3. Descriptions of New Species
Thyreophagus ais Klimov, Kolesnikov, Demard, Vangansbeke sp. n.
urn:lsid:zoobank.org:pub:7C7FEAA7-EB57-4A12-A489-196D2BEEA5D9.
Type material. Holotype: One female—lab culture on bran, harvested 26 February 2021, culture started from specimens originated from the USA, Florida, Fort Pierce, branches on ground in a small wooded area, subcortical, 27°25′34.5″ N 80°24′22.7″ W, 20 November 2020, Emilie Demard coll., BMOC 20-0101-014#S1.1. Paratypes: 3f (S1.2,3; S3.1), 6 HDNs (S4.1; S5.1; S6.1-4)—same data; 2f—original wild sample (see above).
Depository. Holotype, paratypes—University of Michigan, Museum of Zoology, Ann Arbor, Michigan, USA.
Etymology. The new species is named after the Ais people who lived in the eastern coastal area of central Florida (including the type locality of the new species) but went extinct after the arrival of European colonizers [
27].
Habitat. Thyreophagus ais lives under the bark of small fallen branches of deciduous trees in wooded areas. These branches are typically in the initial stages of decomposition, with approximately 80% of their natural sapwood retaining a white color, and 20% displaying brown discoloration, indicating the ongoing decomposition process. Additionally, the bark usually exhibits boreholes from wood-boring beetles.
