Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Protocol
2.2. Detection of Specific Antibodies via VacciCheck
2.3. Statistical Analysis
3. Results
3.1. Cat Population
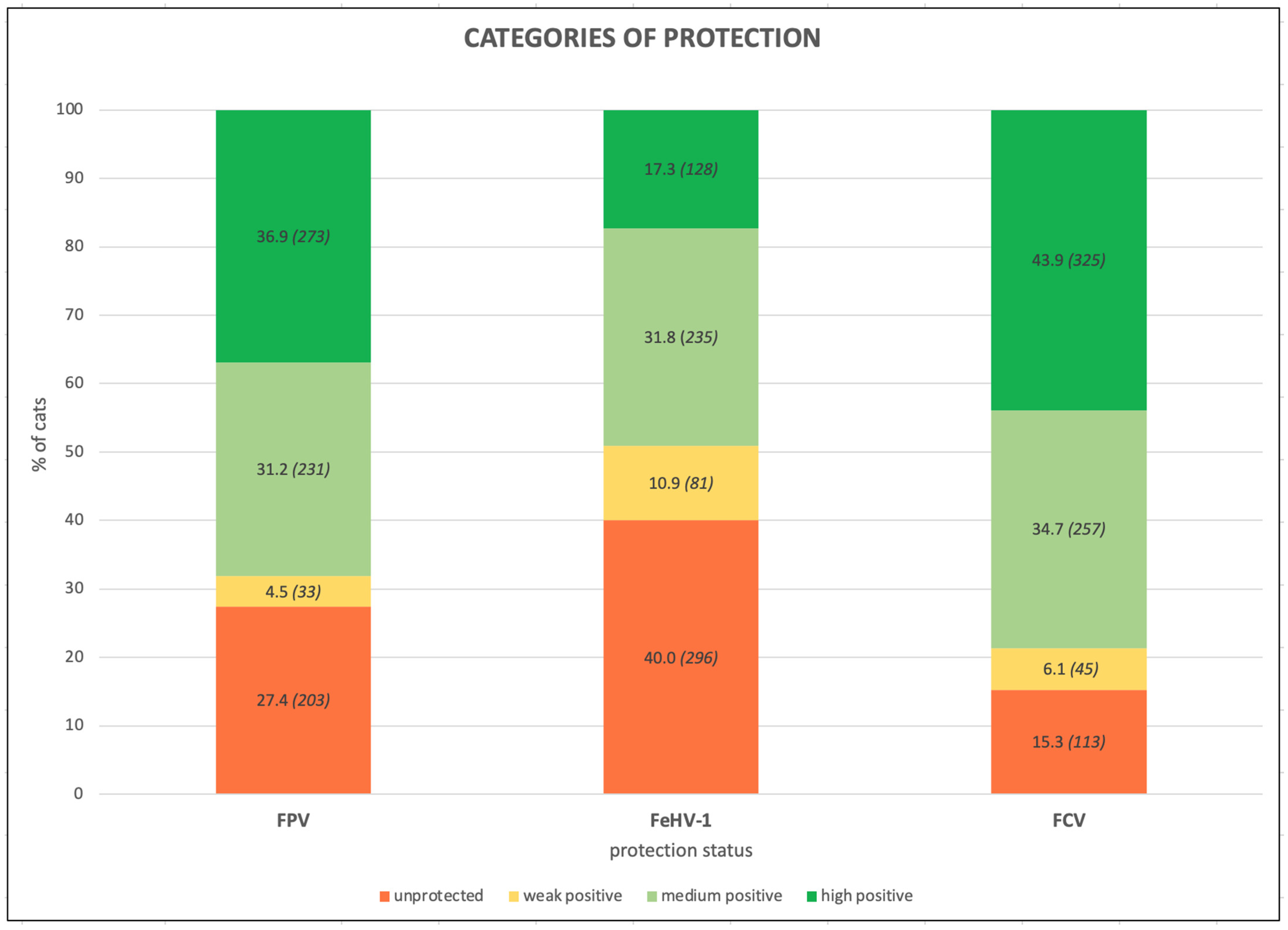
3.2. Antibody Titers and Protection of the Entire Cohort
3.3. Results According to the Different Variables
3.3.1. Origin
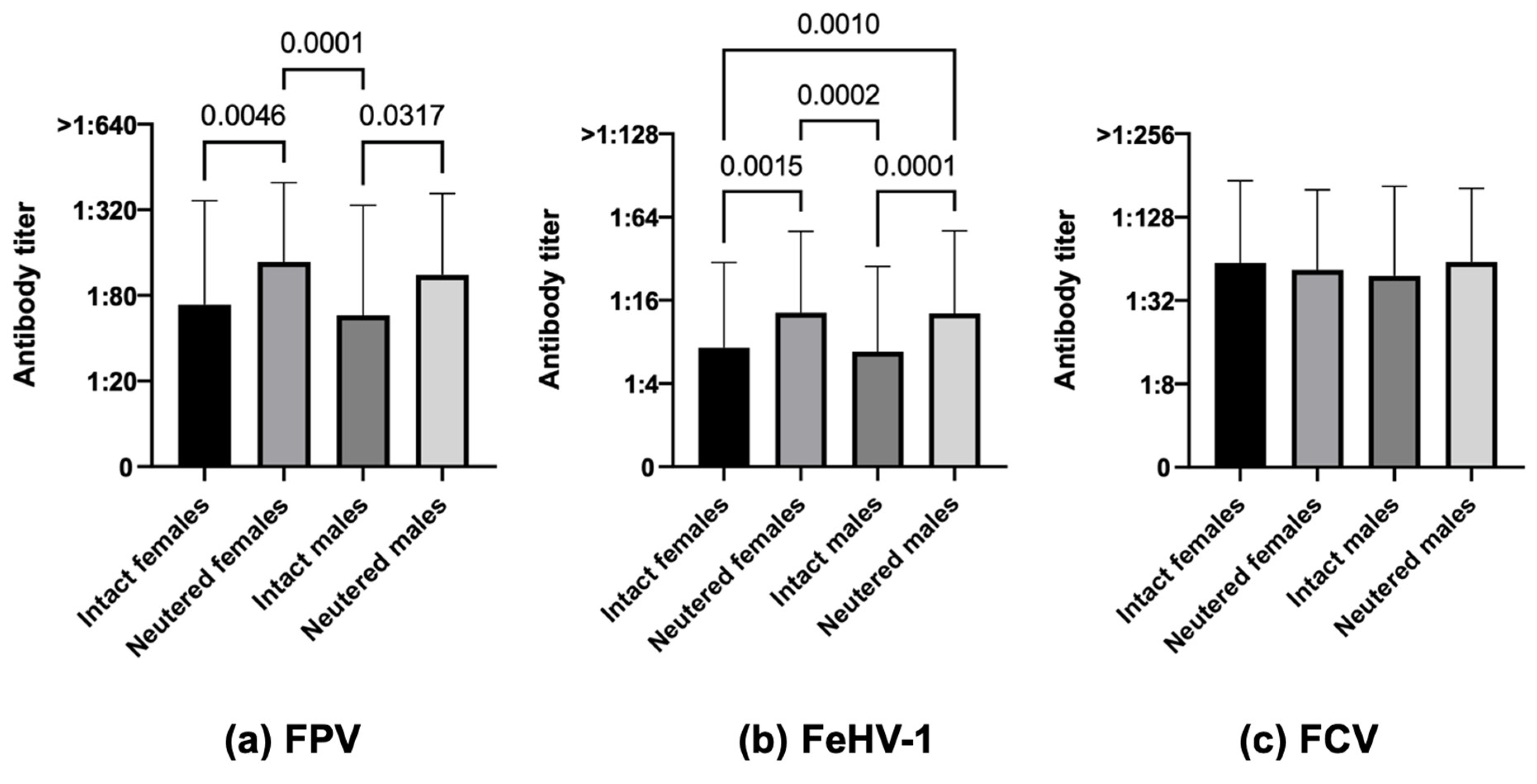
3.3.2. Sex and Reproductive Status
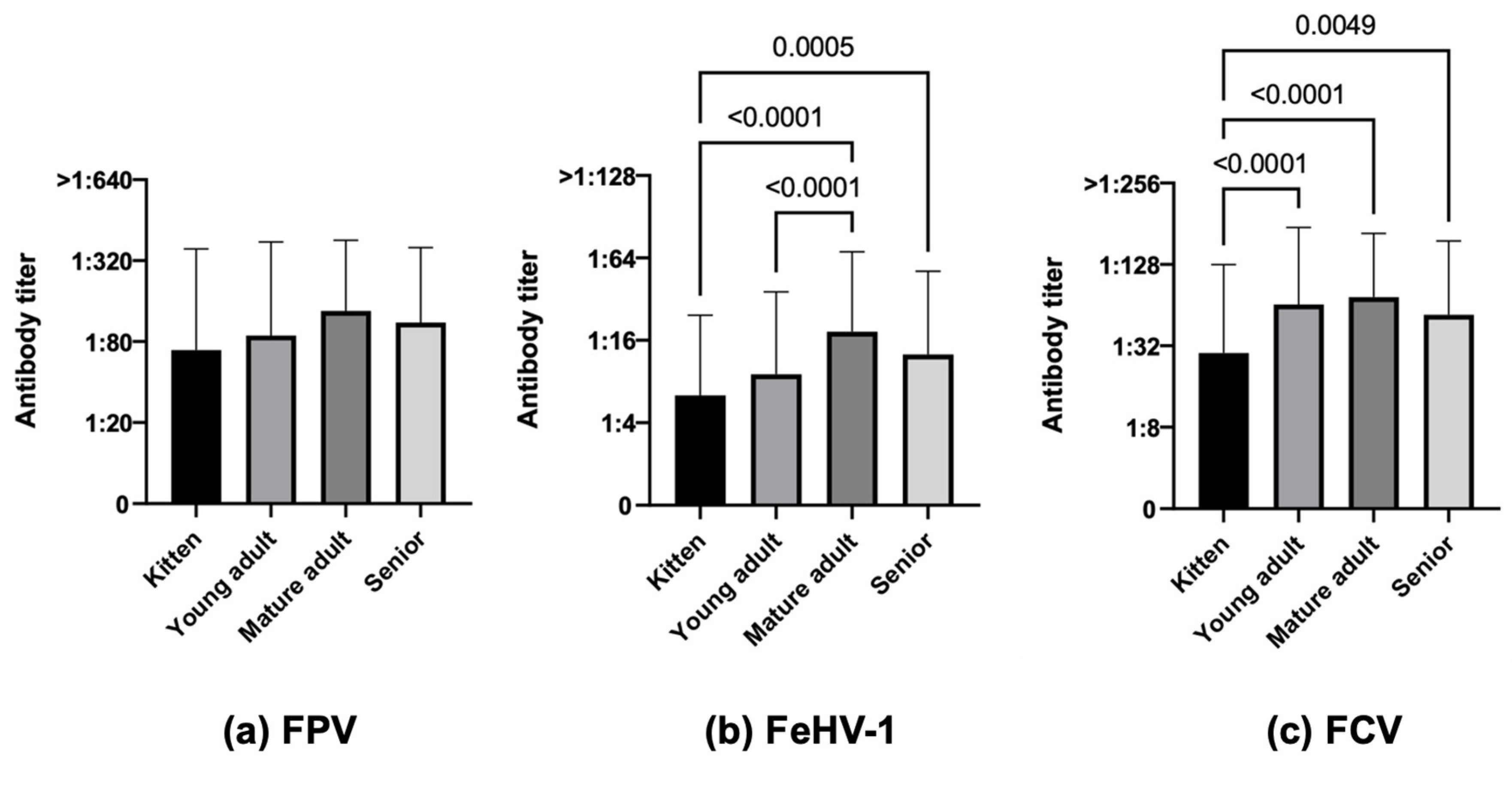
3.3.3. Age
3.3.4. Breed
3.3.5. FIV/FeLV Status
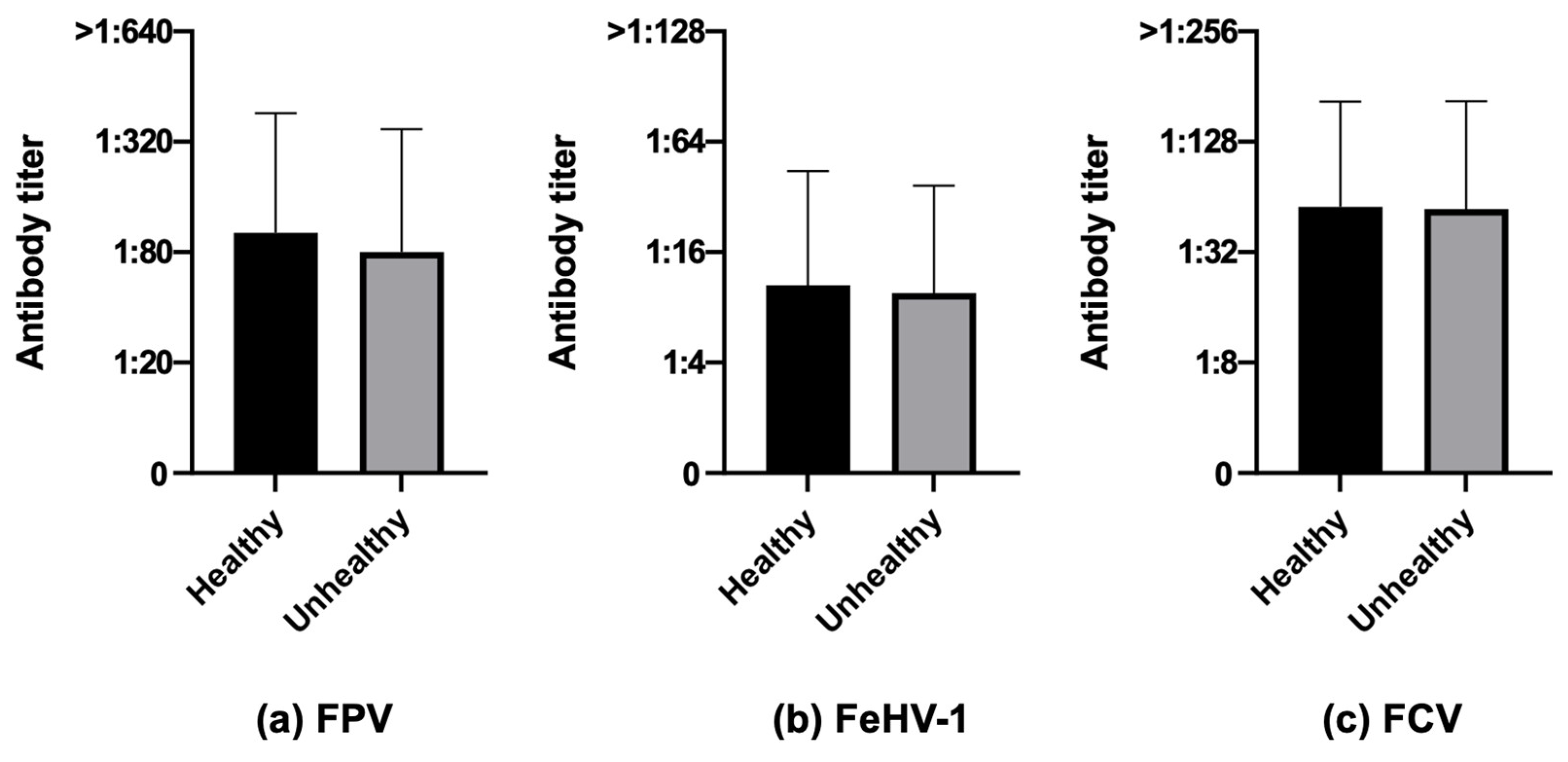
3.3.6. Health Status
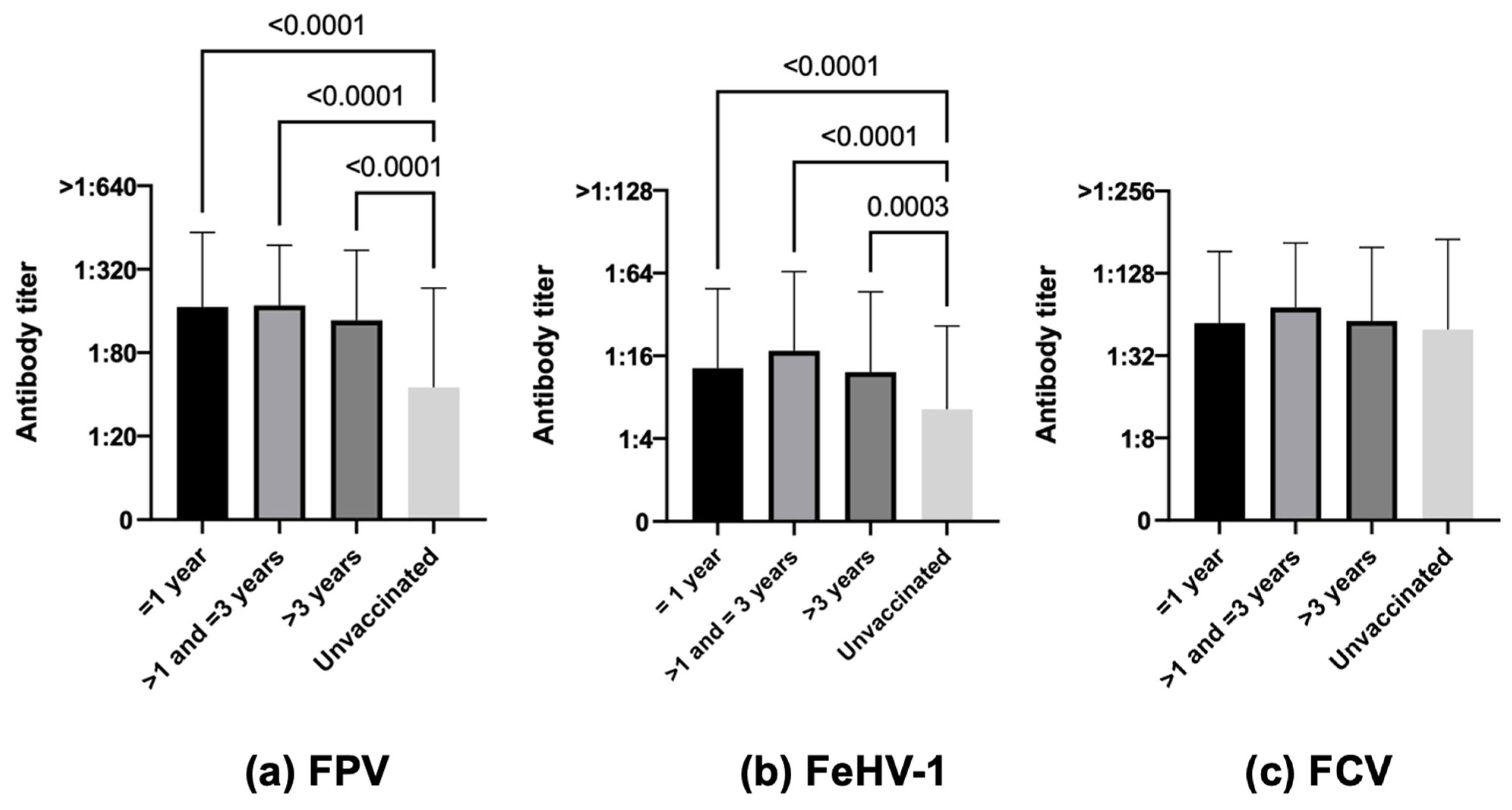
3.3.7. Time Elapsed since Last Vaccination
4. Discussion
Reliability and Usefulness of Assessment of Antibody Titration for Cat Core Vaccines in Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NHS. Why Vaccination Is Important and the Safest Way to Protect Yourself. Available online: https://www.nhs.uk/conditions/vaccinations/why-vaccination-is-important-and-the-safest-way-to-protect-yourself/ (accessed on 12 November 2023).
- Stone, A.E.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; Westman, M.E. 2020 AAHA/AAFP Feline Vaccination Guidelines. J. Feline Med. Surg. 2020, 22, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.R.; Elston, T.H.; Ford, R.B.; Gaskell, R.M.; Hartmann, K.; Hurley, K.F.; Lappin, M.R.; Levy, J.K.; Rodan, I.; Scherk, M.; et al. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel Report. J. Am. Vet. Med. Assoc. 2006, 229, 1405–1441. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Lloret, A.; Lutz, H.; et al. Matrix Vaccination Guidelines. J. Feline Med. Surg. 2015, 17, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Advisory Board on Cat Diseases (ABCD). Vaccination Recommended for Cats According to Lifestyle. Available online: http://www.abcdcatsvets.org/wp-content/uploads/2020/03/Tool_Vaccine-recommendations_Feb2020.pdf (accessed on 5 January 2023).
