Laser Therapy for Vulvar Lichen Sclerosus, a Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Clinical trials that evaluated laser treatment in LS compared to a control group;
- Studies focused on women with proven genital LS;
- Studies reporting the clinical response to this therapy, whether histological, clinical, or quality-of-life scales.
- Other types of studies apart from clinical trials, including other systematic reviews or meta-analyses;
- In vitro or in vivo animal trials;
- Extragenital forms of LS and male patients.
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Synthesis of Results
3. Results
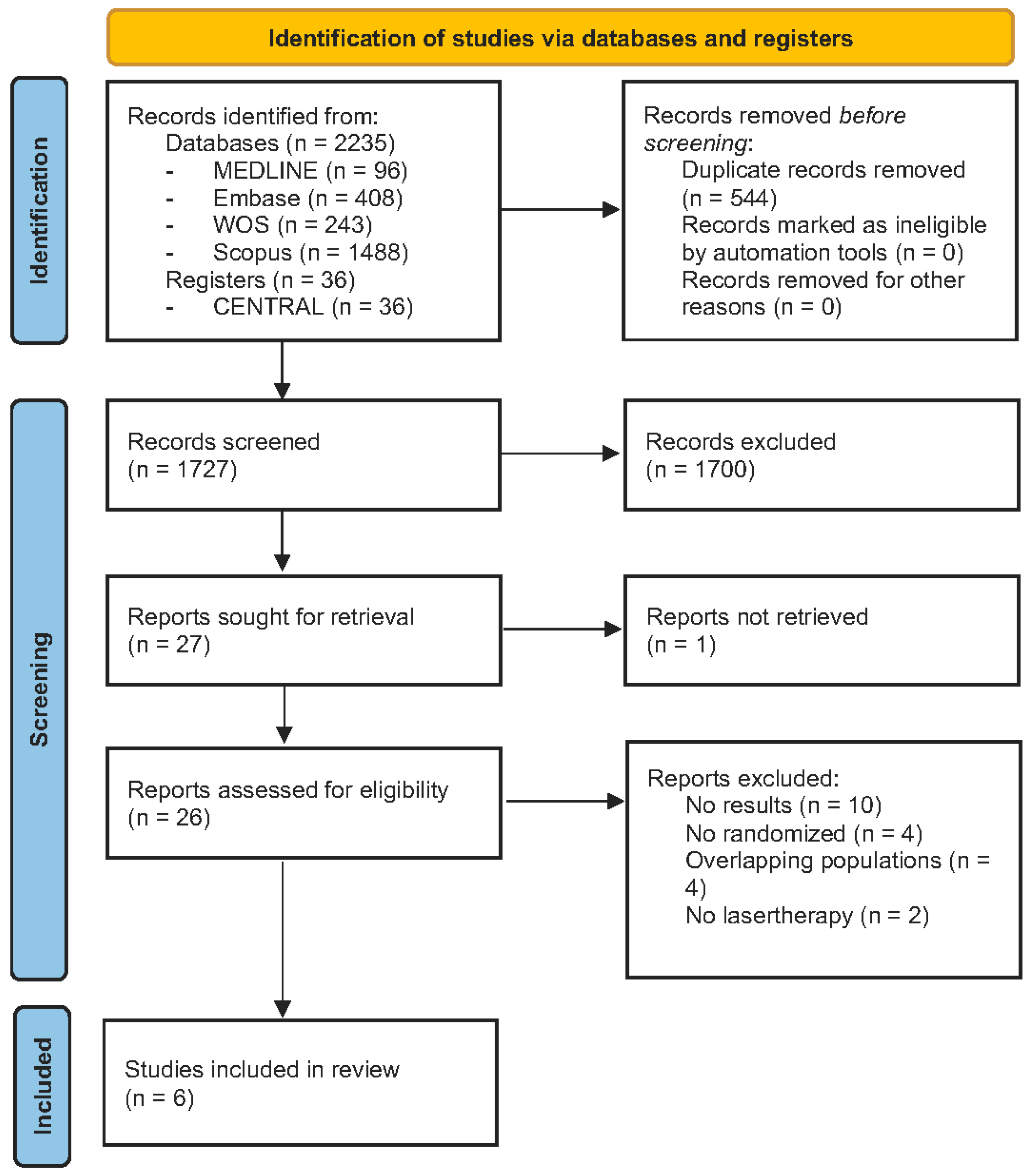
3.1. Study Selection
3.2. Study Characteristics
3.3. Patients Features
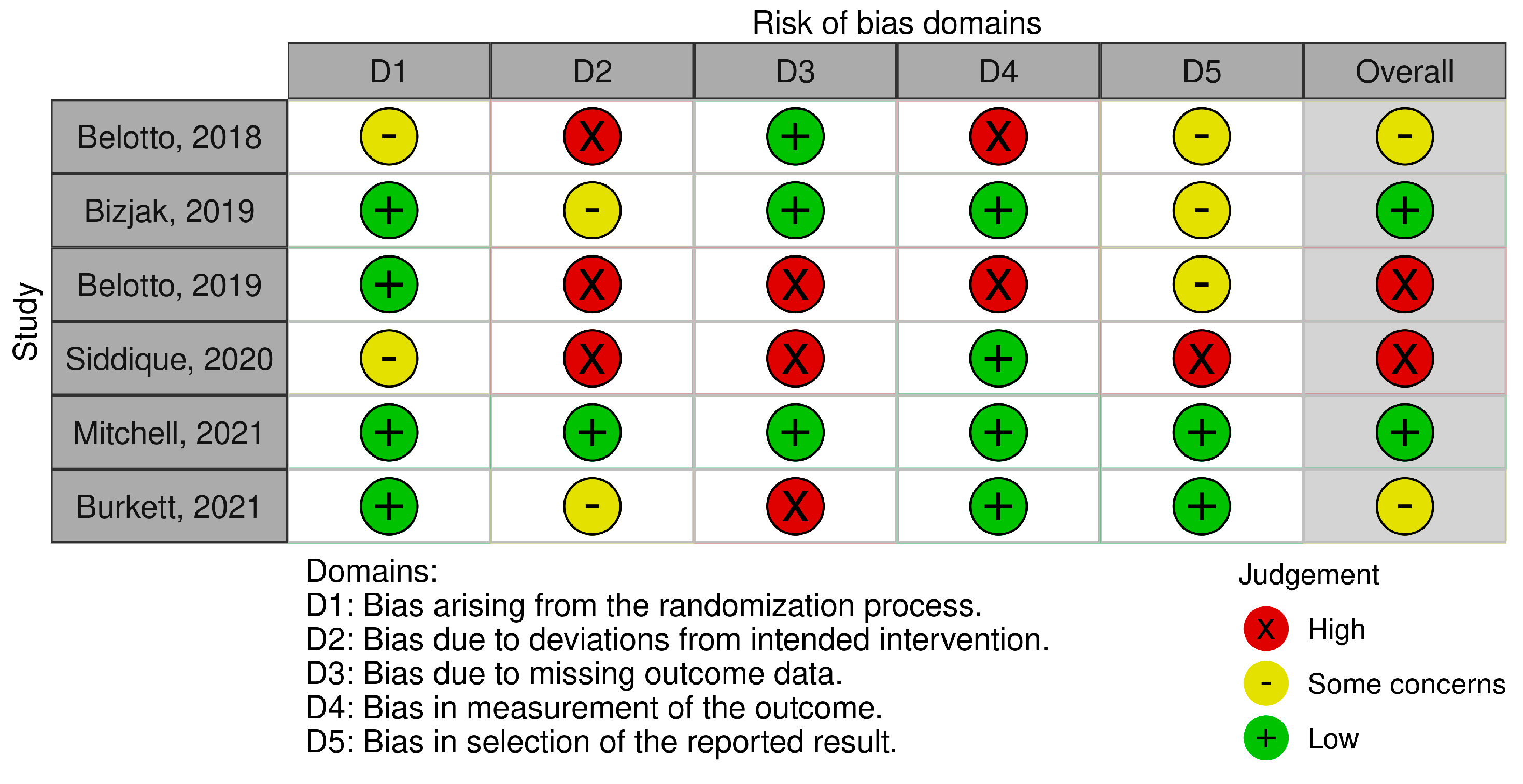
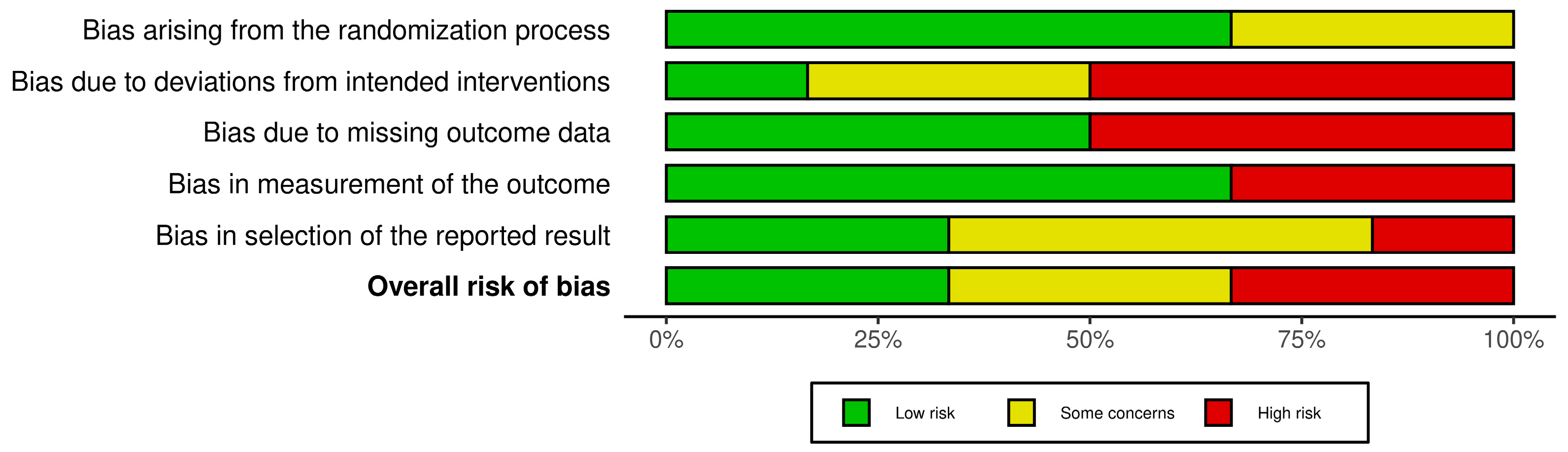
3.4. Risk of Bias Analysis
3.5. Clinical and Histopathological Outcomes
4. Discussion
4.1. Limitations of the Studies
4.2. Outstanding Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Luca, D.A.; Papara, C.; Vorobyev, A.; Staiger, H.; Bieber, K.; Thaçi, D.; Ludwig, R.J. Lichen sclerosus: The 2023 update. Front. Med. 2023, 10, 1106318. [Google Scholar] [CrossRef]
- Tasker, F.; Kirby, L.; Grindlay, D.J.C.; Lewis, F.; Simpson, R.C. Laser therapy for genital lichen sclerosus: A systematic review of the current evidence base. Skin Health Dis. 2021, 1, e52. [Google Scholar] [CrossRef]
- Günthert, A.R.; Duclos, K.; Jahns, B.G.; Krause, E.; Amann, E.; Limacher, A.; Mueller, M.D.; Jüni, P. Clinical scoring system for vulvar lichen sclerosus. J. Sex Med. 2012, 9, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Naswa, S.; Marfatia, Y.S. Physician-administered clinical score of vulvar lichen sclerosus: A study of 36 cases. Indian J. Sex Transm. Dis. AIDS 2015, 36, 174–177. [Google Scholar] [CrossRef]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.G.