Description
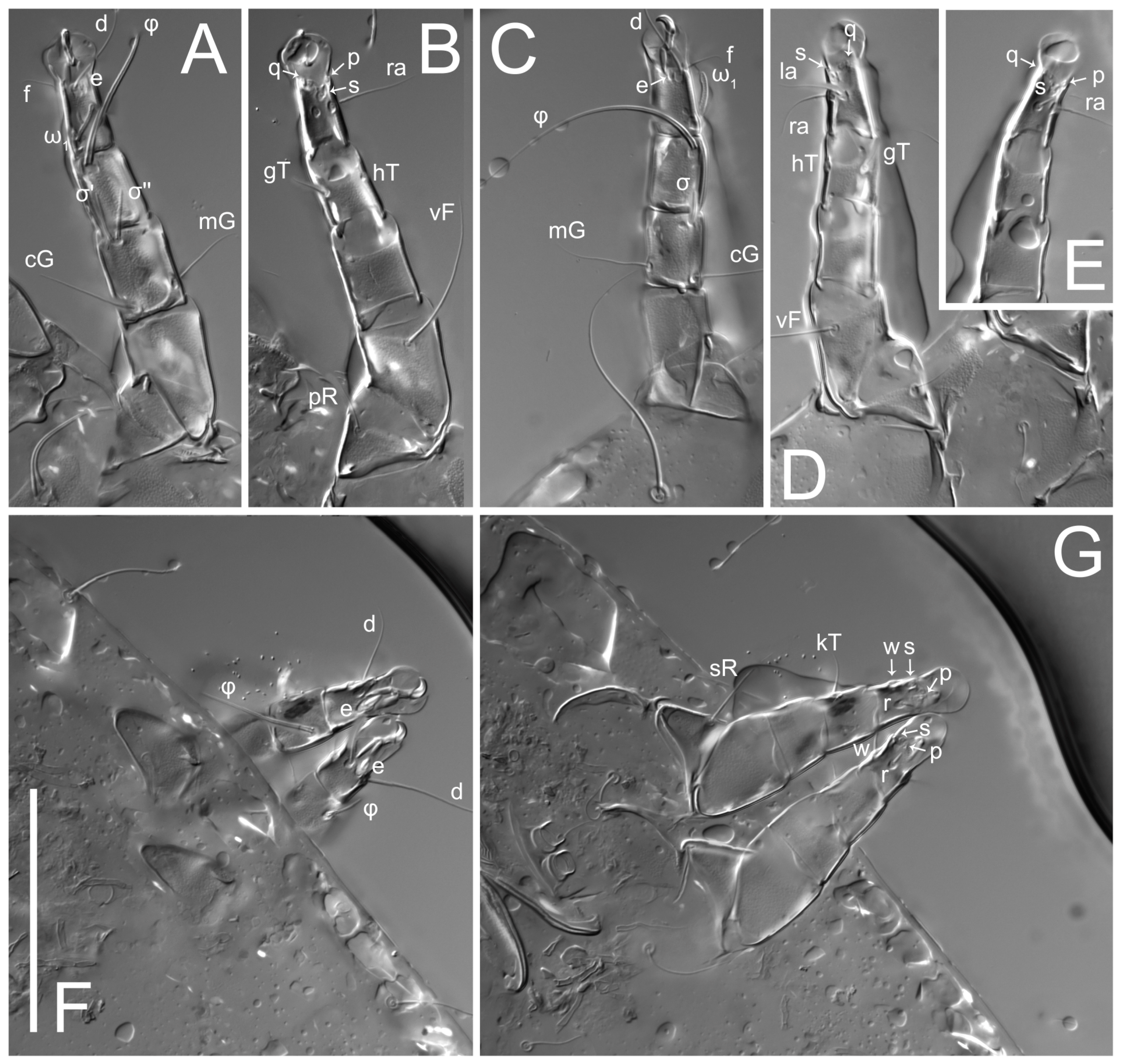
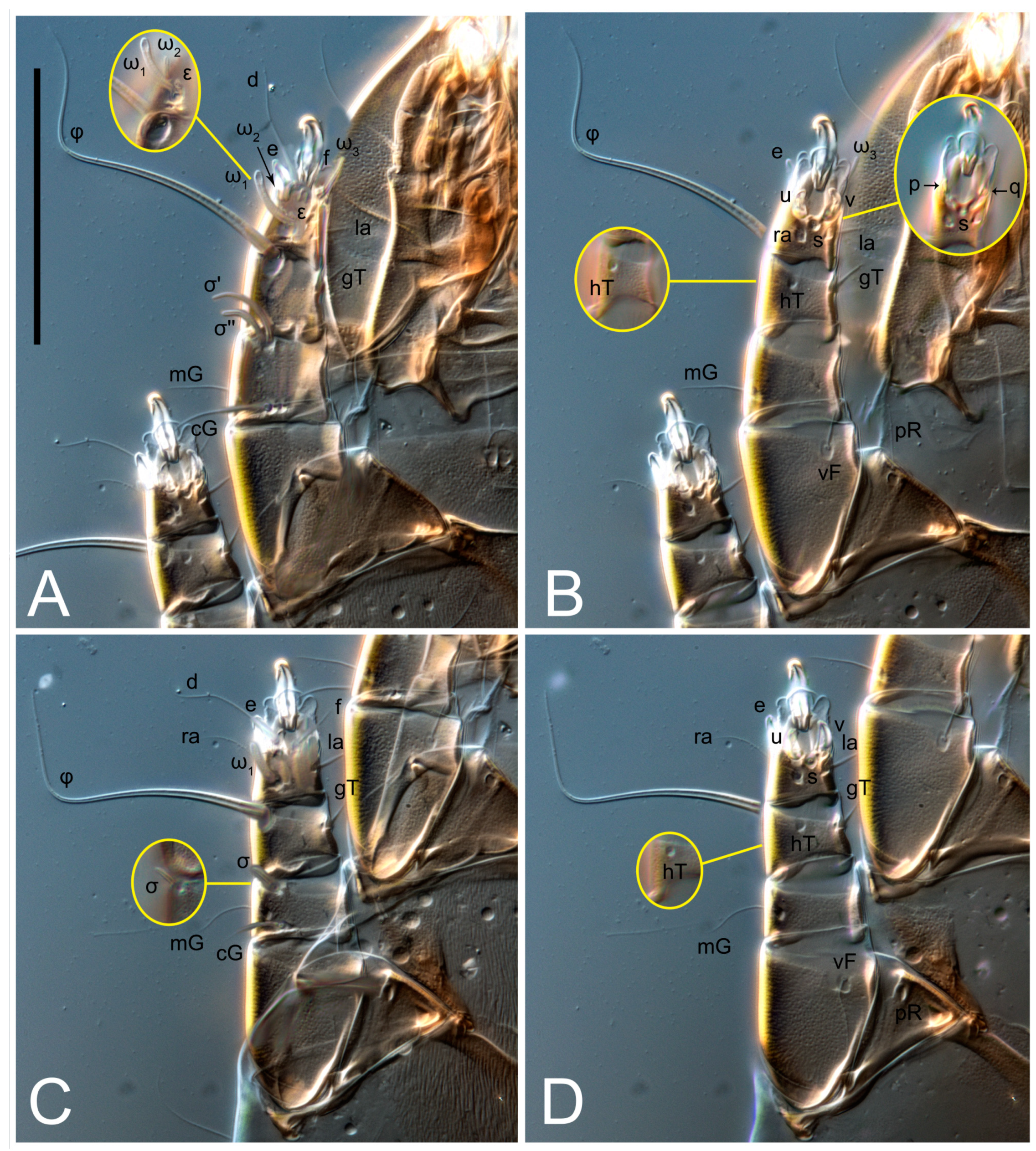
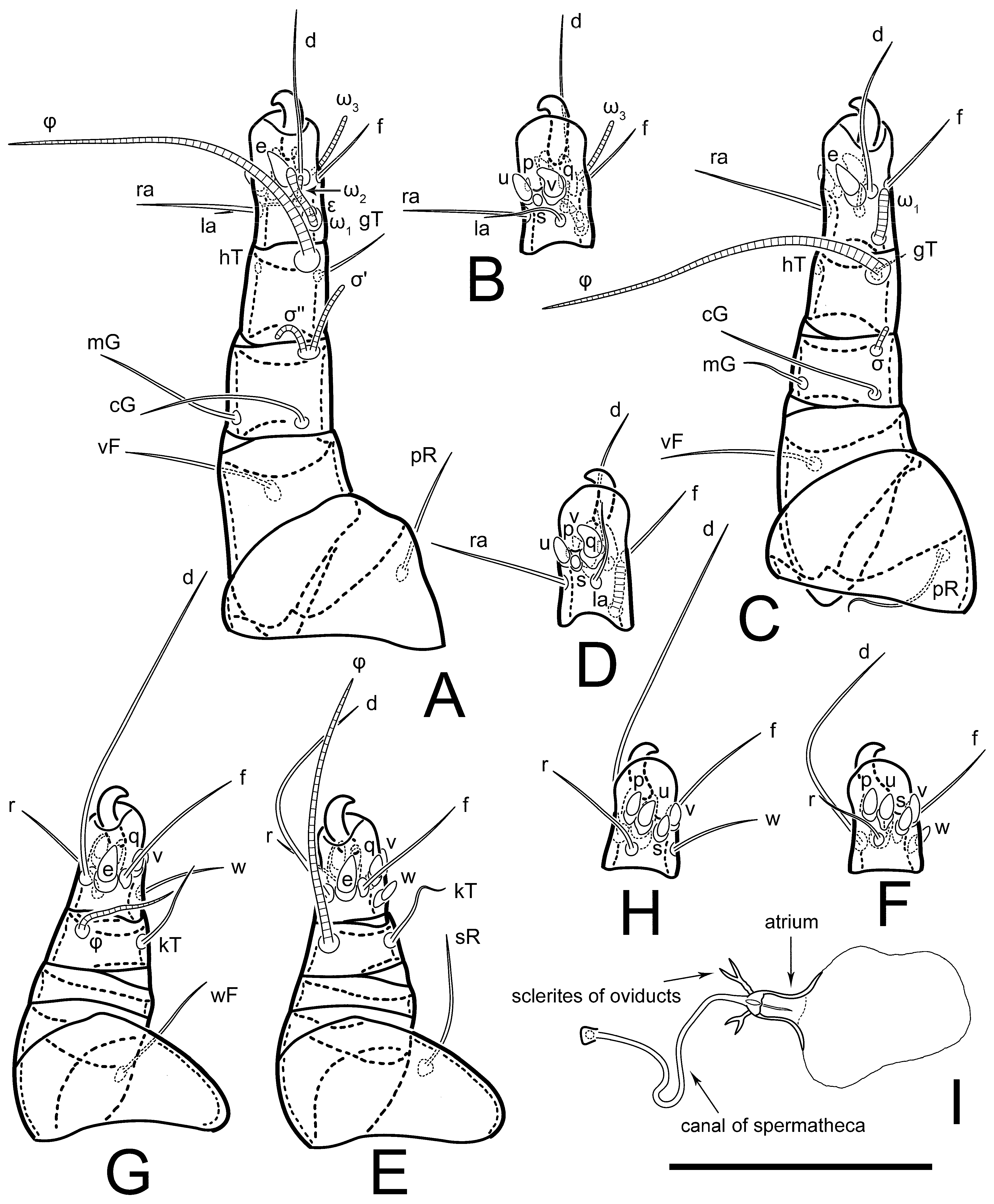
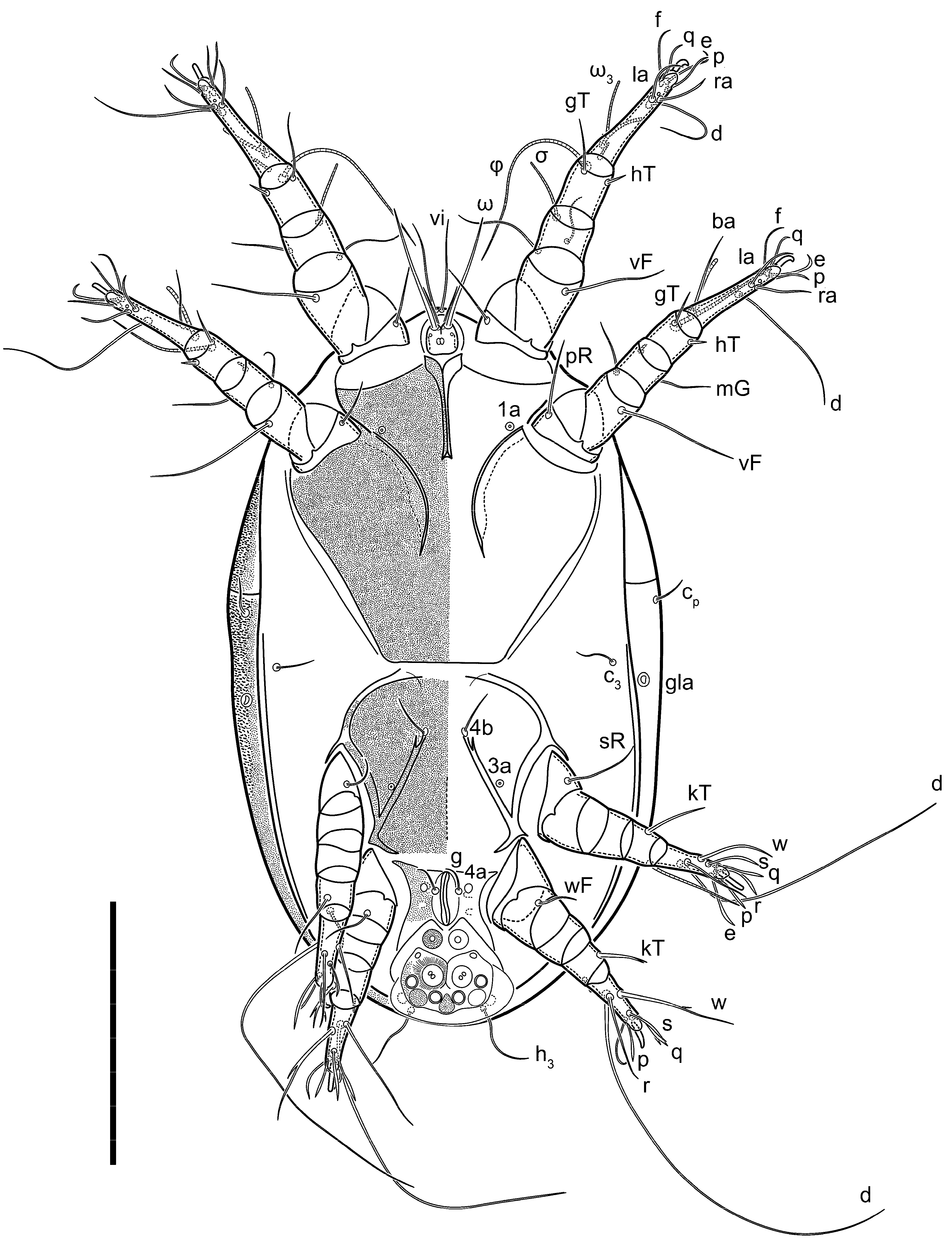
Female (
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5). Idiosoma elongate, 600 × 250 (holotype), 400–620 × 120–230 (paratype,
n = 4), 2.4 (2.6–3.3,
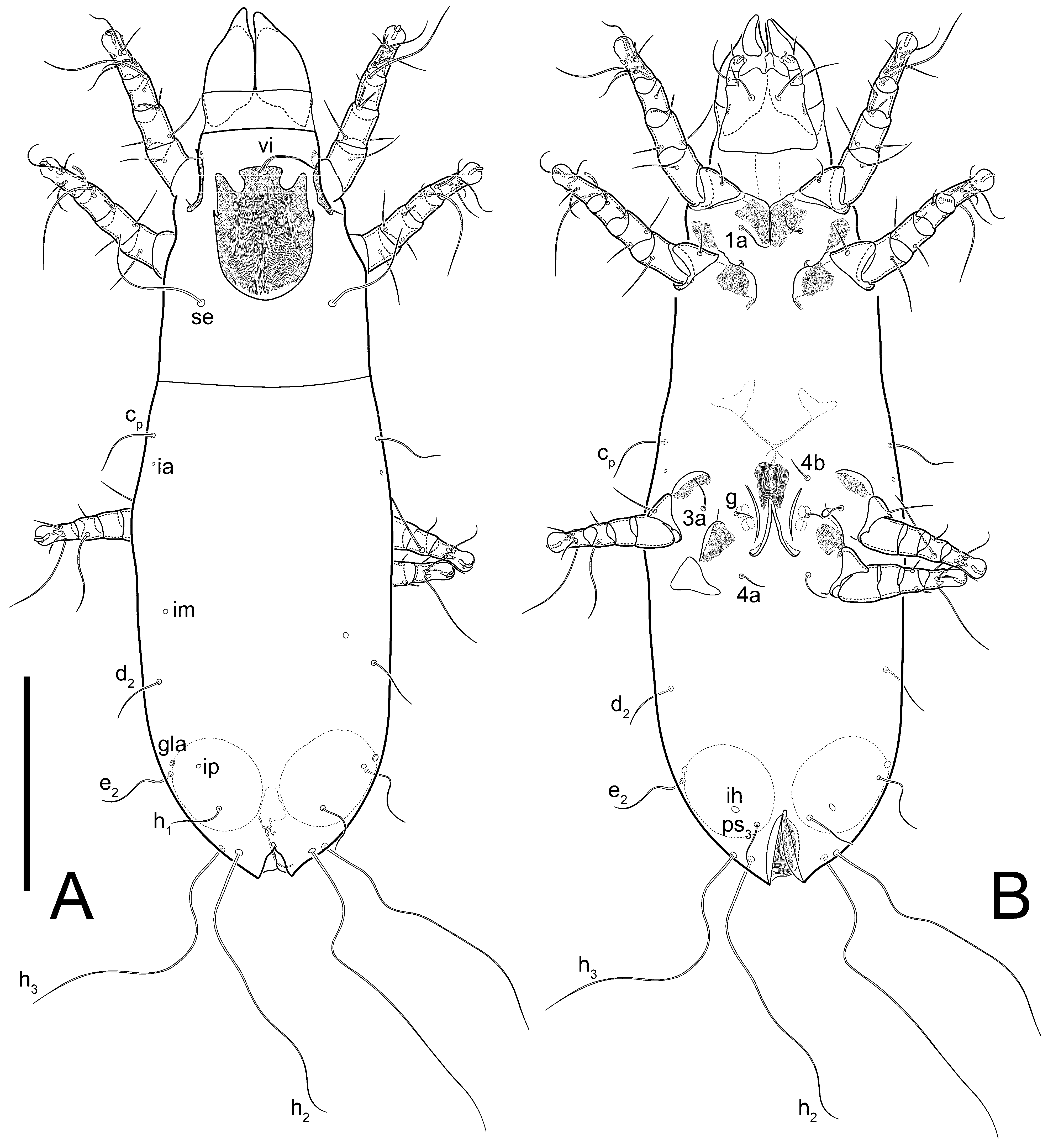
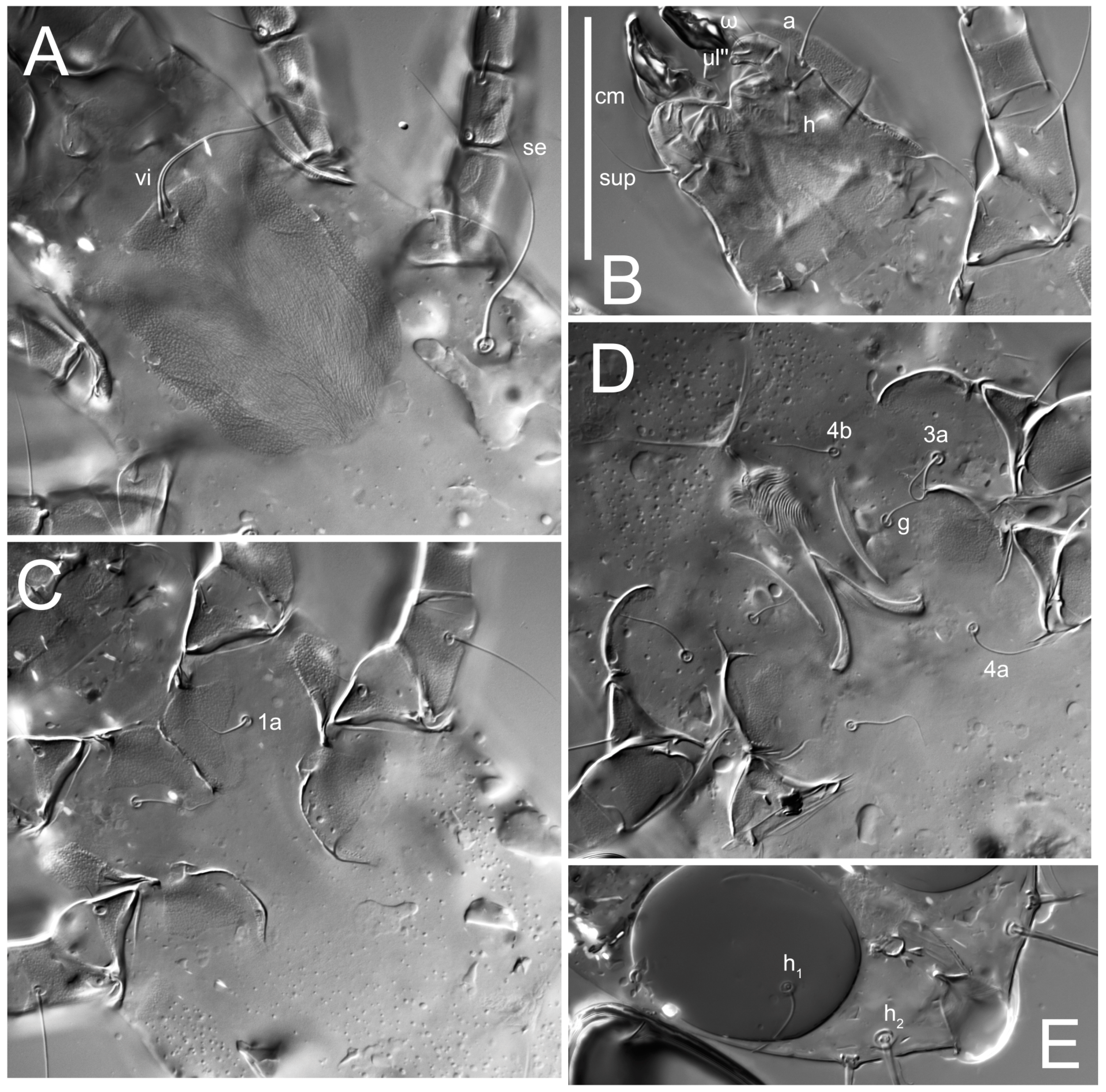
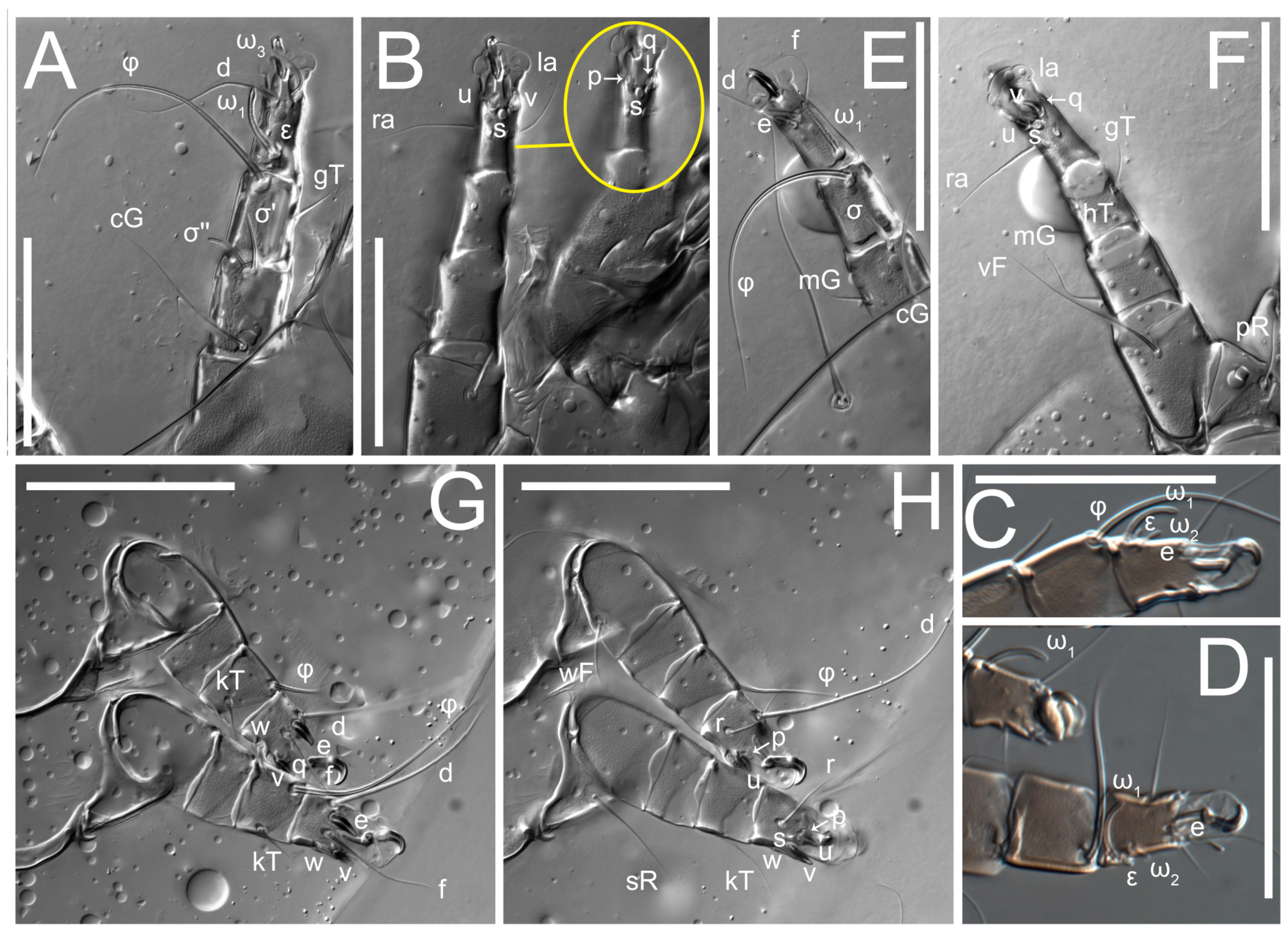
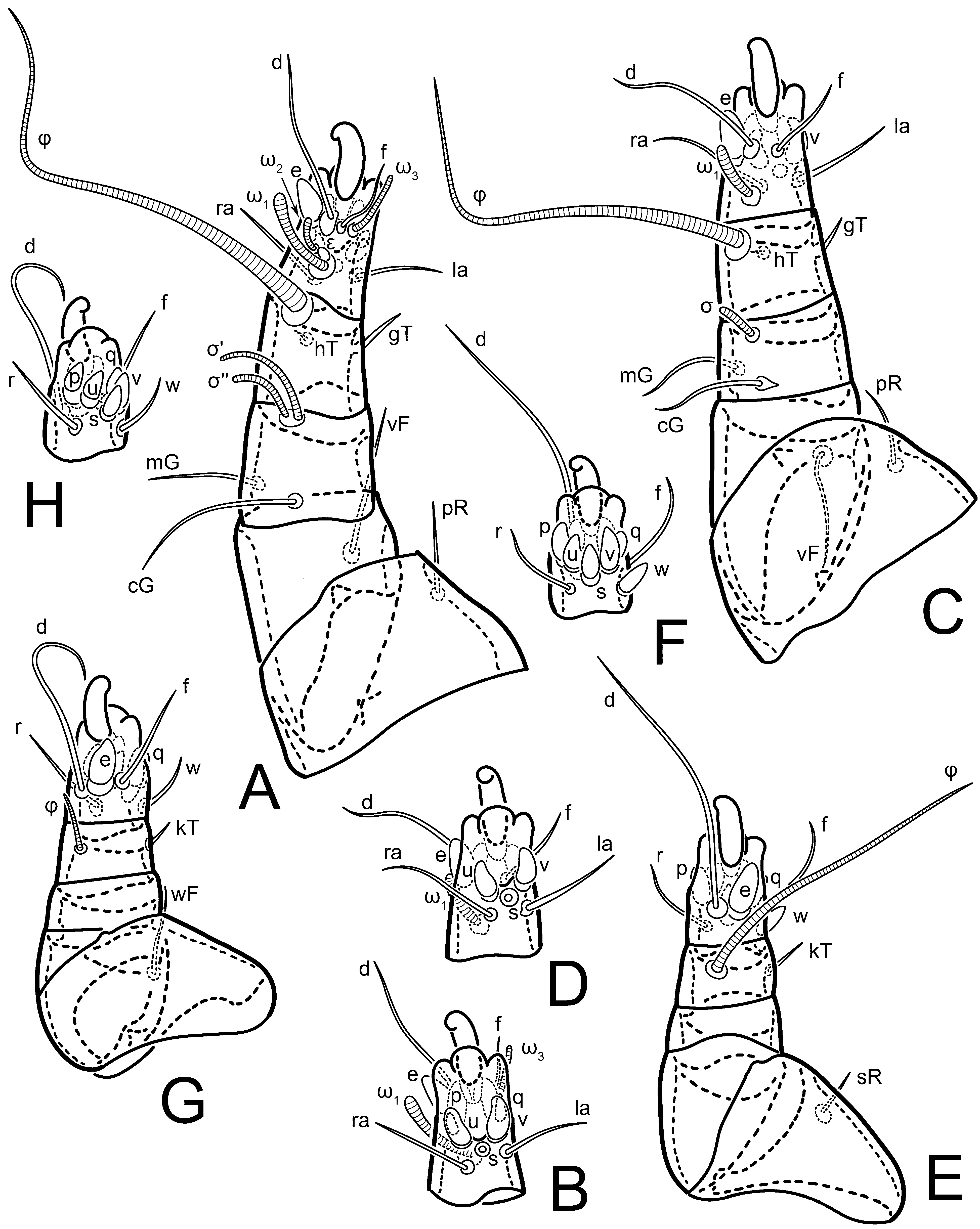
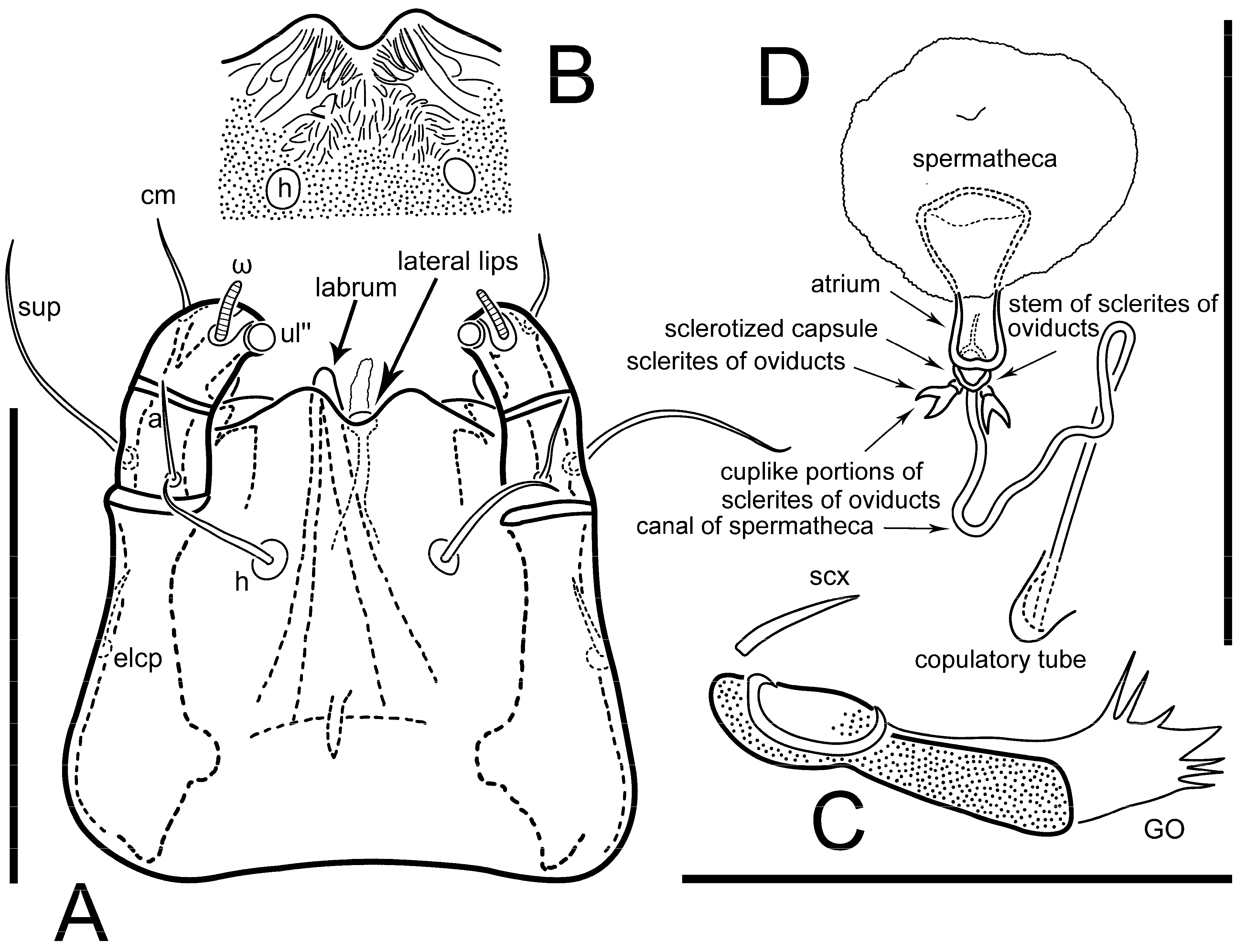
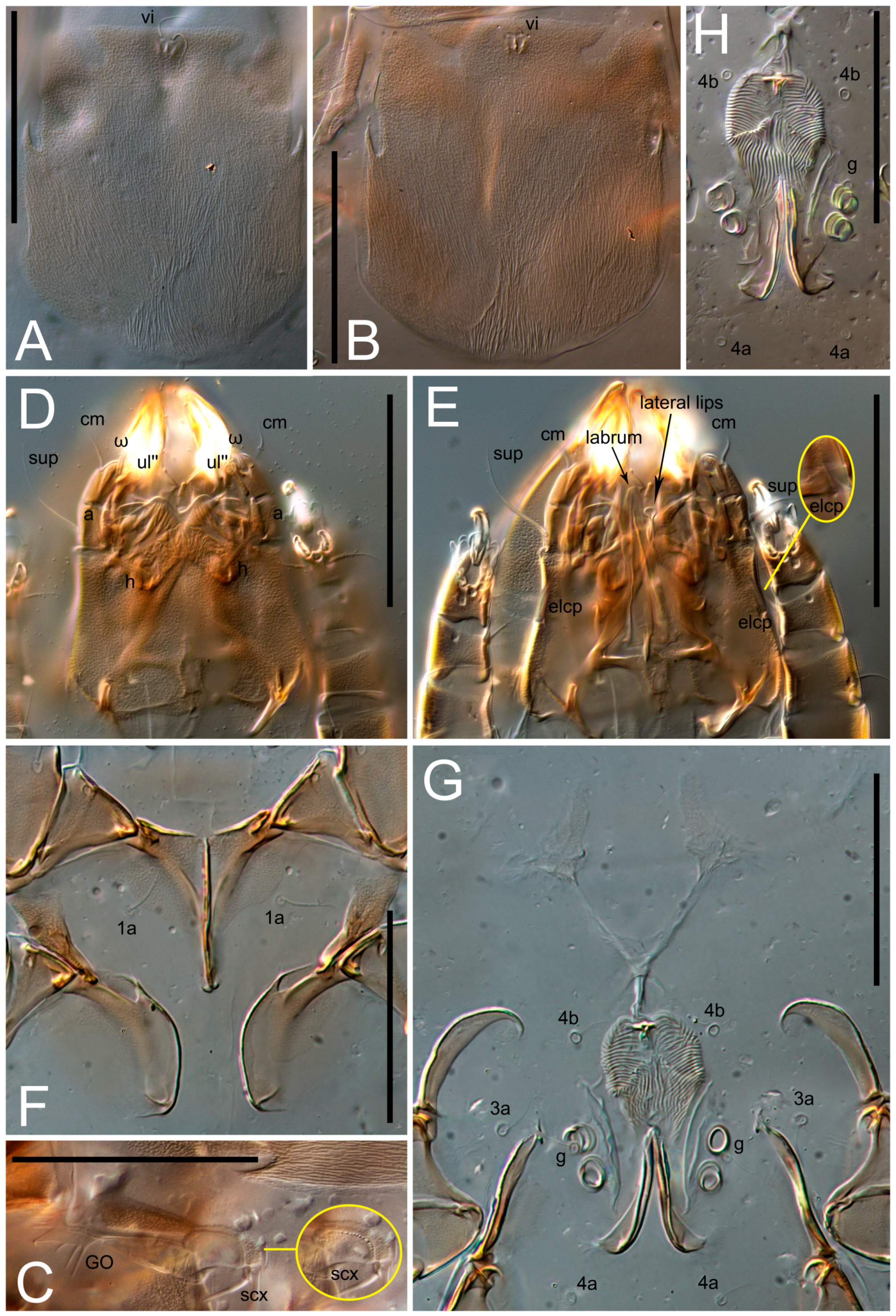
n = 4)-times longer than wide. Idiosomal cuticle smooth. Subcapitular setae (
h) long, widened basally; palp tibial setae (
a), lateral dorsal palp tibial setae (
sup), dorsal palp tarsal seta (
cm) filiform; supracoxal seta
elcp absent; terminal palp tarsal solenidion ω short; external part of eupathidium
ul’’ dome-shaped; terminal eupathidium
ul’ not observed. Prodorsal sclerite 105 (70–94,
n = 4) long, 96 (57–82,
n = 4) wide, 1.1 (1.1–1.2,
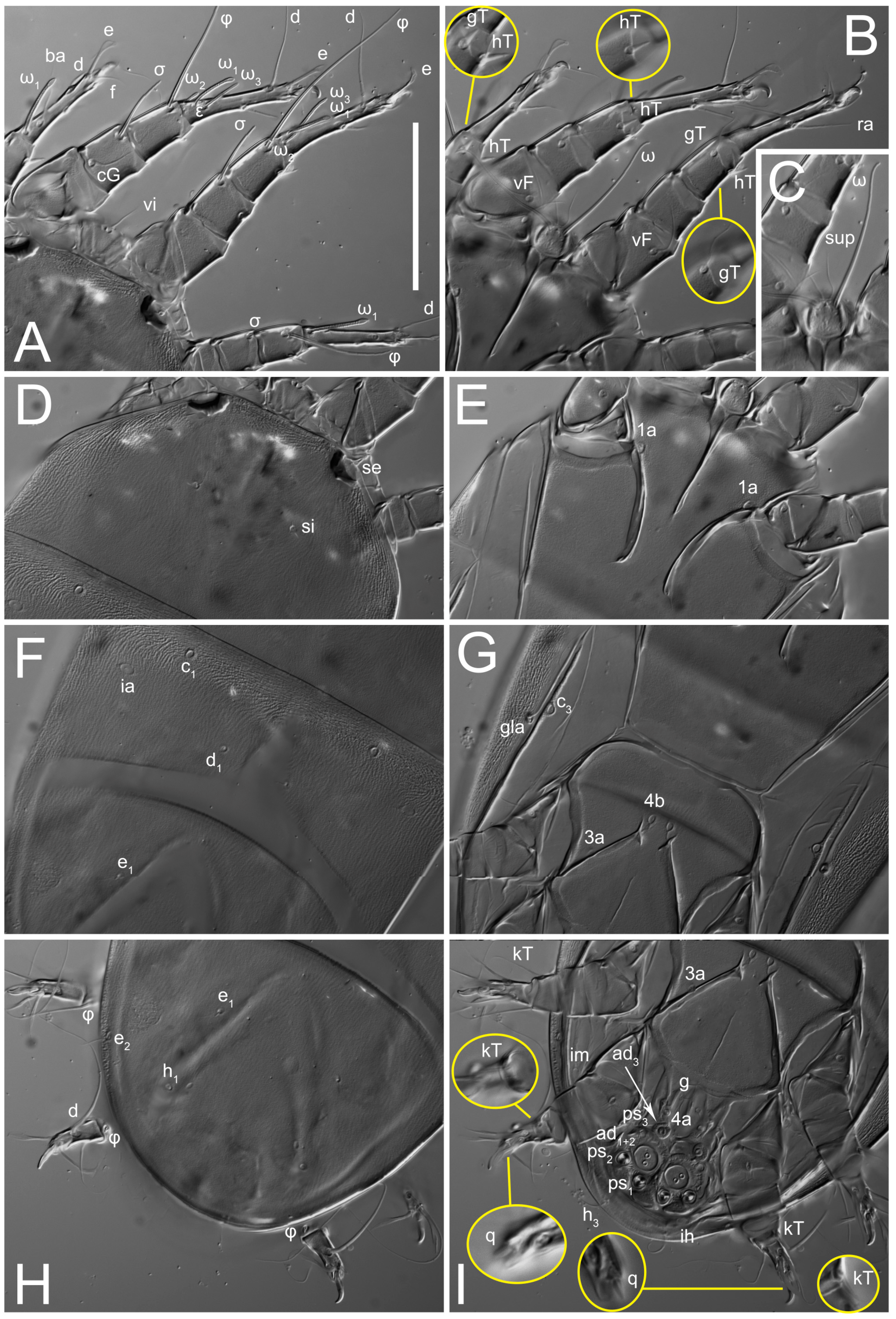
