Second-Line Chemotherapy for Intrahepatic Cholangiocarcinomas: What Is the Real Gain?
Abstract
:1. Introduction
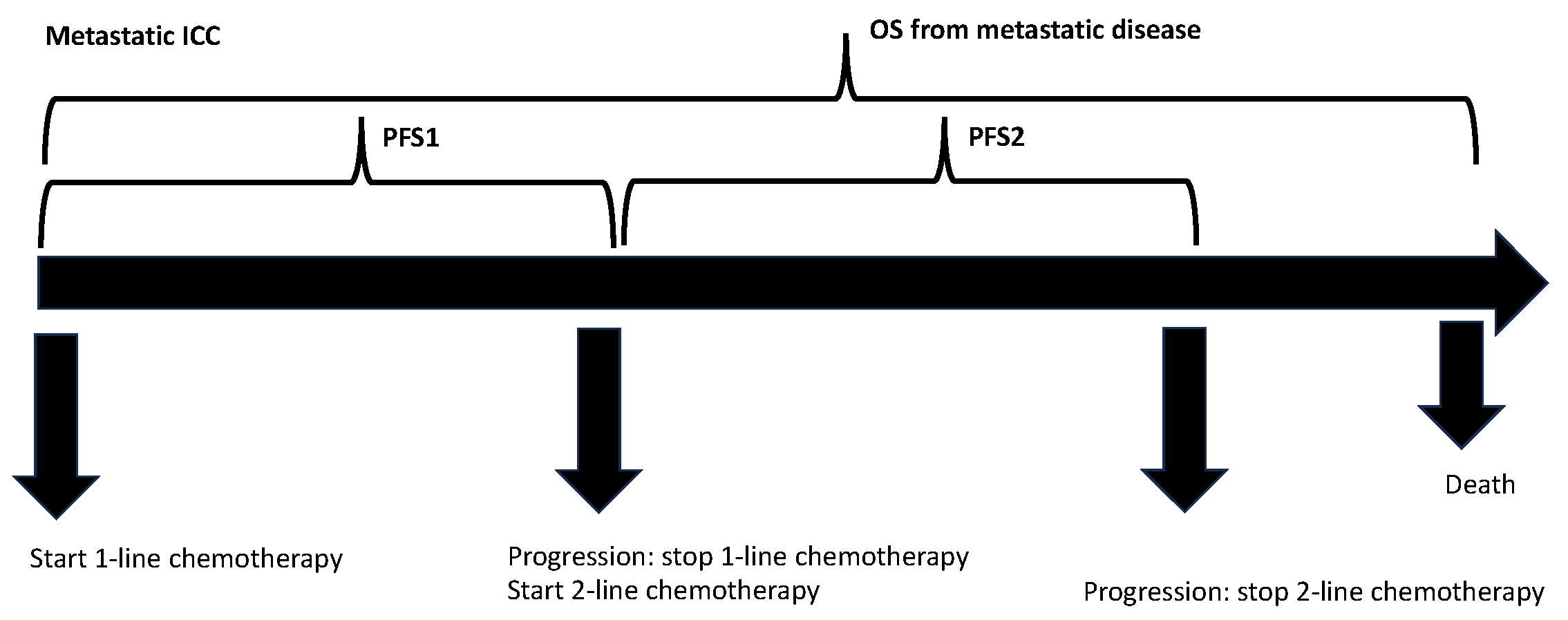
2. Materials and Methods
2.1. Patients, Data Collection, and Follow-Up
2.2. Statistical Analysis
3. Results
3.1. Population Characteristics
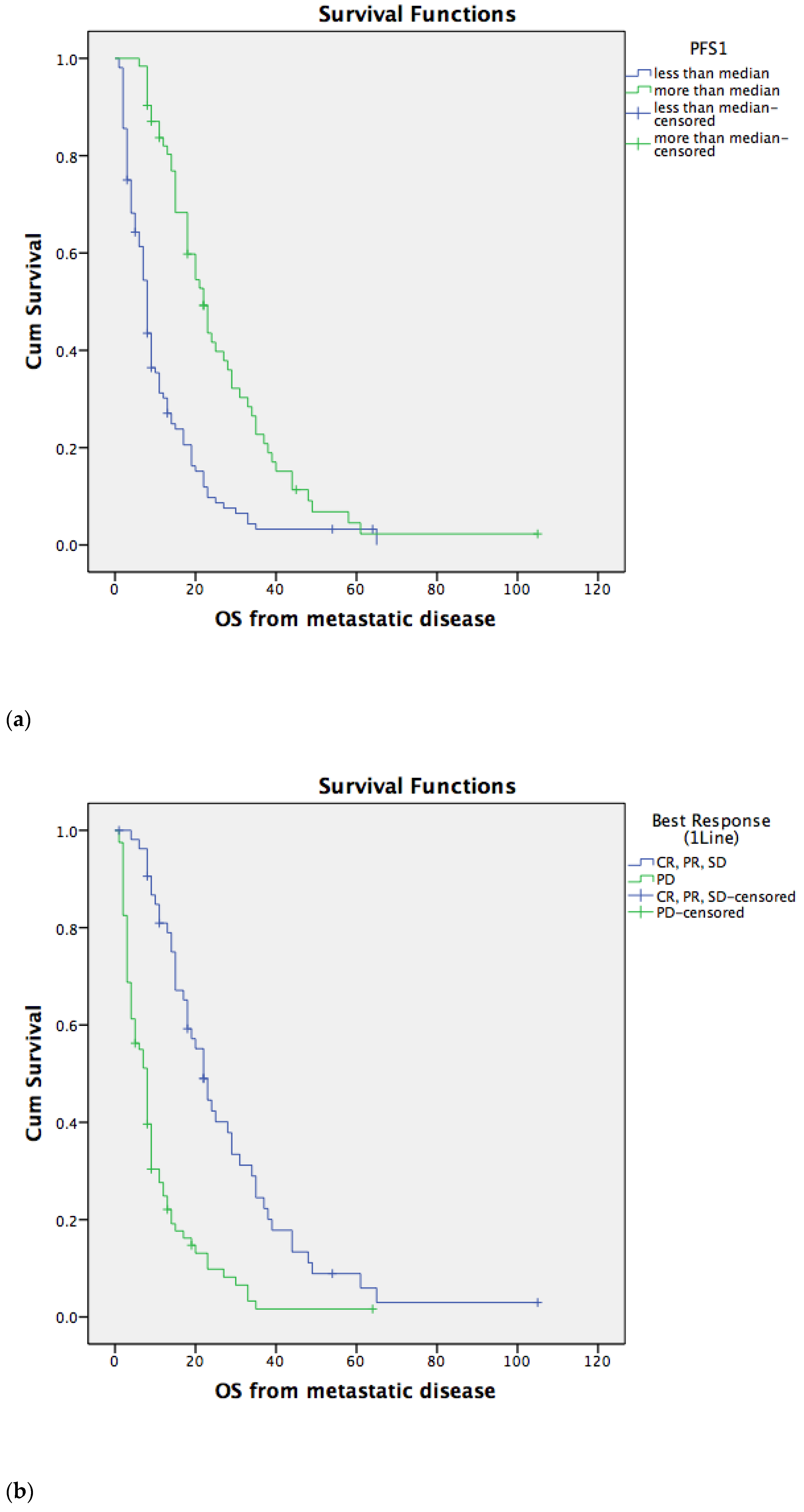
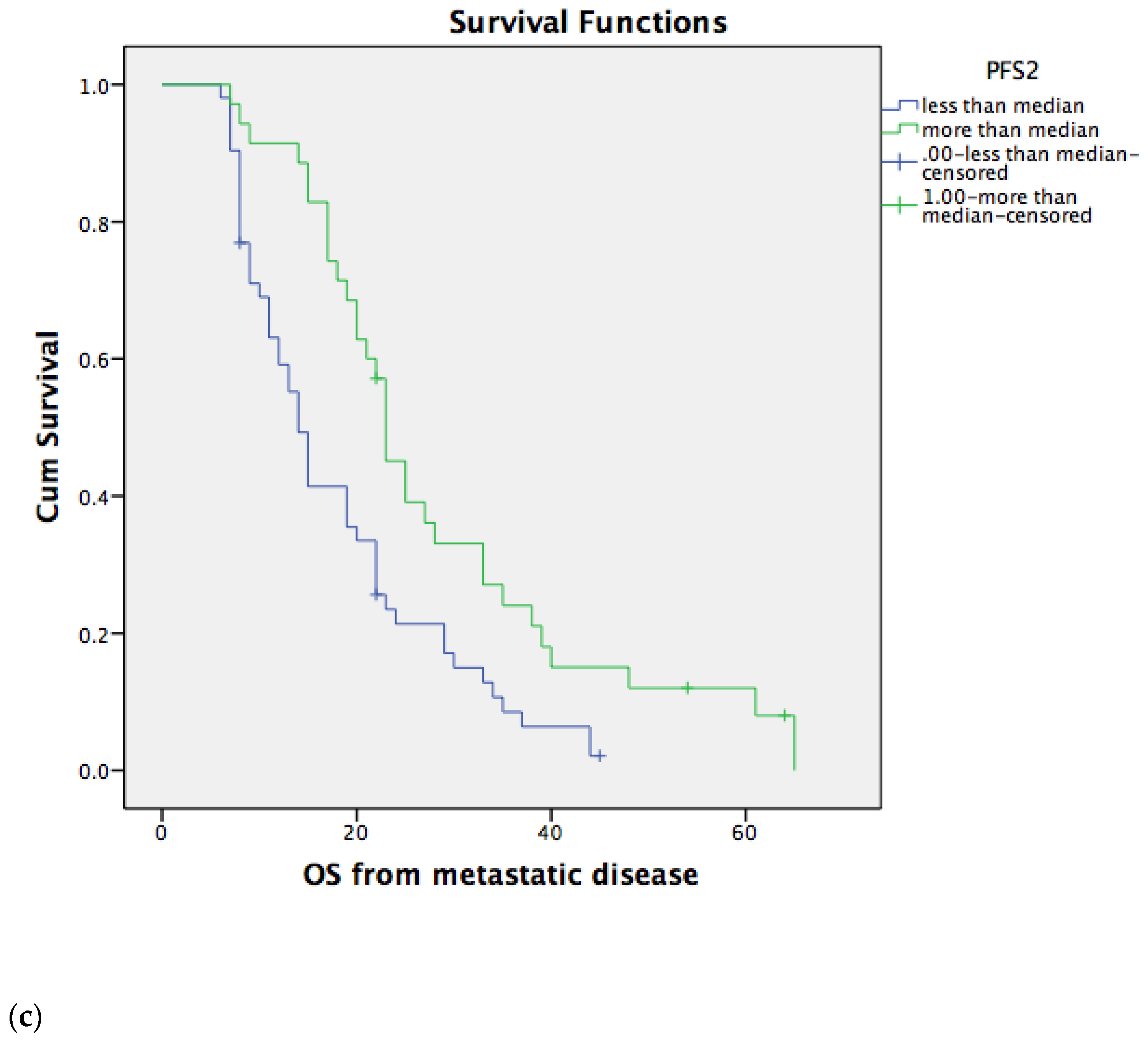
3.2. Overall Survival for Metastatic Disease Depends on PFS1 and PFS2
3.3. Overall Survival for Metastatic Disease Does Not Depend on the Type of Second-Line Chemotherapy
3.4. Univariate and Multivariate Prognostic Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Garajová, I.; Gelsomino, F.; Salati, M.; Leonardi, F.; De Lorenzo, S.; Granito, A.; Tovoli, F. Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis. Curr. Oncol. 2023, 30, 2613–2624. [Google Scholar] [CrossRef]
- Tovoli, F.; Garajová, I.; Gelsomino, F.; Iavarone, M.; Federico, P.; Salati, M.; Corianò, M.; Caputo, F.; De Lorenzo, S.; Granito, A.; et al. Pattern of Progression of Intrahepatic Cholangiocarcinoma: Implications for Second-Line Clinical Trials. Liver Int. 2022, 42, 458–467. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Garajova, I.; Stefanini, B.; Tovoli, F. Targeted Therapies for Gallbladder Cancer: An Overview of Agents in Preclinical and Clinical Development. Expert Opin. Investig. Drugs 2021, 30, 759–772. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination with Gemcitabine plus Cisplatin (GemCis) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40 (Suppl. S4). [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. 56P Updated Overall Survival (OS) from the Phase III TOPAZ-1 Study of Durvalumab (D) or Placebo (PBO) plus Gemcitabine and Cisplatin (+ GC) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC). Ann. Oncol. 2022, 33, S565–S566. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in Combination with Gemcitabine and Cisplatin Compared with Gemcitabine and Cisplatin Alone for Patients with Advanced Biliary Tract Cancer (KEYNOTE-966): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Walter, T.; Horgan, A.M.; McNamara, M.; McKeever, L.; Min, T.; Hedley, D.; Serra, S.; Krzyzanowska, M.K.; Chen, E.; Mackay, H.; et al. Feasibility and Benefits of Second-Line Chemotherapy in Advanced Biliary Tract Cancer: A Large Retrospective Study. Eur. J. Cancer 2013, 49, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-Line Chemotherapy in Advanced Biliary Cancer: A Systematic Review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Goff, L.W.; Keenan, B.P.; Jordan, E.; Wang, R.; Bocobo, A.G.; Chou, J.F.; O’Reilly, E.M.; Harding, J.J.; Kemeny, N.; et al. Second-line Chemotherapy in Advanced Biliary Cancers: A Retrospective, Multicenter Analysis of Outcomes. Cancer 2019, 125, 4426–4434. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-Line FOLFOX Chemotherapy versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Rushbrook, S.M.; Kendall, T.J.; Zen, Y.; Albazaz, R.; Manoharan, P.; Pereira, S.P.; Sturgess, R.; Davidson, B.R.; Malik, H.Z.; Manas, D.; et al. British Society of Gastroenterology guidelines for the diagnosis and management of cholangiocarcinoma. Gut 2023. ahead of print. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Acher, A.W.; Paro, A.; Elfadaly, A.; Tsilimigras, D.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: A Summative Review of Biomarkers and Targeted Therapies. Cancers 2021, 13, 5169. [Google Scholar] [CrossRef]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; Dematteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic Cholangiocarcinoma: Rising Frequency, Improved Survival, and Determinants of Outcome after Resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced Intrahepatic Cholangiocarcinoma: Post Hoc Analysis of the ABC-01, -02, and -03 Clinical Trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef]