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A. WSAVA Guidelines for the Vaccination of Dogs and Cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Karkare, U.; Schultz, R.D.; Squires, R.; Tsujimoto, H. Recommendations on Vaccination for Asian Small Animal Practitioners: A Report of the WSAVA Vaccination Guidelines Group. J. Small Anim. Pract. 2015, 56, 77–95. [Google Scholar] [CrossRef]
- WSAVA. Recomendaciones Sobre Vacunación Para Los Profesionales Latinoamericanos de Pequeños Animales: Un Informe Del Grupo de Directrices de Vacunación de WSAVA. Clínica Vet. 2020, 148, 36–91. [Google Scholar]
- Australian Veterinary Association (AVA). Vaccination of Dogs and Cats. Available online: https://www.ava.com.au/policy-advocacy/policies/companion-animals-health/vaccination-of-dogs-and-cats/ (accessed on 16 January 2023).
- British Veterinary Association (BVA). Getting Your Pet Vaccinated. Available online: https://www.bva.co.uk/media/2649/client_leaflet_9_-_getting_your_pet_vaccinated.pdf (accessed on 4 January 2023).
- Vallée, B. Canadian Veterinary Medical Association Adopts a New Position Statement on Vaccination Protocols for Dogs and Cats. Can. Vet. J. 2008, 49, 362–365, quiz 365. [Google Scholar]
- Joint American Veterinary Medical Association (AVMA)-Federation of Veterinarians of Europe (FVE)-Canadian Veterinary Medical Association (CVMA) Statement on the Benefits of Animal Vaccination Programs in Advancing Animal and Human Health. Available online: https://fve.org/cms/wp-content/uploads/AVMA-CVMA-FVE_vacconation_joint-paper.docx.pdf (accessed on 18 February 2023).
