Comparative Analysis of High-Intensity versus Low-to-Moderate Intensity Statin Therapy in Patients Undergoing Rotational Atherectomy for Calcified Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
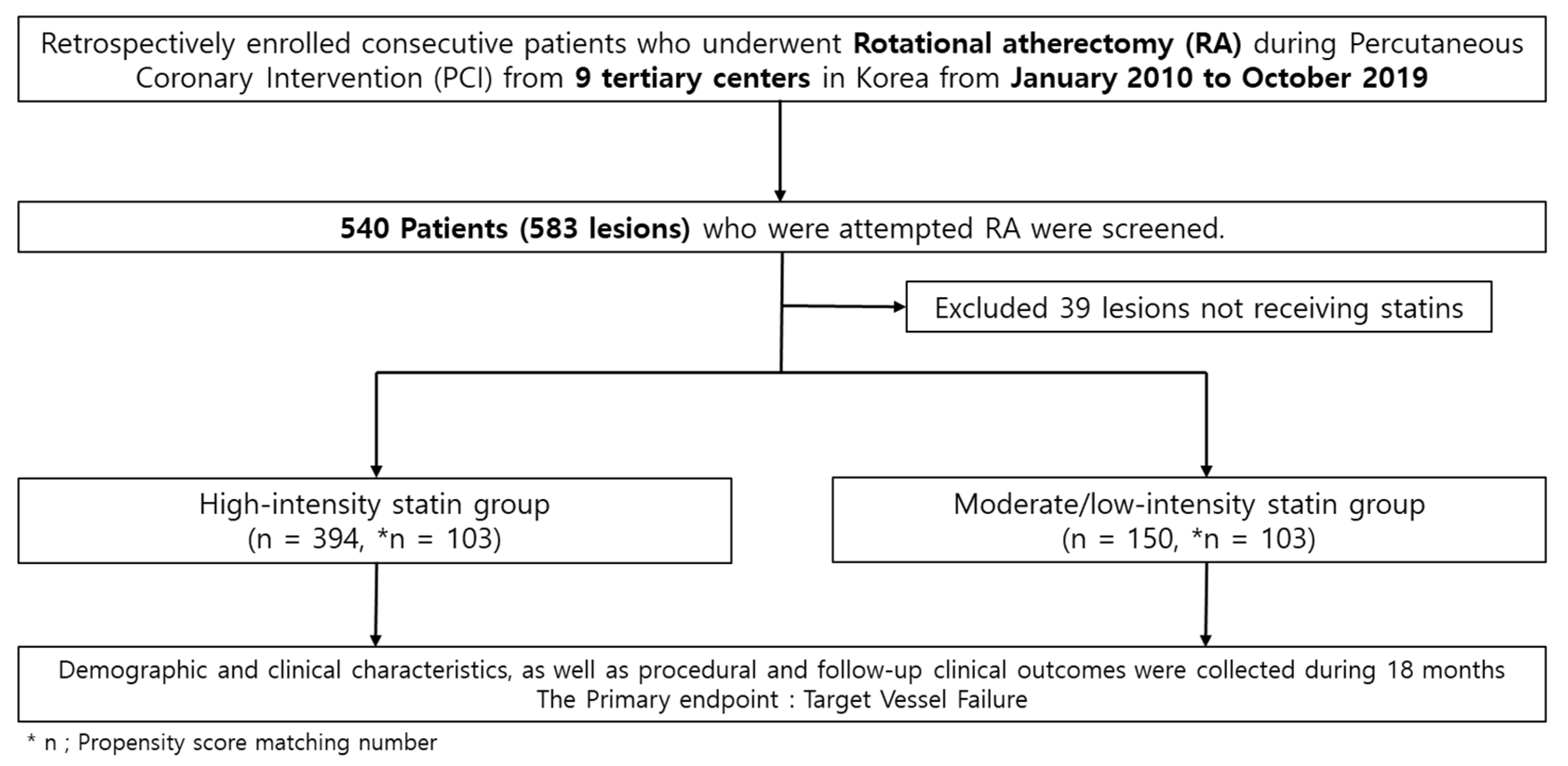
2.1. Study Design and Population
2.2. RA Procedure
2.3. Definition
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okai, I.; Dohi, T.; Okazaki, S.; Jujo, K.; Nakashima, M.; Otsuki, H.; Tanaka, K.; Arashi, H.; Okabe, R.; Nagura, F.; et al. Clinical Characteristics and Long-Term Outcomes of Rotational Atherectomy—J2T Multicenter Registry. Circ. J. 2018, 82, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W.; et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef] [PubMed]
- Barbato, E.; Carrie, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European expert consensus on rotational atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Gordin, J.S.; Stone, G.W.; Sharma, S.K.; Saito, S.; Mahmud, E.; Chambers, J.; Genereux, P.; Shlofmitz, R. Orbital and rotational atherectomy during percutaneous coronary intervention for coronary artery calcification. Catheter. Cardiovasc. Interv. 2018, 92, 61–67. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Banning, A.P. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc. Interv. 2019, 12, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Auth, D.; Marcus, D.R.; Moore, W.S. Removal of focal atheromatous lesions by angioscopically guided high-speed rotary atherectomy. Preliminary experimental observations. J. Vasc. Surg. 1988, 7, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Tomey, M.I.; Kini, A.S.; Sharma, S.K. Current status of rotational atherectomy. JACC Cardiovasc. Interv. 2014, 7, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Asaumi, Y.; Murai, K.; Iwai, T.; Matama, H.; Sawada, K.; Miura, H.; Honda, S.; Fujino, M.; Takagi, K.; et al. Feasibility of rotational atherectomy in patients with acute coronary syndrome: Favorable in-hospital outcomes and clinical importance of complexed coronary atherosclerosis. Heart Vessel. 2023, 38, 1193–1204. [Google Scholar] [CrossRef]