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) Consensus Statements on Pre-invasive Vulvar Lesions. J. Low Genit. Tract. Dis. 2022, 26, 229–244. [Google Scholar] [CrossRef]
- Preti, M.; Borella, F.; Ferretti, S.; Caldarella, A.; Corazza, M.; Micheletti, L.; De Magnis, A.; Borghi, A.; Salvini, C.; Gallio, N.; et al. Genital and extragenital oncological risk in women with vulvar lichen sclerosus: A multi-center Italian study. Maturitas 2023, 175, 107767. [Google Scholar] [CrossRef] [PubMed]
- Krapf, J.M.; Mitchell, L.; Holton, M.A.; Goldstein, A.T. Vulvar Lichen Sclerosus: Current Perspectives. Int. J. Womens Health 2020, 12, 11–20. [Google Scholar] [CrossRef]
- Corazza, M.; Schettini, N.; Zedde, P.; Borghi, A. Vulvar Lichen Sclerosus from Pathophysiology to Therapeutic Approaches: Evidence and Prospects. Biomedicines 2021, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Funaro, D.; Lovett, A.; Leroux, N.; Powell, J. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2014, 71, 84–91. [Google Scholar] [CrossRef]
- Goldstein, A.T.; Creasey, A.; Pfau, R.; Phillips, D.; Burrows, L.J. A double-blind, randomized controlled trial of clobetasol versus pimecrolimus in patients with vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2011, 64, e99–e104. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M.; Minghetti, S.; Virgili, A. Topical tretinoin in the treatment of vulvar lichen sclerosus: An advisable option? Eur. J. Dermatol. 2015, 25, 404–409. [Google Scholar] [CrossRef]