- Sykes, J.; Parrish, C. Feline Panleukopenia Virus Infection and Other Feline Viral Enteritides. In Greene’s Infectious Diseases of the Dog and Cat; Sykes, J.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 352–359. [Google Scholar]
- Carrai, M.; Decaro, N.; Van Brussel, K.; Dall’Ara, P.; Desario, C.; Fracasso, M.; Šlapeta, J.; Colombo, E.; Bo, S.; Beatty, J.A.; et al. Canine Parvovirus Is Shed Infrequently by Cats without Diarrhoea in Multi-Cat Environments. Vet. Microbiol. 2021, 261, 109204. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, D.; Desario, C.; Amorisco, F.; Colaianni, M.L.; Parisi, A.; Terio, V.; Elia, G.; Lucente, M.S.; Cavalli, A.; et al. Characterisation of Canine Parvovirus Strains Isolated from Cats with Feline Panleukopenia. Res. Vet. Sci. 2010, 89, 275–278. [Google Scholar] [CrossRef]
- Stuetzer, B.; Hartmann, K. Feline Parvovirus Infection and Associated Diseases. Vet. J. 2014, 201, 150–155. [Google Scholar] [CrossRef]
- Truyen, U.; Parrish, C.R. Feline Panleukopenia Virus: Its Interesting Evolution and Current Problems in Immunoprophylaxis against a Serious Pathogen. Vet. Microbiol. 2013, 165, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Leisewitz, A. Canine and Feline Parvovirus Infection. In Textbook of Veterinary Internal Medicine; Ettinger, S., Feldman, E., Côté, E., Eds.; Elsevier: St. Louis, MI, USA, 2016; pp. 991–996. [Google Scholar]
- Advisory Board on Cat Diseases (ABCD). Guideline for Feline Panleukopenia. Available online: https://www.abcdcatsvets.org/guideline-for-feline-panleukopenia/ (accessed on 18 August 2023).