- Adeva, J.; Sangro, B.; Salati, M.; Edeline, J.; La Casta, A.; Bittoni, A.; Berardi, R.; Bruix, J.; Valle, J.W. Medical Treatment for Cholangiocarcinoma. Liver Int. 2019, 39, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Salati, M.; Frega, G.; Merz, V.; Caputo, F.; Ricci, A.D.; Palloni, A.; Messina, C.; Spallanzani, A.; Saccoccio, G.; et al. Second-Line Chemotherapy (2L) in Elderly Patients with Advanced Biliary Tract Cancer (ABC): A Multicenter Real-World Study. J. Clin. Oncol. 2021, 39 (Suppl. S3). [Google Scholar] [CrossRef]
- Rizzo, A.; Salati, M.; Frega, G.; Merz, V.; Caputo, F.; Di Federico, A.; Palloni, A.; Carloni, R.; Ricci, A.D.; Gadaleta-Caldarola, G.; et al. Second-Line Chemotherapy in Elderly Patients with Advanced Biliary Tract Cancer: A Multicenter Real-World Study. Medicina 2022, 58, 1543. [Google Scholar] [CrossRef]
- Bridgewater, J.; Palmer, D.; Cunningham, D.; Iveson, T.; Gillmore, R.; Waters, J.; Harrison, M.; Wasan, H.; Corrie, P.; Valle, J. Outcome of Second-Line Chemotherapy for Biliary Tract Cancer. Eur. J. Cancer 2013, 49, 1511. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.p.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal Irinotecan plus Fluorouracil and Leucovorin versus Fluorouracil and Leucovorin for Metastatic Biliary Tract Cancer after Progression on Gemcitabine plus Cisplatin (NIFTY): A Multicentre, Open-Label, Randomised, Phase 2b Study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- Neuzillet, C.; Casadei-Gardini, A.; Brieau, B.; Vivaldi, C.; Brandi, G.; Tougeron, D.; Filippi, R.; Vienot, A.; Silvestris, N.; Pointet, A.L.; et al. Fluropyrimidine Single Agent or Doublet Chemotherapy as Second Line Treatment in Advanced Biliary Tract Cancer. Int. J. Cancer 2020, 147, 3177–3188. [Google Scholar] [CrossRef]
- Kim, B.J.; Yoo, C.; Kim, K.P.; Hyung, J.; Park, S.J.; Ryoo, B.Y.; Chang, H.M. Efficacy of Fluoropyrimidine-Based Chemotherapy in Patients with Advanced Biliary Tract Cancer after Failure of Gemcitabine plus Cisplatin: Retrospective Analysis of 321 Patients. Br. J. Cancer 2017, 116, 561–567. [Google Scholar] [CrossRef]
- Ying, J.; Chen, J. Combination versus Mono-Therapy as Salvage Treatment for Advanced Biliary Tract Cancer: A Comprehensive Meta-Analysis of Published Data. Crit. Rev. Oncol. Hematol. 2019, 139, 134–142. [Google Scholar] [CrossRef]