- Başkan, E.B.; Turan, H.; Tunali, S.; Toker, S.C.; Saricaoglu, H. Open-label trial of cyclosporine for vulvar lichen sclerosus. J. Am. Acad. Dermatol. 2007, 57, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Nayeemuddin, F.; Yates, V.M. Lichen sclerosus et atrophicus responding to methotrexate. Clin. Exp. Dermatol. 2008, 33, 651–652. [Google Scholar] [CrossRef]
- Manuelpillai, N.; Saunders, H.; Veysey, E. Management of severe vulval lichen sclerosus with adalimumab. Australas. J. Dermatol. 2022, 63, 248–250. [Google Scholar] [CrossRef]
- Declercq, A.; Güvenç, C.; De Haes, P. Proposition of standardized protocol for photodynamic therapy for vulvar lichen sclerosus. J. Dermatol. Treat. 2022, 33, 560–568. [Google Scholar] [CrossRef]
- Lee, A.; Bradford, J.; Fischer, G. Long-Term Management of Adult Vulvar Lichen Sclerosus: A Prospective Cohort Study of 507 Women. JAMA Dermatol. 2015, 151, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.; Scurry, J.; Bradford, J.; Lee, G.; Fischer, G. Association of Topical Corticosteroids with Reduced Vulvar Squamous Cell Carcinoma Recurrence in Patients with Vulvar Lichen Sclerosus. JAMA Dermatol. 2020, 156, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Vieira-Baptista, P.; Digesu, G.A.; Bretschneider, C.E.; Damaser, M.; Demirkesen, O.; Heller, D.S.; Mangir, N.; Marchitelli, C.; Mourad, S.; et al. The Clinical Role of LASER for Vulvar and Vaginal Treatments in Gynecology and Female Urology: An ICS/ISSVD Best Practice Consensus Document. J. Low Genit Tract Dis. 2019, 23, 151–160. [Google Scholar] [CrossRef]
- DeBruler, D.M.; Blackstone, B.N.; Baumann, M.E.; McFarland, K.L.; Wulff, B.C.; Wilgus, T.A.; Bailey, J.K.; Supp, D.M.; Powell, H.M. Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg. Med. 2017, 49, 675–685. [Google Scholar] [CrossRef]
