ERK/NF-kB/COX-2 Signaling Pathway Plays a Key Role in Curcumin Protection against Acetaminophen-Induced Liver Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Model and Treatment
2.2. Liver Function Assessment
2.3. GSH Assessment in Liver
2.4. Liver MPO Activity Assay
2.5. Histology
2.6. Quantifying Cytokine Levels in Mouse Liver
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
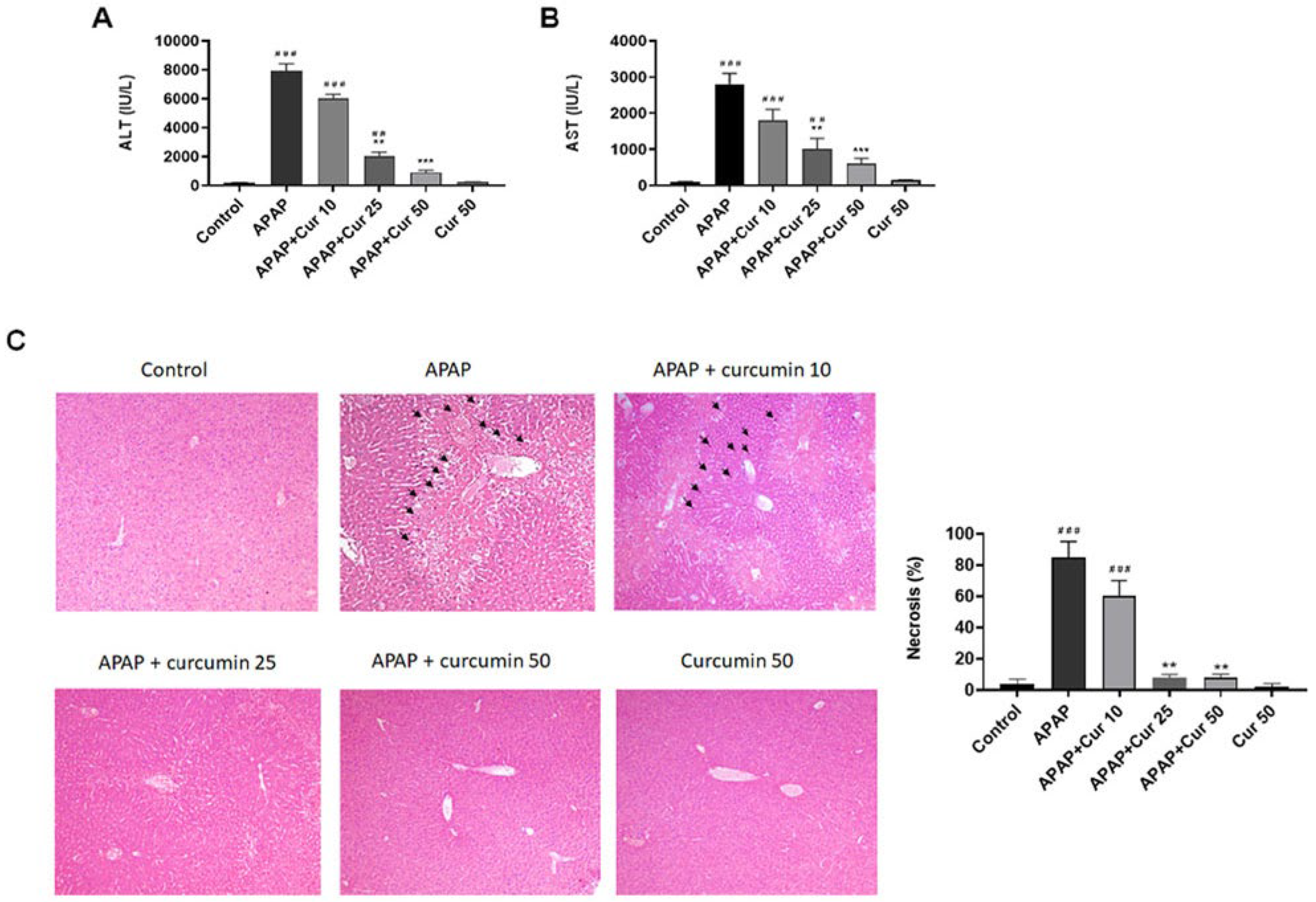
3.1. Protective Effects of Curcumin against APAP-Induced Liver Injury
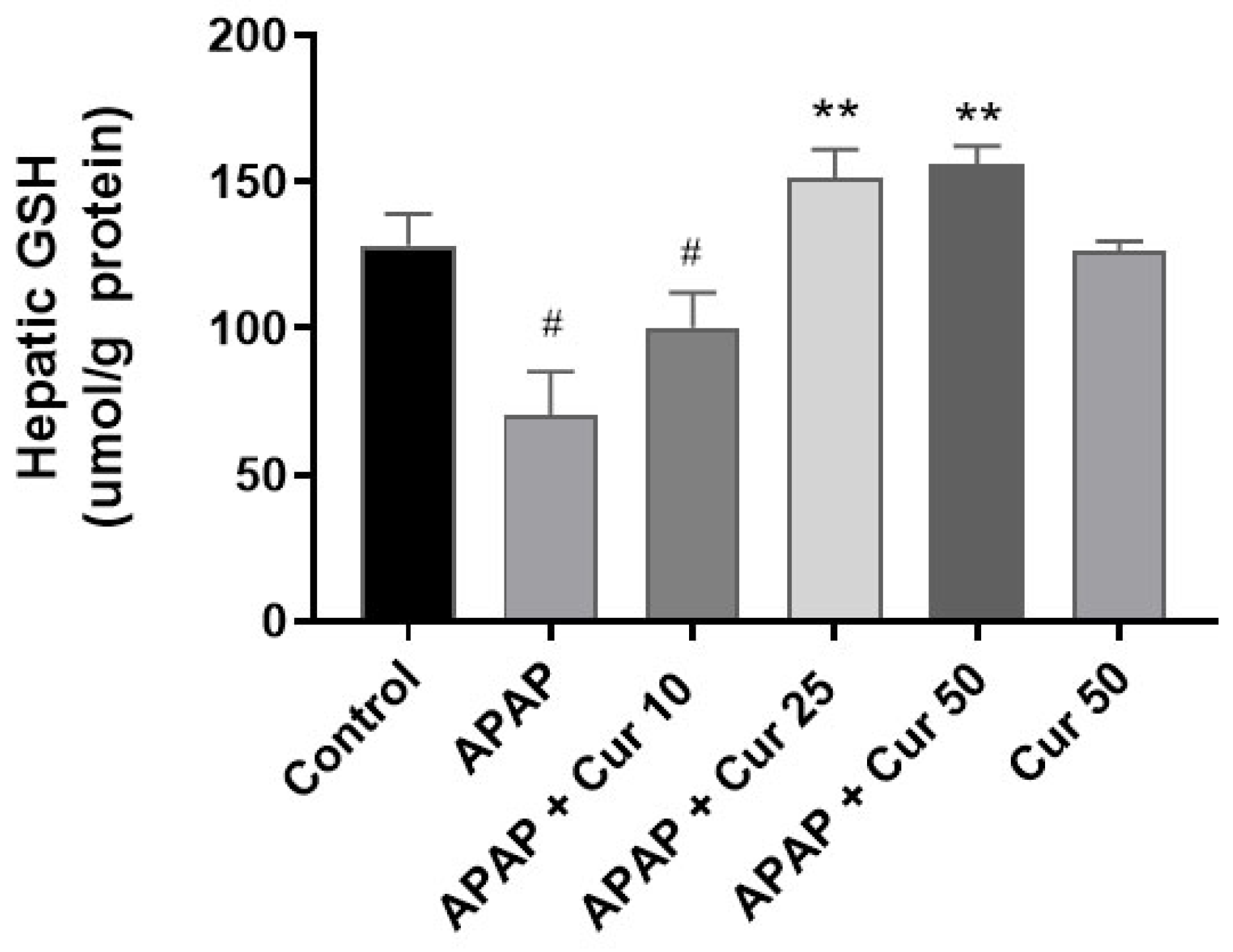
3.2. Effects of Curcumin on Hepatic GSH Expression Levels
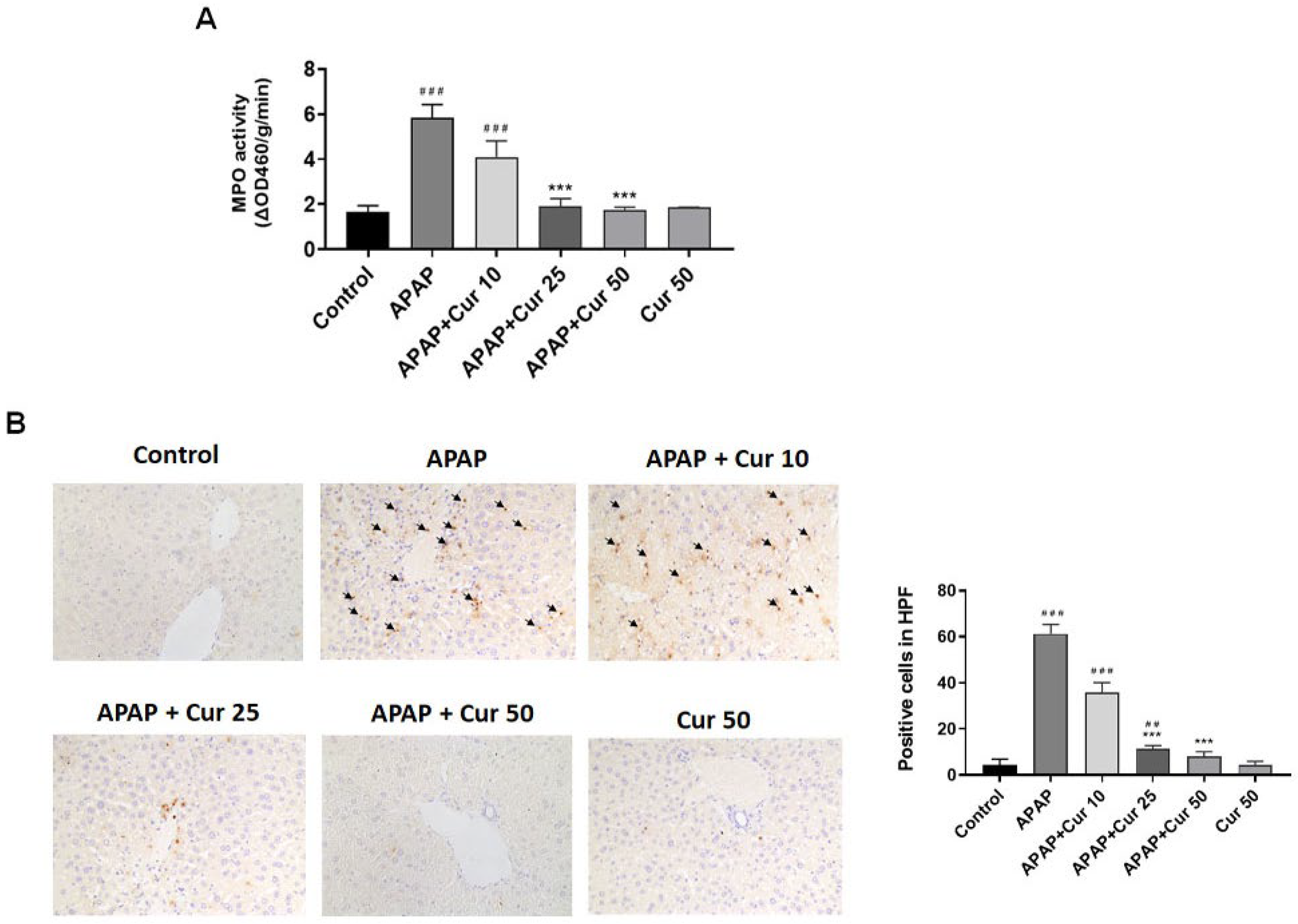
3.3. Effects of Curcumin on MPO Activity and Neutrophil Accumulation in APAP-Induced Liver Injury
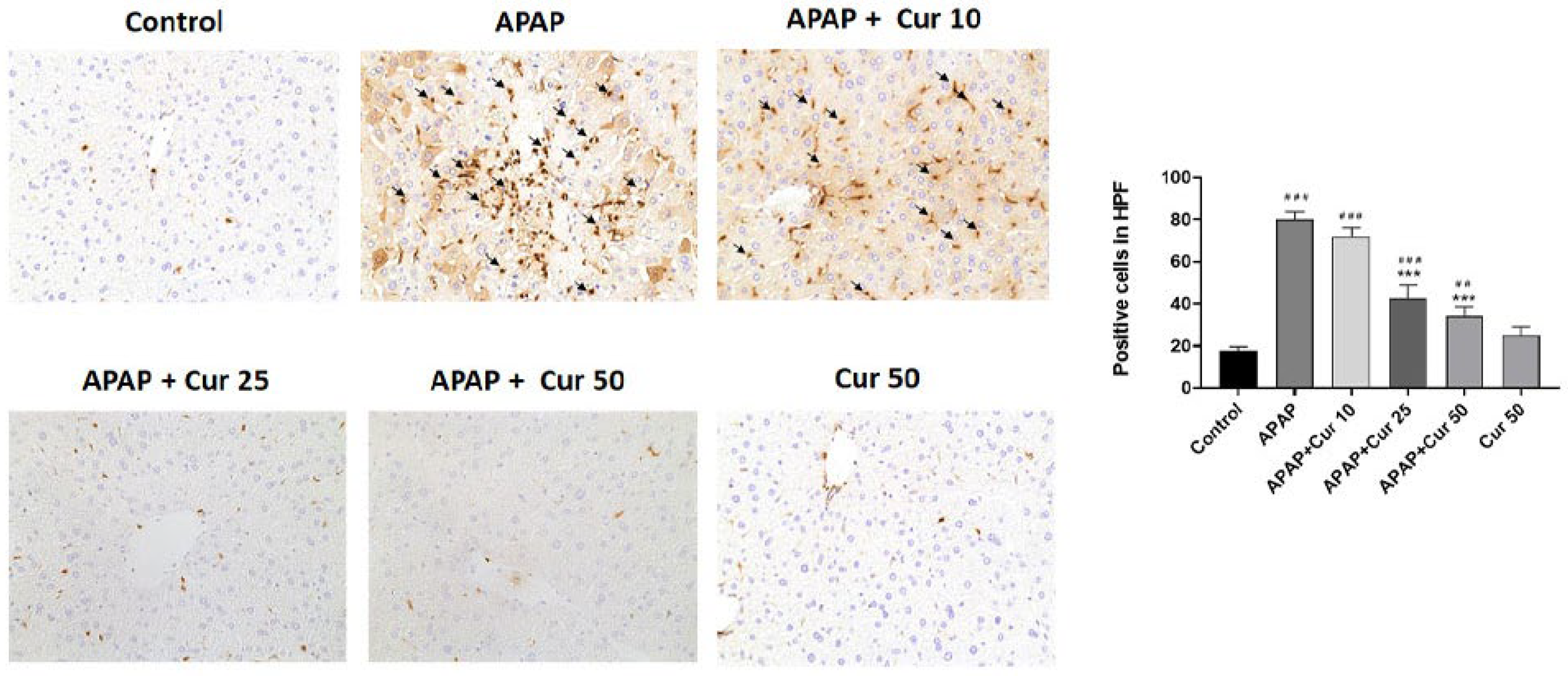
3.4. Effects of Curcumin on Macrophages in APAP-Induced Liver Injury
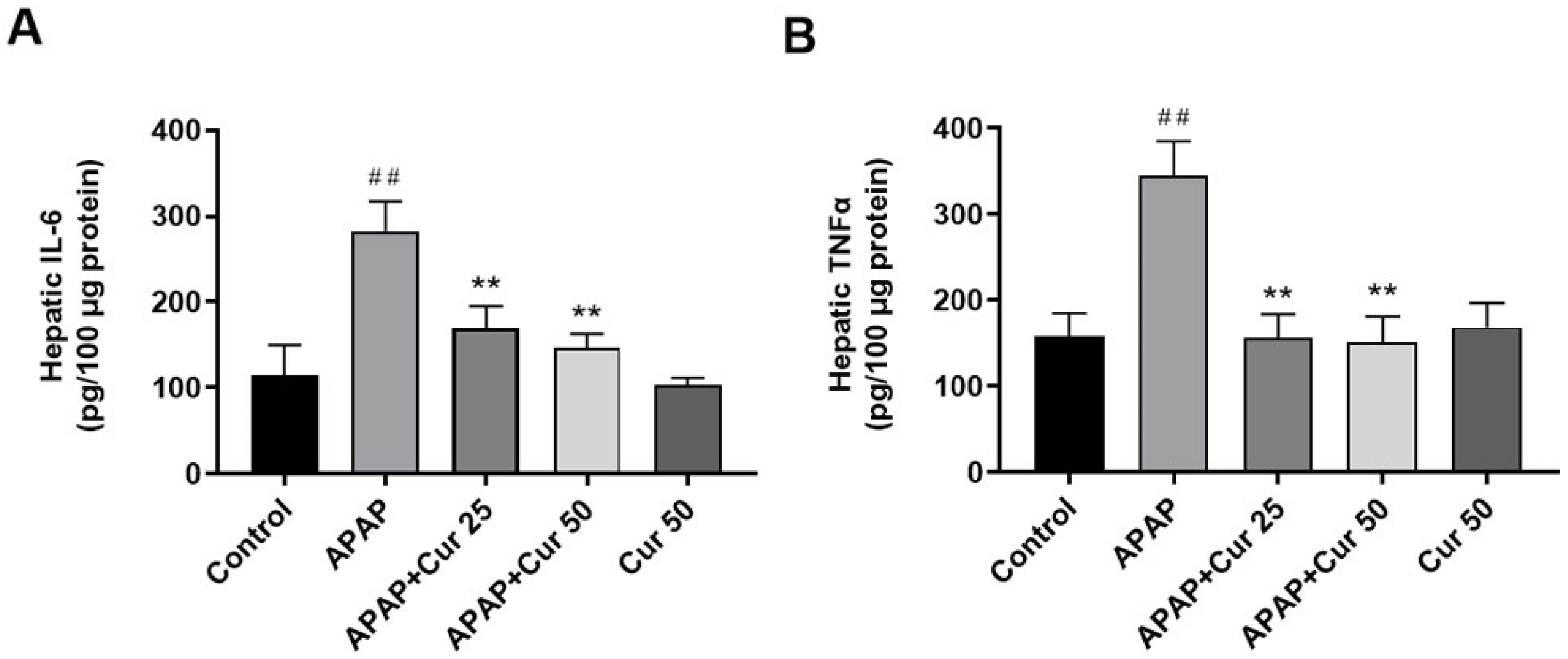
3.5. Effects of Curcumin on Hepatic IL-6 and TNF-α Expression Levels
3.6. Effects of Curcumin on Hepatic ERK, NF-kB, and COX-2 Protein Expression and Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiødt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.; Corey, R.; Leonard, M.; Eghtesad, B. Acetaminophen: Old drug, new warnings. Cleve. Clin. J. Med. 2010, 77, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.G.; Quaglia, A.; Taams, L.S.; Mitry, R.R.; Hussain, M.; Abeles, R.; Possamai, L.A.; Bruce, M.; McPhail, M.; Starling, C.; et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012, 56, 735–746. [Google Scholar] [CrossRef]