- Elbasha, K.; Mankerious, N.; Alawady, M.; Ibrahim, G.; Abdullah, R.; Abdel-Wahab, M.; Hemetsberger, R.; Toelg, R.; Richardt, G.; Allali, A. Long-Term Outcomes after Rotational Atherectomy for Calcified Chronic Total Occlusion versus Nonchronic Total Occlusion Coronary Lesions. J. Interv. Cardiol. 2022, 2022, 2593189. [Google Scholar] [CrossRef]
- Tadros, R.O.; Vouyouka, A.G.; Chung, C.; Malik, R.K.; Krishnan, P.; Ellozy, S.H.; Marin, M.L.; Faries, P.L. The effect of statin use on embolic potential during carotid angioplasty and stenting. Ann. Vasc. Surg. 2013, 27, 96–103. [Google Scholar] [CrossRef]
- Lee, K.; Jung, J.H.; Lee, M.; Kim, D.W.; Park, M.W.; Choi, I.J.; Lee, J.H.; Lee, J.H.; Lee, S.R.; Lee, P.H.; et al. Clinical Outcome of Rotational Atherectomy in Calcified Lesions in Korea-ROCK Registry. Medicina 2021, 57, 694. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J. Am. Coll. Cardiol. 2013, 62, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Fernandez, C. Second-generation drug-eluting stents. Moving the field forward. J. Am. Coll. Cardiol. 2011, 58, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Smith, S.C., Jr.; Stone, N.J.; Grundy, S.M. Secondary Prevention for Atherosclerotic Cardiovascular Disease: Comparing Recent US and European Guidelines on Dyslipidemia. Circulation 2020, 141, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Han, X.; Pan, Y.; Chen, D. A systematic review and meta-analysis of the effect of high-intensity statin on coronary microvascular dysfunction. BMC Cardiovasc. Disord. 2023, 23, 370. [Google Scholar] [CrossRef]
- Ueshima, K.; Itoh, H.; Kanazawa, N.; Komuro, I.; Nagai, R.; Takeuchi, M.; Yamazaki, T.; EMPATHY Study Group. Rationale and Design of the Standard Versus Intensive Statin Therapy for Hypercholesterolemic Patients with Diabetic Retinopathy (EMPATHY) Study: A Randomized Controlled Trial. J. Atheroscler. Thromb. 2016, 23, 976–990. [Google Scholar] [CrossRef][Green Version]
- Schwartz, G.G.; Olsson, A.G.; Ezekowitz, M.D.; Ganz, P.; Oliver, M.F.; Waters, D.; Zeiher, A.; Chaitman, B.R.; Leslie, S.; Stern, T.; et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA 2001, 285, 1711–1718. [Google Scholar] [CrossRef]
