Risk of SARS-CoV-2 Reinfection 3 Years after the Start of the Pandemic: A Population-Level Observational Study
Abstract
:1. Introduction
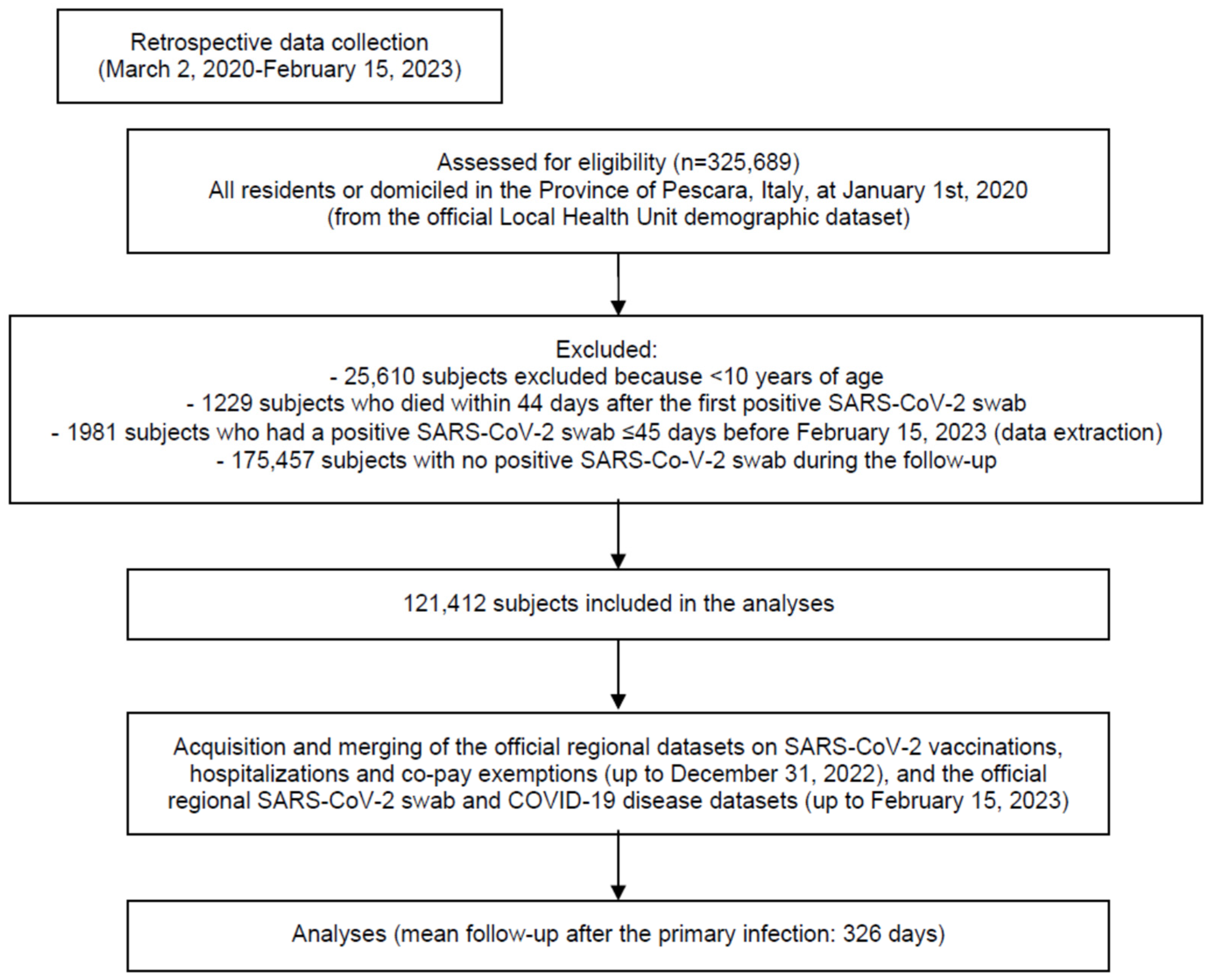
2. Materials and Methods
- -
- COVID-19 vaccines (from 1 January 2021—start of the vaccination campaign—to 31 December 2022);
- -
- SARS-CoV-2 laboratory or pharmacy assays and confirmed COVID-19 cases (up to 15 February 2023 allowing for at least 45 days of follow-up after immunization);
- -
- Demographic (Italian “Anagrafica” up to 15 February 2023).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, L.; Li, P.; Zhang, X.; Jiang, Q.; Turner, D.; Zhou, C.; Gao, Y.; Qian, F.; Zhang, C.; Lu, H.; et al. Risk of SARS-CoV-2 reinfection: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20763. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Izak, M.; Stoyanov, E.; Mandelboim, M.; Perlman, S.; Amir, Y.; Goren, S.; Bialik, A.; Kliker, L.; Atari, N.; et al. Predictors of reinfection with pre-Omicron and Omicron variants of concern among individuals who recovered from COVID-19 in the first year of the pandemic. Int. J. Infect. Dis. 2023, 132, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ukwishaka, J.; Ndayishimiye, Y.; Destine, E.; Danwang, C.; Kirakoya-Samadoulougou, F. Global prevalence of coronavirus disease 2019 reinfection: A systematic review and meta-analysis. BMC Public Health 2023, 23, 778. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. COVID-19 vaccines reduce the risk of SARS-CoV-2 reinfection and hospitalization: Meta-analysis. Front. Med. 2022, 9, 1023507. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. Risk of reinfection and disease after SARS-CoV-2 primary infection: Meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13845. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.D.; Amlag, J.O.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Föh, B.; Schnoor, M.; Balck, A.; Waldeck, F.; Käding, N.; Borsche, M.; Rupp, J.; Katalinic, A.; Klein, C. Transition to endemic: Two-year SARS-CoV-2 surveillance follow-up of the ELISA cohort. ERJ Open Res. 2023, 9. [Google Scholar] [CrossRef]
