Targeted Therapies in Advanced Cholangiocarcinoma
Abstract
1. Introduction
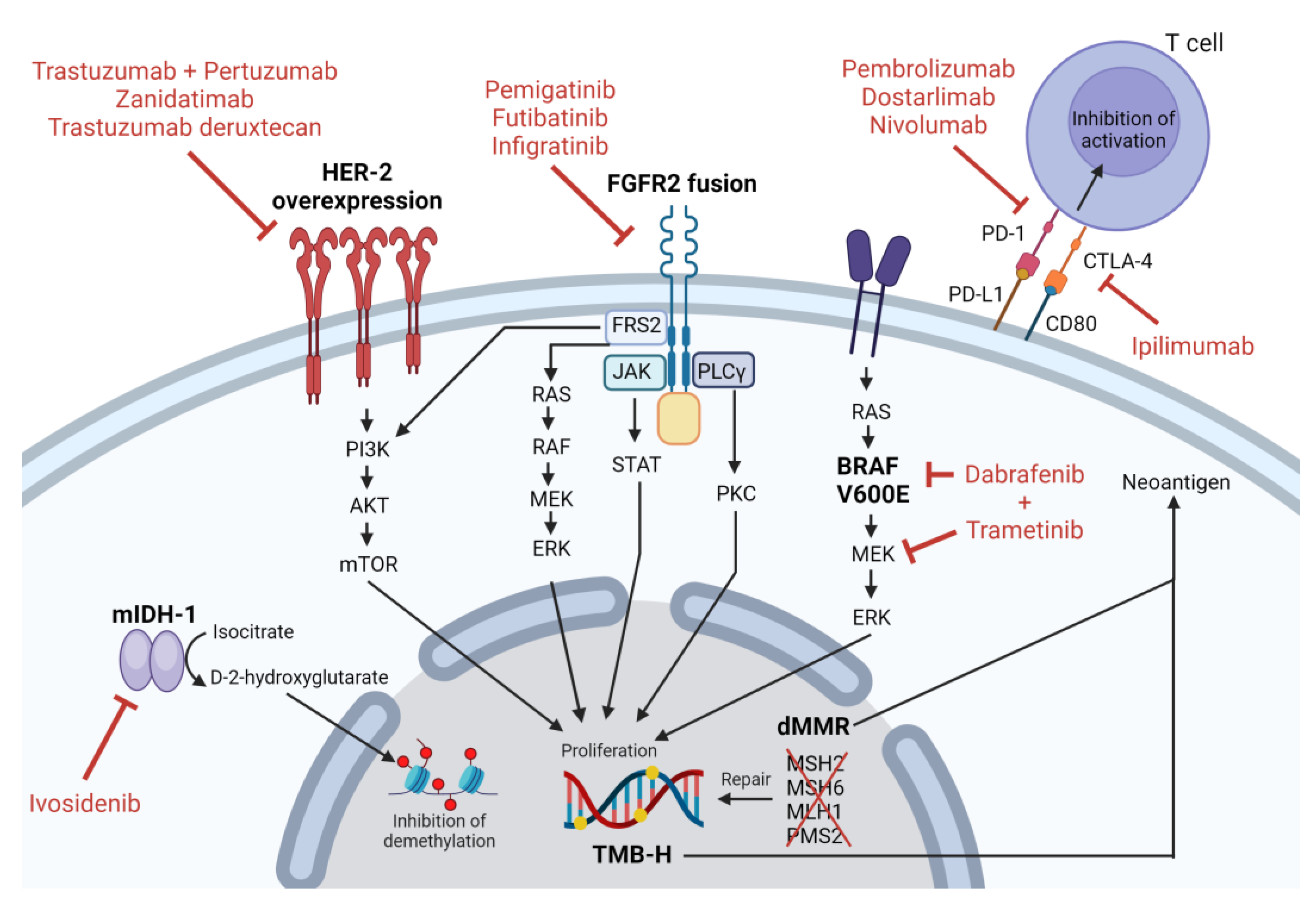
2. IDH-1 Mutation
2.1. IDH-1 Mutation Pathophysiology
2.2. IDH-1 Targeted Therapy
3. HER2 Overexpression and/or Amplification
3.1. HER2 Overexpression and/or Amplification Pathophysiology
3.2. HER2 Overexpression/Amplification Targeted Therapy
4. FGFR2 Fusion or Rearrangement
4.1. FGFR2 Fusion or Rearrangement Pathophysiology
4.2. FGFR2 Fusion or Rearrangement Targeted Therapy
4.2.1. Pemigatinib
4.2.2. Futibatinib
4.2.3. Infigratinib
5. BRAF V600E
5.1. BRAF V600E Pathophysiology
5.2. BRAF V600E Targeted Therapy
6. Deficient Mismatch Repair/High Microsatellite Instability
6.1. Deficient Mismatch Repair/High Microsatellite Instability Pathophysiology
6.2. High Microsatellite Instability Targeted Therapy
6.2.1. Pembrolizumab
6.2.2. Dostarlimab
7. High Tumor Mutation Burden
7.1. High Tumor Mutation Burden Pathophysiology
7.2. High Tumor Mutation Burden Targeted Therapy
8. Other Uncommon/Potential Novel Targets in Cholangiocarcinoma
8.1. NTRK
8.2. RET
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert Consensus Document: Cholangiocarcinoma: Current Knowledge and Future Perspectives Consensus Statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Mukkamalla, S.K.R.; Naseri, H.M.; Kim, B.M.; Katz, S.C.; Armenio, V.A. Trends in Incidence and Factors Affecting Survival of Patients With Cholangiocarcinoma in the United States. J. Natl. Compr. Canc. Netw. 2018, 16, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, M.; Vithayathil, M.; Khan, S.A. Epidemiology of Cholangiocarcinoma. Eur. J. Surg. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and Risk Factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Saad, A.M.; Faisaluddin, M.; Gaman, M.A.; Ruhban, I.A.; Jazieh, K.A.; Al-Husseini, M.J.; Simons-Linares, C.R.; Sonbol, M.B.; Estfan, B.N. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 885–893. [Google Scholar] [CrossRef]
- Kim, D.; Konyn, P.; Cholankeril, G.; Bonham, C.A.; Ahmed, A. Trends in the Mortality of Biliary Tract Cancers Based on Their Anatomical Site in the United States From 2009 to 2018. Am. J. Gastroenterol. 2021, 116, 1053–1062. [Google Scholar] [CrossRef]
- Javle, M.; Lee, S.; Azad, N.S.; Borad, M.J.; Kate Kelley, R.; Sivaraman, S.; Teschemaker, A.; Chopra, I.; Janjan, N.; Parasuraman, S.; et al. Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017. Oncologist 2022, 27, 874–883. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, L.; Li, F.; Li, Q.; Yuan, S.; Huang, S.; Fu, Y.; Yan, X.; Chen, J.; Li, H.; et al. The Epidemiological Trends of Biliary Tract Cancers in the United States of America. BMC Gastroenterol. 2022, 22, 546. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and Cisplatin plus Durvalumab with or without Tremelimumab in Chemotherapy-Naive Patients with Advanced Biliary Tract Cancer: An Open-Label, Single-Centre, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study of Durvalumab in Combination with Gemcitabine plus Cisplatin (GemCis) in Patients (Pts) with Advanced Biliary Tract Cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in Combination with Gemcitabine and Cisplatin Compared with Gemcitabine and Cisplatin Alone for Patients with Advanced Biliary Tract Cancer (KEYNOTE-966): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-Line FOLFOX Chemotherapy versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal Irinotecan plus Fluorouracil and Leucovorin versus Fluorouracil and Leucovorin for Metastatic Biliary Tract Cancer after Progression on Gemcitabine plus Cisplatin (NIFTY): A Multicentre, Open-Label, Randomised, Phase 2b Study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.; Kim, I.; Kim, K.P.; Ryoo, B.Y.; Jeong, J.H.