- Longo, C.; Galimberti, M.; De Pace, B.; Pellacani, G.; Bencini, P.L. Laser skin rejuvenation: Epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Lasers Med. Sci. 2013, 28, 769–776. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Belotto, R.; Fernandes, R.C.M.; de Magalhães, A.C.; Correa, L.; Chavantes, M.C. Vulvar lichen sclerosus: Histopathological evaluation after post photobiomodulation treatment and topical corticosteroid. Lasers Surg. Med. 2018, 50, S44. [Google Scholar] [CrossRef]
- Ogrinc, U.B.; Senčar, S.; Luzar, B.; Lukanović, A. Efficacy of Non-Ablative Laser Therapy for Lichen Sclerosus: A Randomized Controlled Trial. J. Obstet. Gynaecol. Can. 2019, 41, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Belotto, R.; Correa, L.; Martins, W.K.; Fernandes, R.C.M.; Chavantes, M.C. Topic corticosteroid and photobiomodulation treatment impact on vulvar lichen sclerosus: Clinical, inflammatory and reparative analysis. Lasers Surg. Med. 2019, 51, S39–S40. [Google Scholar] [CrossRef]
- Burkett, L.S.; Siddique, M.; Zeymo, A.; Gutman, R.E.; Park, A.J.; Iglesia, C.B. Clobetasol Compared with Fractionated Carbon Dioxide Laser for Lichen Sclerosus: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 968–978. [Google Scholar] [CrossRef]
- Siddique, M.; Burkett, L.S.; Zeymo, A.; Gutman, R.E.; Park, A.J.; Iglesia, C.B. A randomized controlled trial of clobetasol propionate versus fractionated CO2 laser for the treatment of lichen sclerosus-Twelve month follow upand crossover outcomes (ecurls). Female Pelvic Med. Reconstr. Surg. 2020, 26 (Suppl. 10), S6. [Google Scholar] [CrossRef]