- Ray, K.K.; Cannon, C.P. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J. Am. Coll. Cardiol. 2005, 46, 1425–1433. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, M.H.; Kim, H.M.; Ahn, Y.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J. Am. Coll. Cardiol. 2011, 58, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Reed, C.R.; Gargiulo, K.A.; Rodrigues, A.E.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. Anti-fibrotic effects of statin drugs: A review of evidence and mechanisms. Biochem. Pharmacol. 2023, 214, 115644. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Gibson, M.F.; Rimmer, D.M., 3rd; Gibson, T.M.; Sharp, B.R.; Lefer, D.J. Direct vascular and cardioprotective effects of rosuvastatin, a new HMG-CoA reductase inhibitor. J. Am. Coll. Cardiol. 2002, 40, 1172–1178. [Google Scholar] [CrossRef]
- Jones, S.P.; Teshima, Y.; Akao, M.; Marban, E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2003, 93, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Ngamdu, K.S.; Ghosalkar, D.S.; Chung, H.E.; Christensen, J.L.; Lee, C.; Butler, C.A.; Ho, T.; Chu, A.; Heath, J.R.; Baig, M.; et al. Long-term statin therapy is associated with severe coronary artery calcification. PLoS ONE 2023, 18, e0289111. [Google Scholar] [CrossRef]
- Taguchi, I.; Iimuro, S.; Iwata, H.; Takashima, H.; Abe, M.; Amiya, E.; Ogawa, T.; Ozaki, Y.; Sakuma, I.; Nakagawa, Y.; et al. High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial. Circulation 2018, 137, 1997–2009. [Google Scholar] [CrossRef]
- Wang, P. Statin dose in Asians: Is pharmacogenetics relevant? Pharmacogenomics 2011, 12, 1605–1615. [Google Scholar] [CrossRef]
- Park, M.W.; Park, G.M.; Han, S.; Yang, Y.; Kim, Y.G.; Roh, J.H.; Park, H.W.; Suh, J.; Cho, Y.R.; Won, K.B.; et al. Moderate-intensity versus high-intensity statin therapy in Korean patients with angina undergoing percutaneous coronary intervention with drug-eluting stents: A propensity-score matching analysis. PLoS ONE 2018, 13, e0207889. [Google Scholar] [CrossRef]