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; et al. Treatment with Liposomal Irinotecan Plus Fluorouracil and Leucovorin for Patients with Previously Treated Metastatic Biliary Tract Cancer: The Phase 2b NIFTY Randomized Clinical Trial. JAMA Oncol. 2023, 9, 692–699. [Google Scholar] [CrossRef]
- Vogel, A.; Wenzel, P.; Folprecht, G.; Schutt, P.; Wege, H.; Kretzschmar, A.; Jacobasch, L.; Ziegenhagen, N.; Boeck, S.; Kanzler, S.; et al. Nal-IRI and 5-FU/LV Compared to 5-FU/LV in Patients with Cholangio- and Gallbladder Carcinoma Previously Treated with Gemcitabine-Based Therapies (NALIRICC—AIO-HEP-0116). Ann. Oncol. 2022, 33, S19–S26. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kamgar, M.; Mahipal, A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers 2020, 12, 2039. [Google Scholar] [CrossRef]
- Tella, S.H.; Kommalapati, A.; Borad, M.J.; Mahipal, A. Second-Line Therapies in Advanced Biliary Tract Cancers. Lancet Oncol. 2020, 21, e29–e41. [Google Scholar] [CrossRef]
- Dang, L.; Yen, K.; Attar, E.C. IDH Mutations in Cancer and Progress toward Development of Targeted Therapeutics. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 599–608. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified through Broad-Based Tumor Genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef]
- Goyal, L.; Govindan, A.; Sheth, R.A.; Nardi, V.; Blaszkowsky, L.S.; Faris, J.E.; Clark, J.W.; Ryan, D.P.; Kwak, E.L.; Allen, J.N.; et al. Prognosis and Clinicopathologic Features of Patients with Advanced Stage Isocitrate Dehydrogenase (IDH) Mutant and IDH Wild-Type Intrahepatic Cholangiocarcinoma. Oncologist 2015, 20, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and Prognostic Significance of Isocitrate Dehydrogenase 1 Mutations in Cholangiocarcinoma: A Systematic Literature Review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Wintheiser, G.; Zemla, T.; Shi, Q.; Tran, N.; Prasai, K.; Tella, S.H.; Mody, K.; Ahn, D.; Borad, M.; Bekaii-Saab, T.; et al. Isocitrate Dehydrogenase-Mutated Cholangiocarcinoma: Natural History and Clinical Outcomes. JCO Precis. Oncol. 2022, 6, e2100156. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Burris, H.A.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and Activity of Ivosidenib in Patients with IDH1-Mutant Advanced Cholangiocarcinoma: A Phase 1 Study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Gajria, D.; Chandarlapaty, S. HER2-Amplified Breast Cancer: Mechanisms of Trastuzumab Resistance and Novel Targeted Therapies. Expert Rev. Anticancer Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, E.E.I.; Balaji, K.; Raghav, K.; Hess, K.; Javle, M.; Blum-Murphy, M.; Ajani, J.; Kopetz, S.; Broaddus, R.; Routbort, M.; et al. Targeting ERBB2 (HER2) Amplification Identified by Next-Generation Sequencing in Patients With Advanced or Metastatic Solid Tumors Beyond Conventional Indications. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 Expression Status in Diverse Cancers: Review of Results from 37,992 Patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 Pathway in Biliary Tract Malignancies; Systematic Review and Meta-Analysis: A Potential Therapeutic Target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef]