- Mitchell, L.; Goldstein, A.T.; Heller, D.; Mautz, T.; Thorne, C.; Kong, S.Y.J.; Sophocles, M.E.; Tolson, H.; Krapf, J.M. Fractionated Carbon Dioxide Laser for the Treatment of Vulvar Lichen Sclerosus: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.M.; Powell, J.J.; Wojnarowska, F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch. Dermatol. 2004, 140, 702–706. [Google Scholar] [CrossRef]
- Ruanphoo, P.; Bunyavejchevin, S. Treatment for vaginal atrophy using microablative fractional CO2 laser: A randomized double-blinded sham-controlled trial. Menopause 2020, 27, 858–863. [Google Scholar] [CrossRef]
- Cruz, V.L.; Steiner, M.L.; Pompei, L.M.; Strufaldi, R.; Fonseca, F.L.A.; Santiago, L.H.S.; Wajsfeld, T.; Fernandes, C.E. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gunter, J. Genitourinary Syndrome of Menopause and the False Promise of Vaginal Laser Therapy. JAMA Netw. Open 2023, 6, e2255706. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.R.; Connors, T.M.; Chan, W.; Liang, C.S.; Dao, H., Jr.; Vyas, A. Clinical outcomes and adherence to topical corticosteroid therapy in women with vulvar lichen sclerosus: A retrospective cohort study. J. Am. Acad. Dermatol. 2020, 83, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, M.; Lee, G.; Fischer, G. Why do some patients with vulval lichen sclerosus on long-term topical corticosteroid treatment experience ongoing poor quality of life? Australas. J. Dermatol. 2022, 63, 463–472. [Google Scholar] [CrossRef] [PubMed]
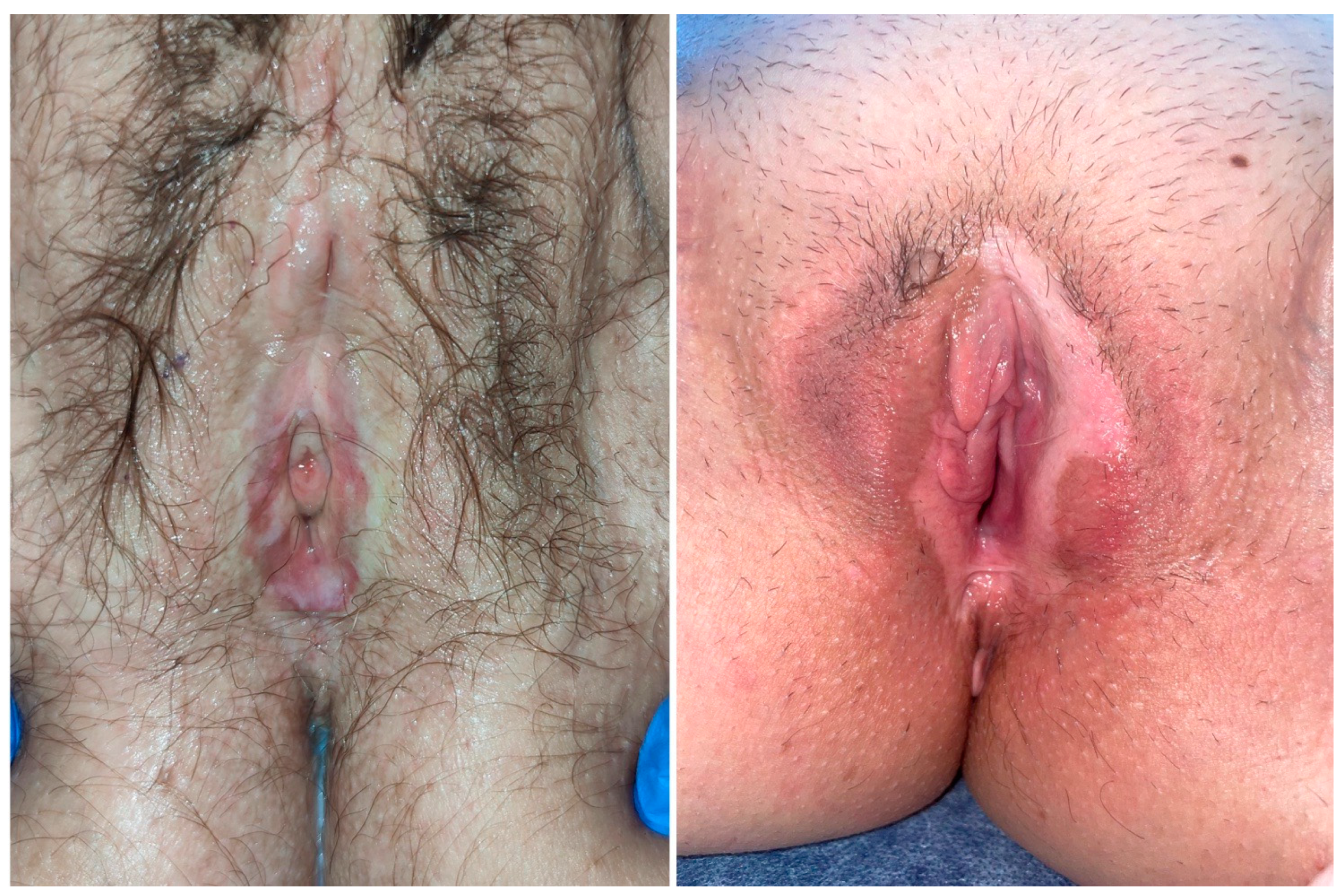
| Grade 1 (Moderate Changes) | Grade 2 (Severe Changes) | |
|---|---|---|
| Erosions | 1–2 small erosions, almost not macroscopically visible | Macroscopically visible and/or more than 2 or confluent lesions. |
| Hyperkertatosis | Affecting the vulva and perineum up to 10% | Affecting the vulva and perineum more than 10% |
