol. 2015, 11, 21–30. [Google Scholar] [CrossRef]
- Gaulin, E.; Drame, N.; Lafitte, C.; Torto-Alalibo, T.; Martinez, Y.; Ameline-Torregrosa, C.; Khatib, M.; Mazarguil, H.; Villalba-Mateos, F.; Kamoun, S.; et al. Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. Plant Cell 2006, 18, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.U.; Liu, M.; Yang, X.; Qiu, D. Fungal Elicitor MoHrip2 Induces Disease Resistance in Rice Leaves, Triggering Stress-Related Pathways. PLoS ONE 2016, 11, e0158112. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Q.; Zi, Q.; Lv, S.; Qiu, D.; Zeng, H. Enhanced disease resistance and drought tolerance in transgenic rice plants overexpressing protein elicitors from Magnaporthe oryzae. PLoS ONE 2017, 12, e0175734. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Z.; Yang, X.; Guo, L.; Qiu, D.; Zeng, H. Transcriptional Profiling of Rice Treated with MoHrip1 Reveal the Function of Protein Elicitor in Enhancement of Disease Resistance and Plant Growth. Front Plant Sci. 2016, 7, 1818. [Google Scholar] [CrossRef]
- Nie, H.Z.; Zhang, L.; Zhuang, H.Q.; Shi, W.J.; Yang, X.F.; Qiu, D.W.; Zeng, H.M. The Secreted Protein MoHrip1 Is Necessary for the Virulence of Magnaporthe oryzae. Int. J. Mol. Sci. 2019, 20, 1643. [Google Scholar] [CrossRef]
- Basit, A.; Hanan, A.; Nazir, T.; Majeed, M.Z.; Qiu, D. Molecular and Functional Characterization of Elicitor PeBC1 Extracted from Botrytis cinerea Involved in the Induction of Resistance against Green Peach Aphid (Myzus persicae) in Common Beans (Phaseolus vulgaris L.). Insects 2019, 10, 35. [Google Scholar] [CrossRef]
- Javed, K.; Humayun, T.; Humayun, A.; Wang, Y.; Javed, H. PeaT1 and PeBC1 Microbial Protein Elicitors Enhanced Resistance against Myzus persicae Sulzer in Chili Capsicum annum L. Microorganisms 2021, 9, 2197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zeng, H.; Guo, L.; Yuan, J.; Qiu, D. Fungal elicitor protein PebC1 from Botrytis cinerea improves disease resistance in Arabidopsis thaliana. Biotechnol. Lett. 2014, 36, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
| Carbohydrates | Proteins/Peptides | |
|---|---|---|
| Endogenous elicitors | xylan, oligo-galacturonic acid, … | glucanase, glutathione, … |
| Exogenous elicitors | peptidoglycan, chitin, glucan, lipopolysaccharides, … | flagellin (flg22), elongation factor Tu (EF-Tu), harpins, elicitins, NLPs, virus coat proteins, virus RNA replicases, … |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, J.; Ma, W.; Li, X. Characteristics, Roles and Applications of Proteinaceous Elicitors from Pathogens in Plant Immunity. Life 2023, 13, 268. https://doi.org/10.3390/life13020268
Li Z, Liu J, Ma W, Li X. Characteristics, Roles and Applications of Proteinaceous Elicitors from Pathogens in Plant Immunity. Life. 2023; 13(2):268. https://doi.org/10.3390/life13020268
Chicago/Turabian StyleLi, Zhangqun, Junnan Liu, Wenting Ma, and Xiaofang Li. 2023. "Characteristics, Roles and Applications of Proteinaceous Elicitors from Pathogens in Plant Immunity" Life 13, no. 2: 268. https://doi.org/10.3390/life13020268
APA StyleLi, Z., Liu, J., Ma, W., & Li, X. (2023). Characteristics, Roles and Applications of Proteinaceous Elicitors from Pathogens in Plant Immunity. Life, 13(2), 268. https://doi.org/10.3390/life13020268