- Vivaldi, C.; Fornaro, L.; Ugolini, C.; Niccoli, C.; Musettini, G.; Pecora, I.; Cacciato Insilla, A.; Salani, F.; Pasquini, G.; Catanese, S.; et al. HER2 Overexpression as a Poor Prognostic Determinant in Resected Biliary Tract Cancer. Oncologist 2020, 25, 886–893. [Google Scholar] [CrossRef]
- Kim, H.; Kim, R.; Kim, H.R.; Jo, H.; Kim, H.; Ha, S.Y.; Park, J.O.; Park, Y.S.; Kim, S.T. HER2 Aberrations as a Novel Marker in Advanced Biliary Tract Cancer. Front. Oncol. 2022, 12, 834104. [Google Scholar] [CrossRef]
- Nami, B.; Maadi, H.; Wang, Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers 2018, 10, 342. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and Trastuzumab for HER2-Positive, Metastatic Biliary Tract Cancer (MyPathway): A Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Harding, J.J.; Fan, J.; Oh, D.-Y.; Choi, H.J.; Kim, J.W.; Chang, H.-M.; Bao, L.; Sun, H.-C.; Macarulla, T.; Xie, F.; et al. Zanidatamab for HER2-Amplified, Unresectable, Locally Advanced or Metastatic Biliary Tract Cancer (HERIZON-BTC-01): A Multicentre, Single-Arm, Phase 2b Study. Lancet Oncol. 2023, 24, 772–782. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.N.; Gonzalez Martin, A.; Jung, K.H.; Lugowska, I.A.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan (T-DXd) in Patients (Pts) with HER2-Expressing Solid Tumors: DESTINY-PanTumor02 (DP-02) Interim Results. J. Clin. Oncol. 2023, 41, LBA3000. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Li, L.; He, Y.; Wei, Y.; Dang, Y.; Nie, S.; Guo, Z. Signaling Pathway and Small-Molecule Drug Discovery of FGFR: A Comprehensive Review. Front. Chem. 2022, 10, 860985. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases: From Basic Concepts to Clinical Applications. Eur. J. Cancer Suppl. 2006, 4, 3–26. [Google Scholar] [CrossRef][Green Version]
- Schlessinger, J. Receptor Tyrosine Kinases: Legacy of the First Two Decades. Cold Spring Harb. Perspect. Biol. 2014, 6, a008912. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr Gene Families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef]
- Dienstmann, R.; Rodon, J.; Prat, A.; Perez-Garcia, J.; Adamo, B.; Felip, E.; Cortes, J.; Iafrate, A.J.; Nuciforo, P.; Tabernero, J. Genomic Aberrations in the FGFR Pathway: Opportunities for Targeted Therapies in Solid Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 552–563. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef]
- Katoh, M. FGFR Inhibitors: Effects on Cancer Cells, Tumor Microenvironment and Whole-Body Homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Storandt, M.H.; Jin, Z.; Mahipal, A. Pemigatinib in Cholangiocarcinoma with a FGFR2 Rearrangement or Fusion. Expert Rev. Anticancer Ther. 2022, 22, 1265–1274. [Google Scholar] [CrossRef]
- Hyung, S.; Han, B.; Jung, J.; Kim, S.T.; Hong, J.Y.; Park, S.H.; Zang, D.Y.; Park, J.O.; Park, Y.S.; Kim, K.M.; et al. Incidence of FGFR2 Amplification and FGFR2 Fusion in Patients with Metastatic Cancer Using Clinical Sequencing. J. Oncol. 2022, 2022, 9714570. [Google Scholar] [CrossRef]