n = 4)-times longer than wide, both smoothly punctated and very finely longitudinally striated, except in anterior 1/5 (smoothly punctate) and posterior 1/4 (ornamented with distinct pattern of broken striae arranged in a triangle). Prodorsal sclerite with setae
vi situated at anterior part of shield (bases touching but separated from each other), rounded anterolateral incisions, and elongate midlateral incisions (corresponding to insertion points of setae
ve).
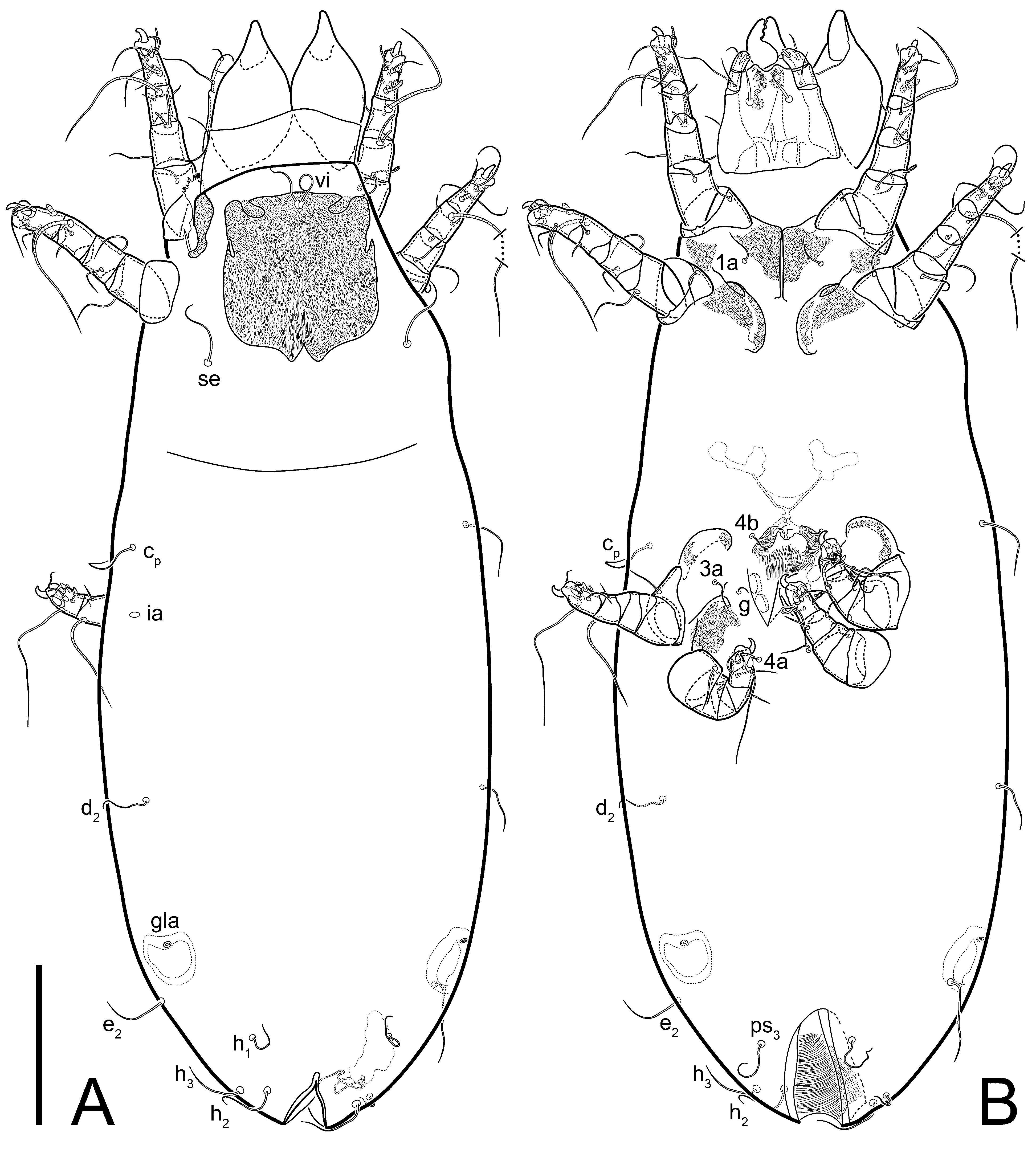
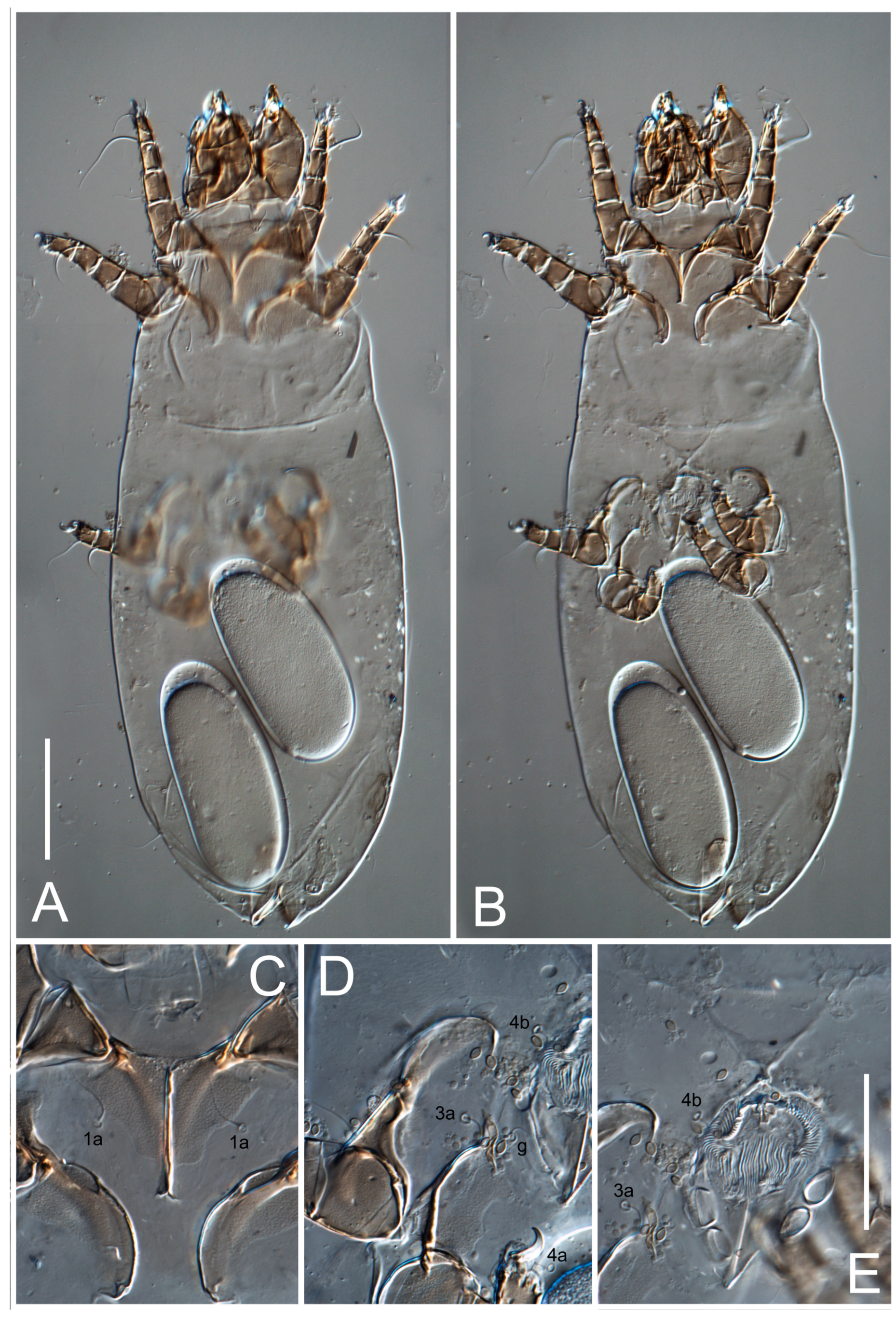
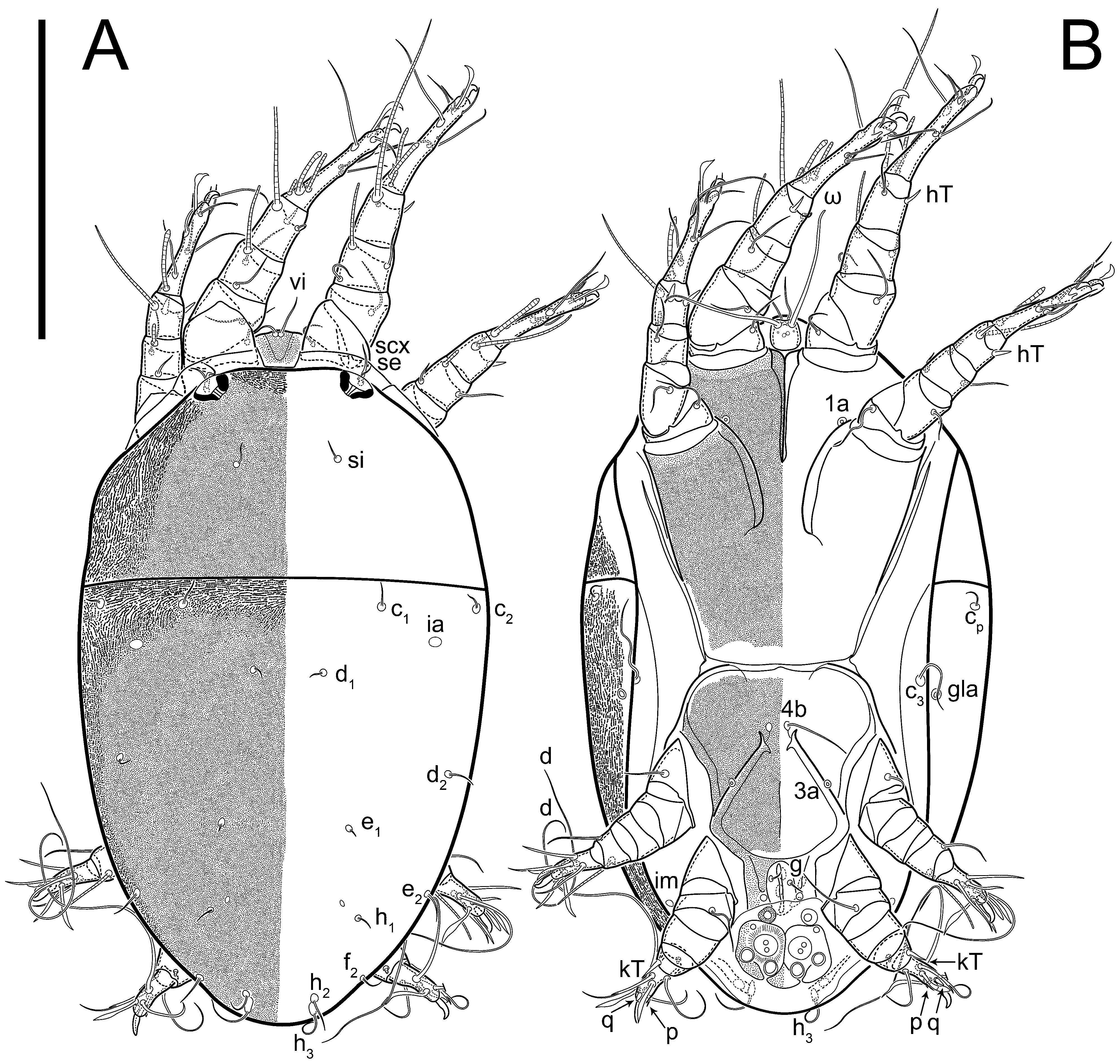
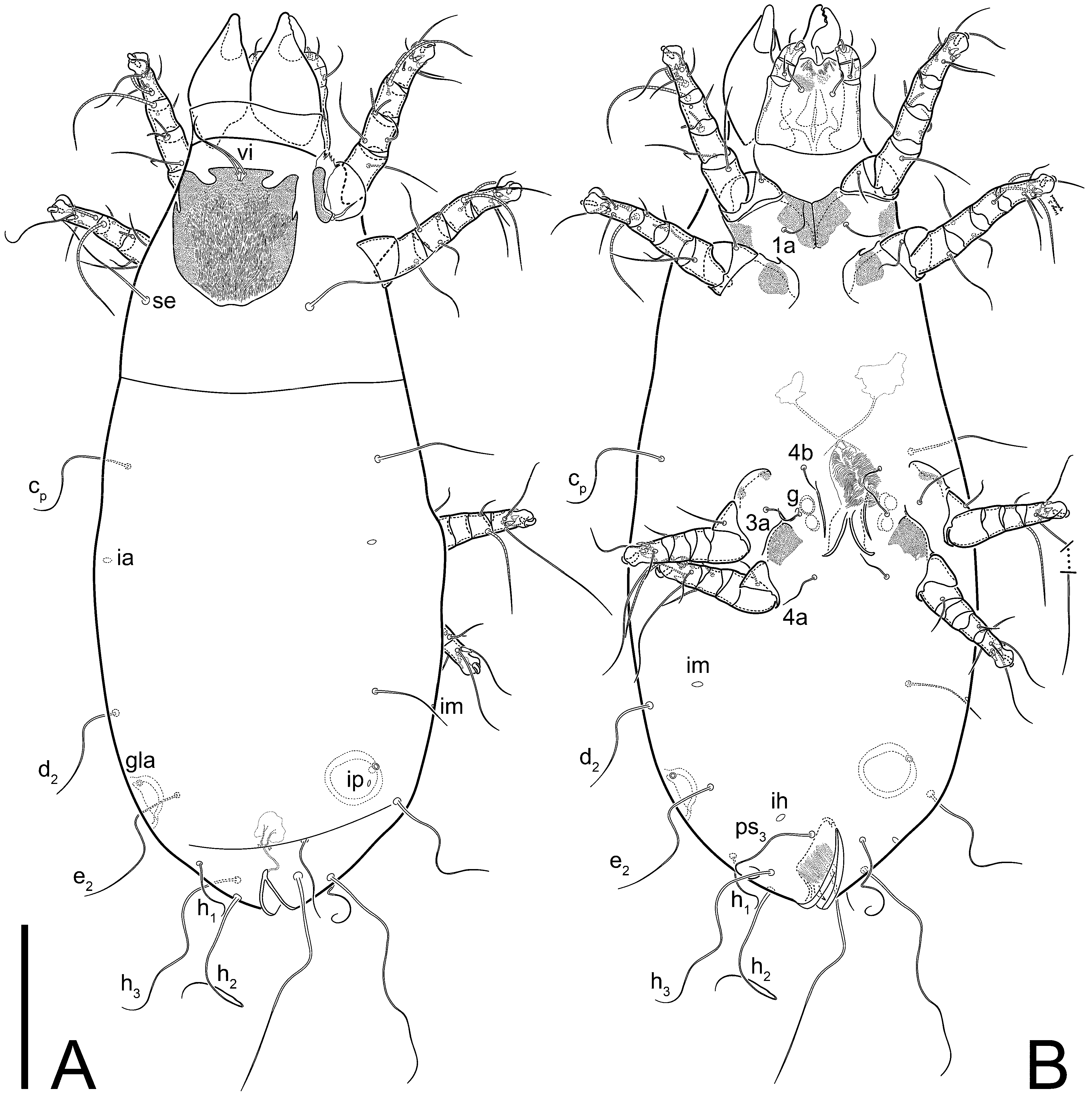
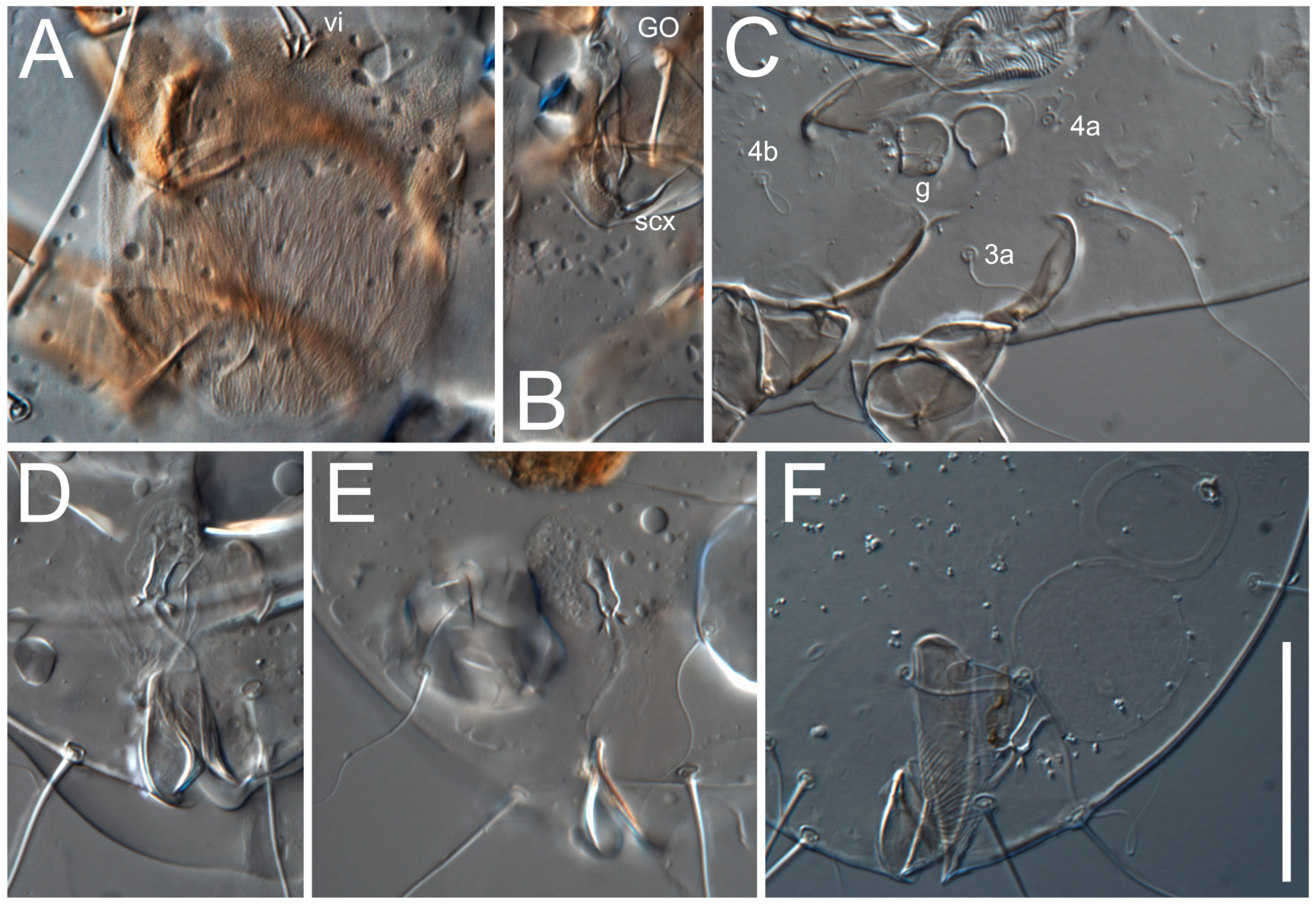
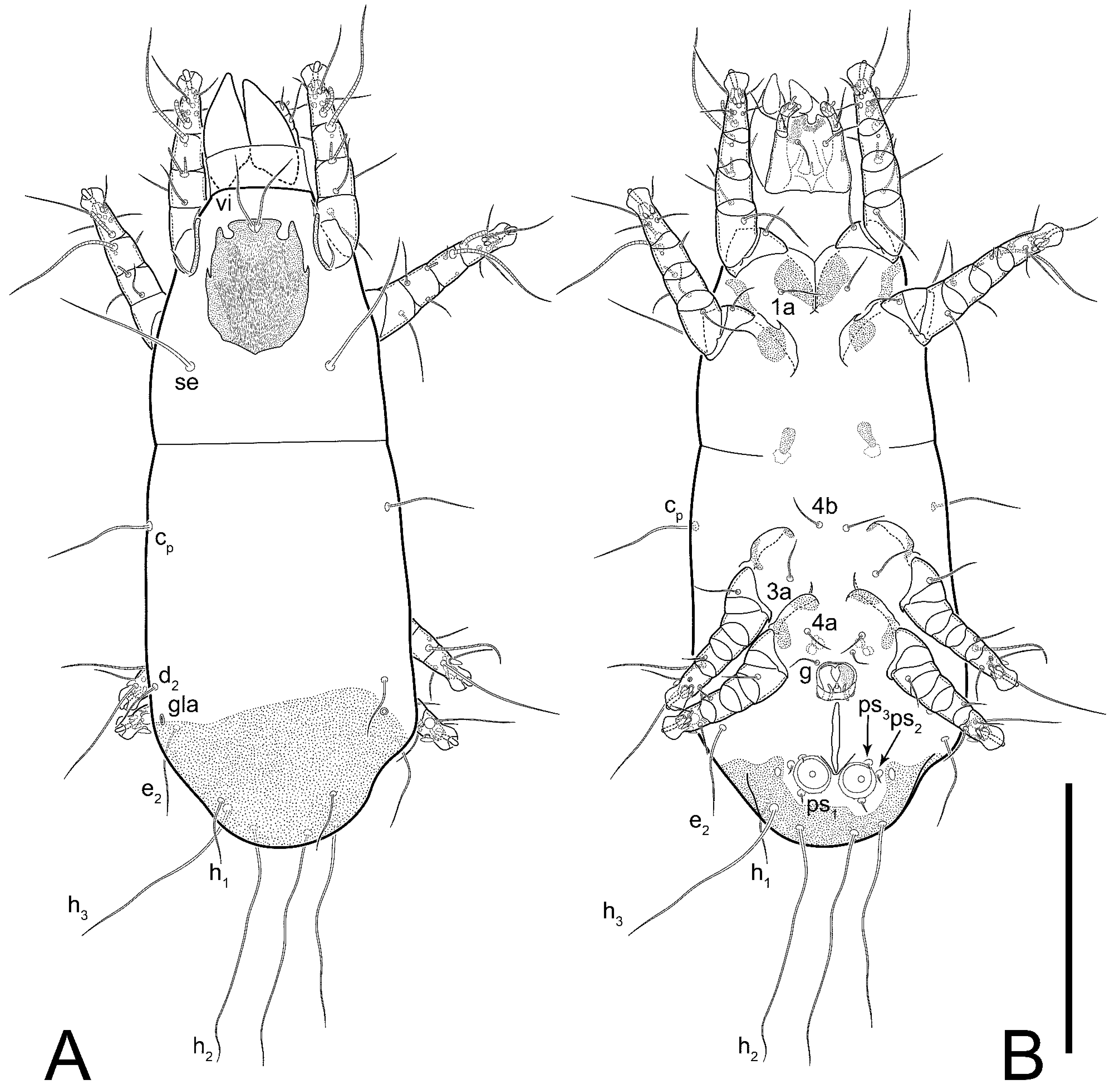
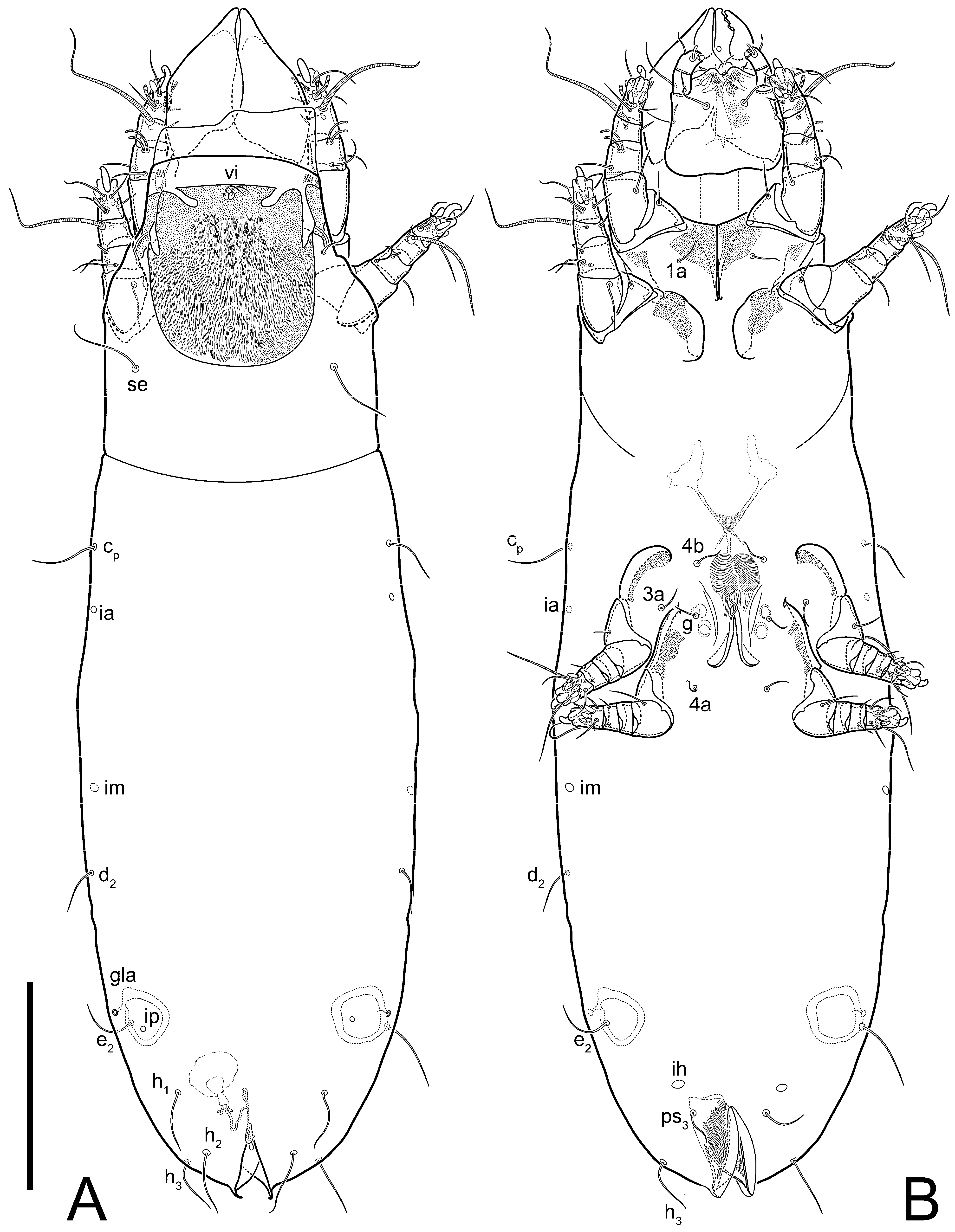
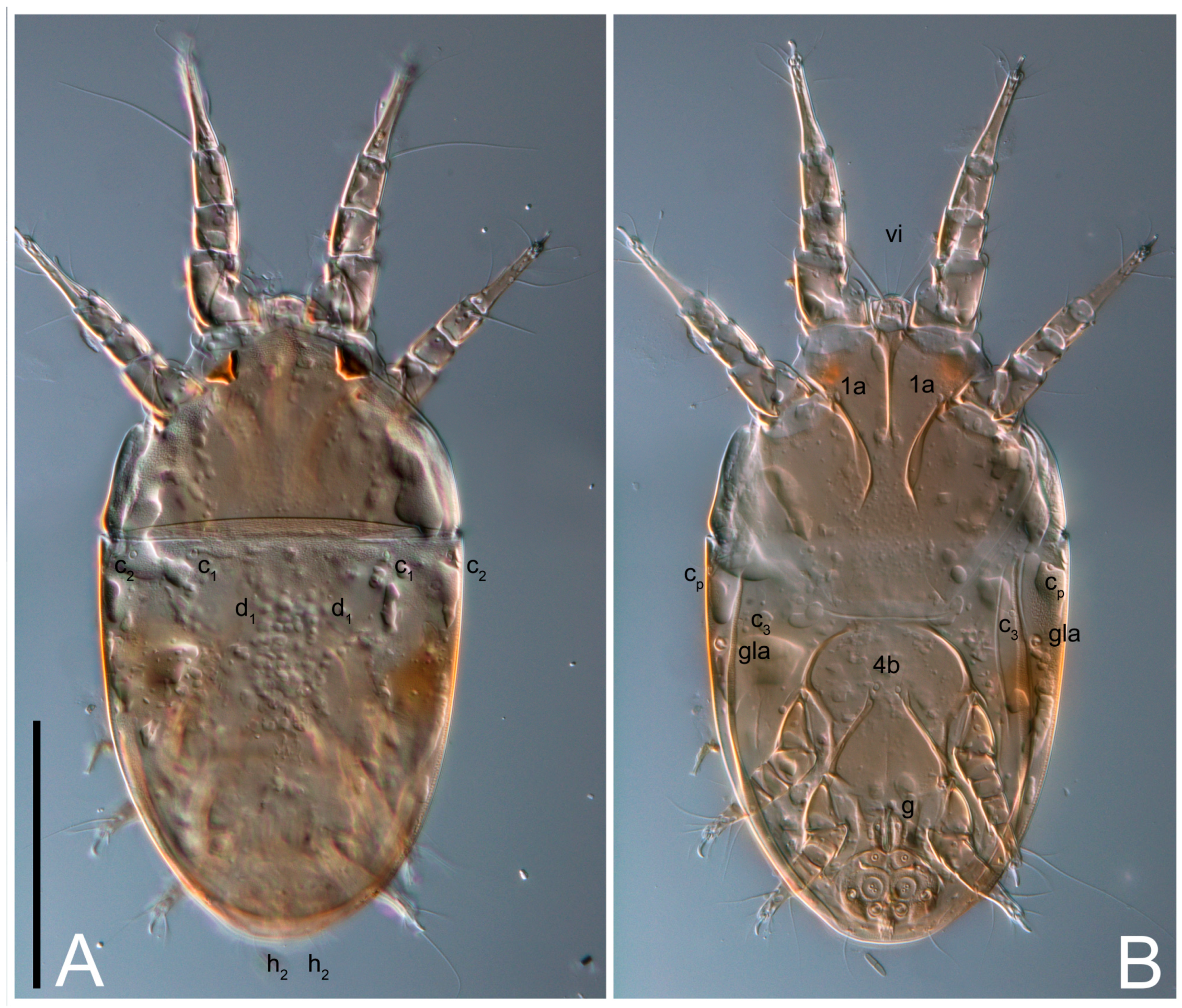
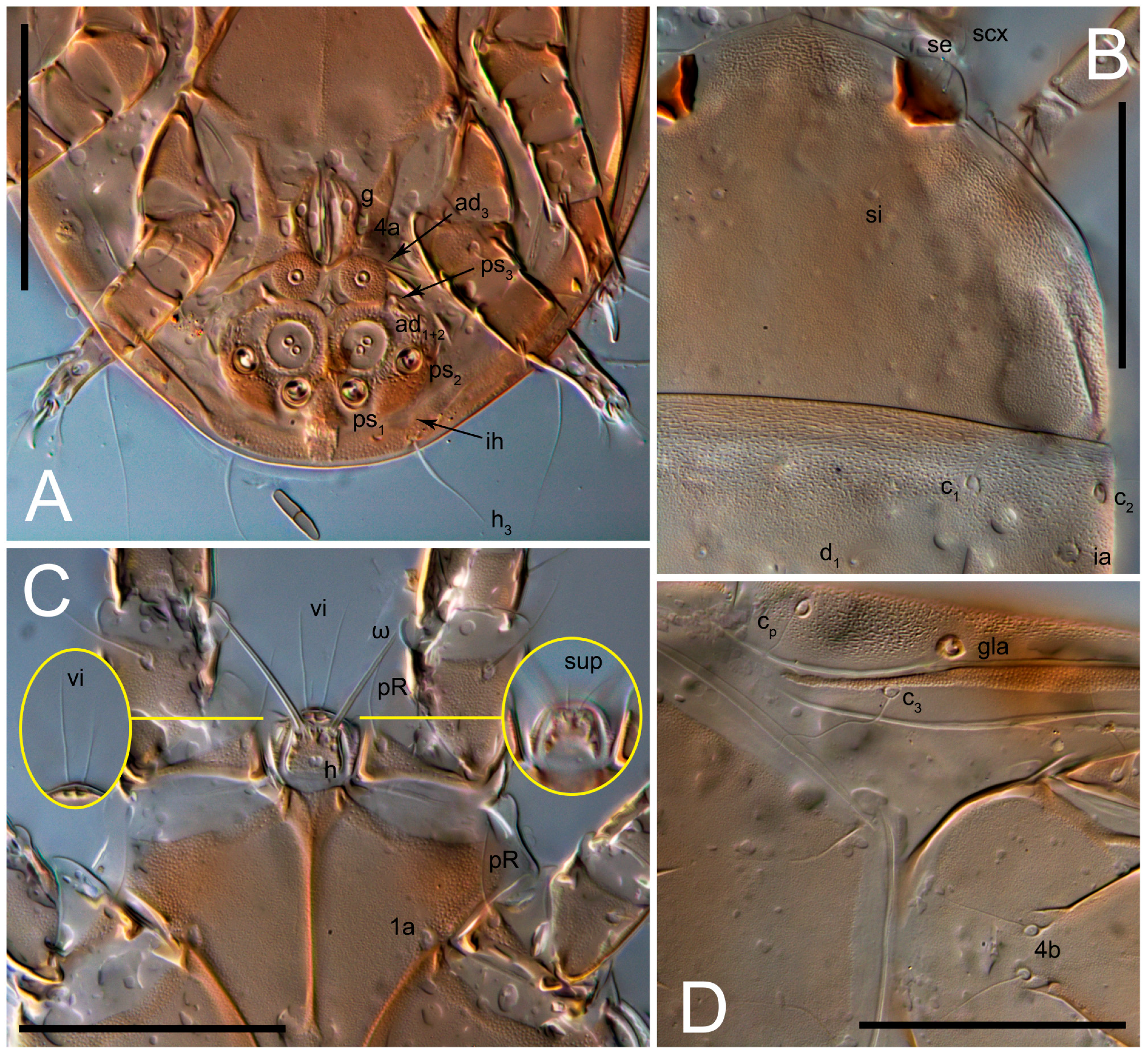
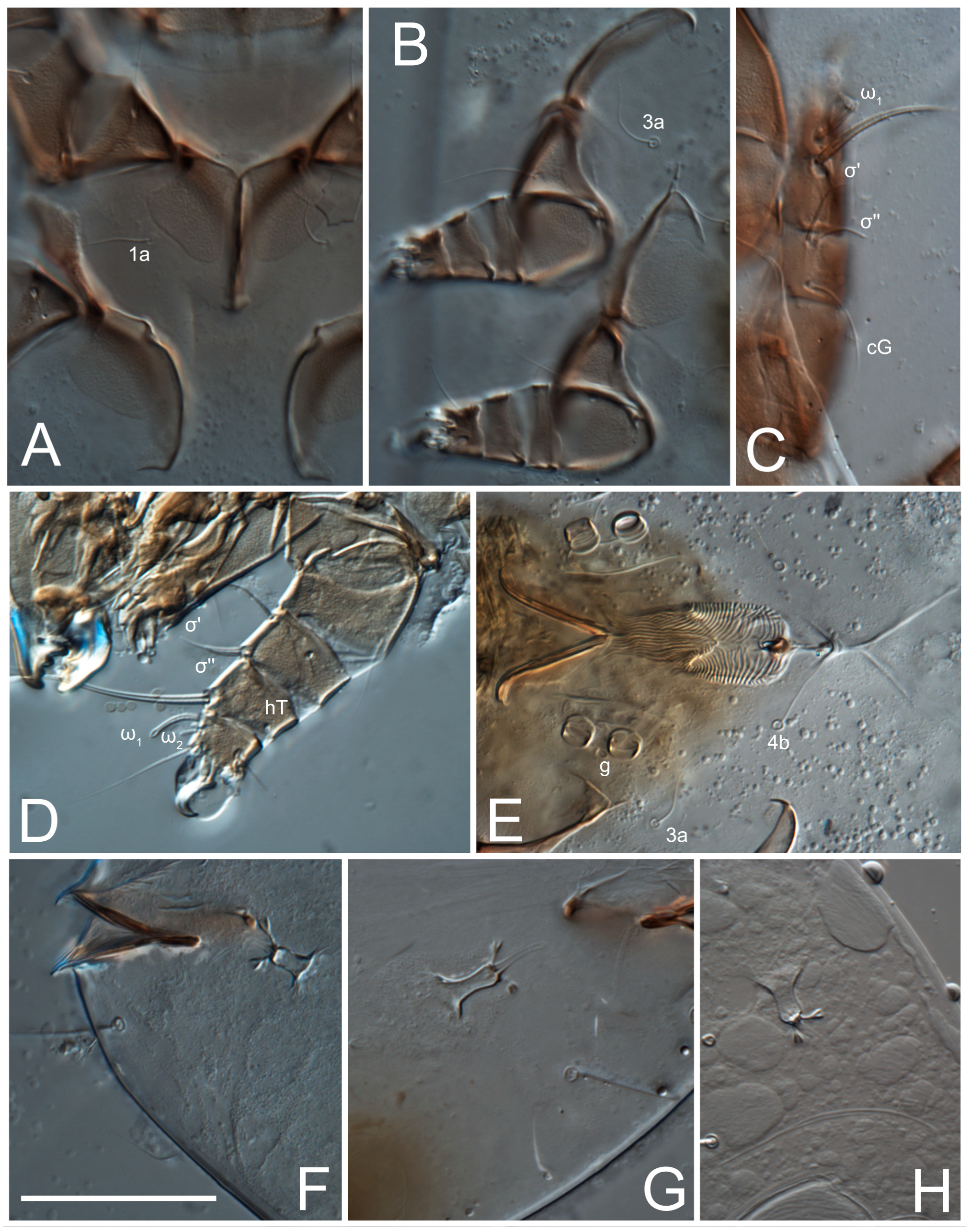
Grandjean’s organ (GO) expanded into 13 membranous finger-like extensions. Supracoxal seta (scx) smooth, sword-shaped, widened, and flattened, tapering at tip. Idiosomal setae (vi, se, cp, d2, e2, h1, h2, h3, ps3) smooth, filiform, and short; opisthosomal gland openings slightly anteriad of setal bases e2. One pair of fundamental cupules (ia) present (other cupules not observed).