- Lancaster, E.M.; Hiatt, J.R.; Zarrinpar, A. Acetaminophen hepatotoxicity: An updated review. Arch. Toxicol. 2015, 89, 193–199. [Google Scholar] [CrossRef]
- Bessems, J.G.; Vermeulen, N.P. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues, and protective approaches. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef]
- James, L.P.; McCullough, S.S.; Knight, T.R.; Jaeschke, H.; Hinson, J.A. Acetaminophen toxicity in mice lacking NADPH oxidase activity: Role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic. Res. 2003, 37, 1289–1297. [Google Scholar] [CrossRef]
- Boulares, A.H.; Ren, T. Mechanism of acetaminophen-induced apoptosis in cultured cells: Roles of caspase-3, DNA fragmentation factor, and the Ca2+ and Mg2+ endonuclease DNAS1L3. Basic. Clin. Pharmacol. Toxicol. 2004, 94, 19–29. [Google Scholar] [CrossRef]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar]
- Williams, C.D.; Bajt, M.L.; Farhood, A.; Jaeschke, H. Acetaminophen induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010, 30, 1280–1292. [Google Scholar] [CrossRef]
- Liu, Z.X.; Govindarajan, S.; Kaplowitz, N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004, 127, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Day, Y.J.; Lee, H.C.; Liou, J.T.; Chou, A.H.; Liu, F.C. ERK Signaling Pathway Plays a Key Role in Baicalin Protection against Acetaminophen-Induced Liver Injury. Am. J. Chin. Med. 2017, 45, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Nat transport, and cisplatin induced apoptosis in renal cells. J. Biol. Chem. 2002, 277, 43377–43388. [Google Scholar] [CrossRef]