- Mahipal, A.; Tella, S.H.; Kommalapati, A.; Anaya, D.; Kim, R. FGFR2 Genomic Aberrations: Achilles Heel in the Management of Advanced Cholangiocarcinoma. Cancer Treat. Rev. 2019, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, M.; Brignola, S.; Munari, G.; Gatti, M.; Dadduzio, V.; Borga, C.; Bergamo, F.; Pellino, A.; Angerilli, V.; Mescoli, C.; et al. Prognostic Impact of FGFR2/3 Alterations in Patients with Biliary Tract Cancers Receiving Systemic Chemotherapy: The BITCOIN Study. Eur. J. Cancer 2022, 166, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.C.; Koblish, H.; Wu, L.; Bowman, K.; Diamond, S.; DiMatteo, D.; Zhang, Y.; Hansbury, M.; Rupar, M.; Wen, X.; et al. INCB054828 (Pemigatinib), a Potent and Selective Inhibitor of Fibroblast Growth Factor Receptors 1, 2, and 3, Displays Activity against Genetically Defined Tumor Models. PLoS ONE 2020, 15, e0231877. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, C.; He, C.; Qian, D.; Lu, L.; Sun, Y.; Xu, M.; Zhuo, J.; Liu, P.C.C.; Klabe, R.; et al. Discovery of Pemigatinib: A Potent and Selective Fibroblast Growth Factor Receptor (FGFR) Inhibitor. J. Med. Chem. 2021, 64, 10666–10679. [Google Scholar] [CrossRef]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.M.; Ji, T.; Saleh, M. FIGHT-101, a First-in-Human Study of Potent and Selective FGFR 1-3 Inhibitor Pemigatinib in Pan-Cancer Patients with FGF/FGFR Alterations and Advanced Malignancies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 522–533. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Shi, G.; Huang, X.; Wen, T.; Song, T.; Kuang, M.; Mou, H.; Bao, L.; Zhao, H.-T.; Zhao, H.; Feng, X.; et al. Pemigatinib in Chinese Patients with Advanced/Metastatic or Surgically Unresectable Cholangiocarcinoma Including FGFR2 Fusion or Rearrangement: Updated Data from an Open-Label, Single-Arm, Multicenter Phase II Study (CIBI375A201 Study). J. Clin. Oncol. 2022, 40, e16183. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.A.; Wasan, H.S.; et al. FIGHT-302: Phase III Study of First-Line (1L) Pemigatinib (PEM) versus Gemcitabine (GEM) plus Cisplatin (CIS) for Cholangiocarcinoma (CCA) with FGFR2 Fusions or Rearrangements. J. Clin. Oncol. 2020, 38, TPS592. [Google Scholar] [CrossRef]
- Kalyukina, M.; Yosaatmadja, Y.; Middleditch, M.J.; Patterson, A.V.; Smaill, J.B.; Squire, C.J. TAS-120 Cancer Target Binding: Defining Reactivity and Revealing the First Fibroblast Growth Factor Receptor 1 (FGFR1) Irreversible Structure. ChemMedChem 2019, 14, 494–500. [Google Scholar] [CrossRef]
- Bahleda, R.; Meric-Bernstam, F.; Goyal, L.; Tran, B.; He, Y.; Yamamiya, I.; Benhadji, K.A.; Matos, I.; Arkenau, H.T. Phase I, First-in-Human Study of Futibatinib, a Highly Selective, Irreversible FGFR1-4 Inhibitor in Patients with Advanced Solid Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1-4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; He, Y.; Soni, N.; et al. FOENIX-CCA2: A Phase II, Open-Label, Multicenter Study of Futibatinib in Patients (Pts) with Intrahepatic Cholangiocarcinoma (ICCA) Harboring FGFR2 Gene Fusions or Other Rearrangements. J. Clin. Oncol. 2020, 38, 108. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.A.; Furuse, J.; et al. Updated Results of the FOENIX-CCA2 Trial: Efficacy and Safety of Futibatinib in Intrahepatic Cholangiocarcinoma (ICCA) Harboring FGFR2 Fusions/Rearrangements. J. Clin. Oncol. 2022, 40, 4009. [Google Scholar] [CrossRef]