Ventral idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g). Shape of coxal sclerites as shown in
Figure 1B,
Figure 3C,D, and
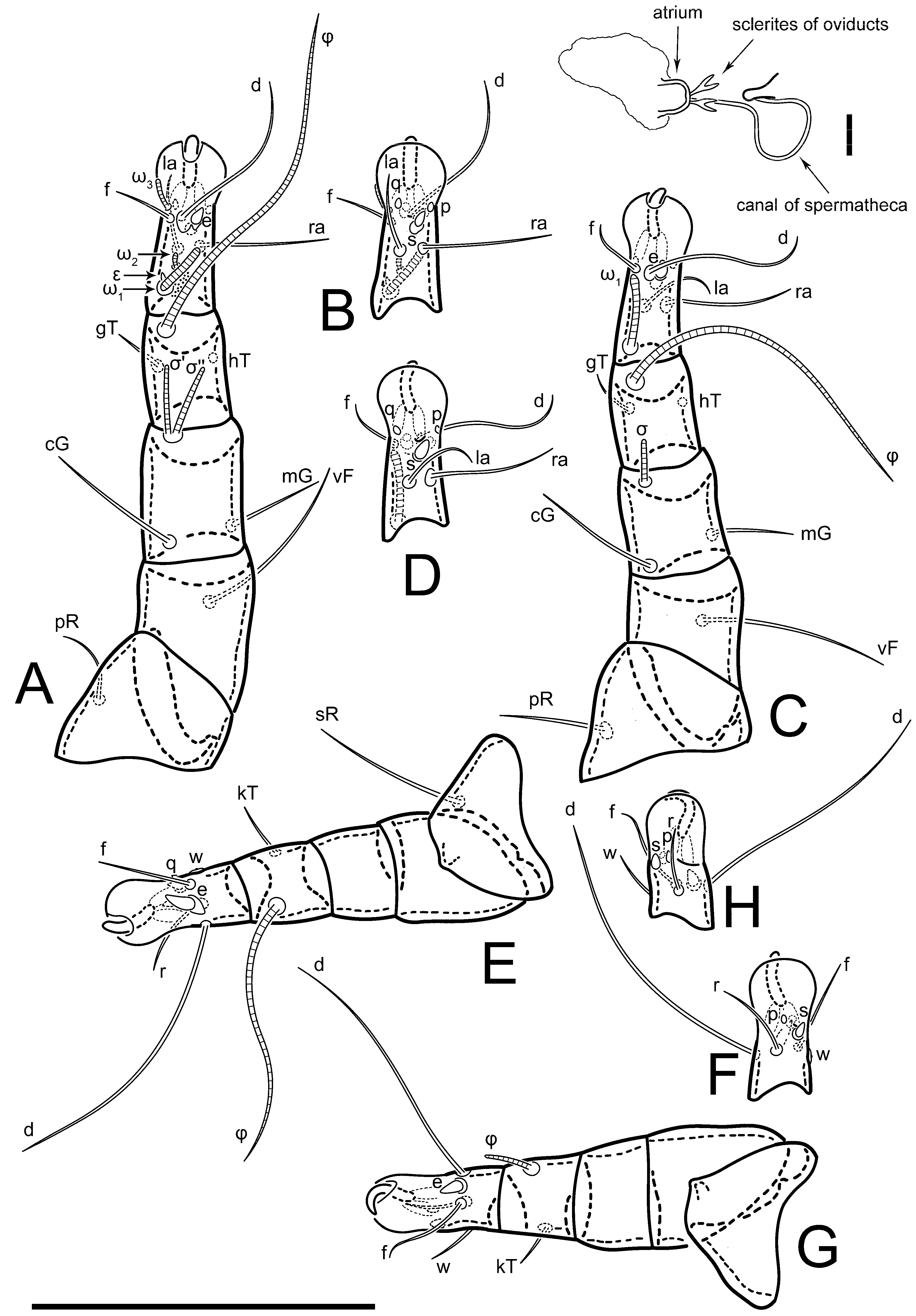
Figure 5E,F. Ovipore situated between coxal fields III and IV; genital valves of ovipore shaped as an inverted Y; epigynal and medial apodemes well-developed. Genital papillae medium-sized, their diameters approximately 5–6-times shorter than the length of genital setae. Anal opening on posterior margin of idiosoma, mostly ventral, with substantial portion situated terminally and dorsally. Copulatory tube weakly developed. Canal of spermatheca long, slender, and uniform in diameter (not widened at entrance to spermatheca), forming a small, well-sclerotized, cup-shaped atrium. Spermatheca simple, with small sclerotized base and small, paired, Y-shaped sclerites of oviducts.
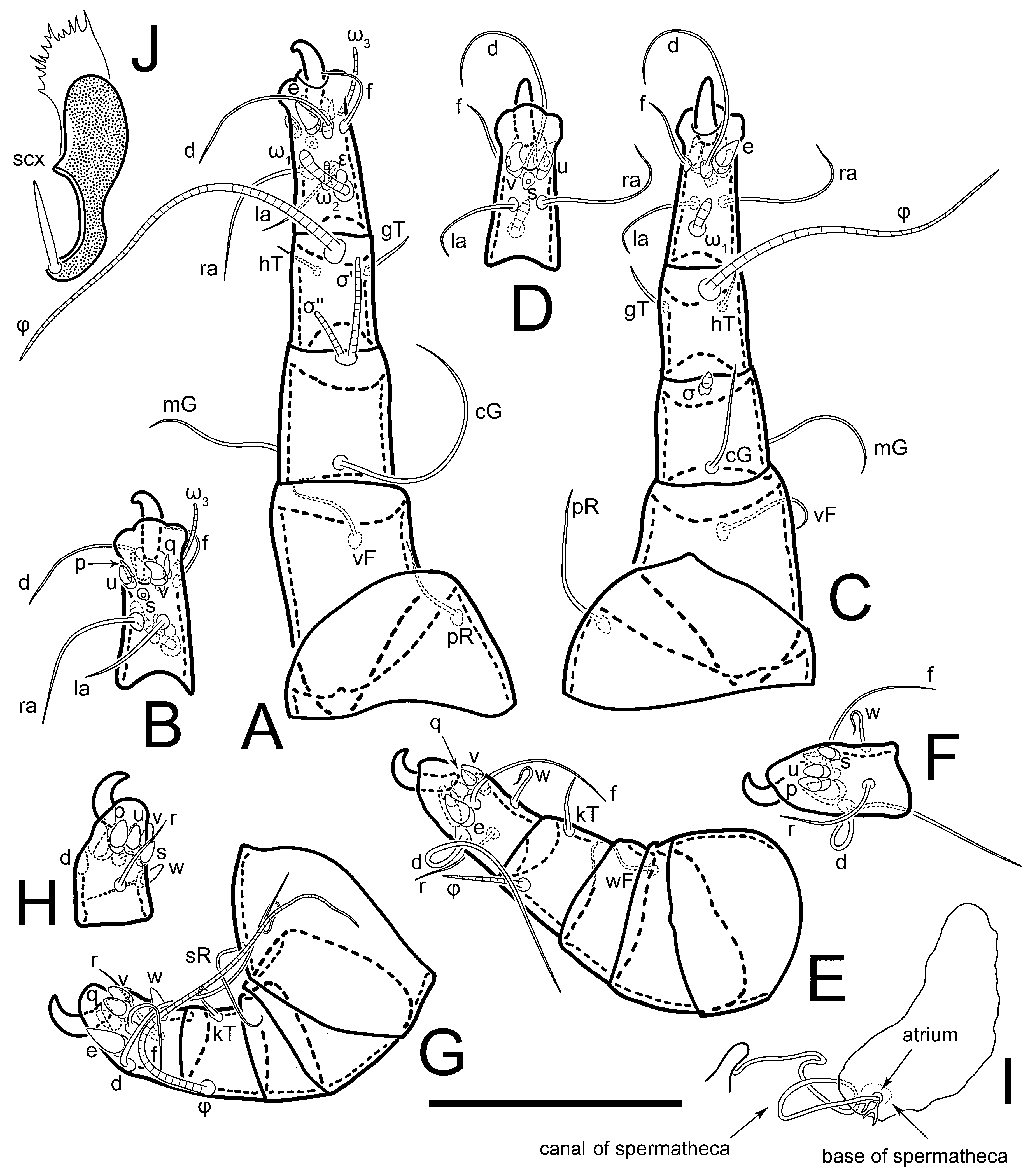
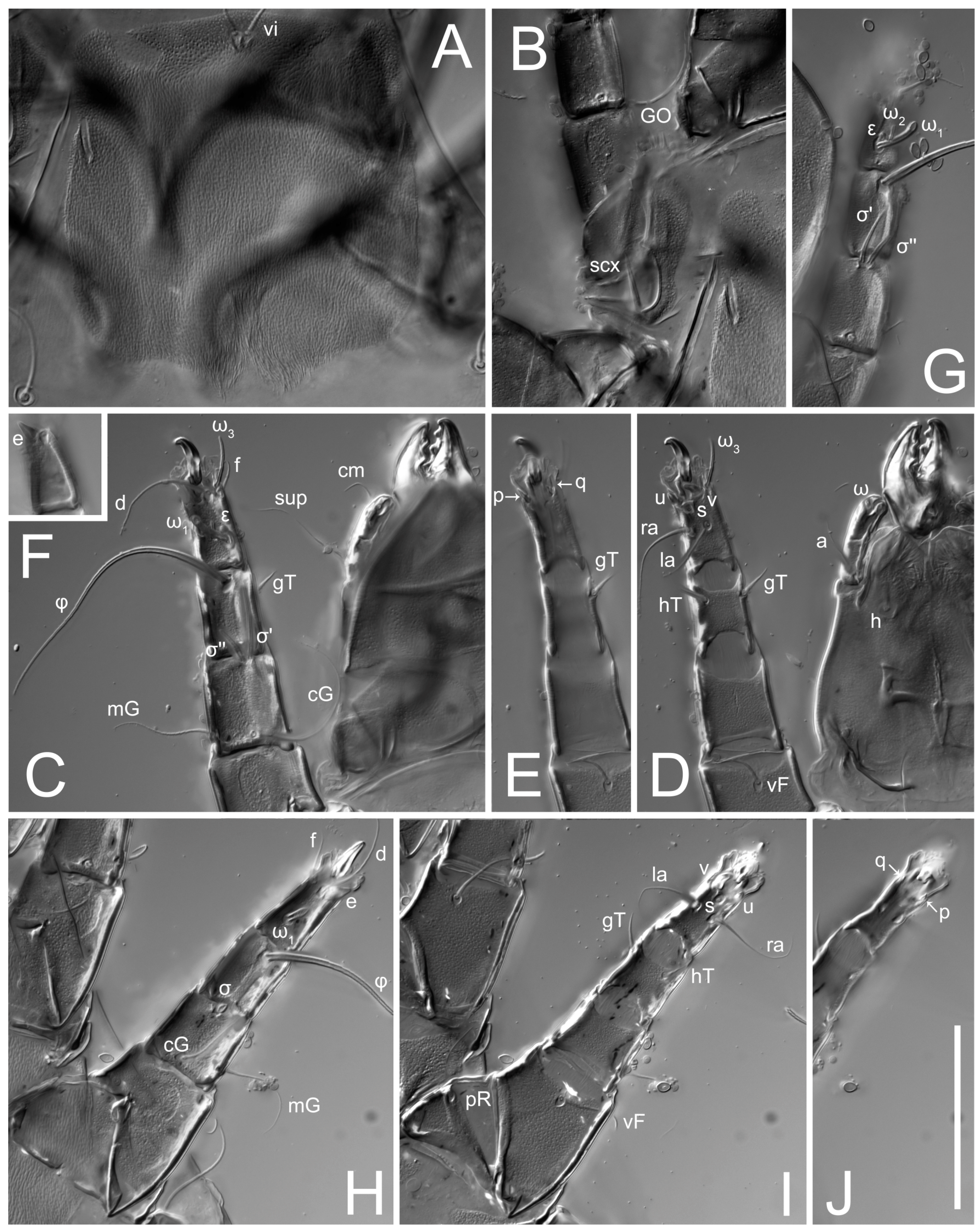
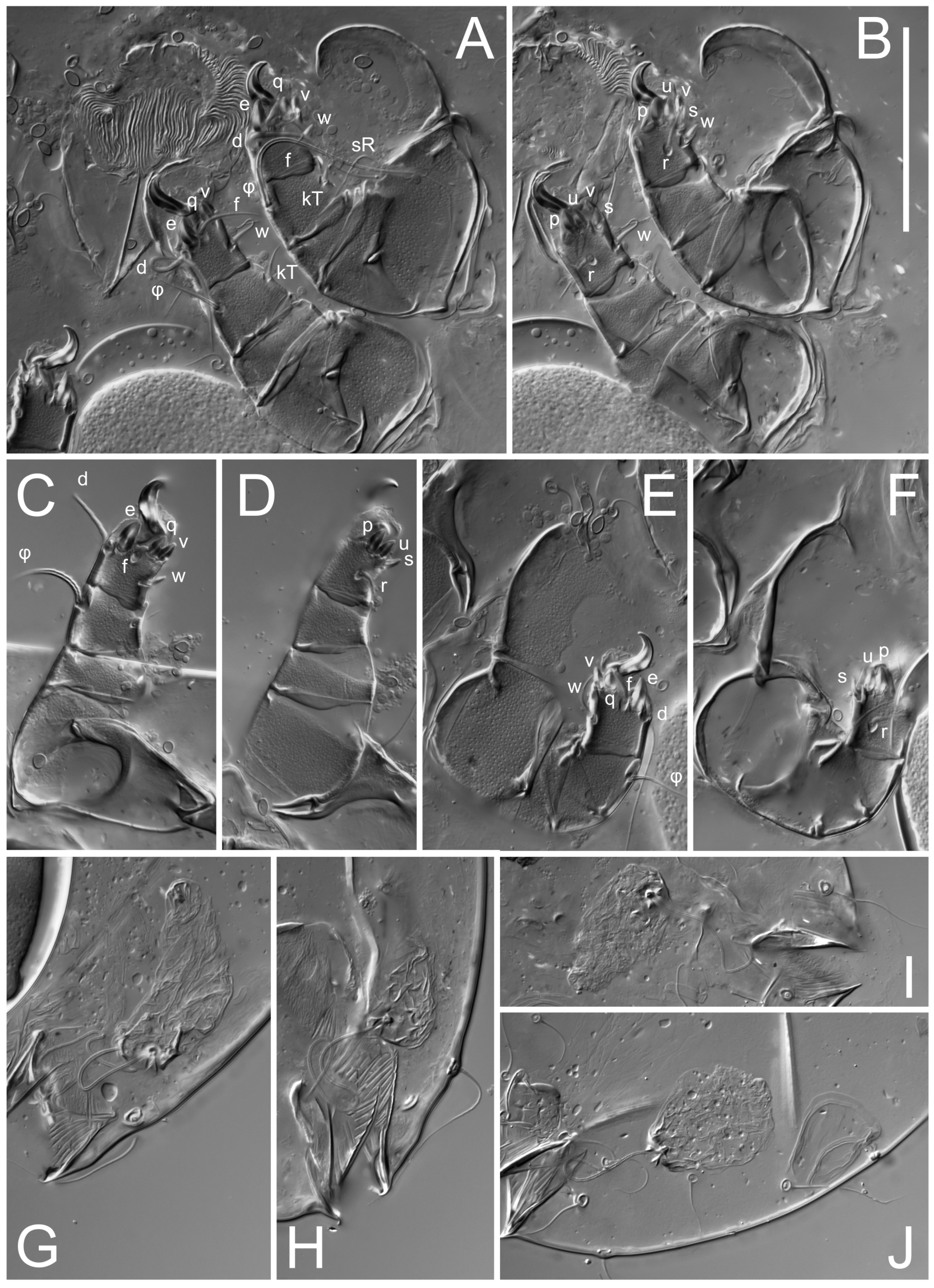
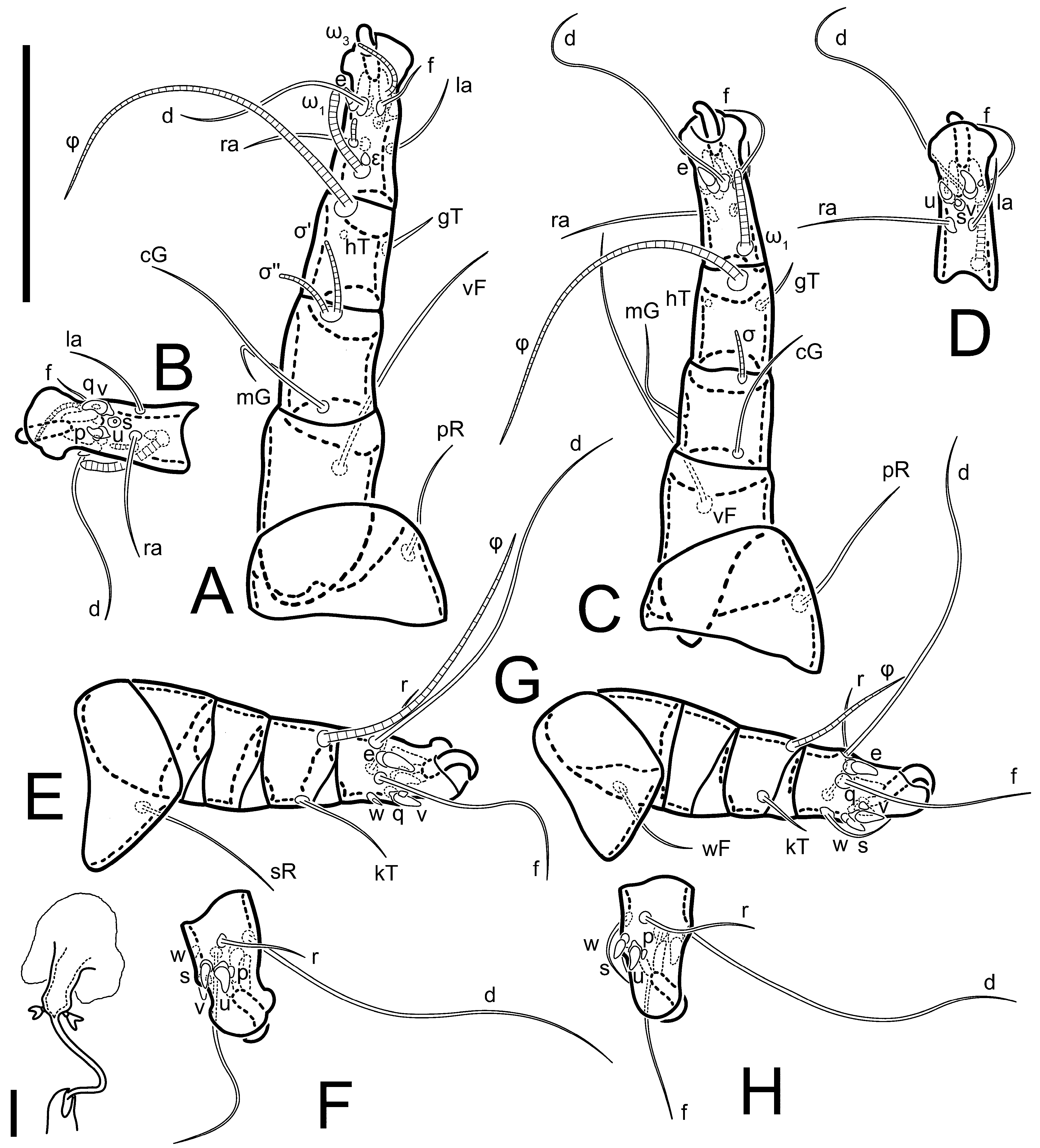
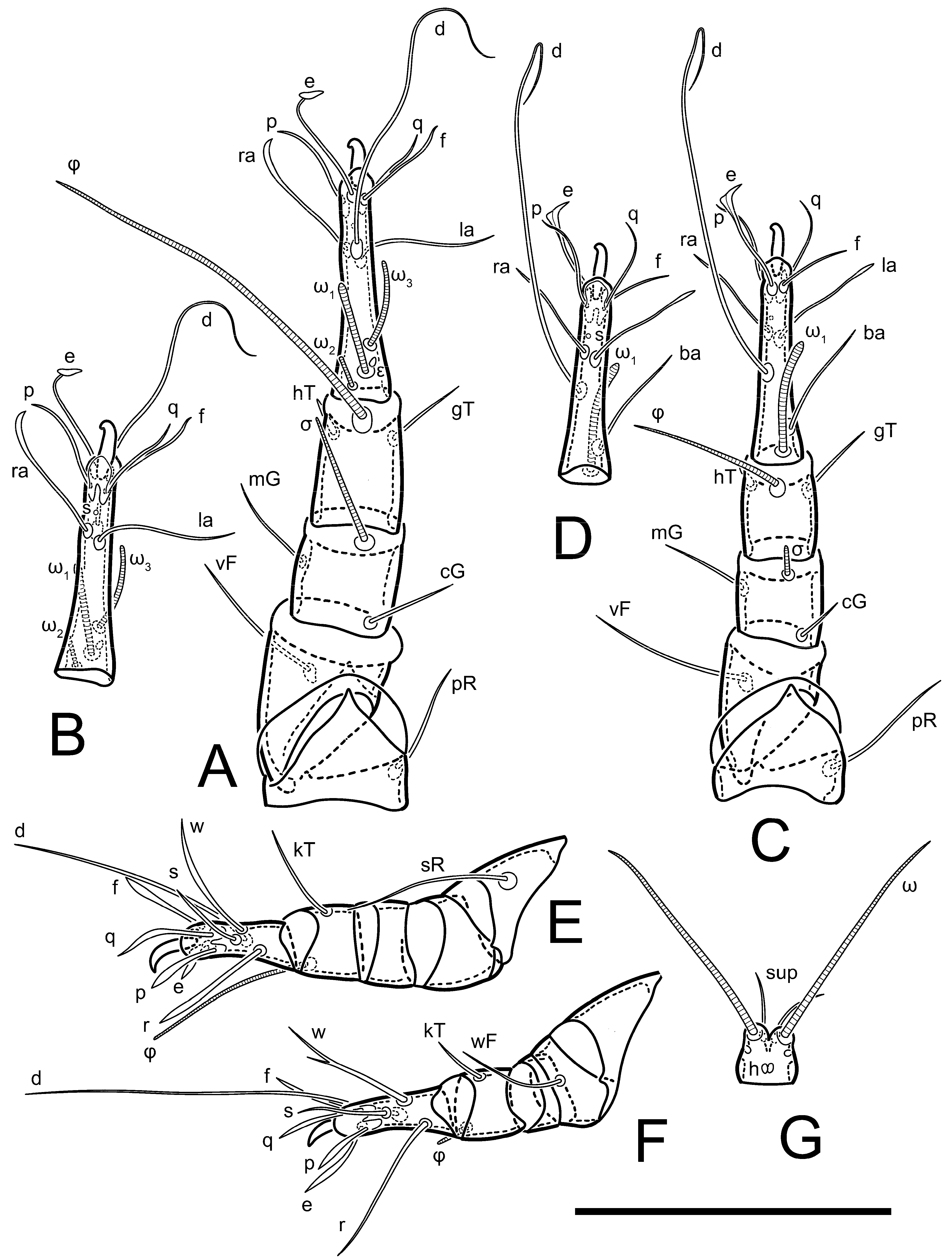
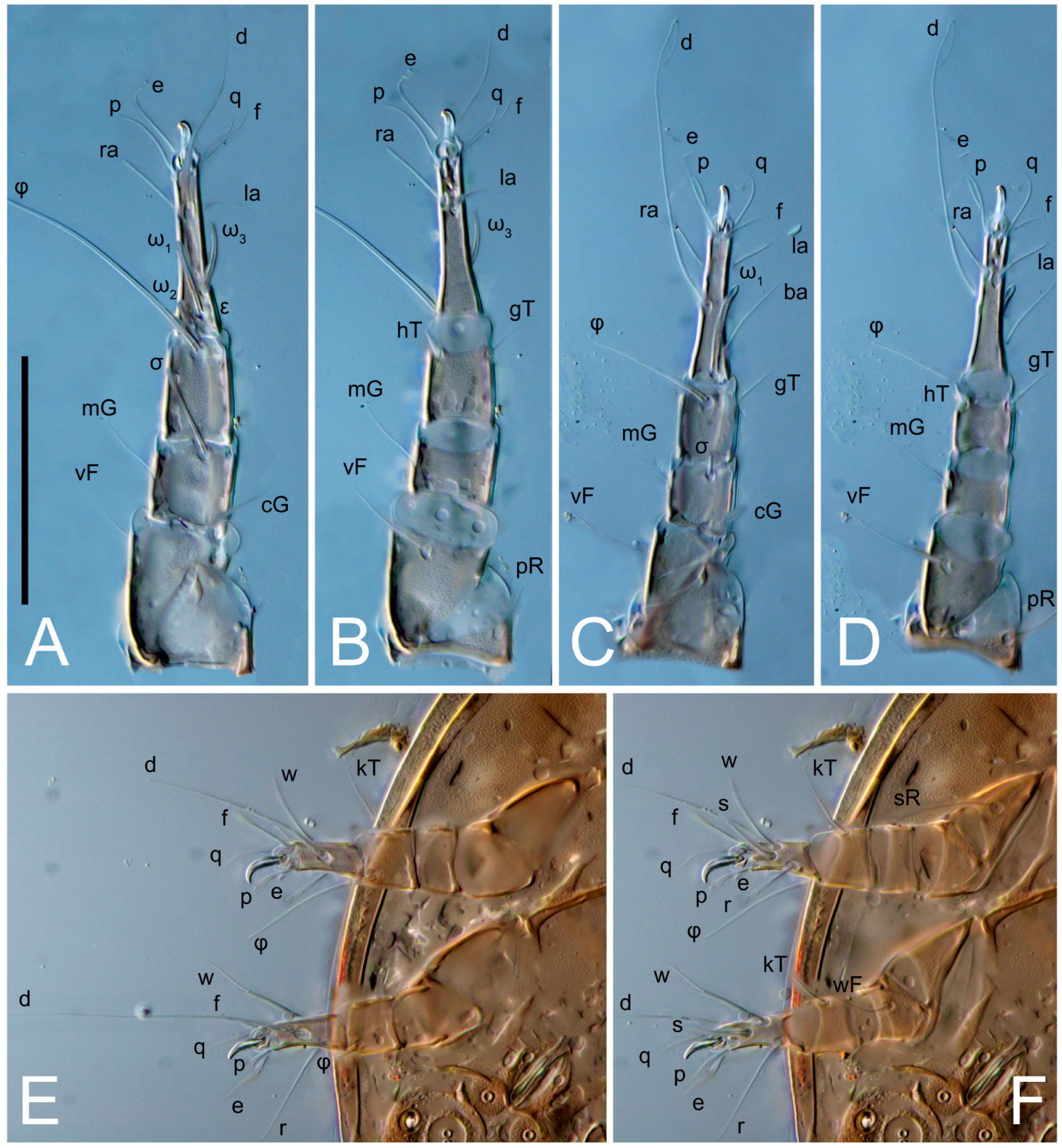
Legs short. Trochanters I–III each with long, filiform seta,
pR I–II,
sR III; trochanter IV without setae. Femoral setation 1-1-0-1; setae
vF I–II and
wF IV long, filiform. Genual setation 2-2-0-0; setae
mG and
cG I–II long, filiform; seta
nG III absent. Tibial setation 2-2-1-1; setae
hT I-II filiform; setae
gT I–II and
kT III–IV elongated, somewhat spiniform. Tarsal setation 10-10-10-10; all pretarsi with hooked empodial claws arising from tarsal apices and short paired condylophores. Tarsus I with 10 setae;
ra,
la,
f, and
d filiform;
e,
u,
v,
p,
q spiniform (
p and
q distinctly shorter than
u and
v);
s flattened, button-shaped; setae
wa absent. Tarsal II setation similar to that of tarsus I, except proral setae (
p,
q) represented by small triangular rudiments (
Figure 4J). Tarsus III with 10 setae;
f,
d,
r filiform;
e,
w,
s,
u,
v,
p,
q spiniform. Tarsus IV similar to tarsus III, except
w filiform. Solenidion ω
1 on tarsus I cylindrical, with clavate apex, slightly curved; solenidion ω
1 on tarsus II simple, cylindrical, with clavate apex, not bent, shorter than ω
1 on tarsus I. Solenidion ω
2 on tarsus I shorter than ω
1, cylindrical, with rounded apex, slightly widened at tip, situated slightly anterior and external to ω
1. Solenidion ω
3 on tarsus I cylindrical, with rounded apex, subequal to ω
1, longer than ω
2. Famulus (ε) of tarsus I wide, spiniform, with broadly rounded apex, widest at middle. Solenidia φ of tibiae I–III elongate, tapering, well-extended beyond apices of respective tarsi with ambulacra; solenidion φ IV shorter, shorter than tarsus IV (with ambulacra). On genu I, solenidion σ’ elongate, tapering, slightly not reaching bases of φ I, about 1.8-times longer than σ’’; σ’’ I slightly wider than σ’, about half the length of tibia I. On genu II, solenidion σ very short and somewhat conical. Solenidion σ of genu III absent.