- Fornaro, L.; Vivaldi, C.; Cereda, S.; Leone, F.; Aprile, G.; Lonardi, S.; Silvestris, N.; Santini, D.; Milella, M.; Caparello, C.; et al. Second-Line Chemotherapy in Advanced Biliary Cancer Progressed to First-Line Platinum-Gemcitabine Combination: A Multicenter Survey and Pooled Analysis with Published Data. J. Exp. Clin. Cancer Res. 2015, 34, 156. [Google Scholar] [CrossRef]
- Neuzillet, C.; Casadei Gardini, A.; Brieau, B.; Vivaldi, C.; Smolenschi, C.; Brandi, G.; Tougeron, D.; Filippi, R.; Vienot, A.; Silvestris, N.; et al. Prediction of Survival with Second-Line Therapy in Biliary Tract Cancer: Actualisation of the AGEO CT2BIL Cohort and European Multicentre Validations. Eur. J. Cancer 2019, 111, 94–106. [Google Scholar] [CrossRef]
- Brieau, B.; Dahan, L.; De Rycke, Y.; Boussaha, T.; Vasseur, P.; Tougeron, D.; Lecomte, T.; Coriat, R.; Bachet, J.B.; Claudez, P.; et al. Second-Line Chemotherapy for Advanced Biliary Tract Cancer after Failure of the Gemcitabine-Platinum Combination: A Large Multicenter Study by the Association Des Gastro-Entérologues Oncologues. Cancer 2015, 121, 3290–3297. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Kelley, R.K.; Roychowdhury, S.; Weiss, K.H.; Abou-Alfa, G.K.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.X.; Goyal, L.; et al. Updated Results from a Phase II Study of Infigratinib (BGJ398), a Selective Pan-FGFR Kinase Inhibitor, in Patients with Previously Treated Advanced Cholangiocarcinoma Containing FGFR2 Fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Bauer, T.M.; Lee, J.J.; Dowlati, A.; Brose, M.S.; Farago, A.F.; Taylor, M.; Shaw, A.T.; Montez, S.; Meric-Bernstam, F.; et al. Larotrectinib in Adult Patients with Solid Tumours: A Multi-Centre, Open-Label, Phase i Dose-Escalation Study. Ann. Oncol. 2019, 30, 325–331. [Google Scholar] [CrossRef]
- Ramjeesingh, R.; Chaudhury, P.; Tam, V.C.; Roberge, D.; Lim, H.J.; Knox, J.J.; Asselah, J.; Doucette, S.; Chhiber, N.; Goodwin, R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Curr. Oncol. 2023, 30, 7132–7150. [Google Scholar] [CrossRef]