- Maes, R. Felid Herpesvirus Type 1 Infection in Cats: A Natural Host Model for Alphaherpesvirus Pathogenesis. ISRN Vet. Sci. 2012, 2012, 495830. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.M.; Gaskell, R.M.; Radford, A. Feline Upper Respiratory Infections. In Textbook of Veterinary Internal Medicine; Ettinger, S., Feldman, E., Côté, E., Eds.; Elsevier: St Louis, MI, USA, 2016; pp. 1013–1015. [Google Scholar]
- Advisory Board on Cat Diseases (ABCD). Guideline for Feline Herpesvirus Infection. Available online: https://www.abcdcatsvets.org/guideline-for-feline-herpesvirus-infection/ (accessed on 18 August 2023).
- Chan, I.; Dowsey, A.; Lait, P.; Tasker, S.; Blackwell, E.; Helps, C.R.; Barker, E.N. Prevalence and Risk Factors for Common Respiratory Pathogens within a Cohort of Pet Cats in the UK. J. Small Anim. Pract. 2023, 64, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.; Lappin, M.; Thomasy, S.; Beatty, J. Feline Herpesvirus Infections. In Greene’s Infectious Diseases of the Dog and Cat; Sykes, J., Ed.; Elsevier: St. Louis, MI, USA, 2023; pp. 301–309. [Google Scholar]
- Advisory Board on Cat Diseses (ABCD). Guideline for Feline Calicivirus Infection. Available online: https://www.abcdcatsvets.org/guideline-for-feline-calicivirus-infection/ (accessed on 18 August 2023).
- Radford, A.; Afonso, M.; Sykes, J. Feline Calicivirus Infections. In Greene’s Infectious Diseases of the Dog and Cat; Sykes, J., Ed.; Elsevier: St. Louis, MI, USA, 2023; pp. 443–454. [Google Scholar]
- Afonso, M.M.; Pinchbeck, G.L.; Smith, S.L.; Daly, J.M.; Gaskell, R.M.; Dawson, S.; Radford, A.D. A Multi-National European Cross-Sectional Study of Feline Calicivirus Epidemiology, Diversity and Vaccine Cross-Reactivity. Vaccine 2017, 35, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Caringella, F.; Elia, G.; Decaro, N.; Martella, V.; Lanave, G.; Varello, K.; Catella, C.; Diakoudi, G.; Carelli, G.; Colaianni, M.L.; et al. Feline Calicivirus Infection in Cats with Virulent Systemic Disease, Italy. Res. Vet. Sci. 2019, 124, 46–51. [Google Scholar] [CrossRef]
- Dall’Ara, P. Vaccini e Vaccinazioni Degli Animali Da Compagnia, 1st ed.; Dall’Ara, P., Ed.; EDRA: Milano, Italy, 2020. [Google Scholar]
- Little, S.; Levy, J.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.; Olah, G.; Denis, K.S. 2020 AAFP Feline Retrovirus Testing and Management Guidelines. J. Feline Med. Surg. 2020, 22, 5–30. [Google Scholar] [CrossRef]
- Jas, D.; Frances-Duvert, V.; Vernes, D.; Guigal, P.-M.; Poulet, H. Three-Year Duration of Immunity for Feline Herpesvirus and Calicivirus Evaluated in a Controlled Vaccination-Challenge Laboratory Trial. Vet. Microbiol. 2015, 177, 123–131. [Google Scholar] [CrossRef]
- Möstl, K. Duration of Vaccine-Induced Immunity. EJCAP 2016, 26, 4–8. [Google Scholar]
- Mitchell, S.; Zwijnenberg, R.; Huang, J.; Hodge, A.; Day, M. Duration of Serological Response to Canine Parvovirus-Type 2, Canine Distemper Virus, Canine Adenovirus Type 1 and Canine Parainfluenza Virus in Client-Owned Dogs in Australia. Aust. Vet. J. 2012, 90, 468–473. [Google Scholar] [CrossRef]
- Ellis, J.; Marziani, E.; Aziz, C.; Brown, C.M.; Cohn, L.A.; Lea, C.; Moore, G.E.; Taneja, N. 2022 AAHA Canine Vaccination Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 213–230. [Google Scholar] [CrossRef]
- Mouzin, D.E.; Lorenzen, M.J.; Haworth, J.D.; King, V.L. Duration of Serologic Response to Three Viral Antigens in Cats. JAVMA 2004, 224, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Chastant-Maillard, S.; Aggouni, C.; Albaret, A.; Fournier, A.; Mila, H. Canine and Feline Colostrum. Reprod. Domest. Anim. 2017, 52, 148–152. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Levy, J.K.; Griffin, B.; Reese, M.J.; Dingman, P.A.; Tucker, S.J.; Dubovi, E.J. Effects of Maternally-Derived Antibodies on Serologic Responses to Vaccination in Kittens. J. Feline Med. Surg. 2012, 14, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Spickler, A.R. Duration of Immunity Induced by Companion Animal Vaccines. Anim. Health Res. Rev. 2010, 11, 165–190. [Google Scholar] [CrossRef]
- Rashid, A.; Rasheed, K.; Akhtar, M. Factors Influencing Vaccine Efficacy—A General Review. J. Anim. Plant Sci. 2009, 19, 22–25. [Google Scholar]
- Egberink, H.; Frymus, T.; Hartmann, K.; Möstl, K.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Hofmann-Lehmann, R.; Lloret, A.; Marsilio, F.; et al. Vaccination and Antibody Testing in Cats. Viruses 2022, 14, 1602. [Google Scholar] [CrossRef]
- Egberink, H.; Hartmann, K. ABCD Guideline for Vaccination and Antibody Titre Testing. Available online: https://www.abcdcatsvets.org/guideline-for-vaccination-and-antibody-titre-testing/ (accessed on 15 August 2023).
- Egberink, H. The Role of Antibody Titer Testing in Vaccination Policy of Dog and Cat. Utrecht Univ. J. 2019, 14, 17–21. [Google Scholar]
- Quimby, J.; Gowland, S.; Carney, H.C.; DePorter, T.; Plummer, P.; Westropp, J. 2021 AAHA/AAFP Feline Life Stage Guidelines. J. Feline Med. Surg. 2021, 23, 211–233. [Google Scholar] [CrossRef]
- Biogal Galed Labs ACS Ltd. VacciCheck® Titer Testing. Available online: https://www.biogal.com/products/vaccicheck/ (accessed on 4 February 2023).
