Advances in Aquaculture Hatchery Techniques of Sea Urchin Sphaerechinus granularis (Lamarck, 1816) (Echinoidea: Toxopneustidae): Broodstock Conditioning and Spawning Induction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broodstock Rearing
2.2. Experiment 1—Gonadosomatic Index (GI)
2.3. Experiment 2—Spawning Induction Technics
2.4. Statistical Analyses
3. Results
3.1. Water Quality
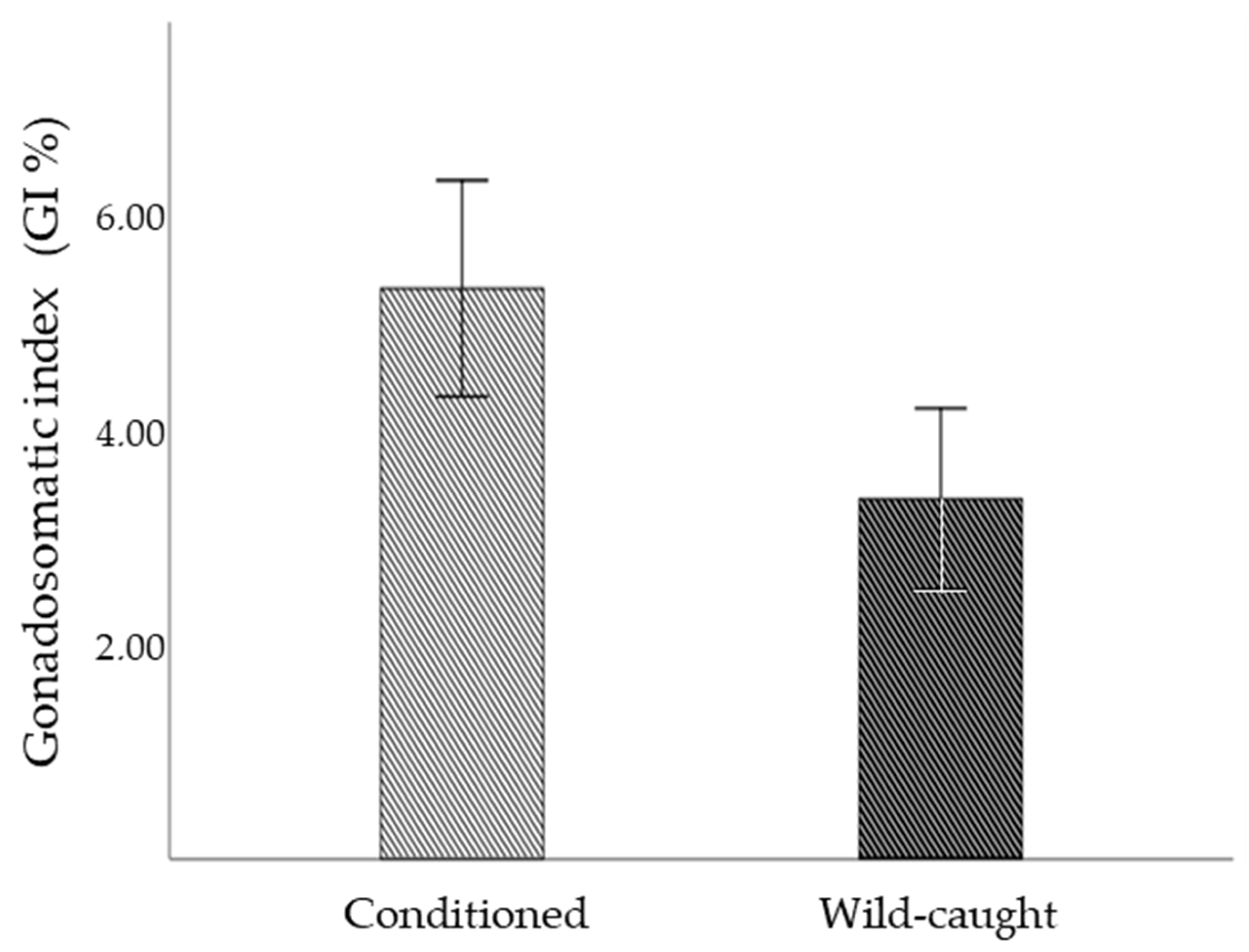
3.2. Gonadosomatic Index (GI)
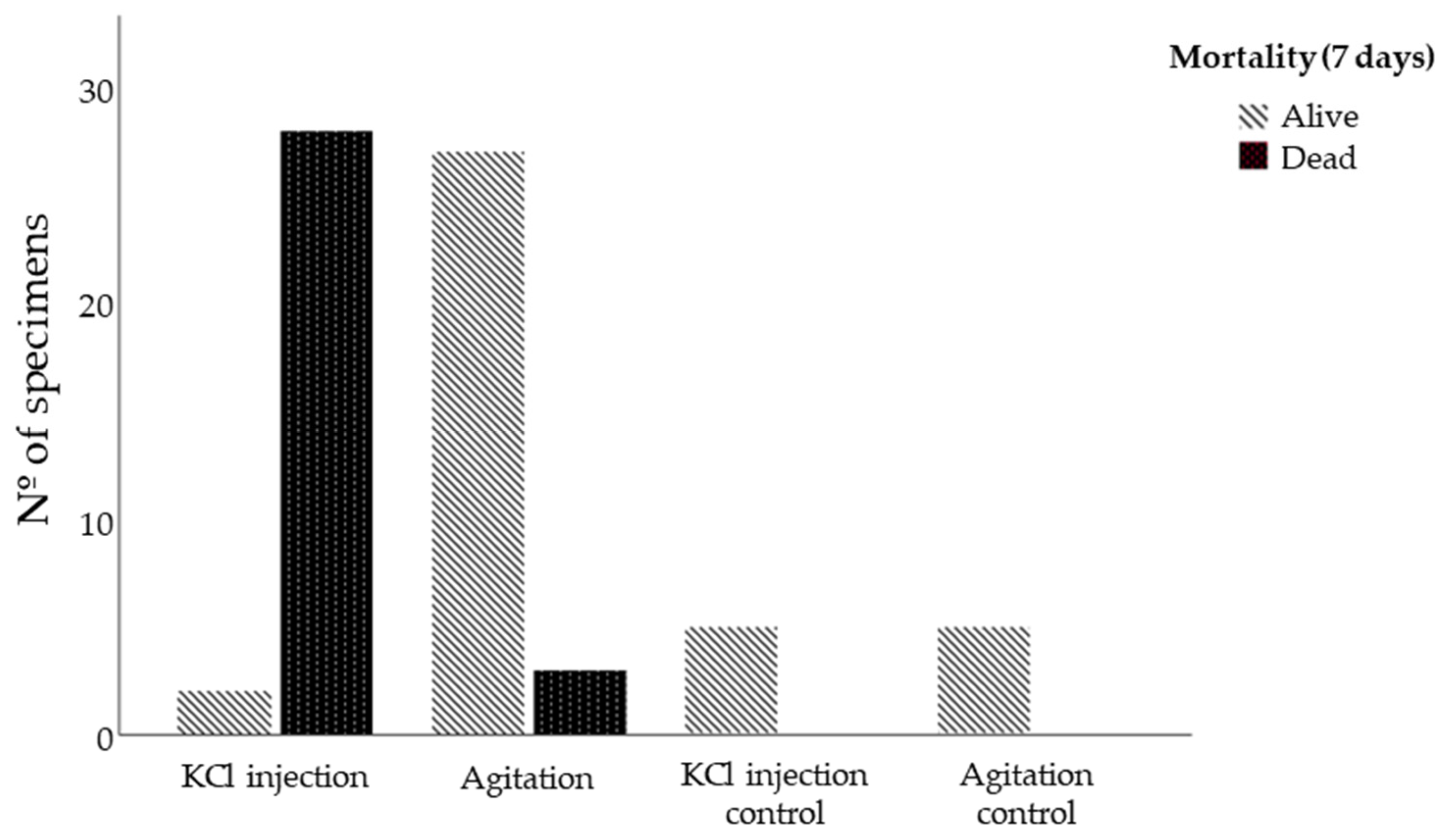
3.3. Spawning Induction Technics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Zhao, C.; Chang, Y.Q. Large-scale production of sea urchin (Strongylocentrotus intermedius) seed in a hatchery in China. Aquac. Int. 2019, 27, 1–7. [Google Scholar] [CrossRef]
- Araújo, J.; Candeias-Mendes, A.; Monteiro, I.; Teixeira, D.; Soares, F.; Pousão-Ferreira, P. The use of diatom Skeletonema costatum on aquaculture-produced purple sea urchin (Paracentrotus lividus) larvae and post-larvae diet. Aquac. Res. 2020, 51, 2545–2554. [Google Scholar] [CrossRef]
- Luís, R.; José, R.; Castro, J.; Andrade, C. A Preliminary Assessment of Microalgal Diets for Echinopluteus Larvae Culture of the Sea Urchin Sphaerechinus granularis (Lamarck, 1816) Echinoidea: Toxopneustidae). J. Mar. Sci. Eng. 2023, 11, 1870. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C.; Ioannidi, V. Population density, size structure, and reproductive cycle of the comestible sea urchin Sphaerechinus granularis (Echinodermata: Echinoidea) in the Pagasitikos gulf (Aegean Sea). Animals 2020, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.; José, R.; Neves, P.; Gois, A.; Cordeiro, N.; Andrade, C.; Ribeiro, C. Population Density, Reproduction Cycle and Nutritional Value of Sphaerechinus granularis (Echinodermata: Echinoidea) in an Oceanic Insular Ecosystem. Front. Mar. Sci. 2022, 8, 699942. [Google Scholar] [CrossRef]