- Wang, A.Y.; Lian, L.H.; Jiang, Y.Z.; Wu, Y.L.; Nan, J.X. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J. Gastroenterol. 2010, 16, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.W.; Kirk, M.A.; Furbee, R.B.; Mehta, N.H.; Skinner, J.R.; Brizendine, E.J. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann. Emerg. Med. 2003, 42, 741–750. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Rajakrishnan, V.; Jayadeep, A.; Arun, O.S.; Sudhakaran, P.R.; Menon, V.P. Changes in the prostaglandin levels in alcohol toxicity: Effect of curcumin and N-acetylcysteine. J. Nutr. Biochem. 2000, 11, 509–514. [Google Scholar]
- EL-Maraghy, S.A.; Rizk, S.M.; El-Sawalhi, M.M. Hepatoprotective potential of crocin and curcumin against iron overload-induced biochemical alterations in rat. Afr. J. Biochem. Res. 2009, 3, 215–221. [Google Scholar]
- Rivera-Espinoza, Y.; Muriel, P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009, 29, 1457–1466. [Google Scholar] [CrossRef]
- Messner, D.J.; Sivam, G.; Kowdley, K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009, 29, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic diseases. J. Parenter. Enteral. Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, X.; Shu, S.; Zhang, W.; Fang, B.; Chen, X.; Zhao, Y.; Liu, Z.; Liang, G. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI). Eur. J. Med. Chem. 2019, 167, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, X.; Hu, X.; Yin, S.; Chen, J.; He, H.; Hong, S.; Yang, B.; Singh, K.K.; Feng, J.; et al. Curcumin analogue C66 attenuates obesity-induced renal injury by inhibiting chronic inflammation. Biomed. Pharmacother. 2021, 137, 111418. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Nagy, L.I.; Berko, A.; Hoffmann, A.; Fehér, L.Z.; Bagyánszki, M.; Kari, B.; Balog, J.A.; Hackler, L., Jr.; Kanizsai, I.; et al. The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis. Molecules 2019, 24, 1546. [Google Scholar] [CrossRef]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: Role of TGF-beta modulation and oxidative stress. Fundam. Clin. Pharmacol. 2008, 22, 417–427. [Google Scholar] [CrossRef]
- Kheradpezhouh, E.; Panjehshahin, M.R.; Miri, R.; Javidnia, K.; Noorafshan, A.; Monabati, A.; Dehpour, A.R. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur. J. Pharmacol. 2010, 628, 274–281. [Google Scholar] [CrossRef]
- Luo, D.D.; Chen, J.F.; Liu, J.J.; Xie, J.H.; Zhang, Z.B.; Gu, J.Y.; Zhuo, J.Y.; Huang, S.; Su, Z.R.; Sun, Z.H. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: Role of CYP2E1 and Keap1-Nrf2 pathway. Food. Chem. Toxicol. 2019, 123, 349–362. [Google Scholar] [CrossRef]
- Chou, A.H.; Liao, C.C.; Lee, H.C.; Liou, J.T.; Liu, F.C. The MAP2K4/JNK/c-Jun Signaling Pathway Plays a Key Role in Dexmedetomidine Protection Against Acetaminophen-Induced Liver Toxicity. Drug. Des. Devel. Ther. 2019, 13, 3887–3898. [Google Scholar] [CrossRef]