- Tam, V.C.; Ramjeesingh, R.; Burkes, R.; Yoshida, E.M.; Doucette, S.; Lim, H.J. Emerging Systemic Therapies in Advanced Unresectable Biliary Tract Cancer: Review and Canadian Perspective †. Curr. Oncol. 2022, 29, 7072–7085. [Google Scholar] [CrossRef]
| Variable | NO Second-Line CT (pts) | YES Second-Line CT (pts) | p |
|---|---|---|---|
| Number of patients | 142 | 113 | |
| Median Age (66.5 years) | |||
| less than median | 62/142 (44%) | 68/113 (60%) | 0.006 |
| more than median | 80/142 (56%) | 45/113 (40%) | |
| Sex | |||
| male | 82/142 (58%) | 59/113 (52%) | 0.215 |
| female | 60/142 (42%) | 54/113 (48%) | |
| ECOG PS | |||
| 0–1 | 98/142 (69%) | 108/113 (96%) | <0.001 |
| >2 | 35/142 (25%) | 4/113 (3%) | |
| unknown | 9/142 (6%) | 1/113 (1%) | |
| Surgery for primary tumor | |||
| yes | 42/142 (30%) | 39/113 (35%) | 0.255 |
| no | 100/142 (70%) | 74/113 (65%) | |
| PFS1 (4 months) | |||
| less than median | 65/142 (46%) | 58/113 (51%) | 0.002 |
| more than median | 25/142 (18%) | 55/113 (49%) | |
| unknown | 52/142 (36%) | ||
| Best Response to 1-line CT | |||
| SD, PR, CR | 14/142 (10%) | 42/113 (37%) | <0.001 |
| PD | 53/142 (37%) | 29/113 (26%) | |
| unknown | 75/142 (53%) | 42/113 (37%) | |
| 2-line CT G vs. FU | |||
| gemcitabine-based | 34/113 (30%) | ||
| FU-based | 66/113 (58%) | ||
| unknown | 13/113 (12%) | ||
| PFS2 (3 months) | |||
| less than median | 62/113 (55%) | ||
| more than median | 42/113 (37%) | ||
| unknown | 9/113 (8%) |
| Variable | HR | 95.0% CI for HR | p |
|---|---|---|---|
| Median age (reference: median age less than the median) | 1.165 | 0.862–1.575 | 0.322 |
| Sex (reference: male sex) | 1.241 | 0.918–1.676 | 0.160 |
| ECOG PS (reference: ECOG PS 0–1 group) | 0.170 | 0.108–0.266 | <0.001 |
| Surgery for primary tumor (reference: no surgery) | 1.099 | 0.801–1.508 | 0.557 |
| PFS1 (reference: PFS1 less than the median) | 2.570 | 1.824–3.620 | <0.001 |
| Best Response to 1-line CT (reference: disease control) | 0.324 | 0.219–0.479 | <0.001 |