- Day, M.J. Small Animal Vaccination: A Practical Guide for Vets in the UK. InPractice 2017, 39, 110–118. [Google Scholar] [CrossRef]
- Day, M. What We Need to Know about Vaccination and Titre Testing. In Proceedings of the British Small Animal Veterinary Association Congress, Birmingham, UK, 11–15 April 2012; pp. 1–7. [Google Scholar]
- Coman, B.; Jones, E.; Westbury, H. Protozoan and Viral Infections of Feral Cats. Aust. Vet. J. 1981, 57, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Yagami, K.; Furukawa, T.; Fukui, M. Serologic and Virologic Surveys on Feline Herpesvirus and Feline Calicivirus Infections in Cats for Experimental Use. Exp. Anim. 1985, 34, 241–248. [Google Scholar] [CrossRef]
- Yamaguchi, N.; MacDonald, D.; Passanisi, W.; Harbour, D.; Hopper, C. Parasite Prevalence in Free-Ranging Farm Cats, Felis Silvestris Catus. Epidemiol. Infect. 1996, 116, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Ikeda, Y.; Nakamura, K.; Naito, R.; Mochizuki, M.; Tohya, Y.; Vu, D.; Mikami, T.; Takahashi, E. Isolation of Feline Parvovirus from Peripheral Blood Mononuclear Cells of Cats in Northern Vietnam. Microbiol. Immunol. 1999, 43, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ikeda, Y.; Miyazawa, T.; Nguyen, N.; Duong, D.; Le, K.; Vo, S.; Phan, L.; Mikami, T.; Takahashi, E. Comparison of Prevalence of Feline Herpesvirus Type 1, Calicivirus and Parvovirus Infections in Domestic and Leopard Cats in Vietnam. J. Vet. Med. Sci. 1999, 61, 1313–1315. [Google Scholar] [CrossRef][Green Version]
- Lappin, M.R.; Andrews, J.; Simpson, D.; Jensen, W.A. Use of Serologic Tests to Predict Resistance to Feline Herpesvirus 1, Feline Calicivirus, and Feline Parvovirus Infection in Cats. JAVMA 2002, 219, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.; Van Vuuren, M.; Lenain, D.; Durand, A. A Serologic Survey of Wild Felids from Central West Saudi Arabia. J. Wildl. Dis. 2003, 39, 696–701. [Google Scholar] [CrossRef]
- Fischer, S.M.; Quest, C.M.; Dubovi, E.J.; Davis, R.D.; Tucker, S.J.; Friary, J.A.; Crawford, P.C.; Ricke, T.A.; Levy, J.K. Response of Feral Cats to Vaccination at the Time of Neutering. JAVMA 2007, 230, 52–58. [Google Scholar] [CrossRef]
- Levy, J.K.; Crawford, P.C.; Lappin, M.R.; Dubovi, E.J.; Levy, M.G.; Alleman, R.; Tucker, S.J.; Clifford, E.L. Infectious Diseases of Dogs and Cats on Isabela Island, Galapagos. J. Vet. Intern. Med. 2008, 22, 60–65. [Google Scholar] [CrossRef]
- Blanco, K.; Prendas, J.; Cortes, R.; Jimenez, C.; Dolz, G. Seroprevalence of Viral Infections in Domestic Cats in Costa Rica. J. Vet. Medial Sci. 2009, 71, 661–663. [Google Scholar] [CrossRef]
- Hellard, E.; Fouchet, D.; Santin-Janin, H.; Tarin, B.; Badol, V.; Coupier, C.; Leblanc, G.; Poulet, H.; Pontier, D. When Cats’ Ways of Life Interact with Their Viruses: A Study in 15 Natural Populations of Owned and Unowned Cats (Felis Silvestris Catus). Prev. Vet. Med. 2011, 101, 250–264. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Gray, L.K.; Levy, J.K.; Dubovi, E.J.; Tucker, S.J. Detection of Protective Antibody Titers against Feline Panleukopenia Virus, Feline Herpesvirus-1, and Feline Calicivirus in Shelter Cats Using a Point-of-Care ELISA. J. Feline Med. Surg. 2011, 13, 912–918. [Google Scholar] [CrossRef] [PubMed]
- DiGangi, B.A.; Levy, J.K.; Griffin, B.; McGorray, S.P.; Dubovi, E.J.; Dingman, P.A.; Tucker, S.J. Prevalence of Serum Antibody Titers against Feline Panleukopenia Virus, Feline Herpesvirus 1, and Feline Calicivirus in Cats Entering a Florida Animal Shelter. JAVMA 2012, 241, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Mende, K.; Stuetzer, B.; Truyen, U.; Hartmann, K. Evaluation of an In-House Dot Enzyme-Linked Immunosorbent Assay to Detect Antibodies against Feline Panleukopenia Virus. J. Feline Med. Surg. 2014, 16, 805–811. [Google Scholar] [CrossRef]
- Mende, K.; Stuetzer, B.; Sauter-Louis, C.; Homeier, T.; Truyen, U.; Hartmann, K. Prevalence of Antibodies against Feline Panleukopenia Virus in Client-Owned Cats in Southern Germany. Vet. J. 2014, 199, 419–423. [Google Scholar] [CrossRef]
- Bergmann, M.; Schwertler, S.; Reese, S.; Speck, S.; Truyen, U.; Hartmann, K. Antibody Response to Feline Panleukopenia Virus Vaccination in Healthy Adult Cats. J. Feline Med. Surg. 2018, 20, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Speck, S.; Rieger, A.; Truyen, U.; Hartmann, K. Antibody Response to Feline Calicivirus Vaccination in Healthy Adult Cats. Viruses 2019, 11, 702. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Labriola, C.; Sala, E.; Spada, E.; Magistrelli, S.; Lauzi, S. Prevalence of Serum Antibody Titres against Feline Panleukopenia, Herpesvirus and Calicivirus Infections in Stray Cats of Milan, Italy. Prev. Vet. Med. 2019, 167, 32–38. [Google Scholar] [CrossRef]
- Bergmann, M.; Speck, S.; Rieger, A.; Truyen, U.; Hartmann, K. Antibody Response to Feline Herpesvirus-1 Vaccination in Healthy Adult Cats. J. Feline Med. Surg. 2020, 22, 329–338. [Google Scholar] [CrossRef]
- Flatt Osborn, J. How Many Cats Are in the World? A Statistical Overview. Available online: https://worldanimalfoundation.org/cats/how-many-cats-are-in-the-world/# (accessed on 21 August 2023).