- Lee, O.S.; Zhang, J.; Jung, S.H.; Kim, H.S.; Lee, M.K.; Lee, H.Y. High-Intensity Statin Therapy Is “Too Much”, Thus Not Indicated for Very Elderly Patients. Pulse 2018, 6, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M.; et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Blazing, M.A.; Wiviott, S.D.; Lewis, E.F.; Fox, K.A.; White, H.D.; Rouleau, J.L.; Pedersen, T.R.; Gardner, L.H.; Mukherjee, R.; et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004, 292, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, W.M.; Jacobs, D.R.; Bloemberg, B.P.; Kromhout, D.; Menotti, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA 1995, 274, 131–136. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ryan, S.; Birmingham, B.; Zalikowski, J.; March, R.; Ambrose, H.; Moore, R.; Lee, C.; Chen, Y.; Schneck, D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 2005, 78, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Kim, Y.; Hyun, S.; Won, H.; Shin, S.Y.; Lee, K.J.; Kim, S.-W.; Kim, T.H.; Kim, C.J. Cholesterol Lowering Effects of Low-dose Statins in Korean Patients. JLA 2014, 3, 21–28. [Google Scholar] [CrossRef]
- Trion, A.; Schutte-Bart, C.; Bax, W.H.; Jukema, J.W.; van der Laarse, A. Modulation of calcification of vascular smooth muscle cells in culture by calcium antagonists, statins, and their combination. Mol. Cell. Biochem. 2008, 308, 25–33. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Andrews, J.; Psaltis, P.J.; Bartolo, B.A.D.; Nicholls, S.J.; Puri, R. Coronary arterial calcification: A review of mechanisms, promoters and imaging. Trends Cardiovasc. Med. 2018, 28, 491–501. [Google Scholar] [CrossRef]
- Vogel, L.H.; Dykun, I.; Raggi, P.; Schmermund, A.; Rassaf, T.; Mahabadi, A.A. High- vs. Low-Intensity Statin Therapy and Changes in Coronary Artery Calcification Density after One Year. J. Clin. Med. 2023, 12, 476. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]

| Overall (n = 544) | Propensity Score Matching Analysis | |||||
|---|---|---|---|---|---|---|
| High | Moderate/Low | High | Moderate/Low | |||
| n = 394 | n = 150 | p-Value | n = 103 | n = 103 | p-Value | |