- Suh, Y.J.; Yun, H.J.; Kim, Y.B.; Kang, E.J.; Choi, J.H.; Choi, Y.K.; Lee, I.B.; Choi, D.H.; Seo, Y.J.; Noh, J.R.; et al. Hepatocyte-Specific Deficiency of DAX-1 Protects Mice from Acetaminophen-Induced Hepatotoxicity by Activating NRF2 Signaling. Int. J. Mol. Sci. 2022, 23, 11786. [Google Scholar] [CrossRef]
- Xia, Y.; Zweier, J.L. Measurement of Myeloperoxidase in Leukocyte-ContainingTissuee. Anal. Biochem. 1997, 245, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Eisei, N.; Akihide, N.; Koji, U.; Hirokazu, T.; Minoru, O.; Toshiro, F.; Sergey, B.; Michael, S.G. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. 2001, 281, F948–F957. [Google Scholar]
- Karamalakova, Y.D.; Nikolova, G.D.; Georgiev, T.K.; Gadjeva, V.G.; Tolekova, A.N. Hepatoprotective properties of Curcuma longa L. extract in bleomycin-induced chronic hepatotoxicity. Drug. Discov. Ther. 2019, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Kuo, J.J.; Chang, H.H.; Tsai, T.H.; Lee, T.Y. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int. J. Mol. Med. 2012, 30, 673–679. [Google Scholar] [CrossRef]
- Wendel, A. Glutathione peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 77, pp. 325–333. [Google Scholar]
- Mendes-Braz, M.; Elias-Miró, M.; Jiménez-Castro, M.B.; Casillas-Ramóırez, A.; Ramalho, F.S.; Peralta, C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J. Biomed. Biotechnol. 2012, 2012, 298657. [Google Scholar] [CrossRef]