- Dall’Ara, P.; Lauzi, S.; Zambarbieri, J.; Servida, F.; Barbieri, L.; Rosenthal, R.; Turin, L.; Scarparo, E.; Filipe, J. Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Dogs. Life 2023, 13, 587. [Google Scholar] [CrossRef]
- Schultz, R.D. Duration of Immunity for Canine and Feline Vaccines: A Review. Vet. Microbiol. 2006, 117, 75–79. [Google Scholar] [CrossRef]
- Schultz, R.D.; Thiel, B.; Mukhtar, E.; Sharp, P.; Larson, L.J. Age and Long-Term Protective Immunity in Dogs and Cats. J. Comp. Pathol. 2010, 142, S102–S108. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.D.; Sommerville, L.; Ryvar, R.; Cox, M.B.; Johnson, D.R.; Dawson, S.; Gaskell, R.M. Endemic Infection of a Cat Colony with a Feline Calicivirus Closely Related to an Isolate Used in Live Attenuated Vaccines. Vaccine 2001, 19, 4358–4362. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R. Feline Panleukopenia: A Re-emergent Disease. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Truyen, U.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Panleukopenia: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.R.; Coyne, K.P.; Dawson, S.; Spibey, N.; Gaskell, R.M.; Radford, A.D. Canine Parvovirus in Asymptomatic Feline Carriers. Vet. Microbiol. 2012, 157, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.R.; Coyne, K.P.; Parker, J.; Dawson, S.; Godsall, S.A.; Pinchbeck, G.; Cripps, P.J.; Gaskell, R.M.; Radford, A.D. Molecular Epidemiology and Phylogeny Reveal Complex Spatial Dynamics in Areas Where Canine Parvovirus Is Endemic. J. Virol. 2011, 85, 7892–7899. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakamoto, M.; Ikeda, Y.; Sato, E.; Kawakami, K.; Miyazawa, T.; Tohya, Y.; Takahashi, E.; Mikami, T.; Mochizuki, M. Pathogenic Potential of Canine Parvovirus Types 2a and 2c in Domestic Cats. Clin. Diagn. Lab. Immunol. 2001, 8, 663–668. [Google Scholar] [CrossRef]
- Munks, M.W.; Montoya, A.M.; Pywell, C.M.; Talmage, G.; Forssen, A.; Campbell, T.L.; Dodge, D.D.; Kappler, J.W.; Marrack, P. The Domestic Cat Antibody Response to Feline Herpesvirus-1 Increases with Age. Vet. Immunol. Immunopathol. 2017, 188, 65–70. [Google Scholar] [CrossRef]
- Dall’Ara, P. Immune System and Ageing in the Dog: Possible Consequences and Control Strategies. Vet. Res. Commun. 2003, 27, 535–542. [Google Scholar] [CrossRef]
- Hartmann, K.; Möstl, K.; Lloret, A.; Thiry, E.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Hofmann-Lehmann, R.; et al. Vaccination of Immunocompromised Cats. Viruses 2022, 14, 923. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Irshad, M.; Dash, S.C. Comparison of Two Schedules of Hepatitis B Vaccination in Patients with Mild, Moderate and Severe Renal Failure. J. Assoc. Physicians India 1999, 47, 183–185. [Google Scholar]
- DaRoza, G.; Loewen, A.; Djurdjev, O.; Love, J.; Kempston, C.; Burnett, S.; Kiaii, M.; Taylor, P.A.; Levin, A. Stage of Chronic Kidney Disease Predicts Seroconversion after Hepatitis B Immunization: Earlier Is Better. Am. J. Kidney Dis. 2003, 42, 1184–1192. [Google Scholar] [CrossRef]
- Lombardi, M.; Pizzarelli, F.; Righi, M.; Cerrai, T.; Dattolo, P.; Nigrelli, S.; Michelassi, S.; Sisca, S.; Alecci, A.; Di Geronimo, P.; et al. Hepatitis B Vaccination in Dialysis Patients and Nutritional Status. Nephron 1992, 61, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.C.; Syme, H.M.; Elliott, J. Risk Factors for Development of Chronic Kidney Disease in Cats. J. Vet. Intern. Med. 2016, 30, 602–610. [Google Scholar] [CrossRef]
- Beck, C.R.; McKenzie, B.C.; Hashim, A.B.; Harris, R.C.; Zanuzdana, A.; Agboado, G.; Orton, E.; Béchard-Evans, L.; Morgan, G.; Stevenson, C.; et al. Influenza Vaccination for Immunocompromised Patients: Summary of a Systematic Review and Meta-analysis. Influenza Other Respir. Viruses 2013, 7, 72–75. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Filipe, J.; Pilastro, C.; Turin, L.; Lauzi, S.; Gariboldi, E.M.; Stefanello, D. Can Chemotherapy Negatively Affect the Specific Antibody Response toward Core Vaccines in Canine Cancer Patients? Vet. Sci. 2023, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.; Geissinger, C. Long-Term Immunity in Cats Vaccinated with an Inactivated Trivalent Vaccine. Am. J. Vet. Res. 1999, 6, 652–658. [Google Scholar]
- Dall’Ara, P.; Lauzi, S.; Filipe, J.; Caseri, R.; Beccaglia, M.; Desario, C.; Cavalli, A.; Aiudi, G.G.; Buonavoglia, C.; Decaro, N. Discrepancy Between In-Clinic and Haemagglutination-Inhibition Tests in Detecting Maternally-Derived Antibodies Against Canine Parvovirus in Puppies. Front. Vet. Sci. 2021, 8, 630809. [Google Scholar] [CrossRef]
- Meazzi, S.; Filipe, J.; Fiore, A.; Di Bella, S.; Mira, F.; Dall’Ara, P. Agreement between In-Clinics and Virus Neutralization Tests in Detecting Antibodies against Canine Distemper Virus (CDV). Viruses 2022, 14, 517. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Lauzi, S.; Turin, L.; Castaldelli, G.; Servida, F.; Filipe, J. Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs. Vet. Sci. 2023, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ara, P.; Vitali, C.; Lasagna, C. Inaffidabilità Di Un Test Rapido per La Valutazione Della Protezione Anticorpale Verso i Vaccini Core Del Cane e Del Gatto. Veterinaria 2022, 36, 129–140. [Google Scholar]
- Reese, M.; Patterson, E.; Tucker, S.; Dubovi, E.; Davis, R.; Crawford, P.; Levy, J. The Effect of Anesthesia and Surgery on Serological Responses to Vaccination in Kittens. JAVMA 2008, 233, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R. Feline Panleukopenia Virus, Feline Herpesvirus-1 and Feline Calicivirus Antibody Responses in Seronegative Specific Pathogen-Free Kittens after Parenteral Administration of an Inactivated FVRCP Vaccine or a Modified Live FVRCP Vaccine. J. Feline Med. Surg. 2012, 14, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.; Willoughby, K.; Gaskell, R.; Wood, G.; Chalmers, W. A Field Trial to Assess the Effect of Vaccination against Feline Herpesvirus, Feline Calicivirus and Feline Panleucopenia Virus in 6-Week-Old Kittens. J. Feline Med. Surg. 2001, 3, 17–22. [Google Scholar] [CrossRef]
- Dodds, J. Vaccination and Antibody Tests: 2018 Update. Available online: https://www.vaccicheck.nl/sites/default/files/documents/serology_testing_clinical_review_dr_dodds_march_2018.pdf (accessed on 8 January 2023).
- Tizard, I.; Ni, Y. Use of Serologic Testing to Assess Immune Status of Companion Animals. J. Am. Vet. Med. Assoc. 1998, 213, 54–60. [Google Scholar]