| Age, years | 71.9 ± 9.7 | 70.1 ± 11.0 | 0.067 | 71.6 ± 8.3 | 70.6 ± 11.3 | 0.453 |
| Male (%) | 232 (58.9) | 100 (66.7) | 0.096 | 70 (68.0) | 64 (62.1) | 0.461 |
| BMI (kg/m²) | 24.0 ± 3.7 | 24.8 ± 4.3 | 0.059 | 24.7 ± 3.9 | 24.6 ± 4.4 | 0.906 |
| SBP (mmHg) | 133.7 ± 24.3 | 128.9 ± 21.3 | 0.034 | 130.3 ± 20.7 | 129.0 ± 21.2 | 0.615 |
| DBP (mmHg) | 74.6 ± 12.7 | 74.4 ± 12.1 | 0.862 | 74.1 ± 12.0 | 75.2 ± 12.8 | 0.515 |
| Smoking (%) | 76 (19.3) | 29 (19.3) | 0.991 | 22 (21.4) | 21 (20.4) | >0.999 |
| HTN (%) | 311 (78.9) | 115 (76.7) | 0.566 | 86 (83.5) | 76 (73.8) | 0.121 |
| DM (%) | 227 (57.6) | 84 (56.0) | 0.734 | 58( 56.3) | 56 (54.4) | 0.892 |
| Hyperlipidemia (%) | 172 (43.7) | 76 (50.7) | 0.142 | 37 (35.9) | 52 (50.5) | 0.053 |
| CKD (%) | 72 (18.3) | 24 (16.0) | 0.534 | 20 (19.4) | 18 (17.5) | 0.864 |
| Dialysis (%) | 34 (8.6) | 15 (10.0) | 0.618 | 9 (8.7) | 11 (10.7) | 0.824 |
| Previous PCI (%) | 101 (25.6) | 43 (28.7) | 0.474 | 35 (34.0) | 29 (28.2) | 0.451 |
| Previous CABG (%) | 16 (4.1) | 10 (6.7) | 0.203 | 4 (3.9) | 7 (6.8) | 0.549 |
| Previous MI (%) | 49( 12.4) | 15 (10.0) | 0.431 | 9 (8.7) | 10 (9.7) | >0.999 |
| CVA (%) | 49( 12.4) | 25 (16.7) | 0.198 | 11 (10.7) | 15 (14.6) | 0.524 |
| PVD (%) | 27 (6.9) | 10 (6.7) | 0.939 | 11 (10.7) | 7 (6.8) | 0.481 |
| Chronic lung disease (%) | 27 (6.9) | 9 (6.0) | 0.721 | 8 (7.8) | 6 (5.8) | 0.791 |
| Heart failure (%) | 50( 12.7) | 29 (19.3) | 0.049 | 22 (21.4) | 20 (19.4) | 0.856 |
| LV_EF (%) | 53.0 ± 13.5 | 52.7 ± 13.6 | 0.810 | 51.9 ± 14.2 | 52.7 ± 14.0 | 0.700 |
| Atrial_fibrillation (%) | 40 (10.2) | 10 (6.7) | 0.209 | 16 (15.5) | 6 (5.8) | 0.053 |
| Clinical_diagnosis | ||||||
| Stable angina (%) | 144 (36.6) | 38 (25.3) | 0.175 | 32 (31.1) | 30 (29.1) | 0.510 |
| Unstable angina (%) | 121 (30.7) | 51 (34.0) | 36 (35.0) | 30 (29.1) | ||
| NSTEMI (%) | 89 (22.6) | 44 (29.3) | 28 (27.2) | 34 (33.0) | ||
| STEMI (%) | 12 (3.1) | 7 (4.7) | 3 (2.9) | 5 (4.9) | ||
| Silent ischemia (%) | 27 (6.9) | 10 (6.7) | 4 (3.9) | 4 (3.9) | ||