- Guillou, M.; Lumingas, L.J.L. The reproductive cycle of the ‘blunt’ sea urchin. Aquac. Int. 1998, 6, 147–160. [Google Scholar] [CrossRef]
- Guillou, M.; Michel, C. Reproduction and growth of Sphaerechinus granularis (echinodermata: Echinoidea) in southern Brittany. J. Mar. Biol. Assoc. UK 1993, 73, 179–192. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquac. 2020, 12, 2080–2090. [Google Scholar] [CrossRef]
- García, E.; Hernández, G.C.; Clemente, S. Robustness of larval development of intertidal sea urchin species to simulated ocean warming and acidification. Mar. Environ. Res. 2018, 139, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gravina, M.; Pagano, G.; Oral, R.; Guida, M.; Toscanesi, M.; Siciliano, T.; Di Nunzio, A.; Burić, P.; Lyons, D.M.; Thomas, P.J.; et al. Heavy rare earth elements affect Sphaerechinus granularis sea urchin early life stages by multiple toxicity endpoints. Bull. Environ. Contam. Toxicol. 2018, 100, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Pruski, A.M.; Nahon, S.; Escande, M.L.; Charles, F. Ultraviolet radiation induces structural and chromatin damage in Mediterranean sea-urchin spermatozoa. Mutat. Res. 2009, 673, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Luís, O.J. Comparison of spawning induction techniques on Paracentrotus lividus (Echinodermata: Echinoidea) broodstock. Aquac. Int. 2011, 19, 181–191. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Brown, N.; Eddy, S. Echinoderm Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-0-470-96038-7. [Google Scholar]