- Borad, M.J.; Bridgewater, J.A.; Morizane, C.; Shroff, R.T.; Oh, D.-Y.; Moehler, M.H.; Furuse, J.; Benhadji, K.A.; He, H.; Valle, J.W. A Phase III Study of Futibatinib (TAS-120) versus Gemcitabine-Cisplatin (Gem-Cis) Chemotherapy as First-Line (1L) Treatment for Patients (Pts) with Advanced (Adv) Cholangiocarcinoma (CCA) Harboring Fibroblast Growth Factor Receptor 2 (FGFR2) Gene Rearrangements (FOENIX-CCA3). J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Guagnano, V.; Kauffmann, A.; Wöhrle, S.; Stamm, C.; Ito, M.; Barys, L.; Pornon, A.; Yao, Y.; Li, F.; Zhang, Y.; et al. FGFR Genetic Alterations Predict for Sensitivity to NVP-BGJ398, a Selective Pan-FGFR Inhibitor. Cancer Discov. 2012, 2, 1118–1133. [Google Scholar] [CrossRef]
- Nogova, L.; Sequist, L.V.; Garcia, J.M.P.; Andre, F.; Delord, J.P.; Hidalgo, M.; Schellens, J.H.M.; Cassier, P.A.; Camidge, D.R.; Schuler, M.; et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients with Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J. Clin. Oncol. 2017, 35, 157–165. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Śmiech, M.; Leszczyński, P.; Kono, H.; Wardell, C.; Taniguchi, H. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef]
- Xin, H.Y.; Sun, R.Q.; Zou, J.X.; Wang, P.C.; Wang, J.Y.; Ye, Y.H.; Liu, K.X.; Hu, Z.Q.; Zhou, Z.J.; Fan, J.; et al. Association of BRAF Variants with Disease Characteristics, Prognosis, and Targeted Therapy Response in Intrahepatic Cholangiocarcinoma. JAMA Netw. Open 2023, 6, E231476. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene Mutational Profiling of Cholangiocarcinomas Identifies Actionable Molecular Subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Spee, B.; Blechacz, B.R.; Avital, I.; Komuta, M.; Barbour, A.; Conner, E.A.; Gillen, M.C.; Roskams, T.; Roberts, L.R.; et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology 2012, 142, 1021–1031. [Google Scholar] [CrossRef]
- Jain, A.; Javle, M. Molecular Profiling of Biliary Tract Cancer: A Target Rich Disease. J. Gastrointest. Oncol. 2016, 7, 797–803. [Google Scholar] [CrossRef]
- Ahn, D.H.; Bekaii-Saab, T. Biliary Cancer: Intrahepatic Cholangiocarcinoma vs. Extrahepatic Cholangiocarcinoma vs. Gallbladder Cancers: Classification and Therapeutic Implications. J. Gastrointest. Oncol. 2017, 8, 239–301. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kwong, L.N.; Javle, M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr. Treat. Options Oncol. 2016, 17, 58. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors with BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Bateman, A.C. DNA Mismatch Repair Proteins: Scientific Update and Practical Guide. J. Clin. Pathol. 2021, 74, 264–268. [Google Scholar] [CrossRef]