| DCMP/ICMP (%) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Overall (n = 544) | Propensity Score Matching Analysis | |||||
|---|---|---|---|---|---|---|
| High | Moderate/Low | High | Moderate/Low | |||
| n = 394 | n = 150 | p-Value | n = 103 | n = 103 | p-Value | |
| Hb | 12.3 ± 1.8 | 12.5 ± 1.9 | 0.275 | 12.6 ± 1.8 | 12.5 ± 1.9 | 0.544 |
| Triglycerides | 115.8 ± 71.6 | 132.6 ± 86.0 | 0.045 | 125.3 ± 81.5 | 122.1 ± 77.9 | 0.740 |
| Total cholesterol | 141.8 ± 37.3 | 146.1 ± 39.8 | 0.245 | 143.5 ± 40.5 | 147.6 ± 41.7 | 0.427 |
| LDL cholesterol | 83.0 ± 40.0 | 87.3 ± 36.9 | 0.284 | 86.0 ± 56.5 | 88.1 ± 39.9 | 0.748 |
| HDL cholesterol | 46.3 ± 13.3 | 45.6 ± 16.1 | 0.636 | 45.9 ± 14.9 | 44.5 ± 13.1 | 0.466 |
| hsCRP | 3.6 ± 13.7 | 2.8 ± 7.0 | 0.180 | 6.9 ± 14.9 | 6.1 ± 8.1 | 0.652 |
| 0.1 (0.3–1.7) | 0.2 (0.4–1.8) | 1.7 (0.1–8.2) | 1.9 (0.3–11.2) | |||
| HbA1c | 6.7 ± 1.3 | 6.7 ± 1.4 | 0.954 | 6.6 ± 1.4 | 6.6 ± 1.5 | 0.738 |
| NOAC | 11 (2.8) | 6 (4.0) | 0.581 | 3 (2.9) | 3 (2.9) | >0.999 |
| DAPT | 380 (96.5) | 150 (100.0) | 0.014 | 103 (100.0) | 103 (100.0) | >0.999 |
| Aspirin | 388 (98.5) | 150 (100.0) | 0.195 | 102 (99.0) | 103 (100.0) | >0.999 |
| P2Y12 inhibitor | 391 (99.2) | 150 (100.0) | 0.565 | 102 (99.0) | 103 (100.0) | >0.999 |
| Cilostazol | 51 (12.9) | 23 (15.3) | 0.468 | 11 (10.7) | 7 (6.8) | 0.481 |
| Beta blocker | 292 (74.1) | 98 (65.3) | 0.042 | 67 (65.1) | 75 (72.8) | 0.215 |
| ACEi/ARB | 253 (64.2) | 97 (64.7) | 0.921 | 64 (62.1) | 63 (61.2) | >0.999 |
| Overall (n = 544) | Propensity Score Matching Analysis | |||||
|---|---|---|---|---|---|---|
| High | Moderate/Low | High | Moderate/Low | |||
| n = 394 | n = 150 | p-Value | n = 103 | n = 103 | p-Value | |
| Lesion classification | ||||||
| A, (%) | 3 (0.8) | 0 (0.0) | 0.465 | 1 (1.0) | 0 (0.0) | 0.957 |
| B1, (%) | 25 (6.4) | 14 (9.3) | 2 (1.9) | 3 (2.9) | ||
| B2, (%) | 41 (10.4) | 15 (10.0) | 8 (7.8) | 6 (5.8) | ||
| C, (%) | 325 (82.5) | 121 (80.7) | 92 (89.3) | 94 (91.3) | ||
| MVD, (%) | 313 (79.4) | 123 (82.0) | 0.504 | 83 (80.6) | 86 (83.5) | 0.728 |
| IVUS (%) | 172 (43.7) | 81 (54.0) | 0.031 | 52 (50.5) | 57 (55.3) | 0.560 |