Male. Absent.
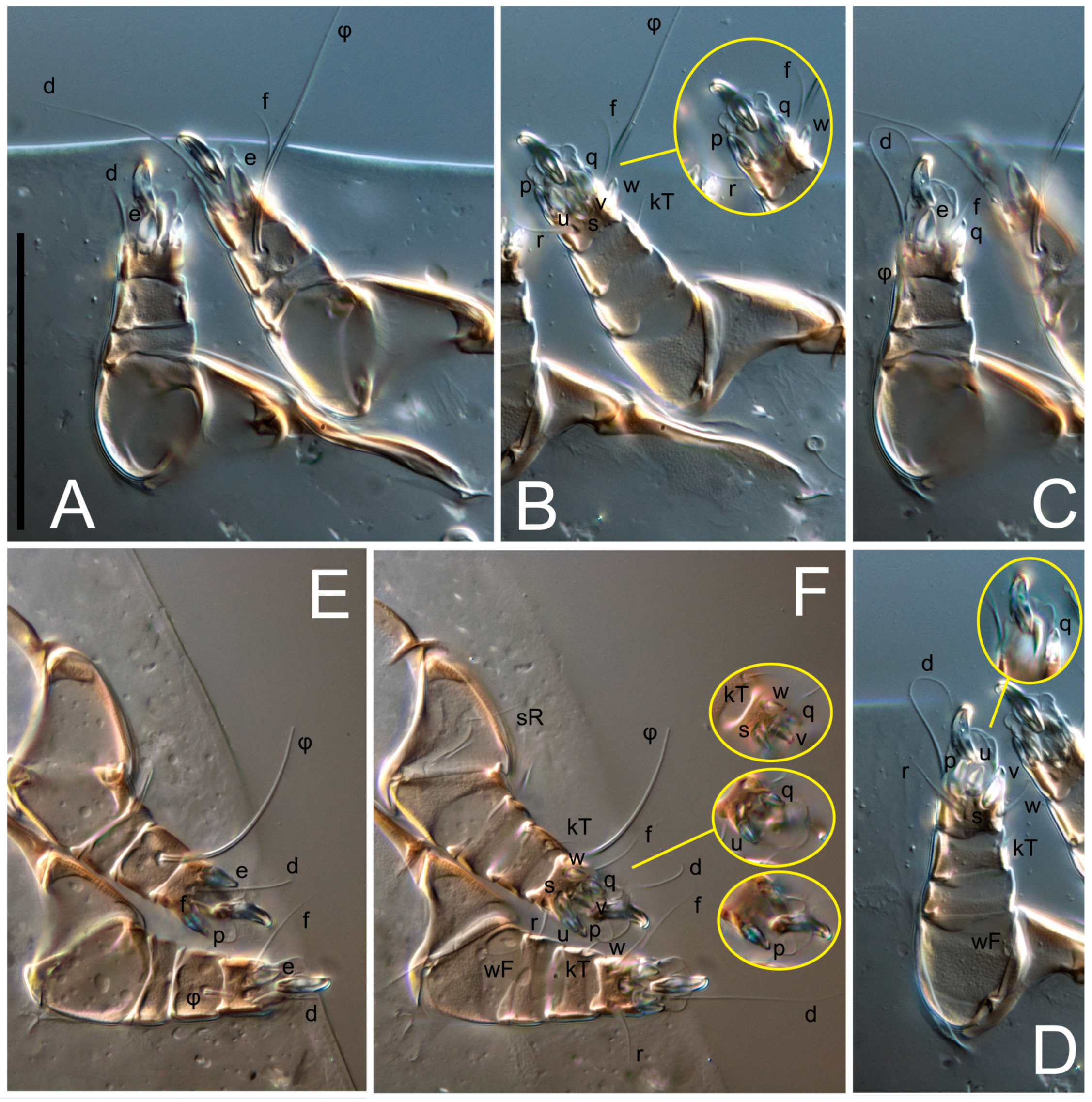
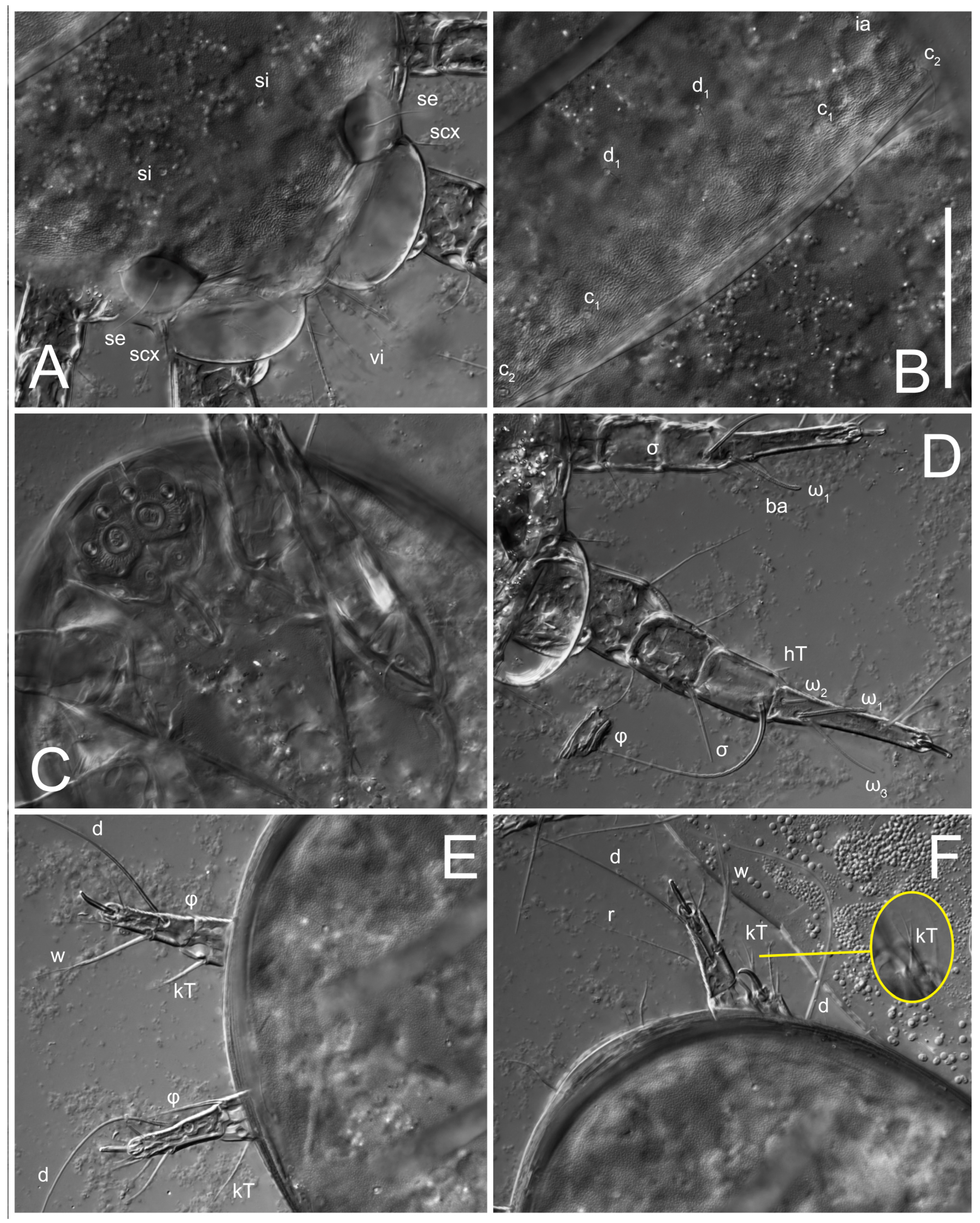
Heteromorphic deutonymph (
Figure 6,
Figure 7 and
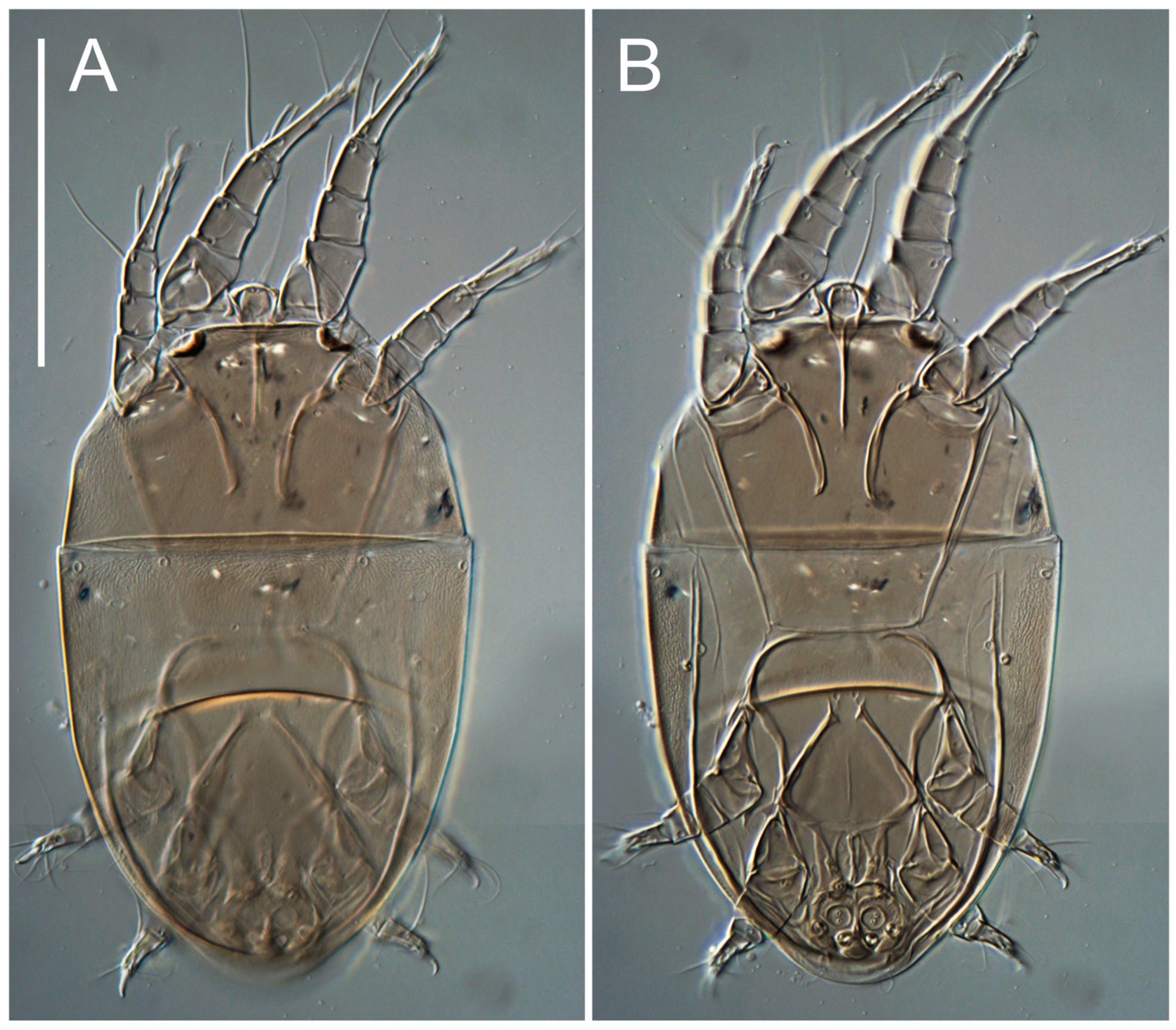
Figure 8). Body elongate, 1.53–1.84-times longer than wide (
n = 4), widest in sejugal region; idiosomal length 210–240,
n = 4 width 130–150,
n = 4. Gnathosoma short, subcapitulum and palp fused, bearing apical palpal solenidia (ω) and filiform apicodorsal setae (
sup); setae
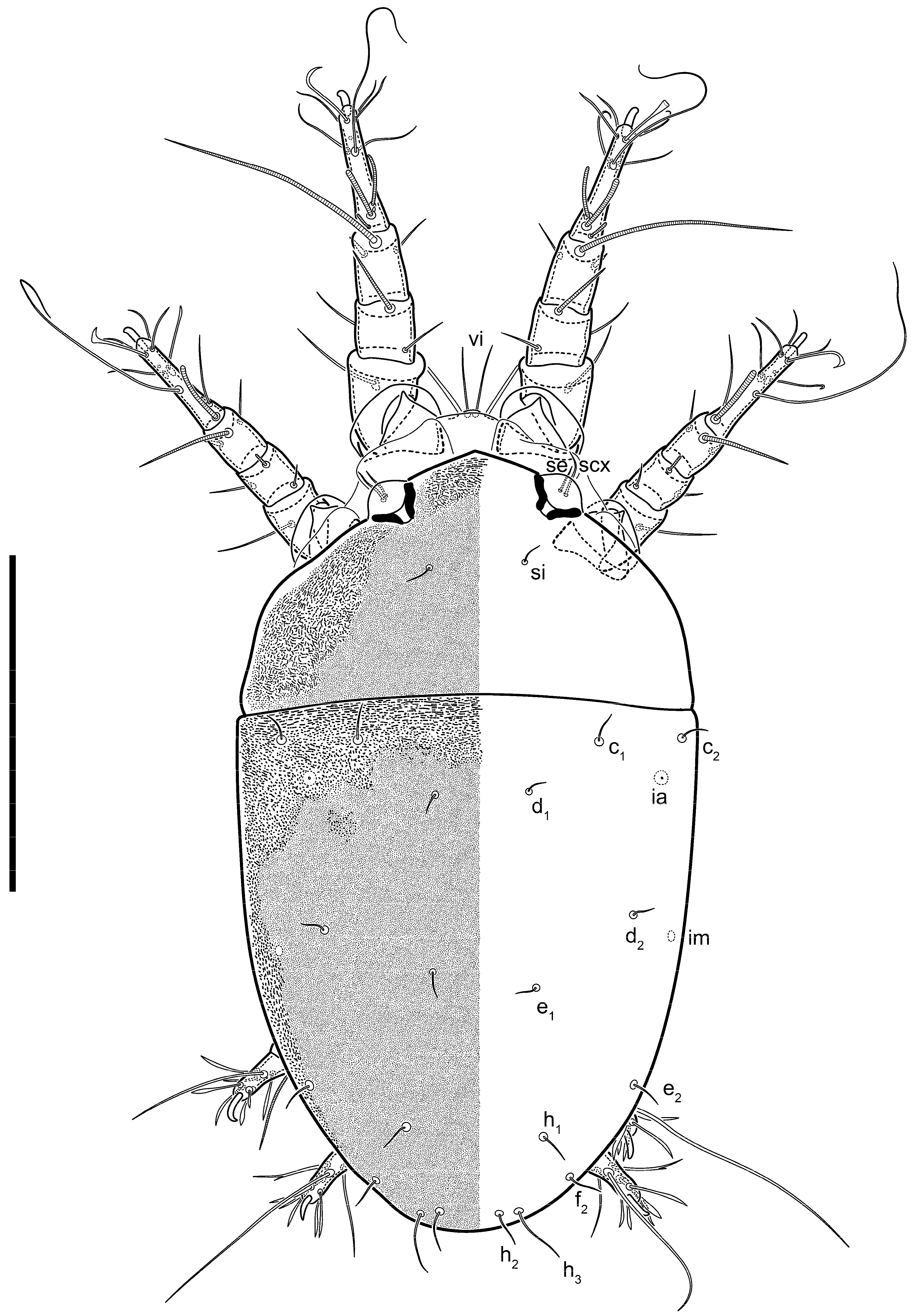
h absent from subcapitular remnant, their positions marked by refractile spots.