- Tham, K.; Studdert, M.J. Antibody and Cell-Mediated Immune Responses to Feline Calicivirus Following Inactivated Vaccine and Challenge. Zentralbl Vet. B 1987, 34, 640–654. [Google Scholar] [CrossRef]
- Spiri, A.M.; Novacco, M.; Meli, M.L.; Stirn, M.; Riond, B.; Fogle, J.E.; Boretti, F.S.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Modified-Live Feline Calicivirus Vaccination Elicits Cellular Immunity against a Current Feline Calicivirus Field Strain in an Experimental Feline Challenge Study. Viruses 2021, 13, 1736. [Google Scholar] [CrossRef]
- Thiry, E.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline Herpesvirus Infection: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 547–555. [Google Scholar] [CrossRef]
- Mouzin, D.E.; Lorenzen, M.J.; Haworth, J.D.; King, V.L. Duration of Serologic Response to Five Viral Antigens in Dogs. JAVMA 2004, 224, 55–60. [Google Scholar] [CrossRef]
- Larson, L.J.; Schultz, R.D. Canine and Feline Vaccinations and Immunology. In Infectious Disease Management in Animal Shelters; Miller, L., Janeczko, S., Hurley, K.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Gore, T.; Lakshmanan, N.; Williams, J.; Jirjis, J.; Chester, S.; Duncan, K.; Coyne, M.; Lum, M.; Sterner, M. Three-Year Duration of Immunity in Cats Following Vaccination against Feline Rhinotracheitis Virus, Feline Calicivirus, and Feline Panleukopenia Virus. Vet. Ther. 2006, 7, 213–222. [Google Scholar]
- Graf, R.; Guscetti, F.; Welle, M.; Meier, D.; Pospischil, A. Feline Injection Site Sarcomas: Data from Switzerland 2009–2014. J. Comp. Pathol. 2018, 163, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Day, M.J.; Thiry, E.; Lloret, A.; Frymus, T.; Addie, D.; Boucraut-Baralon, C.; Egberink, H.; Gruffydd-Jones, T.; Horzinek, M.C.; et al. Feline Injection-Site Sarcoma. J. Feline Med. Surg. 2015, 17, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Egberink, H.; Möstl, K.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; Hofmann-Lehmann, R.; Marsilio, F.; et al. Feline Injection-Site Sarcoma and Other Adverse Reactions to Vaccination in Cats. Viruses 2023, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Jas, D.; Frances-Duvert, V.; Brunet, S.; Oberli, F.; Guigal, P.-M.; Poulet, H. Evaluation of Safety and Immunogenicity of Feline Vaccines with Reduced Volume. Vaccine 2021, 39, 1051–1057. [Google Scholar] [CrossRef]
- McLain Madsen, L. Vaccine Transport, Storage, and Handling. Vet. Tech. 2011, 2011, E1–E4. [Google Scholar]



| Protective Antibody Titers (PATs), % (n. of Cats) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Whole Population (740 Cats) | Vaccinated Cats (435 Cats) | Unvaccinated Cats (305 Cats) | |||||||
| FPV | FeHV-1 | FCV | FPV | FeHV-1 | FCV | FPV | FeHV-1 | FCV | |
| Total Positive | 68.1 (504/740) | 49.1 (363/740) | 78.6 (582/740) | 84.6 (368/435) | 57.9 (252/435) | 82.3 (358/435) | 44.6 (136/305) | 36.4 (111/305) | 73.4 (224/305) |
| Origin | |||||||||
| Owned cats | 75.0 (425/567) | 52.6 (297/567) | 77.4 (438/567) | 84.6 (368/435) | 57.9 (252/435) | 82.3 (358/435) | 43.2 (57/132) | 34.8 (46/132) | 61.4 (81/132) |
| Shelter or colony cats | 45.7 (79/173) | 37.6 (66/173) | 82.7 (144/173) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 45.7 (79/173) | 37.6 (65/173) | 82.7 (143/173) |
| Sex and reproductive status | |||||||||
| Intact females | 55.5 (81/146) | 37.0 (54/146) | 78.8 (115/146) | 84.8 (39/46) | 47.8 (22/46) | 73.9 (34/46) | 42.0 (42/100) | 32.0 (32/100) | 81.0 (81/100) |
| Neutered females | 81.1 (167/206) | 57.3 (118/206) | 80.1 (165/206) | 89.4 (135/151) | 60.9 (92/151) | 84.1 (127/151) | 58.2 (32/55 | 47.3 (26/55) | 69.1 (38/55) |
| Intact males | 49.6 (66/133) | 36.8 (49/133) | 72.2 (96/133) | 80.4 (37/46) | 60.9 (28/46) | 82.6 (38/46) | 33.3 (29/87) | 24.1 (21/87) | 62.1 (54/87) |
| Neutered males | 74.5 (190/255) | 55.7 (142/255) | 80.8 (206/255) | 81.8 (35/192) | 57.3 (110/192) | 82.8 (159/192) | 52.4 (33/63) | 50.8 (32/63) | 74.6 (47/63) |
| Age | |||||||||
| Kittens (4 months–<1 year) | 55.0 (60/109) | 33.9 (37/109) | 58.7 (64/109) | 78.6 (44/56) | 44.6 (25/56) | 73.2 (41/56) | 30.2 (16/53) | 22.6 (12/53) | 43.4 (23/53) |
| Young adults (≥1–<7 years) | 64.8 (228/352) | 44.0 (155/352) | 81.0 (285/352) | 88.4 (152/172) | 54.7 (94/172) | 82.6 (142/172) | 42.2 (76/180) | 33.9 (61/180) | 79.4 (143/180) |
| Mature adults (≥7–<10 years) | 80.4 (90/112) | 69.6 (78/112) | 88.4 (99/112) | 87.0 (67/77) | 74.0 (57/77) | 90.9 (70/77) | 65.7 (23/35) | 60.0 (21/35) | 82.9 (29/35) |
| Seniors (≥10 years) | 75.4 (126/167) | 55.7 (93/167) | 80.2 (134/167) | 80.8 (105/130) | 58.5 (76/130) | 80.8 (105/130) | 56.8 (21/37) | 45.9 (17/37) | 78.4 (29/37) |
| Breed | |||||||||
| Common European | 67.5 (434/643) | 48.8 (314/643) | 78.7 (506/643) | 85.5 (301/352) | 58.2 (205/352) | 82.7 (291/352) | 45.7 (133/291) | 37.5 (109/291) | 73.9 (215/291) |
| Pure breed | 72.2 (70/97) | 50.5 (49/97) | 78.4 (76/97) | 80.7 (67/83) | 56.6 (47/83) | 80.7 (67/83) | 21.4 (3/14) | 14.3 (2/14) | 64.3 (9/14) |
| FIV/FeLV status * | |||||||||
| FIV–FeLV– | 76.1 (239/314) | 49.7 (156/314) | 77.7 (244/314) | 83.2 (198/238) | 53.8 (128/238) | 79.4 (189/238) | 53.9 (41/76) | 36.8 (28/76) | 72.4 (55/76) |
| FIV-positive | 47.1 (8/17) | 47.1 (8/17) | 82.4 (14/17) | 71.4 (5/7) | 42.9 (3/7) | 85.7 (6/7) | 30.0 (3/10) | 50.0 (5/10) | 80.0 (8/10) |
| FeLV-positive | 68.2 (15/22) | 31.8 (7/22) | 86.4 (19/22) | 70.6 (12/17) | 29.4 (5/17) | 88.2 (15/17) | 60.0 (3/5) | 40.0 (2/5) | 80.0 (4/5) |
| FIV- and FeLV-positive | 61.5 (8/13) | 38.5 (5/13) | 92.3 (12/13) | 83.3 (5/6) | 33.3 (2/6) | 100.0 (6/6) | 42.9 (3/7) | 42.9 (3/7) | 85.7 (6/7) |
| Health status | |||||||||
| Healthy | 70.1 (386/551) | 49.9 (275/551) | 78.9 (435/551) | 87.5 (300/343) | 62.1 (213/343) | 83.1 (285/343) | 41.3 (86/208) | 29.8 (62/208) | 72.1 (150/208) |
| Unhealthy | 62.4 (118/189) | 46.6 (88/189) | 77.8 (147/189) | 73.9 (68/92) | 42.4 (39/92) | 79.3 (73/92) | 51.5 (50/97) | 50.5 (49/97) | 76.3 (74/97) |
| Time after vaccination ** | |||||||||
| ≤1 year | // | // | // | 83.5 (142/170) | 55.3 (94/170) | 81.2 (138/170) | // | // | // |
| >1–≤3 years | // | // | // | 88.0 (132/150) | 62.6 (94/150) | 84.7 (127/150) | // | // | // |
| >3 years | // | // | // | 81.7 (94/115) | 55.7 (64/115) | 81.0 (93/115) | // | // | // |