- Olave, M.C.; Graham, R.P. Mismatch Repair Deficiency: The What, How and Why It Is Important. Genes. Chromosomes Cancer 2022, 61, 314–321. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Huang, Q.; Tan, S.; Xiong, X.; Gou, H. Rare DNA Mismatch Repair-Related Protein Loss in Patients with Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma and Their Response to Immunotherapy. Cancer Manag. Res. 2021, 13, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Kumamoto, K.; Matsukawa, H.; Ishikawa, R.; Suto, H.; Oshima, M.; Kamada, H.; Morishita, A.; Kobara, H.; Matsunaga, T.; et al. Low Prevalence of Biliary Tract Cancer with Defective Mismatch Repair Genes in a Japanese Hospital-Based Population. Oncol. Lett. 2022, 23, 4. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch Repair Deficiency/Microsatellite Instability-High as a Predictor for Anti-PD-1/PD-L1 Immunotherapy Efficacy. J. Hematol. Oncol. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Chun, Y.S.; Ikoma, N.; Vauthey, J.N.; Aloia, T.A.; Cuddy, A.; Rodriguez-Bigas, M.A.; Nancy You, Y. Clinical and Genetic Implications of DNA Mismatch Repair Deficiency in Biliary Tract Cancers Associated with Lynch Syndrome. J. Gastrointest. Cancer 2018, 49, 93–96. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Pérez, J.M.T.; Arkenau, H.-T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and Efficacy of Anti–PD-1 Antibody Dostarlimab in Patients (Pts) with Mismatch Repair-Deficient (DMMR) Solid Cancers: Results from GARNET Study. J. Clin. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- Fusco, M.J.; West, H.J.; Walko, C.M. Tumor Mutation Burden and Cancer Treatment. JAMA Oncol. 2021, 7, 316. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Q.; Fu, J.; Jin, Z.; Su, J.; Zhang, J.; Chen, C.; Geng, Z.; Zhang, D. Comprehensive Analysis of Genomic Mutation Signature and Tumor Mutation Burden for Prognosis of Intrahepatic Cholangiocarcinoma. BMC Cancer 2021, 21. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Song, J.P.; Liu, X.Z.; Chen, Q.; Liu, Y.F. High Tumor Mutation Burden Indicates a Poor Prognosis in Patients with Intrahepatic Cholangiocarcinoma. World J. Clin. Cases 2022, 10, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Kim, R.; Jo, H.; Kim, H.R.; Hong, J.; Park, J.O.; Park, Y.S.; Kim, S.T. Tumor Mutational Burden as a Biomarker for Advanced Biliary Tract Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211062324. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Schenker, M.; Burotto, M.; Richardet, M.; Ciuleanu, T.; Goncalves, A.; Steeghs, N.; Schöffski, P.; Ascierto, P.A.; Maio, M.; Lugowska, I.; et al. Abstract CT022: CheckMate 848: A Randomized, Open-Label, Phase 2 Study of Nivolumab in Combination with Ipilimumab or Nivolumab Monotherapy in Patients with Advanced or Metastatic Solid Tumors of High Tumor Mutational Burden. Cancer Res. 2022, 82, CT022. [Google Scholar] [CrossRef]
- Manea, C.A.; Badiu, D.C.; Ploscaru, I.C.; Zgura, A.; Bacinschi, X.; Smarandache, C.G.; Serban, D.; Popescu, C.G.; Grigorean, V.T.; Botnarciuc, V. A Review of NTRK Fusions in Cancer. Ann. Med. Surg. 2022, 79, 103893. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Demols, A.; Rocq, L.; Perez-Casanova, L.; Charry, M.; De Nève, N.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Maris, C.; Closset, J.; et al. A Two-Step Diagnostic Approach for NTRK Gene Fusion Detection in Biliary Tract and Pancreatic Adenocarcinomas. Oncologist 2023, 28, e520–e525. [Google Scholar] [CrossRef]