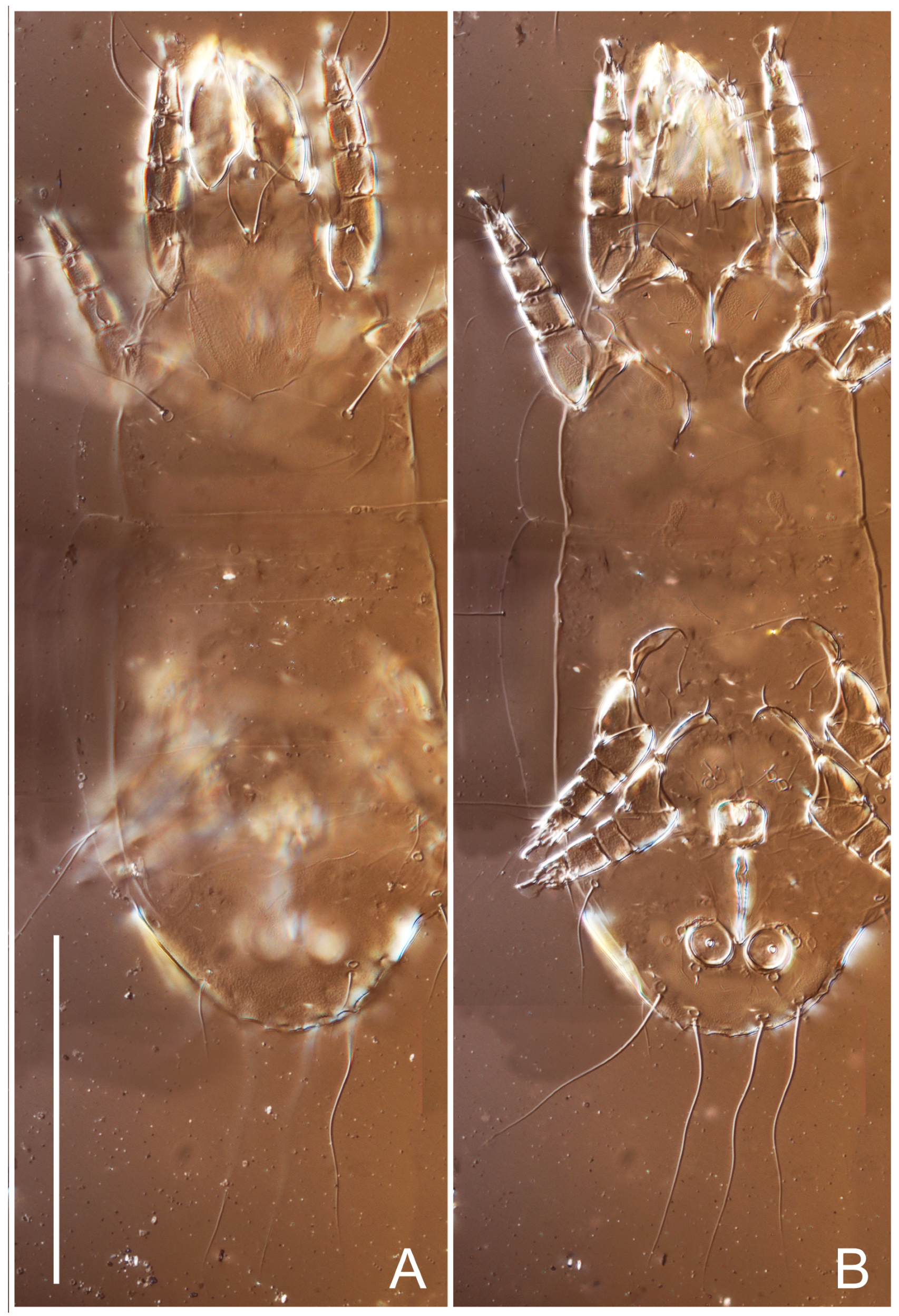
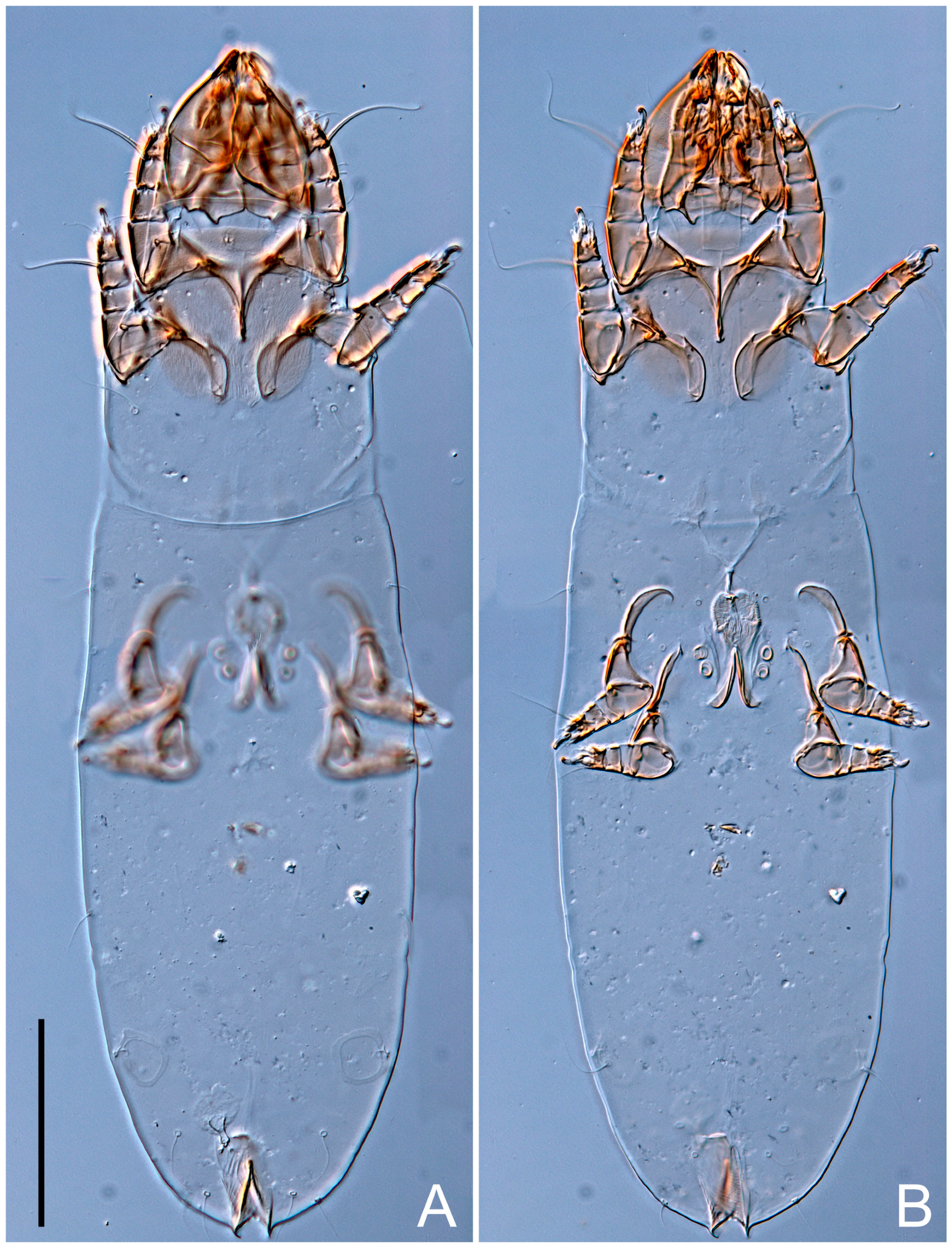
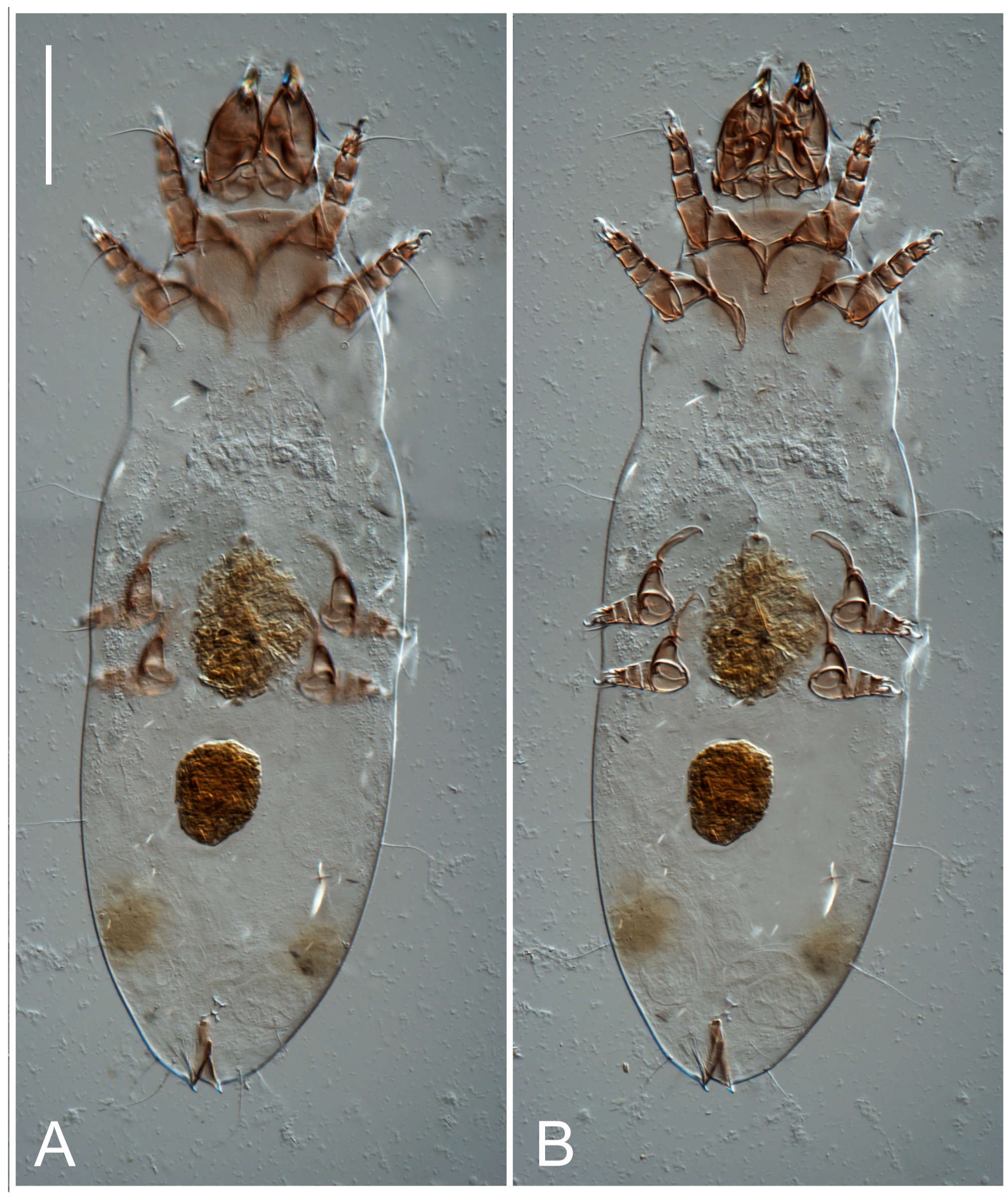
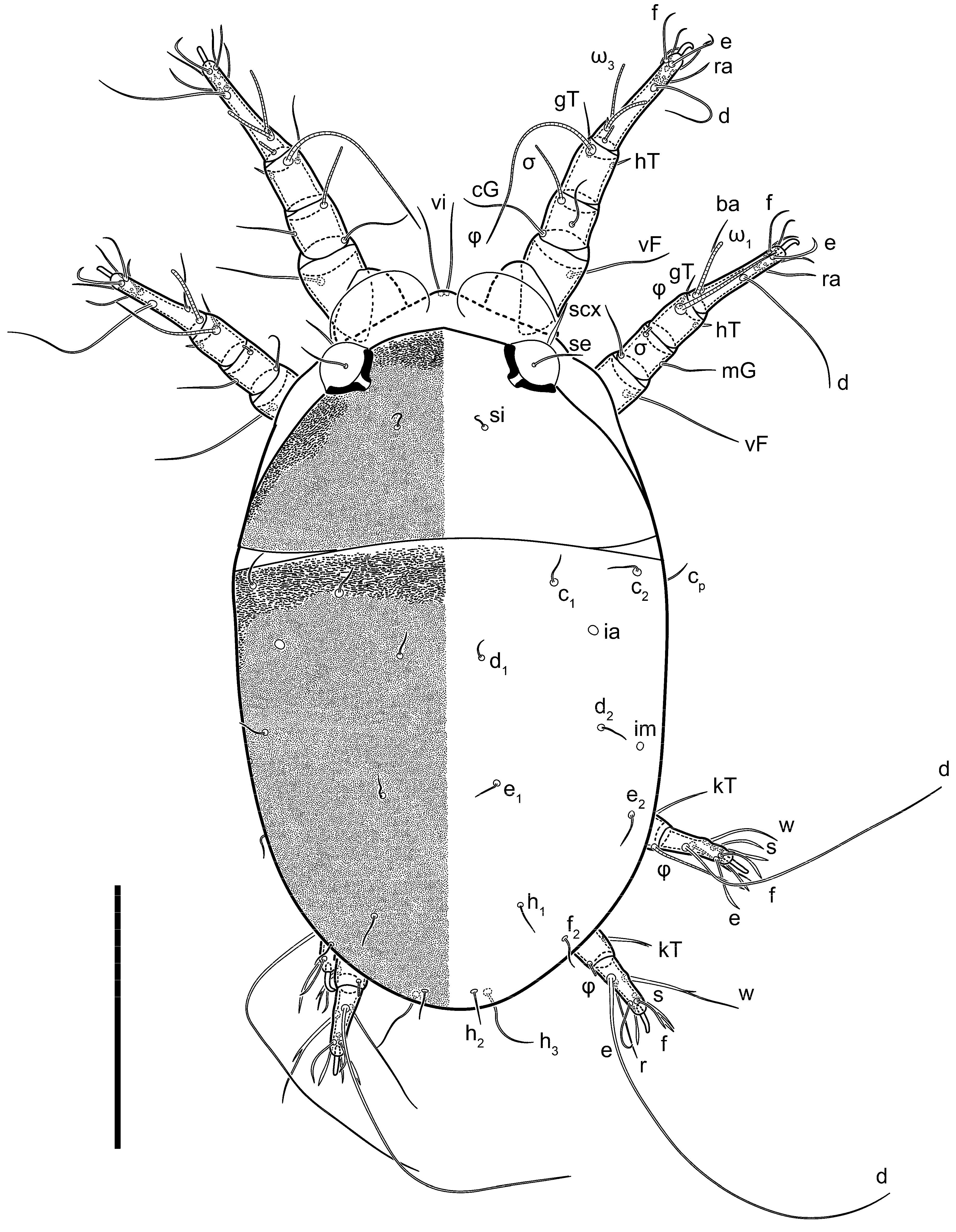
Dorsum. Propodosoma and hysterosoma each covered by smoothly punctate shields; distinct linear pattern present on anterior and lateral sides of propodosomal sclerite and hysterosomal shield. Anterior propodosoma with internal vertical setae (vi) (bases separated) and a single sclerite. A pair of lateral ocelli present on propodosoma, widely separated from each other (distance 35–42, n = 4); lenses and pigmented spots present; maximal diameter of lenses 12–14, n = 4. External vertical setae (ve) absent; external scapular setae se situated just below eye lenses; internal scapular setae (si) distinctly posterior and medial to external scapulars (se). Supracoxal setae of legs I (scx) filiform, with extended base, satiated below se. Sejugal furrow well-developed. Propodosomal sclerite 65–80, n = 4, hysterosomal shield 139–160, n = 4, ratio hysterosomal shield/propodosomal sclerite = 2.0–2.13. Hysterosomal shield with 11 pairs of simple, filiform setae (c1, c2, cp, d1, d2, e1, e2, f2, h1, h2, h3), setae h3 distinctly longer than others. Opisthonotal gland openings (gla) ventral, situated on hysterosomal shield, slightly posterior to setae c3. Of four fundamental pairs of cupules, three pairs observed: ia posteriomediad of setae c2, im ventral, lateral to trochanter IV, ih ventral, lateral to posterior portion of attachment organ.
Venter. Coxal fields sclerotized, smoothly punctate. Anterior apodemes of coxal fields I fused forming sternum; sternum not reaching posterior border of sternal shield by distance exceeding its length. Posterior border of sternal shield not sclerotized. Anterior apodemes of coxal fields II curved medially. Posterior apodemes of coxal fields II weakly developed, thin. Sternal and ventral shield adjacent. Anterior apodemes of coxal fields III free, connected by thin transverse sclerotization. Posterior medial apodeme present in area of coxal fields IV, well-separated from anterior apodemes IV and genital opening. Posterior apodemes IV absent. Subhumeral setae (c3) filiform, situated on ventral surface between legs II–III, adjacent to region separating sternal and ventral shields. Coxal setae 1a, 3a reduced, represented by very small structures each within an alveolus. Coxal setae (4b) filiform, situated at tips anterior to coxal apodemes IV; 4a in form of small, rounded conoids. Progenital region in posterior portion of coxal fields IV; genital opening elongate; two pairs of genital papillae within genital atrium; genital papillae two-segmented, with rounded apices; genital setae (g) filiform, situated laterad of genital opening. Attachment organ posterior to coxal fields IV. Anterior suckers (ad3) round, median suckers (ad1+2) distinctly larger, with paired vestigial alveoli; pair of small refractile spots anterolateral to median suckers (ps3); lateral conoidal setae of attachment organ (ps2) situated distinctly posterior to line joining centers of median suckers, slightly anterior conoids (ps1) and slightly posterior to median suckers (ad1+2); anterior and posterior lateral and posterior median cuticular conoids well-developed; anus situated between anterior suckers (ad3).
Legs. Legs elongate, all segments free. Trochanters I–III each with long, filiform seta,
pR I–II,
sR III. Femoral setation 1-1-0-1; setae
vF I–II and
wF IV long, filiform. Genual setation 2-2-0-0; setae
mG and
cG I–II filiform, seta
nG III absent. Tibial setation 2-2-1-1; setae
hT I somewhat spiniform; setae
gT I filiform (
Figure 8B); setae
gT and
hT II spiniform; setae
kT III filiform; setae
kT IV spiniform. Tarsal setation 7-8-8-8. All pretarsi consisting of hooked empodial claws arising from tarsal apices attached to short, paired condylophores within tarsal apices. Tarsus I with six filiform setae (
ra,
la,
p,
q,
d, and
f), setae
d elongate, their bases situated at level anterior to bases of setae
ra and
la; one spoon-shaped seta
e; setae
s alveolar, setae
wa,
aa and
ba I absent; tarsus II similar to tarsus I except seta
ba present and filiform, close to ω
1. Tarsus III with eight setae (
w,
r,
s,
p,
q,
e,
f,
d) smooth; all setae, except
d III more or less foliate; seta
d equal to or longer than leg III. Tarsus IV similar to tarsus III, except setae
q and
p short, spiniform, seta
r longer, filiform, seta
w filiform and with distinct prong, seta
d distinctly longer than legs IV. Solenidia ω
1 on tarsi I–II cylindrical, with slightly clavate apices; ω
3 on tarsus I slightly shorter than ω1, with rounded apex, positioned slightly anterior to ω
1; ω
1 and ω
3 separated by bulbous famulus (ε); solenidion ω
2 of tarsus I slightly widened apically, situated somewhat more basal and posterior to ω
1 + ε+ω
3 group; solenidia φ of tibiae I–III elongate, tapering; φ I longer than tarsus I; φ II shorter than tarsus II; φ III reaching tip of tarsus III without ambulacrum; φ IV short; σ of genu I elongate, tapering slightly, nearly reaching tip of tibia I; σ of genu II shorter, cylindrical, not reaching midlength of tibia II; σ of genu III absent.
Diagnosis
Female.
Thyreophagus ais is close to
Th. calusorum,
Th. mauritianus (Fain, 1982),
Th. gallegoi Portus and Gomez, 1979 and
Th. vermicularis Fain and Lukoschus, 1982 in having a very short solenidion σ II.
Th. ais is similar to
Th. calusorum and
Th. mauritianus by the shape of solenidion σ II, which has convex sides (sides straight and not convex in
Th. gallegoi and
Th. vermicularis), but it differs from
Th. calusorum by the following: sclerites of oviducts are Y-shaped (V-shaped in
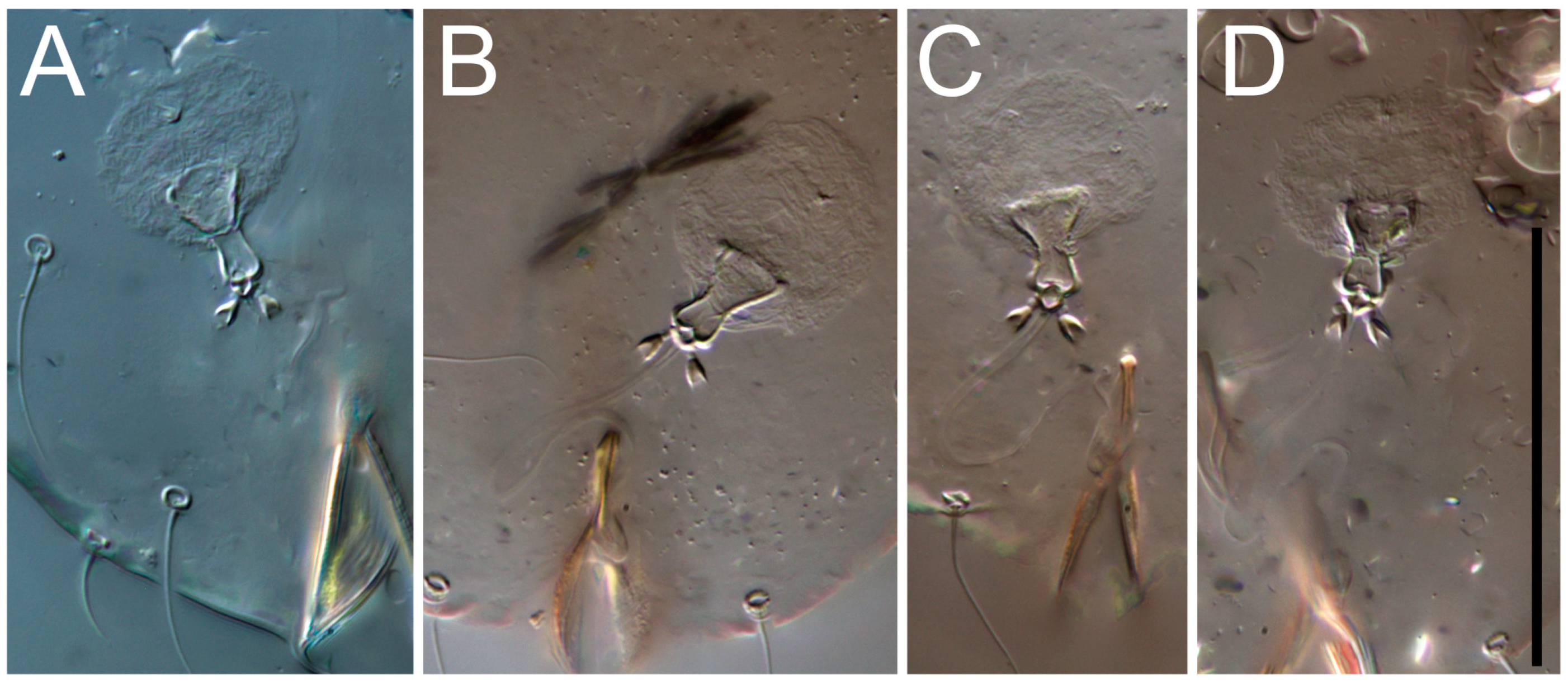
Th. calusorum); the canal of spermatheca at the entrance to spermatheca is not widened (
Figure 2I) vs. widened in
Th. calusorum [
8] (
Figure 9); the canal of spermatheca forms a small, well-sclerotized, cup-shaped atrium (
Figure 2I and
Figure 5G–J) vs. canal uniform in appearance, atrium absent [
8] (
Figure 9); bases of setae
vi are touching but separated (in common area in
Th. calusorum).
Th. ais differs from
Th. mauritianus as follows: the cuplike portions of the sclerites of oviducts are subequal to their stems (
Figure 5G–J vs. shorter than stems in
Th. mauritianus [
8] (Figure 31B–D); solenidion σ’ is distinctly longer than σ’’ (subequal in
Th. mauritianus); solenidion ω
1 II is three-times longer than its width (five-times longer in
Th. mauritianus); and solenidion φ IV is nearly reaching the bases of setae
d IV (reaching the middle of tarsus IV in
Th. mauritianus).
Th. ais differs from
Th. gallegoi by the absence of linear sclerites near the typical sclerites of oviducts (present in
Th. gallegoi) and from
Th. vermicularis by the Y-shaped paired sclerites of oviducts, which are at least three-times longer than their width (V-shaped, more than five-times longer than their width in
Th. vermicularis).
Heteromorphic deutonymph. Th. ais is very similar to Th. calusorum, but differs by short, spiniform setae q and p IV (foliate in Th. calusorum), setae d III are longer than leg III (shorter in Th. calusorum), d IV distinctly longer than leg IV (subequal to leg IV in Th. calusorum). Setae hT I and kT IV are always spiniform in Th. ais, but it can be variable, either spiniform or filiform in Th. calusorum (Klimov et al., 2022).
Thyreophagus hobe Klimov, Kolesnikov, Demard, Vangansbeke, sp. n.
urn:lsid:zoobank.org:pub:7C7FEAA7-EB57-4A12-A489-196D2BEEA5D9.
Type material. Holotype: female—USA: Florida, Fort Pierce, branches on ground in a small, wooded area, subcortical, stick3, 27°25′34.5″ N 80°24′22.7″ W, 12 October 2020, Emilie Demard, BMOC 20-0101-008. Culture maintained until 26 February 2021 but then was accidentally lost (no specimens from these cultures were preserved).
Depository. Holotype—University of Michigan, Museum of Zoology, Ann Arbor, Michigan, USA.
Etymology. The new species is named after the Hobe Indians who lived in the eastern coastal area of central Florida (between St. Lucie and Jupiter inlets), but went extinct after the arrival of European colonizers [
27]. The men of the Hobe Indians had long hair
6, which is reminiscent of the long dorsal setae of
Thyreophagus hobe.
Habitat. Thyreophagus hobe lives under the bark of small fallen branches found in wooded areas. These branches are typically in the initial stages of decomposition, with approximately 80% of their natural sapwood retaining a white color, and 20% displaying brown discoloration, indicating the ongoing decomposition process.
Description
Female (
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13). Idiosoma elongate, 350 × 120 (holotype), 2.9-times longer than wide. Idiosomal cuticle smooth. Subcapitular setae (
h) long, widened basally; palp tibial setae (
a), lateral dorsal palp tibial setae (
sup), dorsal palp tarsal seta (
cm) filiform; supracoxal seta
elcp present, slightly widened basally; terminal palp tarsal solenidion ω short; external part of terminal eupathidium
ul’’ dome-shaped; terminal eupathidium
ul’ not observed. Prodorsal sclerite 63 long, 46 wide, 1.4-times longer than wide, with setae
vi (situated at anterior part of shield, bases in common area of unsclerotized cuticle), rounded anterolateral incisions, and elongate midlateral incisions (corresponding to insertion points of setae
ve); shield punctate, 3/4 of posterior central region with linear pattern. Grandjean’s organ (GO) expanded anteriorly into membranous finger-shaped extensions (exact number cannot be observed on the single specimen). Supracoxal seta (
scx) smooth, sword-shaped, widened and flattened, tapering at tip. Idiosomal setae (
vi,
se,
cp,
d2,
e2,
h1,
h2,
h3,
ps3) smooth, filiform, setae
h2 and
h3 very long (2.3-times longer than length of prodorsal shield); opisthosomal gland openings slightly anteriad of setal bases
e2. Four pairs of fundamental cupules (
ia,
im,
ip,
ih) present.
Ventral surface of idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g), seta
4b unpaired in holotype (abnormality). Shape of coxal sclerites as shown in
Figure 1B and
Figure 4C,D. Genital region between coxal fields III and IV; genital valves shaped in an inverted Y; epigynal and medial apodemes well-developed. Genital papillae medium-sized, their diameter approximately 5–6-times shorter than length of genital setae. Anal opening situated on posterior margin of idiosoma, mostly ventral, with substantial portion positioned terminally and dorsally. Copulatory tube weakly developed. Canal of spermatheca medium-sized, slender, uniformly wide, not widening at entrance to spermatheca. Spermatheca simple, with sclerotized, dome-shaped atrium (length and width subequal), small, paired Y-shaped sclerites of oviducts, and large sperm storage sac.
Legs short, all segments free. Trochanters I–III each with long, filiform seta, pR I–II, sR III; trochanter IV without setae. Femoral setation 1-1-0-0; setae vF I–II long, filiform, wF IV absent. Genual setation 2-2-0-0; setae mG and cG I–II long, filiform; seta nG III absent. Tibial setation 2-2-1-1; setae hT I-II represented by alveoli; setae gT I–II and kT III–IV elongated, filiform. Tarsal setation 8-8-8-8; all pretarsi have hooked empodial claws arising from tarsal apices and attached to short paired condylophores. Tarsus I with eight setae; ra, la, f, and d filiform; e, s, p, and q spiniform, small; u and v absent (represented by weakly developed rounded structures), setae wa absent. Tarsus II setation similar to that of tarsus I. Tarsus III with eight setae; f, d, r filiform; e and s spiniform; p, q, u, and v similar to these of tarsus I–II; seta w flattened, button-shaped. Tarsus IV similar to tarsus III, except w filiform. Solenidion ω1 on tarsus I cylindrical, with clavate apex, slightly curved; solenidion ω1 on tarsus II simple, cylindrical, with clavate apex, not bent, longer than ω1 on tarsus I. Solenidion ω2 on tarsus I shorter than ω1, cylindrical, with rounded apex, slightly expanded at tip, situated slightly anterior and external to ω1. Solenidion ω3 on tarsus I cylindrical, with rounded apex, shorter than ω1 I. Famulus (ε) of tarsus I spiniform, with pointed apex. Solenidia φ of tibiae I–III elongate, tapering, well extending beyond apices of respective tarsi with ambulacra; solenidion φ IV shorter than tarsus IV (with ambulacra). Genual solenidia σ’ and σ’’ I elongate, tapering, subequal, slightly not reaching bases of φ I. Genual solenidion σ II 7–9-times longer than its width, with rounded tip. Genual solenidion σ III absent.
Male. Absent.
Heteromorphic deutonymph. Unknown.
Diagnosis
Female. Thyreophagus hobe differs from all known species of Thyreophagus by the absence of femoral setae wF IV and unguinal setae u, v I–IV. Like Th. australis Clark, 2009, Th. hobe has very long setae h2 and h3 (2.3-times longer than the length of the prodorsal shield in Th. hobe (2.5–2.8-times longer than the length of the prodorsal sclerite in Th. australis), but in Th. hobe, setae h1 five-times shorter than h2 and h3 (vs. h1, h2, and h3 are subequal in Th. australis).
Thyreophagus ojibwe Klimov, Kolesnikov, Vangansbeke, sp. n.
urn:lsid:zoobank.org:pub:7C7FEAA7-EB57-4A12-A489-196D2BEEA5D9.