- Townsend, J.P.; Hassler, H.B.; Wang, Z.; Miura, S.; Singh, J.; Kumar, S.; Ruddle, N.H.; Galvani, A.P.; Dornburg, A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe 2021, 2, e666–e675. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Ma, K.C.; Dorabawila, V.; León, T.M.; Henry, H.; Johnson, A.G.; Rosenberg, E.; Mansfield, J.A.; Midgley, C.M.; Plumb, I.D.; Aiken, J.; et al. Trends in Laboratory-Confirmed SARS-CoV-2 Reinfections and Associated Hospitalizations and Deaths Among Adults Aged ≥ 18 Years–18 U.S. Jurisdictions, September 2021–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 683–689. [Google Scholar] [CrossRef]
- Chisale, M.R.O.; Sinyiza, F.W.; Kaseka, P.U.; Chimbatata, C.S.; Mbakaya, B.C.; Wu, T.J.; Nyambalo, B.W.; Chauma-Mwale, A.; Chilima, B.; Yu, K.J.; et al. Coronavirus Disease 2019 (COVID-19) Reinfection Rates in Malawi: A Possible Tool to Guide Vaccine Prioritisation and Immunisation Policies. Vaccines 2023, 30, 1185. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Khera, T.; Gethings, O.; Diamond, I.; Studley, R.; Taylor, N.; Peto, T.E.A.; Walker, A.S.; et al. Risk of SARS-CoV-2 reinfection during multiple Omicron variant waves in the UK general population. medRxiv 2023. [Google Scholar] [CrossRef]
- Acuña-Castillo, C.; Barrera-Avalos, C.; Bachelet, V.C.; Milla, L.A.; Inostroza-Molina, A.; Vidal, M.; Luraschi, R.; Vallejos-Vidal, E.; Mella-Torres, A.; Valdés, D.; et al. An ecological study on reinfection rates using a large dataset of RT-qPCR tests for SARS-CoV-2 in Santiago of Chile. Front. Public Health 2023, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Acuti Martellucci, C.; Soldato, G.; Carota, R.; Fazii, P.; Caponetti, A.; Manzoli, L. Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian province: A cohort study. J. Public Health 2022, 44, e475–e478. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 2022, 10, 884121. [Google Scholar] [CrossRef]
- Riccardo, F.; Andrianou, X.; Bella, A.; Del Manso, M.; Urdiales, A.M.; Fabiani, M.; Bellino, S.; Boros, S.; D’Ancona, F.; Rota, M.C.; et al. COVID-19 Integrated Surveillance System; Italian National Institute of Health: Rome, Italy, 2020; Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza (accessed on 7 January 2022).