| 2-line CT G vs. FU (reference: 2-line CT G) | 1.006 | 0.603–1.677 | 0.983 |
| PFS2 (reference: PFS2 less than the median) | 1.987 | 1.249–3.161 | 0.004 |
| Variable | HR | 95.0% CI for HR | p |
|---|---|---|---|
| Median age (reference: median age less than the median) | 1.785 | 0.962–3.313 | 0.06 |
| Sex (reference: male sex) | 1.651 | 0.883–3.089 | 0.11 |
| ECOG PS (reference: ECOG PS 0–1 group) | 0.051 | 0.009–0.292 | 0.001 |
| Surgery for primary tumor (reference: no surgery) | 3.812 | 1.584–9.174 | 0.003 |
| PFS1 (reference: PFS1 less than the median) | 0.571 | 0.228–1.430 | 0.23 |
| Best Response to 1-line CT (reference: disease control) | 0.203 | 0.073–0.560 | 0.002 |
| 2-line CT G vs. FU (reference: 2-line CT G) | 1.008 | 0.463–2.190 | 0.98 |
| PFS2 (reference: PFS2 less than the median) | 3.494 | 1.679–7.269 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garajová, I.; Gelsomino, F.; Salati, M.; Mingozzi, A.; Peroni, M.; De Lorenzo, S.; Granito, A.; Tovoli, F.; Leonardi, F. Second-Line Chemotherapy for Intrahepatic Cholangiocarcinomas: What Is the Real Gain? Life 2023, 13, 2170. https://doi.org/10.3390/life13112170
Garajová I, Gelsomino F, Salati M, Mingozzi A, Peroni M, De Lorenzo S, Granito A, Tovoli F, Leonardi F. Second-Line Chemotherapy for Intrahepatic Cholangiocarcinomas: What Is the Real Gain? Life. 2023; 13(11):2170. https://doi.org/10.3390/life13112170
Chicago/Turabian StyleGarajová, Ingrid, Fabio Gelsomino, Massimiliano Salati, Anna Mingozzi, Marianna Peroni, Stefania De Lorenzo, Alessandro Granito, Francesco Tovoli, and Francesco Leonardi. 2023. "Second-Line Chemotherapy for Intrahepatic Cholangiocarcinomas: What Is the Real Gain?" Life 13, no. 11: 2170. https://doi.org/10.3390/life13112170
APA StyleGarajová, I., Gelsomino, F., Salati, M., Mingozzi, A., Peroni, M., De Lorenzo, S., Granito, A., Tovoli, F., & Leonardi, F. (2023). Second-Line Chemotherapy for Intrahepatic Cholangiocarcinomas: What Is the Real Gain? Life, 13(11), 2170. https://doi.org/10.3390/life13112170