- Bertola, A.; Park, O.; Gao, B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: A critical role for E-selectin. Hepatology 2013, 58, 1814–1823. [Google Scholar] [CrossRef]
- Liu, Z.X.; Han, D.; Gunawan, B.; Kaplowitz, N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006, 43, 1220–1230. [Google Scholar] [CrossRef]
- Holt, M.P.; Cheng, L.; Ju, C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008, 84, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Warner, T.D. Cyclo-oxygenase 2: Pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999, 128, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Frejborg, E.; Salo, T.; Salem, A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 9246. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARgamma-NF-kappaB signaling pathway. Food. Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef]
- Yu, S.M.; Kim, S.J. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int. J. Mol. Med. 2015, 35, 325–332. [Google Scholar] [CrossRef]
- Jo, S.K.; Cho, W.Y.; Sung, S.A.; Kim, H.K.; Won, N.H. MEK inhibitor, U0126, attenuates cisplatin induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005, 67, 458–466. [Google Scholar] [CrossRef]
- Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Gang, G.T.; Kim, K.S.; Lee, I.K.; Yun, B.S.; Lee, C.H. Davallialactone protects against acetaminophen overdose-induced liver injuries in mice. Food Chem. Toxicol. 2013, 58, 14–21. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Tung, W.H.; Hsieh, H.L.; Yang, C.M. Enterovirus 71 induces COX-2 expression via MAPKs, NF-kappaB, and AP-1 in SK-N-SH cells: Role of PGE (2) in viral replication. Cell Signal. 2010, 22, 234–246. [Google Scholar] [CrossRef]