- Demols, A.; Perez-Casanova, L.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Maris, C.; et al. 71P NTRK Gene Fusions in Bilio-Pancreatic Cancers. Ann. Oncol. 2020, 31, S268. [Google Scholar] [CrossRef]
- Iyer, R.; Wehrmann, L.; Golden, R.L.; Naraparaju, K.; Croucher, J.L.; MacFarland, S.P.; Guan, P.; Kolla, V.; Wei, G.; Cam, N.; et al. Entrectinib Is a Potent Inhibitor of Trk-Driven Neuroblastomas in a Xenograft Mouse Model. Cancer Lett. 2016, 372, 179–186. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ritz, J.; Cooper, G.M. Activation of a Novel Human Transforming Gene, Ret, by DNA Rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kawai, K.; Asai, N. Roles of the RET Proto-Oncogene in Cancer and Development. JMA J. 2020, 3, 175–181. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-Cancer Efficacy of Pralsetinib in Patients with RET Fusion-Positive Solid Tumors from the Phase 1/2 ARROW Trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-Agnostic Efficacy and Safety of Selpercatinib in Patients with RET Fusion-Positive Solid Tumours Other than Lung or Thyroid Tumours (LIBRETTO-001): A Phase 1/2, Open-Label, Basket Trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Lu, G.; Hu, Z.; Chen, Q.; Du, X. FGF/FGFR Signaling Pathway Involved Resistance in Various Cancer Types. J. Cancer 2020, 11, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Pei, H.Z.; Li, T.; Huang, J.; Guo, Y.; Zhao, Y.; Yang, M.; Zhang, D.; Chang, Z.; Zhang, Q.; et al. The Molecular Mechanisms of Resistance to IDH Inhibitors in Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 931462. [Google Scholar] [CrossRef]
- Zhang, Y.; Esmail, A.; Mazzaferro, V.; Abdelrahim, M. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers 2022, 14, 5074. [Google Scholar] [CrossRef] [PubMed]
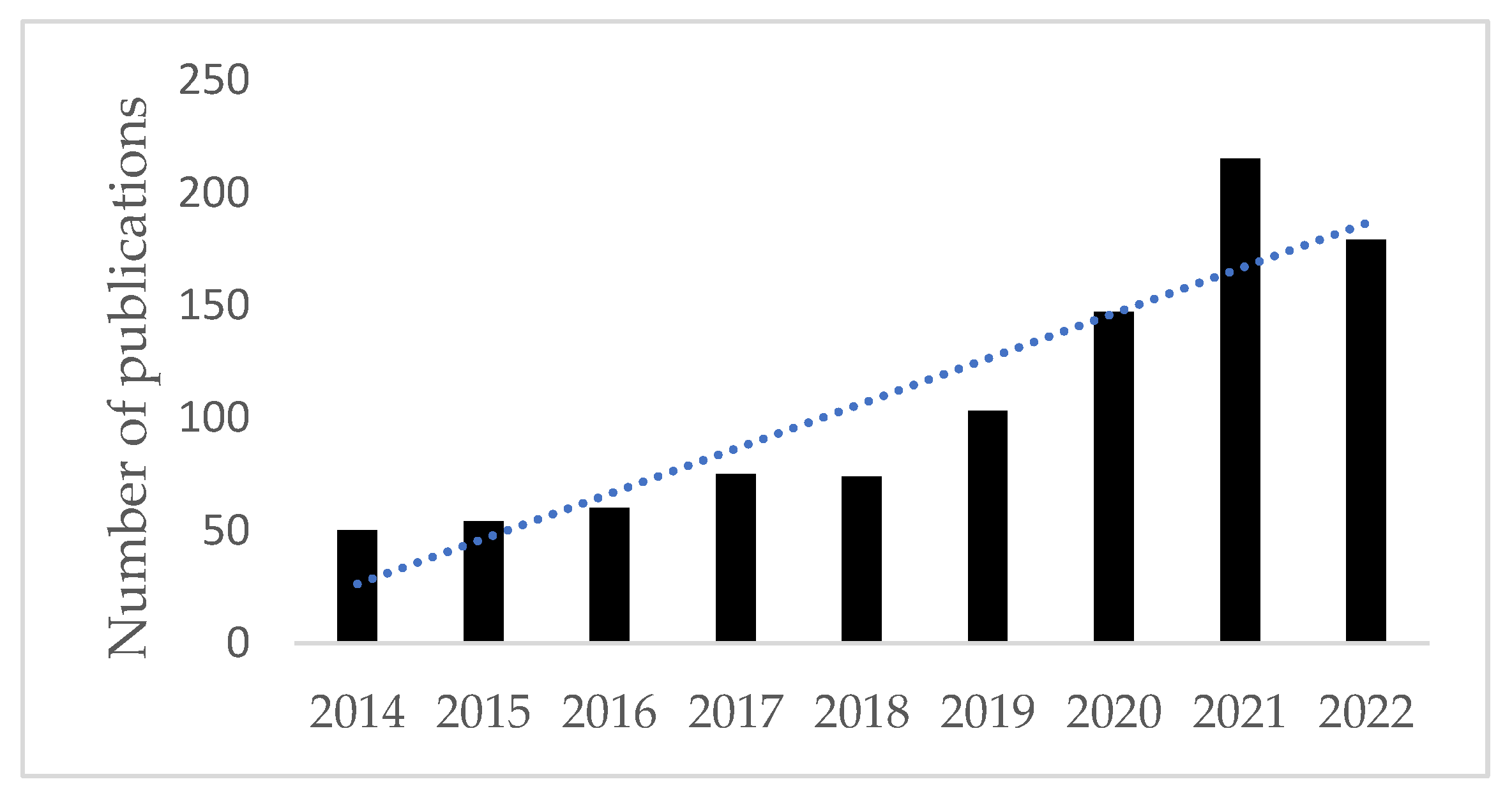
| Trial Name | Study Arm | Control Arm | Trial Phase | Line of Therapy | Patient Number | Primary EndPoint(s) | ORR | mPFS (Months) | HR, 95% CI (mPFS) | p (mPFS) | mOS (Months) | HR, 95% CI (mOS) | p (mOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOPAZ-1 [12] | Gemcitabine + cisplatin + durvalumab | Gemcitabine + cisplatin | 3 | First | 685 | OS | 26.7% | 7.2 vs. 5.7 | 0.75, 0.64–0.89 | 0.001 | 12.8 vs. 11.5 | 0.80, 0.66–0.97 | 0.021 |
| KEYNOTE-966 [13] | Gemcitabine + cisplatin + pembrolizumab | Gemcitabine + cisplatin | 3 | First | 1069 | OS | 29% | 6.5 vs. 5.6 | 0.86, 0.75–1.00 | 0.023 | 12.7 vs. 10.9 | 0.83, 0.72–0.95 | 0.0034 |
| ABC-06 [14] | FOLFOX | Best supportive care | 3 | Second | 162 | OS | 5% | 4.0 (FOLFOX arm) | NR | NR | 6.2 vs. 5.3 | 0.69, 0.50–0.97 | 0.031 |
| NIFTY [15,16] | 5-FU + leucovorin + liposomal irinotecan | 5-FU + leucovorin | 2 | Second | 178 | PFS | 12.5% | 4.2 vs. 1.7 | 0.61, 0.44–0.86 | 0.004 | 8.6 vs. 5.3 | 0.68, 0.48–0.95 | 0.02 |
| NALIRICC-AIO-HEP-0116 [17] | 5-FU + leucovorin + liposomal irinotecan | 5-FU + leucovorin | 2 | Second | 100 | PFS | 14.3% | 2.8 vs. 2.3 | NR | NR | 6.9 vs. 8.2 | NR | NR |
| Incidence | ||
|---|---|---|
| Target | iCCA | eCCA |
| IDH-1 mutation [23,24,25] | 20–25% | <1% |
| HER2 overexpression/amplification [35] | 5% | 17% |
| FGFR2 fusion or rearrangement [50] | 15% | <1% |
| BRAF V600E [71,72,73,74,75,76] | 1–5% | <1% |
| Deficient mismatch repair/high microsatellite instability [81,82,83] | 2–3% | 2–3% |
| High tumor mutation burden [90,91] | 3.5% | 2% |