- Abou ElMaaty, E.E.; Hanafy, M.H.; Yassien, M.H.; Ghobashy, A.F.A.; Ahmed, M.I.; Baeta, M. Induced Spawning and Stocking Density of Tripneustus gratilla for aquaculture Proposes in the Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2023, 27, 381–397. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Asare, O.E.; Megwalu, F.O.; Molla, M.H.R.; Alom, M.Z. Evaluation of growth and production performances of the white sea urchin, Salmacis sphaeroides (Linnaeus, 1758) in a captive aqua-rearing system. Aust. J. Sci. Technol. 2019, 3, 1–8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, R.; José, R.; Castro, J.; Andrade, C. Advances in Aquaculture Hatchery Techniques of Sea Urchin Sphaerechinus granularis (Lamarck, 1816) (Echinoidea: Toxopneustidae): Broodstock Conditioning and Spawning Induction. Life 2023, 13, 2233. https://doi.org/10.3390/life13112233
Luís R, José R, Castro J, Andrade C. Advances in Aquaculture Hatchery Techniques of Sea Urchin Sphaerechinus granularis (Lamarck, 1816) (Echinoidea: Toxopneustidae): Broodstock Conditioning and Spawning Induction. Life. 2023; 13(11):2233. https://doi.org/10.3390/life13112233
Chicago/Turabian StyleLuís, Ricardo, Ricardo José, João Castro, and Carlos Andrade. 2023. "Advances in Aquaculture Hatchery Techniques of Sea Urchin Sphaerechinus granularis (Lamarck, 1816) (Echinoidea: Toxopneustidae): Broodstock Conditioning and Spawning Induction" Life 13, no. 11: 2233. https://doi.org/10.3390/life13112233
APA StyleLuís, R., José, R., Castro, J., & Andrade, C. (2023). Advances in Aquaculture Hatchery Techniques of Sea Urchin Sphaerechinus granularis (Lamarck, 1816) (Echinoidea: Toxopneustidae): Broodstock Conditioning and Spawning Induction. Life, 13(11), 2233. https://doi.org/10.3390/life13112233