- Kim, S.J.; Jeong, H.J.; Moon, P.D.; Myung, N.Y.; Kim, M.C.; Kang, T.H.; Lee, K.M.; Park, R.K.; So, H.S.; Kim, E.C.; et al. The COX-2 inhibitor SC-236 exerts anti-inflammatory effects by suppressing phosphorylation of ERK in a murine model. Life Sci. 2007, 81, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, A.-H.; Lee, H.-C.; Liao, C.-C.; Yu, H.-P.; Liu, F.-C. ERK/NF-kB/COX-2 Signaling Pathway Plays a Key Role in Curcumin Protection against Acetaminophen-Induced Liver Injury. Life 2023, 13, 2150. https://doi.org/10.3390/life13112150
Chou A-H, Lee H-C, Liao C-C, Yu H-P, Liu F-C. ERK/NF-kB/COX-2 Signaling Pathway Plays a Key Role in Curcumin Protection against Acetaminophen-Induced Liver Injury. Life. 2023; 13(11):2150. https://doi.org/10.3390/life13112150
Chicago/Turabian StyleChou, An-Hsun, Hung-Chen Lee, Chia-Chih Liao, Huang-Ping Yu, and Fu-Chao Liu. 2023. "ERK/NF-kB/COX-2 Signaling Pathway Plays a Key Role in Curcumin Protection against Acetaminophen-Induced Liver Injury" Life 13, no. 11: 2150. https://doi.org/10.3390/life13112150
APA StyleChou, A.-H., Lee, H.-C., Liao, C.-C., Yu, H.-P., & Liu, F.-C. (2023). ERK/NF-kB/COX-2 Signaling Pathway Plays a Key Role in Curcumin Protection against Acetaminophen-Induced Liver Injury. Life, 13(11), 2150. https://doi.org/10.3390/life13112150