| Target | Trial Name | Study ARM | Control Arm | Trial Phase | Cohort | Patient Number | Primary EndPoint(s) | ORR | mPFS (Months) | HR, 95% CI (mPFS) | p (mPFS) | mOS (MONTHs) | HR, 95% CI (mOS) | p (mOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IDH-1 mutation | ClarIDHy [29,30] | Ivosidenib | Placebo | 3 | mIDH-1 CCA w/progression on prior therapy | 230 | PFS | 2% | 2.7 vs. 1.4 | 0.37, 0.25-0.54 | <0.0001 | 10.3 vs. 7.5 | 0.79, 0.56–1.12 | 0.09 |
| HER2 overexpression/amplification | MyPathway [39] | Pertuzumab plus trastuzumab | N/A | 2a | HER2+, previously treated BTC | 39 | ORR | 23% | 2.6 (iCCA), 6.8 (eCCA) | N/A | N/A | 3.9 (iCCA), 8.0 (eCCA) | N/A | N/A |
| HERIZON-BTC-01 [40] | Zanidatimab | N/A | 2b | HER2 2+/3+ (Cohort 1), previously treated BTC | 80 | ORR | 41.3% | 5.5 | N/A | N/A | Not reported | N/A | N/A | |
| DESTINY-PanTumor02 [41] | Trastuzumab deruxtecan ** | N/A | 2 | HER2+, previously treated solid malignancies | 267 (41 w/BTC) | ORR | 37.1%(22.0% among all BTC) | Not reported | N/A | N/A | Not reported | N/A | N/A | |
| FGFR2 fusion/rearrangement | FIGHT-202 [57] | Pemigatinib | N/A | 2 | FGFR2 fusion/rearrangement | 107 | ORR | 35.5% | 6.9 | N/A | N/A | 21.1 | N/A | N/A |
| FGFR2 mutation | 20 | 0 | 2.1 | 6.7 | ||||||||||
| No FGFR2 abnormality | 18 | 0 | 1.7 | 4 | ||||||||||
| FOENIX-CCA2 [63,64] | Futibatinib | N/A | 2 | iCCA w/FGFR2 fusion/rearrangement | 103 | ORR | 41.7% | 8.9 | N/A | N/A | 20.0 | N/A | N/A | |
| Javle et al. [68] | Infigratinib | N/A | 2 | CCA w/FGFR2 fusion or other alteration | 61 | ORR | 14.80% | 5.8 | N/A | N/A | Not reported | N/A | N/A | |
| Javle et al. [69] | N/A | 2 | CCA w/FGFR2 fusion/rearrangement w/progression on gemcitabine | 122 | ORR | 23.10% | 7.3 | N/A | N/A | 12.2 | N/A | N/A | ||
| BRAF V600E | ROAR [78] | Dabrafenib plus trametinib | N/A | 2 | BTC w/BRAF V600E | 43 | ORR | 51% | 9 | N/A | N/A | 14 | N/A | N/A |
| MSI-H | KEYNOTE-158 [86,87] | Pembrolizumab | N/A | 2 | Advanced non-colorectal dMMR/MSI-H tumors | 351 (22 w/CCA) | ORR | 30.80% | 3.5 | N/A | N/A | 20.1 | N/A | N/A |
| TMB-H | KEYNOTE-158 (subgroup analysis) * [94] | Pembrolizumab | N/A | 2 | Advanced non-colorectal TMB-H tumors | 102 | ORR | 29% | 2.1 | N/A | N/A | 11.7 | N/A | N/A |
| Advanced non-colorectal non- TMB-H tumors | 688 | 6% | 2.1 | N/A | N/A | 12.8 | N/A | N/A | ||||||
| CheckMate 848 [95] | Ipilimumab plus nivolumab | N/A | 2 | Advanced, TMB-H solid tumors | 148 | ORR | 22.5-35.3% | 2.8-4.1 | N/A | N/A | 8.5-14.5 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storandt, M.H.; Kurniali, P.C.; Mahipal, A.; Jin, Z. Targeted Therapies in Advanced Cholangiocarcinoma. Life 2023, 13, 2066. https://doi.org/10.3390/life13102066
Storandt MH, Kurniali PC, Mahipal A, Jin Z. Targeted Therapies in Advanced Cholangiocarcinoma. Life. 2023; 13(10):2066. https://doi.org/10.3390/life13102066
Chicago/Turabian StyleStorandt, Michael H., Peter C. Kurniali, Amit Mahipal, and Zhaohui Jin. 2023. "Targeted Therapies in Advanced Cholangiocarcinoma" Life 13, no. 10: 2066. https://doi.org/10.3390/life13102066
APA StyleStorandt, M. H., Kurniali, P. C., Mahipal, A., & Jin, Z. (2023). Targeted Therapies in Advanced Cholangiocarcinoma. Life, 13(10), 2066. https://doi.org/10.3390/life13102066