| FPV | FeHV-1 | FCV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protected % (Number) | Protected % (Number) | Protected % (Number) | ||||||||
| Statistical Variable (Number) | YES | NO | p-Value | YES | NO | p-Value | YES | NO | p-Value | |
| Origin | Owned (567) | 75.0 (425) | 25.0 (142) | <0.0001 | 52.6 (297) | 47.4 (270) | 0.0007 | 77.4 (438) | 22.6 (129) | 0.1681 |
| Shelter/colony (173) | 45.7 (79) | 54.3 (94) | 37.6 (66) | 62.4 (107) | 82.7 (144) | 17.3 (29) | ||||
| Sex | Intact females (146) | 55.5 (81) | 44.5 (65) | <0.0001 | 37.0 (54) | 63.0 (92) | <0.0001 | 78.8 (115) | 21.2 (31) | 0.2397 |
| Neutered females (206) | 81.1 (167) | 18.9 (39) | 57.3 (118) | 42.7 (88) | 80.1 (165) | 19.9 (41) | ||||
| Intact males (133) | 49.6 (66) | 50.4 (67) | 36.8 (49) | 63.2 (84) | 72.2 (96) | 12.8 (37) | ||||
| Neutered males (255) | 74.5 (190) | 25.5 (65) | 55.7 (142) | 44.3 (113) | 80.8 (206) | 19.3 (49) | ||||
| Age | Kittens (109) | 55.0 (60) | 45.0 (49) | <0.0001 | 33.9 (37) | 66.1 (72) | <0.0001 | 58.7 (64) | 41.3 (45) | <0.0001 |
| Young adults (352) | 64.8 (228) | 35.2 (124) | 44.0 (155) | 56.0 (197) | 81.0 (285) | 19.0 (67) | ||||
| Mature adults (112) | 81.4 (91) | 18.9 (21) | 69.6 (78) | 30.6 (34) | 88.4 (99) | 11.7 (13) | ||||
| Seniors (167) | 75.4 (126) | 24.6 (41) | 55.7 (93) | 44.3 (74) | 80.2 (134) | 19.8 (33) | ||||
| Breed | Common European (643) | 67.5 (434) | 32.5 (209) | 0.4138 | 48.8 (314) | 51.2 (329) | 0.8276 | 78.7 (506) | 21.3 (137) | 0.9999 |
| Pure breed (97) | 72.2 (70) | 27.8 (27) | 50.5 (49) | 49.5 (48) | 78.4 (76) | 21.6 (21) | ||||
| FIV/FeLV status | FIV–FeLV– (314) | 76.1 (239) | 23.9 (75) | 0.0364 | 49.7 (156) | 50.3 (158) | 0.2602 | 77.7 (244) | 22.3 (70) | 0.4691 |
| FIV+ (17) | 47.1 (8) | 52.9 (9) | 47.1 (8) | 52.9 (9) | 82.4 (14) | 17.6 (3) | ||||
| FeLV+ (22) | 68.2 (15) | 31.8 (7) | 31.8 (7) | 68.2 (15) | 86.4 (19) | 13.6 (3) | ||||
| FIV+FeLV+ (13) | 61.5 (8) | 38.5 (5) | 38.5 (5) | 61.5 (8) | 92.3 (12) | 7.7 (1) | ||||
| Health status | Healthy (551) | 70.1 (386) | 29.9 (165) | 0.0712 | 49.9 (275) | 50.1 (276) | 0.4507 | 78.9 (435) | 21.1 (116) | 0.6822 |
| Unhealthy (189) | 62.4 (118) | 37.6 (71) | 46.6 (88) | 53.4 (101) | 77.8 (147) | 22.2 (42) | ||||
| Time after vaccination | ≤1 year (170) | 83.5 (142) | 16.5 (28) | <0.0001 | 55.3 (94) | 44.7 (76) | <0.0001 | 81.2 (138) | 18.8 (32) | 0.0258 |
| >1–≤3 years (150) | 88.0 (132) | 12.0 (18) | 62.6 (94) | 37.4 (56) | 84.7 (127) | 15.3 (23) | ||||
| >3 years (115) | 81.7 (94) | 18.3 (21) | 55.7 (64) | 44.3 (51) | 81.0 (93) | 19.1 (22) | ||||
| Unvaccinated (305) | 44.6 (136) | 55.4 (169) | 36.4 (111) | 63.6 (194) | 73.4 (224) | 26.6 (81) | ||||
| Seronegative Cats | Whole Population (740 Cats) % (n. of Cats) | Vaccinated Cats (435 Cats) % (n. of Cats) | Unvaccinated Cats (305 Cats) % (n. of Cats) |
|---|---|---|---|
| Only for FPV | 8.4 (62) | 3.2 (14) | 15.7 (48) |
| Only for FeHV-1 | 13.2 (98) | 12.2 (53) | 14.8 (45) |
| Only for FCV | 1.5 (12) | 1.6 (7) | 1.6 (5) |
| For FPV and FeHV-1 | 8.8 (65) | 1.8 (8) | 18.7 (57) |
| For FPV and FCV | 1.1 (8) | 0.2 (1) | 2.3 (7) |
| For FeHV-1 and FCV | 4.1 (30) | 3.9 (17) | 4.3 (13) |
| For FPV, FeHV-1 and FCV | 6.8 (50) | 2.8 (12) | 12.5 (38) |
| Total | 43.9 (325) | 25.7 (112) | 69.8 (213) |
| For FPV (alone or with one or both other viruses) | 25.0 (185) | 8.0 (35) | 49.2 (150) |
| For FeHV-1 (alone or with one or both other viruses) | 32.8 (243) | 20.7 (90) | 50.2 (153) |
| For FCV (alone or with one or both other viruses) | 13.5 (100) | 8.5 (37) | 20.7 (63) |
| % of Protection | ||||||
|---|---|---|---|---|---|---|
| Authors (year) | Reference | Country | No. of Cats | FPV | FeHV-1 | FCV |
| Coman et al. (1981) | [47] | Australia | * 300 | 79.0 | 11.0 | 77.0 |
| Yagami et al. (1985) | [48] | Japan | ** 507 | // | 20.1 | 81.3 |
| Yamaguchi et al. (1996) | [49] | UK | * 45 | 96.0 | 100.0 | 100.0 |
| Miyazawa et al. (1999) | [50] | Vietnam | * 69 | 53.6 | 1.4 | 39.1 |
| Nakamura et al. (1999) | [51] | Vietnam | * 50 | 44.0 | 44.0 | 74.0 |
| Lappin et al. (2002) | [52] | USA | 276 | 68.5 | 70.7 | 92.4 |
| Ostrowski et al. (2003) | [53] | Saudi Arabia | * 13 | 8.0 | 15.0 | 39.0 |
| Mouzin et al. (2004) | [35] | USA | 272 | 96.7 | 88.2 | 97.8 |
| Fischer et al. (2007) | [54] | Florida (USA) | * 61 | 33.0 | 21.0 | 64.0 |
| Levy et al. (2008) | [55] | Galapagos | 52 | 67.0 | 10.0 | 44.0 |
| Blanco et al. (2009) | [56] | Costa Rica | 96 | 92.8 | 71.9 | // |
| Hellard et al. (2011) | [57] | France | 273 | 36.6 | 54.2 | 77.6 |
| * 219 | 15.9 | 67.8 | 86.6 | |||
| DiGangi et al. (2011) | [58] | Florida (USA) | * 356 | 41.0 | 10.0 | 36.0 |
| DiGangi et al. (2012) | [59] | Florida (USA) | * 347 | 39.8 | 11.0 | 36.6 |
| Mende et al. (2014) | [60] | Germany | 347 | 63.0 | // | // |
| Mende et al. (2014) | [61] | Germany | 350 | 70.6 | // | // |
| Bergmann et al. (2018) | [62] | Germany | 112 | 64.3 | // | // |
| Bergmann et al. (2019) | [63] | Germany | 111 | // | // | ° 62.2 |
| °° 77.2 | ||||||
| Dall’Ara et al. (2019) | [64] | Italy | * 151 | 45.6 | 37.0 | 85.4 |
| Bergmann et al. (2020) | [65] | Germany | 110 | // | 40.9 | // |
| Our study (2023) | // | Italy | 567 | 75.0 | 52.6 | 77.4 |
| * 173 | 45.7 | 37.6 | 82.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Ara, P.; Lauzi, S.; Turin, L.; Servida, F.; Barbieri, L.; Zambarbieri, J.; Mazzotti, G.; Granatiero, F.; Scarparo, E.; Mirabile, A.; et al. Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Cats. Life 2023, 13, 2249. https://doi.org/10.3390/life13122249
Dall’Ara P, Lauzi S, Turin L, Servida F, Barbieri L, Zambarbieri J, Mazzotti G, Granatiero F, Scarparo E, Mirabile A, et al. Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Cats. Life. 2023; 13(12):2249. https://doi.org/10.3390/life13122249
Chicago/Turabian StyleDall’Ara, Paola, Stefania Lauzi, Lauretta Turin, Francesco Servida, Laura Barbieri, Jari Zambarbieri, Giulia Mazzotti, Federico Granatiero, Elena Scarparo, Aurora Mirabile, and et al. 2023. "Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Cats" Life 13, no. 12: 2249. https://doi.org/10.3390/life13122249
APA StyleDall’Ara, P., Lauzi, S., Turin, L., Servida, F., Barbieri, L., Zambarbieri, J., Mazzotti, G., Granatiero, F., Scarparo, E., Mirabile, A., Bo, S., & Filipe, J. (2023). Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Cats. Life, 13(12), 2249. https://doi.org/10.3390/life13122249