| Fissures | Rhagades affecting the posterior introitus | Generalized vulvar rhagades |
| Agglutination | Partially affecting preputium clitoridis and labia minora | Complete agglutination of both |
| Stenosis | Narrowing of the introitus that could still be passed by two fingers | A narrowing that could be passed by less than two fingers |
| Atrophy | Shrinkage of labia minora and clitoris | A narrowing that could be passed by less than two fingers |
| Study | Design | n | Intervention | Scales/Parameters | Results | Follow Up | Conflicts of Interest |
|---|---|---|---|---|---|---|---|
| Belotto R., 2018 [23] | RCT | 30 ♀ |
| Histological changes (VLS1 y VLS2) | Both groups show inflammatory reduction and epithelial aspect improvement post-treatment. LG 86.7% vs. 33.3% VSL2 pre- and post-treatment; CG 86.7% vs. 26.7%. | 8 weeks, Control biopsy 90 days after | No stated |
| Bizjak Ogrinc U., 2019 [24] | RCT | 40 ♀ |
| VAS (burning, itching, pain) Tolerability VAS Histological Clinical photographs Patient satisfaction (0–3) |
| 1, 3, 6 months | No stated |
| Belotto R., 2019 [25] | RCT | 12 ♀ |
| VAS Histological Thermography |
| No stated | No stated |
| Burkett L., 2021 [26] | RCT | 52 ♀ |
| Skindex-29 Patient satisfaction: PGI-S PGI-I VAS VSQ VHI | Fifty-two participants, 27 pts each group, one dropout in steroid group.
| 6 months | No conflicts of interest |
| Mitchell L., 2021 [28] | RCT | 40 ♀ |
| CSS patient + investigator’s impressions. Biopsy (0–6 scale) Photos | Twenty patients in each group, three women excluded for not having control biopsy.
| 24-week treatment period + 8-week follow-up | Additional funding for this study was supplied by El.En Group, Florence, Italy, the manufacturer of the laser used in this study. In addition, El.En Group supplied the laser used in the study. |