| Mean stent diameter, mm | 3.0 ± 0.4 | 3.0 ± 0.4 | 0.137 | 3.1 ± 0.4 | 3.1 ± 0.4 | 0.988 |
| Total number of stents | 2.3 ± 1.1 | 2.5 ± 1.3 | 0.221 | 2.5 ± 1.3 | 2.5 ± 1.2 | 0.660 |
| Total stent length, mm | 68.5 ± 34.0 | 70.3 ± 39.5 | 0.636 | 70.5 ± 36.8 | 70.5 ± 36.5 | 0.963 |
| Procedure success (%) | 385 (97.7) | 140 (93.3) | 0.013 | 103 (100.0) | 100 (97.1) | 0.250 |
| Overall | Propensity Score Matching | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Moderate/Low | Multivariate ** | High | Moderate/Low | Multivariate ** | |||||||
| n = 394 | n = 150 | log-Rank p-Value | HR | 95%CI | p-Value | n = 103 | n = 103 | log-Rank p-Value | HR | 95%CI | p-Value | |
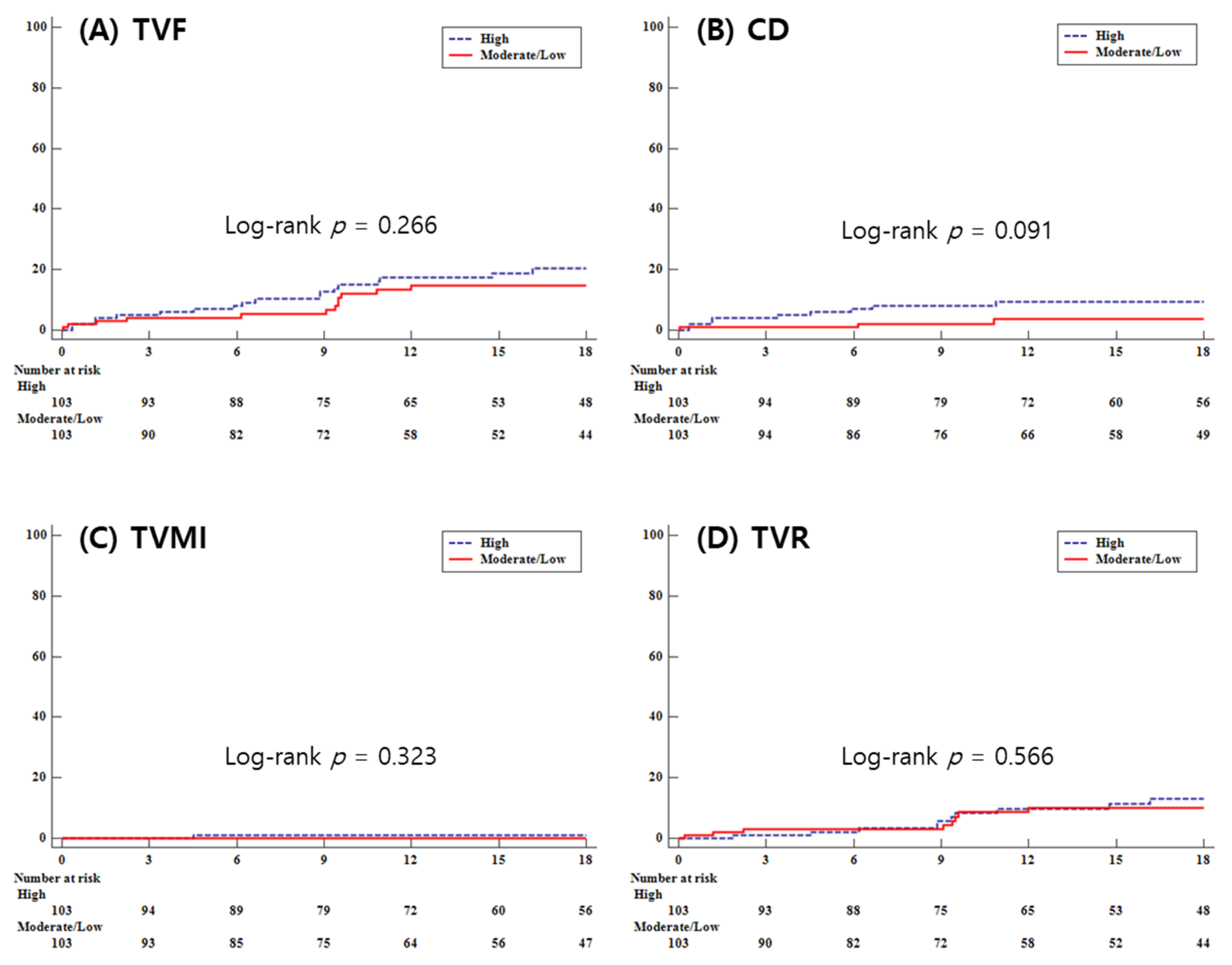
| Target vessel failure | 44 (11.2) | 19 (12.7) | 0.501 | 0.952 | 0.539–1.683 | 0.867 | 19 (18.5) | 12 (11.7) | 0.266 | 0.666 | 0.323–1.371 | 0.270 |
| All-cause death | 26 (6.6) | 7 (4.7) | 0.437 | 0.702 | 0.290–1.698 | 0.432 | 10 (9.7) | 5 (4.9) | 0.214 | 0.513 | 0.175–1.500 | 0.222 |
| Cardiac death | 20 (5.1) | 4 (2.7) | 0.247 | 0.439 | 0.141–1.373 | 0.157 | 9 (8.7) | 3 (2.9) | 0.091 | 0.342 | 0.093–1.264 | 0.108 |
| MI | 11 (2.8) | 5 (3.3) | 0.679 | 1.144 | 0.377–3.475 | 0.812 | 2 (1.9) | 2 (1.9) | 0.942 | 1.075 | 0.151–7.639 | 0.942 |
| Target vessel MI | 5 (1.3) | 2 (1.3) | 0.921 | 0.887 | 0.154–5.115 | 0.894 | 1 (1.0) | 0 (0.0) | 0.323 | - | - | - |
| Any revascularization | 34 (8.6) | 16 (10.7) | 0.379 | 1.063 | 0.575–1.966 | 0.845 | 13 (12.6) | 10 (9.7) | 0.627 | 0.815 | 0.358–1.860 | 0.628 |
| Target vessel revascularization | 25 (6.4) | 13 (8.7) | 0.283 | 1.146 | 0.569–2.311 | 0.703 | 11 (10.7) | 8 (7.8) | 0.566 | 0.767 | 0.308–1.906 | 0.568 |
| Target lesion revascularization | 21 (5.3) | 10 (6.7) | 0.476 | 0.967 | 0.437–2.137 | 0.933 | 9 (8.7) | 5 (4.9) | 0.318 | 0.577 | 0.193–1.722 | 0.324 |
| Non-target lesion revascularization | 13 (3.3) | 6 (4.0) | 0.634 | 1.198 | 0.429–3.345 | 0.730 | 3 (2.9) | 4 (3.9) | 0.580 | 1.521 | 0.340–6.800 | 0.583 |
| CVA | 6 (1.5) | 4 (2.7) | 0.369 | 1.637 | 0.404–6.632 | 0.490 | 1 (1.0) | 0 (0.0) | 0.551 | 2.045 | 0.185–22.549 | 0.559 |