Type material. Holotype female (f1), paratype female (f2)—USA: Michigan, Michigan State University Chestnut Plantation, Castanea dentata (Fagales: Fagaceae), branch on ground (tree1.stick1), 42°09′06.6″ N 84°25′33.2″ W, 23 November 2020, P. Klimov, BMOC 20-0101-010#slide1; 1 paratype female—same data, slide 2; 1 paratype female—same data, C. dentata blight tissues, BMOC 20-0101-012#slide3.
Non-type material. One male, two females—CANADA: Ontario C.E.F., Ottawa, under bark of dead willow twigs, Salix sp. (Malpighiales: Salicaceae), O. Peck, 1974 CNC788949 (Canadian National Collection) (studied as high-resolution images, provided by Fred Beaulieu, Canadian National Collection of Insects, Arachnids & Nematodes (CNC)).
Depository. Holotype, paratypes—University of Michigan, Museum of Zoology, Ann Arbor, Michigan, USA.
Etymology. The new species is named after the Ojibwe, North American indigenous people from what is currently southern Canada and Midwestern United States, including the state of Michigan.
Habitat. Thyreophagus ojibwe lives under the bark of small fallen branches typically found around American chestnut trees. These branches are generally in the initial stages of decomposition. Additionally, mites were found on the blight-infected tissues, suggesting that these mites can likely feed on this harmful fungus and potentially transmit hypoviruses.
Description
Female (
Figure 14,
Figure 15,
Figure 16,
Figure 17 and
Figure 18). Idiosoma elongate, 460 × 200 (holotype), 420-450 × 180-200 (paratype,
n = 3), 2.3 (2.3,
n = 3)-times longer than wide. Idiosomal cuticle smooth. Subcapitular setae (
h) long, widened basally; palp tibial setae (
a), lateral dorsal palp tibial setae (
sup), dorsal palp tarsal seta (
cm) filiform; supracoxal seta
elcp present, slightly widened basally; terminal palp tarsal solenidion ω short; external part of terminal eupathidium
ul’’ dome-shaped; terminal eupathidium
ul’ not observed. Prodorsal sclerite 85 (71–80,
n = 3) long, 75 (60–68,
n = 3) wide, 1.1 (1.1–1.2,
n = 3)-times longer than wide, with setae
vi (situated at anterior part of sclerite, bases touching but distinctly separated), rounded anterolateral incisions, and elongate midlateral incisions (corresponding to insertion points of setae
ve); shield smoothly punctate, 3/4 of posterior central region with linear pattern. Grandjean’s organ (GO) with 8 membranous, finger-shaped extensions. Supracoxal seta (
scx) smooth, sword-shaped, widened and flattened, tapering at tip. Idiosomal setae (
vi,
se,
cp,
d2,
e2,
h1,
h2,
h3,
ps3) smooth, filiform; setae
h2 and
h3 long, 1.75-times longer than length of prodorsal sclerite; opisthosomal gland openings slightly anteriad of setal bases of
e2. Four pairs of fundamental cupules (
ia,
im,
ip,
ih) present.
Ventral surface of idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g). Shape of coxal sclerites as shown in
Figure 1B. Genital region between coxal fields III and IV; genital valves shaped as an inverted Y; epigynal and medial apodemes well-developed. Genital papillae large, their diameters approximately four-times shorter than length of genital setae. Anal opening on posterior margin of idiosoma, mostly ventral, with substantial portion positioned terminally and dorsally. Canal of spermatheca short, slender tube, uniformly wide, slightly expanded at entrance to spermatheca. Spermatheca with elongated, vase-shaped atrium (longer than width); paired Y-shaped sclerites of oviducts small (their stems short, shorter than atrium in its narrowest part); and large sperm storage sac.
Legs short, all segments free. Trochanters I–III each with long, filiform seta, pR I–II, sR III; trochanter IV without setae. Femoral setation 1-1-0-1; setae vF I–II and wF IV long, filiform. Genual setation 2-2-0-0; setae mG and cG I–II long, filiform; seta nG III absent. Tibial setation 2-2-1-1; setae hT I-II alveolar; setae gT I–II elongated, somewhat spiniform; setae kT III–IV filiform. Tarsal setation 10-10-10-10; all pretarsi with hooked empodial claws attached to short, paired condylophores. Tarsus I with 10 setae; ra, la, f, and d filiform; e, u, v spiniform; p and q represented by small triangular rudiments; s flattened, button-shaped; setae wa absent. Tarsus II setation similar to that of tarsus I, except seta s small, spiniform. Tarsus III with 10 setae, f, d, r filiform, e, s, u, v spiniform, w small spiniform, p and q represented by small triangular rudiments. Tarsus IV similar to tarsus III, except w filiform. Solenidia ω1 on tarsi I and II cylindrical, with clavate apices, slightly curved, almost reaching tip of tarsus (without ambulacra). Solenidion ω2 on tarsus I shorter than ω1, cylindrical, with rounded apex, slightly widened at tip, situated slightly anterior and external to ω1. Solenidion ω3 on tarsus I cylindrical, with rounded apex, shorter than ω1, longer than ω2. Famulus (ε) of tarsus I wide, spiniform, with broadly rounded apex, widest at middle. Solenidia φ of tibiae I–III elongate, tapering, well extending beyond apices of respective tarsi with ambulacra; solenidion φ IV shorter, almost reaching tip of tarsus IV (without ambulacra). Genual solenidia σ’ and σ’’ I elongate, tapering, subequal, slightly not reaching bases of φ I. Genual solenidion σ II 10-times longer than its width) and somewhat conical. Genual solenidion σ III absent.
Male (
n = 1) (
Figure 19 and
Figure 20). Idiosoma elongate, 250 × 120, 2.1-times longer than wide. Idiosomal cuticle smooth. Gnathosoma as in female. Prodorsal sclerite 50 long, 38 wide, 1.3-times longer than wide, with setae
vi, incisions and ornamented as in female.
Grandjean’s organ (GO) and supracoxal seta (scx) as in female. Idiosomal setae (vi, se, cp, d2, e2, h1, h2, h3) smooth, filiform, setae h2 and h3 long (1.8-times longer than length of prodorsal shield); opisthosomal gland openings slightly anteriad of setal bases e2. Four pairs of fundamental cupules (ia, im, ip, ih) present. Opisthonotal shield solid, whole, smoothly punctated; ventral part of shield extends to anal suckers.
Ventral surface of idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g). Shape of coxal sclerites shown in
Figure 19. Genital region between coxal fields IV; arms of genital capsule rounded; aedeagus short, not protruding beyond anterior edge of supporting sclerite. Genital papillae medium-sized, their diameter approximately 4–5-times shorter than genital setae. Anal sucker rounded in outline. Setae
ps1-3 very short.
Legs I–III as in female, except solenidion ω3 on tarsus I very short, truncated and solenidion σ’’ about two-times longer than σ’. Trochanter and genua IV without setae, femur IV with setae wF IV long, filiform, tibia IV with kT IV elongated, somewhat spiniform. Tarsus IV with 10 setae; f, r, w filiform; d and e represented by suckers; u, v, p, q spiniform; s flattened, button-shaped or minute, spiniform. Solenidion φ on tibia IV short and wider.
Heteromorphic deutonymph. Unknown.
Diagnosis
Female. Th. ojibwe is very similar to Th. corticalis, but differs as follows: setae h2 and h3 are 1.75-times longer than the length of the prodorsal sclerite (1.1–1.25 times in Th. corticalis); the diameter of the genital papillae is approximately four-times shorter than the length of genital setae (six-times shorter in Th. corticalis); the stem of the Y-shaped sclerites of oviducts is about 3–4-times shorter than the length of Y-shaped sclerites of oviducts (subequal in Th. corticalis); ventral apical spines p and q of tarsi III–IV are shorter than spines u and v III–IV (subequal in Th. corticalis).
Male. Th. ojibwe differs from Th. corticalis by the longer setae h2 and h3 (their length is 1.8-times longer than the length of prodorsal sclerite (1.1–1.25-times longer in Th. corticalis) and by spines p and q on tarsi III–IV, which are shorter than spines u and v (subequal in Th. corticalis).
Thyreophagus potawatomorum Klimov, Kolesnikov, Vangansbeke, sp. n.
(
Figure 21,
Figure 22,
Figure 23,
Figure 24,
Figure 25,
Figure 26,
Figure 27,
Figure 28,
Figure 29,
Figure 30,
Figure 31,
Figure 32,
Figure 33 and
Figure 34).
urn:lsid:zoobank.org:pub:7C7FEAA7-EB57-4A12-A489-196D2BEEA5D9.
Type material. Holotype female (f2) and one paratype female (f1)—USA: Michigan, Ann Arbor, Hansen Nature Area, stick 5, 42°16′03.8″ N 83°46′53.2″ W, 14 October 2020, P. Klimov, BMOC 20-0101-004#slide 1; four paratype females—same data (culture, harvested 26 February 2021), slide 2; six paratype females—same data, slide 3; two paratype HDNs—same data, slide 5; one paratype HDN—same data, slide 6; one paratype HDN—same data, slide 8.
Non-type material. Two DNs, one pharate DN—same data, slide 7.
Depository. Holotype, paratypes—University of Michigan, Museum of Zoology, Ann Arbor, Michigan, USA.
Etymology. This species is named after Potawatomi, native American people of the Great Plains, upper Mississippi River, and western Great Lakes region (including the state of Michigan).
Habitat. Thyreophagus potawatomorum lives under the bark of small fallen branches of deciduous trees in wooded areas. These branches are typically in the initial stages of decomposition, with approximately 80% of their natural sapwood retaining a white color, and 20% displaying brown discoloration, indicating the ongoing decomposition process. Additionally, the bark usually exhibits boreholes from wood-boring beetles.
Description
Female (
Figure 21,
Figure 22,
Figure 23,
Figure 24,
Figure 25,
Figure 26,
Figure 27 and
Figure 28). Idiosoma elongate, 580 (holotype), 164 wide, 3.5-times longer than wide. Idiosomal cuticle smooth. Subcapitular setae (
h) long, widened basally; palp tibial setae (
a), lateral dorsal palp tibial setae (
sup), dorsal palp tarsal seta (
cm) filiform; supracoxal seta
elcp present, widened basally; terminal palp tarsal solenidion ω short, bacilliform; external part of terminal eupathidium
ul’’ dome-shaped; terminal eupathidium
ul’ not observed. Prodorsal sclerite 88 (77–82,
n = 6) long, 80 (65–70,
n = 6) wide, 1.1 (1.1–1.2,
n = 6)-times longer than wide, with setae
vi (situated at anterior part of sclerite, bases separate but touching each other), rounded anterolateral incisions, and elongate midlateral incisions (insertion points of setae
ve). Prodorsal sclerite punctate, with longitudinal linear pattern extending anteriorly from posterior end of sclerite and covering area exceeding 75% of sclerite; anterior lateral and medial areas have only punctate patterns; lines form a triangle at posterior end of sclerite. Grandjean’s organ (GO) with seven membranous finger-like processes; central process distinctly wider than remaining processes. Supracoxal seta (
scx) smooth, sword-shaped, widened and flattened, tapering at tip, slightly curved. Idiosomal setae (
vi,
se,
cp,
d2,
e2,
h1,
h2,
h3,
ps3) smooth, filiform, short and slender; opisthosomal gland openings slightly anteriad of setal bases
e2. Four pairs of fundamental cupules (
ia,
im,
ip,
ih) present.
Ventral idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g). Shape of coxal sclerites as shown in
Figure 1B and
Figure 5F,G. Ovipore between coxal fields III and IV; genital valves shaped as an inverted Y; epigynal and medial apodemes well-developed. Genital papillae medium-sized, their diameter approximately 5–6-times shorter than length of genital setae. Anal opening on posterior margin of idiosoma, mostly ventral, with substantial portion positioned terminally and dorsally. Copulatory tube situated anteriad of dorsal end of anus. Canal of spermatheca long, slender tube of uniform width, not widened at entrance to spermatheca; atrium of spermatheca vase-shaped (length greater than width) with a rounded capsule at junction with canal of spermatheca. Small, paired Y-shaped sclerites of oviducts with short stems.
Legs short, all segments free. Trochanters I–III each with filiform seta, pR I–II, sR III; trochanter IV without setae. Femoral setation 1-1-0-1; setae vF I–II and wF IV long, filiform. Genual setation 2-2-0-0; setae mG and cG I–II long, filiform; seta nG III absent. Tibial setation 2-2-1-1; setae hT I-II small, short, thin; setae gT I–II elongated, somewhat spiniform; setae kT III–IV elongated, somewhat spiniform. Tarsal setation 10-10-10-10; all pretarsi with hooked empodial claws and short, paired condylophores. Tarsus I with 10 setae; ra, la, f, and d filiform; e, u, v, p, q spiniform; s flattened, button-shaped; setae wa absent. Tarsus II setation similar to that of tarsus I, except proral setae (p, q) represented by small triangular rudiments. Tarsus III with 10 setae; f, d, r filiform; e, w, s, u, v, p, q spiniform. Tarsus IV similar to tarsus III, except w filiform. Solenidion ω1 on tarsus I cylindrical, with clavate apex, bent and pointed outward, to posterior side of tarsus; solenidion ω1 on tarsus II simple, cylindrical, with clavate apex, not bent, shorter than ω1 on tarsus I. Solenidion ω2 on tarsus I shorter than ω1, cylindrical, with rounded apex, slightly widened at tip, situated slightly anterior and external to ω1. Solenidion ω3 on tarsus I cylindrical, with rounded apex, shorter than ω1, longer than ω2. Famulus (ε) of tarsus I wide, spiniform, with broadly rounded apex. Solenidia φ of tibiae I–III elongate, tapering, well extending beyond apices of respective tarsi with ambulacra; solenidion φ IV shorter than tarsus IV (with ambulacra). On genu I, solenidion σ’ elongate, with rounded tip, slightly not reaching bases of φ I, about 1.5-times longer than σ’’; σ’’ I distinctly wider than σ’, about half the length of tibia I. On genu II, solenidion σ short (more than three-times longer than its width), with rounded tip. Solenidia σ III and IV absent.
Male. Absent.
Heteromorphic deutonymph (
n = 1) (
Figure 29,
Figure 30,
Figure 31,
Figure 32,
Figure 33 and
Figure 34). Body elongate, 1.67-times longer than wide, widest in sejugal region; idiosomal length 235, width 140. Gnathosoma short, subcapitulum and palp fused, with apical palpal solenidia ω and filiform apicodorsal setae
(sup); setae
h absent from subcapitular remnant, their positions marked by somewhat refractile spots.
Dorsum. Propodosomal and hysterosomal smoothly punctate; distinct linear pattern present on anterior and lateral sides of propodosomal sclerite and hysterosomal shield. Apex of propodosomal sclerite shaped as obtuse triangle. Internal vertical setae (vi), sitiated on apex of propodosoma, long, bases separated. A pair of lateral ocelli present on propodosoma; widely separated from each other (distance 36, n = 1); lenses and pigmented spots present, maximal diameter of lenses 16. External vertical setae (ve) absent; external scapular setae se situated just below eye lenses; internal scapular setae (si) distinctly posterior and medial to external scapulars (se). Supracoxal setae of legs I (scx) filiform, situated below se. Sejugal furrow well-developed. Propodosomal sclerite 73, hysterosomal shield 160, ratio hysterosoma shield/propodosomal sclerite, length = 2.19. Hysterosoma with 11 pairs of simple, filiform setae on hysterosomal shield (c1, c2, cp, d1, d2, e1, e2, f2, h1, h2, h3), setae h3 distinctly longer than others. Opisthonotal gland openings (gla) ventral; situated slightly posterior to setae c3 off hysterosomal shield. Of four fundamental pairs of cupules, only three pairs observed: ia posteriomedial of setae c2, im posterior of d2 level and ih ventral, lateral to posterior sides of attachment organ.
Venter. Coxal fields sclerotized, smoothly punctate. Anterior apodemes of coxal fields I fused forming sternum. Sternum not reaching posterior border of sternal shield by distance exceeding its length. Posterior border of sternal shield weakly sclerotized. Anterior apodemes of coxal fields II curved medially. Posterior apodemes of coxal fields II weakly developed, thin. Sternal and ventral shields adjacent. Anterior apodemes of coxal fields III free. Posterior medial apodeme present between coxal fields IV, well-separated from anterior apodemes IV and genital opening. Posterior apodemes IV absent. Dorsal hysterosomal shield separated from ventral surface by a distinct suture on each side. Subhumeral setae (c3) long, filiform, positioned on ventral surface between legs II–III, adjacent to region separating sternal and ventral shields. Coxal setae 1a, 3a reduced, represented by minute structures each situated in an alveolus. Setae 4b, g filiform; 4a in form of small, rounded conoids, 4b longer than g. Genital region in posterior portion of coxal fields IV; genital opening elongate, there are two pairs of genital papillae inside progenital atrium; papillae two-segmented, with rounded apices. Coxal setae (4b) situated at tips anterior to coxal apodemes IV; genital setae (g) situated laterad of genital opening. Attachment organ posterior to coxal fields IV. Anterior suckers (ad3) round, median suckers (ad1+2) distinctly larger, with paired vestigial alveoli; pair of small refractile spots anterolateral to median suckers (ps3); lateral conoidal setae of attachment organ (ps2) situated slightly posterior to a line joining centers of median suckers, distinctly anterior conoidal setae (ps1) and slightly posterior to median suckers (ad1+2); anterior and posterior lateral and posterior median cuticular conoids well-developed; anus positioned between anterior suckers (ad3).
Legs. Legs elongate, all segments free. Trochanters I–III each with long, filiform seta, pR I–II, sR III. Femoral setation 1-1-0-1; setae vF I–II and wF IV long, filiform. Genual setation 2-2-0-0; setae mG and cG I–II filiform, seta nG III absent. Tibial setation 2-2-1-1; setae hT I and II spiniform; setae gT I filiform; setae kT III filiform; kT IV somewhat spiniform. Tarsal setation 7-8-8-8. All pretarsi consisting of hooked empodial claws arising from tarsal apices, attached to short paired condylophores within tarsal apices. Tarsus I with three filiform setae (p, q and d), three slightly foliate seate (ra, la and f), and one spoon-shaped seta e. Seta d I elongate, longer than tarsus (its base situated at level of setae ra and la); seta s I alveolar; setae wa, aa and ba I absent. Tarsus II similar to tarsus I except seta ba present and filiform, base of seta d posterior to level of setae ra and la. Tarsus III with eight setae (w, r, s, p, q, e, f, d) smooth, all setae, except d III foliate. Tarsus IV similar to tarsus III, except seta r longer, filiform, and setae w filiform and has a distinct prong. Solenidia ω1 on tarsi I–II cylindrical, with slightly clavate apices, ω1 II longer than ω1 I. Solenidion ω3 on tarsus I slightly shorter than ω1, with rounded apex, positioned slightly anterior to ω1; famulus (ε) bulbous, situated between ω1 and ω3. Solenidion ω2 of tarsus I thin, with rounded apex, positioned somewhat more basal and posterior to ω1+ε+ ω3 group. Solenidia φ of tibiae I–III elongate, tapering; φ I and III, longer than tarsus I and III, respectively; φ II shorter than tarsus II; φ IV short. Solenidion σ of genu I elongate, slightly tapering, nearly reaching tip of tibia I; σ of genu II shorter, cylindrical, not reaching midlength of tibia II; σ of genu III absent.
Diagnosis
Females. Thyreophagus potawatomorum is similar to Th. spinitarsis (Fain, 1982) by the linear striations of the prodorsal sclerite extending over at least 75% of the sclerite length and by tarsi III having seven spiniform setae (e, u, v, p, q, s, w), but differs by the widened, vase-shaped atrium (dome-shaped in Th. spinitarsis). Th. potawatomorum is very close to Th. berxi sp. n., but differs by the following character states: setae vi are not extending beyond the anterior margin of the prodorsum (extending in Th. berxi); bases of vi touch each other (distinctly separated in Th. berxi), lines of the prodorsal sclerite are longer than the diameter of the bases of vi (shorter in Th. berxi); on the prodorsal sclerite, the anterior medial striated area differs from the posterior medial striated area (medial striated area has a uniform pattern in Th. berxi); opisthosomal setae h2 and h3 are shorter than the anus (distinctly longer in Th. berxi); a rounded capsule is present between the canal of spermatheca and the atrium (absent in Th. berxi).
Heteromorphic deutonymph. Th. potawatomorum is close to Th. corticalis and Th. berxi by the following character states: body elongate (more than 1.7-times longer than wide), setae hT and gT I–II are present, different in shape, bases of setae d are at the level with bases of setae of ra and la on tarsus I. Th. potawatomorum differs from Th. corticalis and Th. berxi by the following character states: posterior median apodeme present, well-developed (absent in Th. corticalis and weakly developed in Th. berxi); the diameter of ocelli is about 16 (19 in Th. corticalis; 23 in Th. berxi); the distance between the ocelli is 36 (60 in Th. corticalis; 50 in Th. berxi or); setae wa I are absent (present in Th. corticalis; absent in Th. berxi); on tibia IV, seta kT IV without distinct prong (with a distinct prong in Th. berxi; without in Th. corticalis).
Thyreophagus berxi Klimov, Kolesnikov, Wäckers, Merckx, Duarte, Vangansbeke, sp. n.
urn:lsid:zoobank.org:pub:7C7FEAA7-EB57-4A12-A489-196D2BEEA5D9.
Type material. Holotype female, five paratype females—BELGIUM: East Flanders, Gentbrugge, Moscou, Fagus sylvatica (Fagales: Fagaceae) twig, subcortical, 51°01′50.7″ N 3°44′54.6″ E, 16 November 2019, Dominiek Vangansbeke, PBK 22-0905-033#slide1; one paratype female, same data, slide 2. One paratype female—BELGIUM: Flemish Brabant, Arenberg Castle, Leuven, Fagus sylvatica (label on slide = “19-1-20 Tr. Sp. LA Arenberg beech 1”), 50°51′32.6″ N 4°39′54.4″ E, 19 January 2020, Jonas Merckx, PBK 22-0905-034#slide1. Six paratype females, one paratype HDN—GERMANY: Saxony, Dresden, Betula (Fagales: Betulaceae) (original label says: “birch twig, Felix white bag” comment = most likely birch twig”), 51°05’38.7″ N 13°50’35.6″ E, 22 November 2020, Felix Wäckers (received via Dominiek Vangansbeke), PBK 22-0905-035#slide1. Six paratype females, three paratype HDNs—FRANCE: Grand Est, Verzenay, Marcus Duarte, 8 April 2023, DV_2023-037.
Depository. Holotype, paratypes (Belgium, Germany)—University of Michigan, Museum of Zoology, Ann Arbor, Michigan, USA. Paratypes (France)—Royal Belgian Institute of Natural Sciences, Brussels, Belgium.
Etymology. The new species is named after the Belgian entomologist Peter Berx.
Habitat. Thyreophagus berxi lives under the bark of small fallen branches of deciduous trees, such as European beech and birch trees.
Description
Female (
Figure 35,
Figure 36,
Figure 37,
Figure 38 and
Figure 39). Idiosoma elongate, 670 (600–630,
n = 2) (holotype, range), 230 (200–240,
n = 2) wide, 2.9 (2.5–3.1,
n = 2)-times longer than wide. Idiosomal cuticle smooth. Subcapitular setae (
h) long, widened basally; palp tibial setae (
a), lateral dorsal palp tibial setae (
sup), dorsal palp tarsal seta (
cm) filiform; supracoxal seta
elcp present, widened basally; terminal palp tarsal solenidion ω short, bacilliform; external part of terminal eupathidium
ul’’ dome-shaped; terminal eupathidium
ul’ small, dome-shaped. Prodorsal sclerite 98 (100) long, 94 (96) wide, 1.1 (1.1,
n = 1)-times longer than wide, with setae
vi (situated at anterior part of shield, bases distinctly separated), rounded anterolateral incisions, and elongate midlateral incisions (insertion points of setae
ve). Prodorsal sclerite has three types of patterns: smoothly punctated (anterior lateral 1/3 and anterior medial 1/2 parts of sclerite), longitudinal lines (posterior medial part of sclerite), smoothly punctated smaller lines (posterior lateral part). Grandjean’s organ (GO) with 9–10 membranous finger-like extensions. Supracoxal seta (
scx) smooth, sword-shaped, widened and flattened, tapering at tip, slightly curved. Idiosomal setae (
vi,
se,
cp,
d2,
e2,
h1,
h2,
h3,
ps3) smooth, filiform, medium length and slender; opisthosomal gland openings slightly anteriad of setal bases
e2. Only one pair of fundamental cupules (
ih) observed.
Ventral surface of idiosoma with four pairs of coxal setae (
1a,
3a,
4a, and
4b) and one pair of genital setae (
g). Shape of coxal sclerites as in
Figure 1B and
Figure 5A,B. Ovipore between coxal fields III and IV; genital valves shaped as an inverted Y; epigynal and medial apodeme well-developed. Genital papillae medium-sized, their diameter approximately 0.3–0.4-times the length of coxal and genital setae. Anal opening on posterior margin of idiosoma, mostly ventral, with substantial portion situated terminally and dorsally. Copulatory tube present, short, situated anteriad of dorsal end of anus, with opening developed. Canal of spermatheca long, slender, uniformly wide, except slightly widened at entrance to atrium of spermatheca. Atrium vase-shaped, length greater than width. A capsule between atrium and canal of spermatheca absent. Y-shaped sclerites of oviducts with long stems.