| Siddique M., 2020 [27] | RCT + crossed over | 55 ♀ | Continuation of the previous study. After 6 moths, the patients still symptomatic crossed over: Steroid to Laser group (StLG), Laser to Steroid groups (LtSG) | Skindex-29 Patient satisfaction PGI-S PGI-I VAS VSQ VHI | Fifty-five participants, 48 had 12-month follow-up, 7 losses. Of the 48, 21 crossed over (11 from steroid to laser, 10 from laser to steroid).
| 6, 12 months | R. E. Gutman: Boston Scientific: Consultant and Grant/Research Support, Up To Date: Royalty, Johnson & Johnson: Expert Witness Sling Class Action; A. Park: Allergan: Speakers’ Bureau; C.B. Iglesia: Foundation for Female health Awareness made payable to MedStar Health Research Institute: Grant/Research Support. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Villalba, A.; Ayen-Rodriguez, A.; Naranjo-Diaz, M.J.; Ruiz-Villaverde, R. Laser Therapy for Vulvar Lichen Sclerosus, a Systematic Review. Life 2023, 13, 2146. https://doi.org/10.3390/life13112146
Gil-Villalba A, Ayen-Rodriguez A, Naranjo-Diaz MJ, Ruiz-Villaverde R. Laser Therapy for Vulvar Lichen Sclerosus, a Systematic Review. Life. 2023; 13(11):2146. https://doi.org/10.3390/life13112146
Chicago/Turabian StyleGil-Villalba, Ana, Angela Ayen-Rodriguez, Maria Jose Naranjo-Diaz, and Ricardo Ruiz-Villaverde. 2023. "Laser Therapy for Vulvar Lichen Sclerosus, a Systematic Review" Life 13, no. 11: 2146. https://doi.org/10.3390/life13112146
APA StyleGil-Villalba, A., Ayen-Rodriguez, A., Naranjo-Diaz, M. J., & Ruiz-Villaverde, R. (2023). Laser Therapy for Vulvar Lichen Sclerosus, a Systematic Review. Life, 13(11), 2146. https://doi.org/10.3390/life13112146