| Stent thrombosis | 3 (0.8) | 2 (1.3) | 0.525 | 0.914 | 0.116–7.232 | 0.932 | 1 (1.0) | 1 (1.0) | 0.970 | 1.054 | 0.066–16.869 | 0.970 |
| Total Bleeding | 21(5.3) | 9 (6.0) | 0.724 | 1.255 | 0.559–2.820 | 0.582 | 4 (3.9) | 6 (5.8) | 0.468 | 1.592 | 0.449–5.643 | 0.472 |
| Minor Bleeding | 15 (3.8) | 3 (2.0) | 0.320 | 0.494 | 0.139–1.752 | 0.275 | 3 (2.9) | 3 (2.9) | 0.938 | 1.066 | 0.215–5.285 | 0.938 |
| Major Bleeding | 6 (1.5) | 6 (4.0) | 0.076 | 3.775 | 1.095–13.011 | 0.035 | 1 (1.0) | 3 (2.9) | 0.293 | 3.162 | 0.329–30.420 | 0.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-S.; Jung, J.; Her, S.-H.; Kim, K.; Kim, Y.; Lee, K.; Yoo, K.-D.; Moon, K.-W.; Moon, D.; Lee, S.-N.; et al. Comparative Analysis of High-Intensity versus Low-to-Moderate Intensity Statin Therapy in Patients Undergoing Rotational Atherectomy for Calcified Coronary Artery Disease. Life 2023, 13, 2232. https://doi.org/10.3390/life13112232
Choi S-S, Jung J, Her S-H, Kim K, Kim Y, Lee K, Yoo K-D, Moon K-W, Moon D, Lee S-N, et al. Comparative Analysis of High-Intensity versus Low-to-Moderate Intensity Statin Therapy in Patients Undergoing Rotational Atherectomy for Calcified Coronary Artery Disease. Life. 2023; 13(11):2232. https://doi.org/10.3390/life13112232
Chicago/Turabian StyleChoi, Sang-Suk, Jin Jung, Sung-Ho Her, Kyunyeon Kim, Youngmin Kim, Kyusup Lee, Ki-Dong Yoo, Keon-Woong Moon, Donggyu Moon, Su-Nam Lee, and et al. 2023. "Comparative Analysis of High-Intensity versus Low-to-Moderate Intensity Statin Therapy in Patients Undergoing Rotational Atherectomy for Calcified Coronary Artery Disease" Life 13, no. 11: 2232. https://doi.org/10.3390/life13112232
APA StyleChoi, S.-S., Jung, J., Her, S.-H., Kim, K., Kim, Y., Lee, K., Yoo, K.-D., Moon, K.-W., Moon, D., Lee, S.-N., Jang, W.-Y., Choi, I.-J., Lee, J.-H., Lee, J.-H., Lee, S.-R., Lee, S.-W., Yun, K.-H., & Lee, H.-J. (2023). Comparative Analysis of High-Intensity versus Low-to-Moderate Intensity Statin Therapy in Patients Undergoing Rotational Atherectomy for Calcified Coronary Artery Disease. Life, 13(11), 2232. https://doi.org/10.3390/life13112232