Legs short, all segments free. Trochanters I–III each with filiform seta, pR I–II, sR III; trochanter IV without setae. Femoral setation 1-1-0-1; setae vF I–II and wF IV long, filiform. Genual setation 2-2-0-0; setae mG and cG I–II long, filiform; seta nG III absent. Tibial setation 2-2-1-1; setae hT I–II alveoli or small, short, and thin; setae gT I–II elongated, somewhat spiniform; setae kT III–IV elongated, somewhat spiniform. Tarsal setation 10-10-10-10; all pretarsi with hooked empodial claws and short paired condylophores. Tarsus I and II with 10 setae; ra, la, f, and d filiform; e, u, v, spiniform; s flattened, button-shaped; proral setae (p, q) represented by small triangular rudiments; setae wa absent. Tarsus III with 10 setae; f, d, r filiform; e, w, s, u, v, p, q spiniform. Tarsus IV similar to tarsus III, except w filiform. Solenidion ω1 on tarsus I cylindrical, with clavate apex, bent and pointed outward, to posterior side of tarsus; solenidion ω1 on tarsus II simple, cylindrical, with clavate apex, not bent, shorter than ω1 on tarsus I. Solenidion ω2 on tarsus I shorter than ω1, cylindrical, with rounded apex, slightly widened at tip, situated slightly anterior and external to ω1. Solenidion ω3 on tarsus I cylindrical, with rounded apex, as long as ω1, longer than ω2. Famulus (ε) of tarsus I wide, spiniform, with broadly rounded apex. Solenidia φ of tibiae I–III elongate, tapering, well extending beyond apices of respective tarsi with ambulacra; solenidion φ IV shorter, shorter than tarsus IV (with ambulacra). On genu I, solenidion σ’ elongate, with rounded tip, reaching bases of φ I; σ’’ I distinctly wider than σ’, slightly not reaching bases of φ I. On genu II, solenidion σ short (more than three-times longer than its width), with rounded tip. Solenidion σ of genu III and IV absent.
Male. Absent.
Heteromorphic deutonymph (
n = 1) (
Figure 40,
Figure 41 and
Figure 42). Body elongate, 1.7-times longer than wide, widest in sejugal region; idiosomal length 270, width 160. Gnathosoma short, subcapitulum and palp fused, with apical palpal solenidia (ω) and filiform apicodorsal setae (
sup); setae
h absent from subcapitular remnant, their positions marked by somewhat refractile spots.
Dorsum. Propodosomal sclerite and hysterosomal shield smoothly punctate; distinct linear pattern present on anterior and lateral sides of propodosomal sclerite. A small area of linear pattern present on hysterosomal shield. Anterior end p of propodosoma shaped as obtuse triangle. Internal vertical setae (vi) apical, long, bases separated. A pair of lateral ocelli present on propodosoma; ocelli widely separated from each other (distance 50); lenses and pigmented spots present, maximum diameter of lenses 23. External vertical setae (ve) absent; external scapular setae se situated just below eye lenses; internal scapular setae (si) distinctly posterior and medial to external scapulars (se). Supracoxal setae of legs I (scx) filiform, situated below setae se. Sejugal furrow well-developed. Propodosomal sclerite 80, hysterosomal shield 180, ratio hysterosomal shield/propodosomal sclerite length = 2.25. Hysterosomal shield with 11 pairs of simple, filiform setae (c1, c2, cp, d1, d2, e1, e2, f2, h1, h2, h3), setae h3 distinctly longer than others. Opisthonotal gland openings (gla) ventral; situated ventrally on hysterosomal shield, slightly posterior to setae c3. Of four fundamental pairs of cupules, only three pairs observed: ia posteriomedial of setae c2, im posterior to level of d2 and ih ventral, lateral to posterior sides of attachment organ.
Venter. Coxal fields sclerotized, smoothly punctate. Anterior apodemes of coxal fields I fused forming sternum. Sternum not reaching posterior border of sternal shield by distance exceeding its length. Posterior border of sternal shield weakly sclerotized. Anterior apodemes of coxal fields II curved medially. Posterior apodemes of coxal fields II weakly developed, thin, sternal and ventral shield adjacent. Anterior apodemes of coxal fields III free. Posterior medial apodeme in area of coxal fields IV weakly expressed. Posterior apodemes IV absent. Subhumeral setae (c3) long, filiform, situated ventrally between legs II–III, adjacent to region separating sternal and ventral shields. Coxal setae 1a, 3a reduced, represented by minute structures each situated in an alveolus. Setae 4b, g filiform; 4a small, rounded conoids, 4b longer than g. Genital region in posterior portion of coxal fields IV; genital opening elongate; there are two pairs of genital papillae; genital papillae two-segmented, with rounded apices. Coxal setae (4b) situated at anterior tips of coxal apodemes IV; genital setae (g) laterad of genital opening. Attachment organ posterior to coxal fields IV. Anterior suckers (ad3) round, median suckers (ad1+2) distinctly larger, with paired vestigial alveoli (not situated on a common sclerite); pair of small refractile spots anterolateral to median suckers (ps3); lateral conoidal setae of attachment organ (ps2) situated slightly posterior to line joining centers of median suckers, distinctly anterior conoidal setae (ps1) and slightly posterior to median suckers (ad1+2); anterior and posterior lateral and posterior median cuticular conoids well-developed; anus situated between anterior suckers (ad3).
Legs. Legs elongate, all segments free. Trochanters I–III each with long, filiform seta, pR I–II, sR III. Femoral setation 1-1-0-1; setae vF I–II and wF IV long, filiform. Genual setation 2-2-0-0; setae mG and cG I–II filiform, seta nG III absent. Tibial setation 2-2-1-1; setae hT I and II spiniform; setae gT I filiform, long (reaching the base ω3 I); gT II shorter, filiform; setae kT III somewhat spiniform, kT IV and has a distinct prong. Tarsal setation 7-8-8-8. All pretarsi consisting of hooked empodial claws arising from tarsal apices, and short, paired condylophores within tarsal apices. Tarsus I with three filiform setae (p, q, and d), three slightly foliate (ra, la, and f), and one spoon-shaped seta e; seta d elongated, longer than tarsus (its base situated at level of bases ra and la); seta s alveolar; setae wa, aa, and ba I absent; tarsus II similar to tarsus I except seta ba present and filiform, base of seta d posterior of bases ra and la. Tarsus III with eight setae (w, r, s, p, q, e, f, d) smooth; all setae, except d III foliate. Tarsus IV similar to tarsus III, except seta w filiform, with a distinct prong. Solenidia ω1 on tarsi I–II cylindrical, with slightly clavate apices, ω1 II longer than ω1 I. Solenidion ω3 on tarsus I longer and thinner than ω1, with rounded apex, positioned slightly anterior to ω1; ω1 and ω3 separated by bulbous famulus (ε). Solenidion ω2 of tarsus I thin, slightly widened apically, situated somewhat more basal and posterior to ω1+ε+ ω3 group. Solenidia φ of tibiae I–III elongate, tapering; φ I and III longer than tarsus I and III, respectively; φ II shorter than tarsus II; φ IV short. Solenidia σ of genu I elongate, slightly tapering, nearly reaching tip of tibia I; σ of genu II shorter, cylindrical, not reaching midlength of tibia II; σ of genu III absent.
Diagnosis
Female. Thyreophagus berxi is close to Th. spinitarsis (Fain, 1982) and Th. potawatomorum sp. n. by tarsi III with seven spiniform setae (e, u, v, p, q, s, w) and the prodorsal sclerite with linear striation extending over at least 75% of its length. Th. berxi differs from Th. spinitarsis by the vase-shaped atrium of the spermatheca, with the width in the central part two-times shorter than the width at the entrance to spermatheca (dome-shaped, width in central part vs. basal part is subequal in Th. spinitarsis). See above for the differences from Th. potawatomorum.
Heteromorphic deutonymph. Th. berxi is close to Th. corticalis and Th. potawatomorum by the following characters: body elongate (more than 1.7-times longer than wide), setae hT and gT I–II present, different in shape, bases of seta d are at the level of bases of ra and la on tarsus I. Th. berxi differs from Th. corticalis by the following character states: the posterior medial apodeme is weakly developed (absent in Th. corticalis); the diameter of ocelli is about 23 (19 in Th. corticalis); setae wa I are absent (present in Th. corticalis); seta kT IV with a distinct prong (vs. prong absent in Th. corticalis). See above for the differences from Th. potawatomorum.
4. Keys to Species of Thyreophagus of the World
Females
Adults of the following species are unknown: Th. africanus (Mahunka, 1974), Th. javensis (Oudemans, 1911), Th. sminthurus (Fain and Johnston, 1974), Th. johnstoni (Fain, 1982), Th. leclercqi (Fain, 1982), Th. rwandanus (Fain, 1982).
Not included (species inquirendae): Th. aleurophagus (Sicher, 1894), Th. angustus (Banks, 1906), Th. berlesianus (Zachvatkin, 1941), Th. entomophagus nominalis (Kadzhaya, 1973), Th. lignieri (Zachvatkin, 1953), Th. magnus (Berlese, 1910), Th. polezhaevi (Zachvatkin, 1953), Th. ponticus (Kadzhaya, 1973).
1 Very large species, body length > 1500 μm. Egypt………………………………………………………………………………Th. cynododactylon (El-Bishlawy, 1990).
- Smaller species, body length < 700 μm………………………………………………………………………………………………………………………………………2
2 Setae u and v on tarsi III–IV vestigial; seta wF IV absent. USA (Florida) …………………………………………………………………………………Th. hobe sp. n.
- Setae u and v on tarsi III–IV well-developed, spiniform; seta wF IV present……….……………………………………………………………………………………3.
3 Tarsus III with three ventral apical spiniform setae (s, u and v) well-developed, proral setae p and q vestigial or absent…………………………………………4.
- Tarsus III with five ventral apical spiniform setae well-developed (s, u, v, p, and q)……………………………………………………………………………………7.
4 Prodorsal sclerite wider than long, almost entirely punctate, with a few short longitudinal striations in posteromedian region; atrium of spermatheca in form of an inverted bell, with base 18–20 μm wide; seta w III filiform. Widespread…………………………………………………………………………Th. entomophagus (Laboulbène and Robin, 1862).
- Prodorsal shield distinctly longer than wide, almost entirely covered with fine longitudinal striations; atrium of spermatheca smaller, not in form of a bell; seta w III small spine or absent…………………………………………………………………………………………………………………………………………………………………………………5
5 Prodorsal sclerite with linear striation in posterior half of shield. Italy……………………………………………………………………Th. italicus (Vacante, 1989).
- Prodorsal sclerite with linear striation extending over at least 75% of its length………………………………………………………………………………………6
6 Idiosomal length 270–360 μm, width 87–150 μm; atrium of spermatheca dome-shaped, wider (6 μm) than long (5 μm) and not narrowed toward its center; seta w III a very short spine; seta se not longer than prodorsal shield. Morocco………………………………………………………………………………………………………Th. cooremani (Fain, 1982).
- Idiosomal length 525–675 μm, width 210–280 μm; atrium of spermatheca vase-shaped, 12 μm long, maximum width 12 μm, narrowed toward the center where it 5 μm wide; narrowed toward middle and widened in its proximal part; seta w III vestigial; seta se longer than prodorsal sclerite. Europe…………………………..Th. odyneri (Fain, 1982).
7 Seta w III filiform…………………………………………………………………………………………………………………………………………………………………8
- Seta w III spiniform or vestigial…………………………………………………………………………………………………………………………………………………13
8 Anterior margin of prodorsal sclerite without paired indentations…………………………………………………………………………………………………………9
- Anterior margin of prodorsal sclerite with paired indentations……………………………………………………………………………………………………………10
9 Atrium of spermatheca weakly developed; sclerotized portion of spermatheca in form of a broad arc, much wider than long. Cuba …Th. passerinus (Cruz, 1990).
- Atrium of spermatheca well-developed, vase-shaped. Kenya…………………………………………………Th. plocepasseri (Klimov, Mwangi, Vangansbeke, 2020).
10 Seta wa I absent. Ukraine…………………………………………………………………………………………………………Th. annae (Sevastianov and Kivganov, 1992).
- Seta wa I present, spiniform or vestigial………………………………………………………………………………………………………………………………………11
11 Solenidion σ’ I longer than σ’’ I; prodorsal sclerite at most 1.3-times as long as wide...................................................................................................................12
- Solenidion σ’ I shorter than σ’’ I; prodorsal sclerite 1.5-times longer than wide. Ireland……………………………………………………………Th. evansi (Fain, 1982).
12 Long terminal setae h2 and h3 with bases inflated, conical; base of spermatheca forming thin sclerotized arc that divided anteriorly into four short, fine sclerotized lines; genu I with solenidion σ’ short, 8 μm long, σ” 6 μm long (ratio 1.4:1); Great Britain ……………………………………………………………………………Th. macfarlanei (Fain, 1982).
- Long terminal setae h2 and h3 with very thin bases; atrium U-shaped with thick sides, 6 μm long, 5 μm wide; genu I with solenidion σ’ thin, 18–20 μm, σ” slightly thickened, 12 μm long (ratio 1.58: 1); Morocco …………………………………………………………………………………………………………………………………Th. athiasae (Fain, 1982).
13 Seta w III well-developed, spiniform…………………………………………………………………………………………………………………………………………14
- Seta w III vestigial………………………………………………………………………………………………………………………………………………………………25
14 Solenidion σ II very short, 2–3-times longer than its width; atrium absent or minute, much shorter than sclerites of oviducts (in Th. ais). Base of spermatheca in form of a broad arc, much wider than long, or small and rounded …………………………………………………………………………………………………………………………………………15
- Solenidion σ II longer; atrium, well-developed, longer than wide. Base of spermatheca variable …………………………………………………………………19
15 Solenidion σ II short and filiform or nearly conical, but with sides straight and not convex………………………………………………………………………16
- Solenidion σ II with convex sides…………………………………………………………………………………………………………………………………………17
16 Solenidion σ II short and filiform or nearly conical, but with sides straight and not convex; linear sclerites near the typical sclerites of oviducts present; paired sclerites of oviducts Y-shaped, not elongated, at least three-times longer than its width. Widespread ………………………………………………………...Th. gallegoi (Portus and Gomez, 1979).
- Solenidion σ II short and filiform; linear sclerites near the typical sclerites of oviducts absent; paired sclerites of oviducts elongated, more than five-times longer than their width, V-shaped. Great Britain ……………………………………………………………………………………………………………………………………Th. vermicularis (Fain and Lukoschus, 1982).
17 Paired sclerites of oviducts V-shaped; canal of spermatheca at entrance to spermatheca widened; bases of setae vi nearly touching, situated in common unsclerotized area. USA (Florida)…………………………………………………………………………………Th. Calusorum (Klimov, Demard, Stinson, Duarte, Wäckers, and Vangansbeke, 2022).
- Paired sclerites of oviducts Y-shaped; canal of spermatheca at entrance to spermatheca, uniform in width, not widened; bases of setae vi, separated, not in common area …………………………………………………………………………………………………………………………………………………………………………………………18
18 Cuplike portions of sclerites of oviducts distinctly shorter than their stems; solenidia σ’ and σ’’ subequal; solenidion ω1 II five-times longer than its width; solenidion φ IV reaching middle of tarsus IV. Mauritius ………………………………………………………………………………………………………………………………Th. mauritianus (Fain, 1982).
- Cuplike portions of sclerites of oviducts subequal; solenidia σ’ distinctly longer than σ’’; solenidion ω1 II three-times longer than its width; solenidion φ IV longer, nearly reaching bases of setae d IV. USA (Florida) ……………………………………………………………………………………………………………………………………………Th. ais sp. n.
19 Seta wa I present…………………………………………………………………………………………………………………………………………………………………20
- Seta wa I absent…………………………………………………………………………………………………………………………………………………………………………21
20 Anterior margin of prodorsal shield without paired indentations; prodorsal shield smoothly punctated; posterior hysterosomal seta h1 less than half of length of h2. Colombia………………………………………………………………………………………………………………………………………Th. incanus (Fain and Rack, 1987).
- Anterior margin of prodorsal shield with paired indentations; prodorsal shield with linear striation extending over at least 75% of its length; posterior hysterosomal seta h1 half of length of h2. Europe……………………………………………………………………………………………………………………………………………………Th. spinitarsis (Fain, 1982).
21 With one pair of large, sclerotized, funnel-like, internal structures near posterior end of body (not to be confused with sclerites oviducts) ………………………22
- Without paired, funnel-like structures in posterior body……………………………………………………………………………………………………………………………23
22 Solenidion φ of tibia IV very short (4 μm); USA (California) …………………………………………………………………………Th. tridens (Fain and Lukoschus, 1986).
- Solenidion φ of tibia IV longer (14 μm). Brazil…………………………………………………………………………Th. cracentiseta Barbosa, (OConnor and Moraes, 2016).
23 Setae h1, h2, and h3 very long (2.5–2.8-times longer than length of prodorsal shield), similar in length. New Zealand……………………………Th. australis (Clark, 2009).
- Setae h2 and h3 less than 2.5-times longer than length of prodorsal shield, seta h1 less than half of length of h2………………………………………………………………24
24 Setae vi short, not extending beyond anterior margin of prodorsum; bases of vi touching; medial area of prodorsal shield with non-uniform pattern of striations in its anterior and posterior parts; setae h2 and h3 shorter than anus; rounded sclerotized capsule at junction of canal of spermatheca and atrium present. USA (Michigan)………………………………………………………………………………………………………………………………………………………Th. potawatomorum sp. n.
- Setae vi long, extending beyond anterior margin of prodorsum; bases of setae vi distinctly separated; entire medial area of prodorsal shield uniformly striated; setae h2 and h3 longer than anus; rounded capsule at junction of canal of spermatheca and atrium absent. France, Belgium, Germany……………………………………………………………Th. berxi sp. n.
25 Setae h2 and h3 1.1–1.25-times longer than length of prodorsal shield; diameter of genital papillae approximately six-times shorter than length of genital setae. Palaearctic ………………………………………………………………………………………………………………………………………………………………………Th. corticalis (Michael, 1885).
- Setae h2 and h3, long, their lengths 1.75-times longer than length of prodorsal shield; diameter of genital papillae approximately four-times shorter than length of genital setae. USA (Michigan), Canada (Ontario) …………………………………………………………………………………………………………………………………………Th. ojibwe sp. n.
Males (modified after [
1])
Adults of the following species are unknown: Th. africanus (Mahunka, 1974), Th. javensis (Oudemans, 1911), Th. sminthurus (Fain and Johnston, 1974), Th. johnstoni (Fain, 1982), Th. leclercqi (Fain, 1982), and Th. rwandanus (Fain, 1982).
Not included (species inquirendae): Th. aleurophagus (Sicher, 1894), Th. angustus (Banks, 1906), Th. berlesianus (Zachvatkin, 1941), Th. entomophagus nominalis (Kadzhaya, 1973), Th. lignieri (Zachvatkin, 1953), Th. magnus (Berlese, 1910), Th. polezhaevi (Zachvatkin, 1953), and Th. ponticus (Kadzhaya, 1973).
Males are unknown in Th. athiasae, Th. plocepasseri, Th. polezhaevi, Th. cooremani, Th. calusorum, Th. evansi, Th. macfarlanei, Th. spinitarsis, Th. tridens, Th. vermicularis, Th. ais, Th. berxi, Th. hobe, Th. potawatomorum, and Th. mauritianus.
Not included (species inquirendae): Th. aleurophagus, Th. angustus, Th. berlesianus, Th. italicus, Th. lignieri, Th. magnus, and Th. ponticus.
1 Prodorsal sclerite smoothly punctulate, without longitudinal striations……………………………………………………………………………………………………………2
- Prodorsal sclerite with short longitudinal striations, at least near posterior margin…………………………………………………………………………………………………5
2 Posterior venter with sclerotized projection very poorly developed or absent. Colombia…………………………………………………….Th. incanus (Fain and Rack, 1987).
- Posterior venter with sclerotized projection well-developed…………………………………………………………………………………………………………………………3
3 Body elongate, six-times longer than wide; large species, length > 700 μm. Egypt……………………………………………….Th. cynododactylon (El-Bishlawy, 1990).
- Body ovoid, 1.5–2-times longer than wide; small species, length < 500 μm. Widespread……………………………………………………………………………………………...4
4 Tarsus IV with five spine-like setae (s, u, v, p, q), three filiform setae (f, r, w), and two suckers (d, e). Ireland…………………………………………………….Th. evansi (Fain, 1982).
- Tarsus IV with three spine-like setae (s, u, v), three filiform setae (f, r, w), and two suckers (d, e). Widespread………………Th. entomophagus (Laboulbène and Robin, 1862).
5 Posterior venter with distinct rounded, sclerotized projection…………………………………………………………………………………………………………………………6
- Posterior body smoothly rounded, without ventral projection…………………………………………………………………………………………………………………………8
6 Entire width of prodorsal sclerite covered by longitudinal striation. Europe……………………………………………………………………………Th. odyneri (Fain, 1982).
- Longitudinal striation on prodorsal sclerite restricted to median region, lateral areas simply punctulate……………………………………………………………………………7
7 Setae h2 and h3 1.1–1.25-times longer than length of prodorsal shield; spines p, q, and u, v III–IV subequal. Palaearctic…………………………..Th. corticalis (Michael, 1885).
- Setae h2 and h3 long, their lengths are 1.8-times longer than the length of the prodorsal shield; spines p and q shorter than spines u and v on tarsi III–IV. USA (Michigan), Canada (Ontario). …………………………………………………………………………………………………………………………………………………………………………………Th. ojibwe sp. n.
8 Posterior hysterosoma with a large sclerotized area extending posteriad from level of setae e2. Ukraine……………………………..Th. annae (Sevastyanov and Kivganov, 1992).
- Posterior hysterosoma unsclerotized or at most with short terminal sclerotization posterior to setae h1…………………………………………………………………………9
9 Posterior idiosoma with short sclerotized area posterior to setae h1…………………………………………………………………………………………………………………10
- Posterior idiosoma unsclerotized…………………………………………………………………………………………………………………………………………………………11
10 Genu I with solenidia σ’ and σ” approximately equal in length. Widespread……………………………………………………………Th. gallegoi (Portus and Gomez, 1979).
- Genu I with solenidion σ’ only half of length of σ”. Cuba………………………………………………………………………………………………Th. passerinus (Cruz, 1990).
11 Dorsal hysterosomal setae relatively long, setae d2 and e2 much longer than distance between their alveoli. New Zealand………………………Th. australis (Clark, 2009).
- Dorsal hysterosomal setae much shorter, setae d2 and e2 shorter than distance between their alveoli. Brazil……….Th. cracentiseta (Barbosa, Oconnor, and Moraes, 2016).
Heteromorphic deutonymphs
Unknown for the following species: Th. aleurophagus, Th. angustus, Th. annae, Th. athiasae, Th. cooremani, Th. cracentiseta, Th. cynododactylon, Th. entomophagus nominalis, Th. evansi, Th. gallegoi, Th. hobe, Th. incanus, Th. italicus, Th. macfarlanei, Th. magnus, Th. mauritianus, Th. ojibwe, Th. odyneri, Th. passerinus, Th. plocepasseri, Th. polezhaevi, Th. ponticus, Th. spinitarsis, Th. tridens, and Th. vermicularis.
Not included (species inquirendae): Th. berlesianus, Th. lignieri.
Published measurements of T. corticalis (distance between ocelli) were corrected.
1 Dorsal surface completely striated. Afrotropical………………………………………………………………………………………………………………Th. africanus (Mahunka, 1974).
- Dorsal surface smoothly punctate, without striations…………………………………………………………………………………………………………………………………………2
2 Body ovoid, 1.3–1.5-times longer than wide…………………………………………………………………………………………………………………………………………………3
- Body elongate, more than 1.7-times longer than wide…………………………………………………………………………………………………………………………………………5
3 Seta hT I more than half the length of gT I. Europe………………………………………………………………………………………………………………….Th. leclercqi (Fain, 1982).
- Seta hT I less than half the length of gT I……………………………………………………………………………………………………………………………………………………4
4 Opisthonotal gland openings approximately equidistant from setae c3 and cp; seta kT III filiform; setae wa I–II absent. Widespread…Th. entomophagus (Laboulbène and Robin, 1862).
- Opisthonotal gland openings much closer to ventral seta c3 than to dorsolateral seta cp; seta kT III with distinct prong; setae wa I-II present. New Zealand…Th. australis (Clark, 2009).
5 Hysterosomal sclerite about 1.7-times longer than prodorsal sclerite…………………………………………………………………………………………………………………6
- Hysterosomal sclerite about two-times longer than prodorsal sclerite…………………………………………………………………………………………………………………7
6 Seta hT I absent; posterior medial apodeme in area of coxal fields IV present; seta kT III with distinct prong. Great Britain, USA (Washington) …Th. sminthurus (Fain and Johnston, 1974).
- Seta hT I present; posterior medial apodeme in area of coxal fields IV absent; seta kT III without prong, filiform. USA (Maryland)………………………Th. johnstoni (Fain, 1982).
7 Setae hT II and gT II subequal ………………………………………………………………………………………………………………………………………………………………………8
- Setae hT II twice the length of gT II…………………………………………………………………………………………………………………………………………………………………9
8 Setae hT II and gT II filiform, both subequal to tibia II. Java………………………………………………………………………………………………Th. javensis (Oudemans, 1911).
- Setae hT II and gT II spiniform, shorter than half the length of tibia. Afrotropical………………………………………………………………………………Th. rwandanus (Fain, 1982).
9 On tarsus I, bases of setae d situated at the same level with bases of setae ra and la; diameter of ocellus 16–23 μm …………………………………………………………………10
- On tarsus I, bases of setae d distal to bases of setae ra and la; diameter of ocellus 10–14 μm …………………………………………………………………………………………….12
10 Width of ocellus 16 μm, distance between ocelli 36 μm; posterior medial apodeme in area of coxal fields IV present. USA (Michigan)………………..Th. potawatomorum sp. n.
- Width of ocellus about 18–23 μm, distance between ocelli about 40–50 μm; posterior medial apodeme in area of coxal fields IV weakly developed or absent………………….11
11 On tibia IV, seta kT IV with distinct prong; width of ocellus 23 μm, distance between ocelli 50 μm; posterior medial apodeme in area of coxal fields IV weakly developed. Europe……………………………………………………………………………………………………………………………………………………………………………………Th. berxi sp. n.
- On tibia IV, seta kT IV without prong; width of ocellus about 18–19 μm, distance between ocelli about 40–42 μm; posterior medial apodeme in area of coxal fields IV absent. Widespread………………………………………………………………………………………………………………………………………………………………Th. corticalis (Michael, 1885).
12 Tarsal setae p and q on tarsus IV foliate; seta d III shorter than leg III; setae d IV shorter or slightly longer than leg IV; diameter of ocellus 10–13. USA (Florida)……………………………………………………………………………………………………………………Th. calusorum (Klimov, Demard, Stinson, Duarte, Wäckers and Vangansbeke, 2022)
- Tarsal setae p and q on tarsus IV short, spiniform; setae d III longer than leg III, d IV distinctly longer than leg IV; diameter of ocellus 12–14. USA (Florida)……….Th. ais sp. n.