- Center for Disease Control. Common Investigation Protocol for Investigating Suspected SARS-CoV-2 Reinfection; Center for Disease Control: Atlanta, GA, USA, 2020.
- UK Health Security Agency. Technical Summary-UKHSA Data Series on Deaths in People with COVID-19; UK Health Security Agency: London, UK, 2022.
- Rosso, A.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Acuti Martellucci, C.; Carota, R.; De Benedictis, M.; Di Marco, G.; Di Luzio, R.; Fiore, M.; et al. COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up. Vaccines 2023, 11, 1325. [Google Scholar] [CrossRef]
- Nishiura, H.; Ito, K.; Anzai, A.; Kobayashi, T.; Piantham, C.; Rodríguez-Morales, A.J. Relative Reproduction Number of SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta Variant in South Africa. J. Clin. Med. 2021, 11, 30. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Houhamdi, L.; Hoang, V.T.; Stoupan, D.; Fournier, P.E.; Raoult, D.; Colson, P.; Gautret, P. High rate of reinfection with the SARS-CoV-2 Omicron variant. J. Infect. 2022, 85, 174–211. [Google Scholar] [CrossRef]
- Jian, F.; Yu, Y.; Song, W.; Yisimayi, A.; Yu, L.; Gao, Y.; Zhang, N.; Wang, Y.; Shao, F.; Hao, X.; et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA.4 and BA.5 subvariants. Lancet Infect. Dis. 2022, 22, 1535–1537. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Zhang, Y.; Kappel, M.; Rathi, S.; Watkins, K.; Zhang, L.; Lockett, C. Rapid Increase in Suspected SARS-CoV-2 Reinfections, Clark County, Nevada, USA, December 2021. Emerg. Infect. Dis. 2022, 28, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Al-Najjar, Y.; Sharma, O.; Kamal, I.; Javed, A.; Gohil, H.S.; Paul, P.; Al-Khalifa, A.M.; Laws, S.; Zakaria, D. Role of previous infection with SARS-CoV-2 in protecting against omicron reinfections and severe complications of COVID-19 compared to pre-omicron variants: A systematic review. BMC Infect. Dis. 2023, 23, 432. [Google Scholar] [CrossRef] [PubMed]
- GISAID. hCoV-19 Variants Dashboard. 2023. Available online: https://gisaid.org/hcov-19-variants-dashboard/ (accessed on 17 July 2023).
- Ma, W.; Fu, H.; Jian, F.; Cao, Y.; Li, M. Immune evasion and ACE2 binding affinity contribute to SARS-CoV-2 evolution. Nat. Ecol. Evol. 2023, 7, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Severity and Outcomes of SARS-CoV-2 Reinfection Compared with Primary Infection: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3335. [Google Scholar] [CrossRef] [PubMed]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health–Eur. 2022, 20, 100453. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.A.; Lacy, J.; Stowe, J.; Seghezzo, G.; Sachdeva, R.; Simmons, R.; Bukasa, A.; O’Boyle, S.; Andrews, N.; Ramsay, M.; et al. Disease severity during SARS-CoV-2 reinfection: A nationwide study. J. Infect. 2022, 84, 542–550. [Google Scholar] [CrossRef]
- Davies, M.-A.; Morden, E.; Rousseau, P.; Arendse, J.; Bam, J.-L.; Boloko, L.; Cloete, K.; Cohen, C.; Chetty, N.; Dane, P.; et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa. Int. J. Infect. Dis. 2023, 127, 63–68. [Google Scholar] [CrossRef]
- Axfors, C.; Ioannidis, J.P.A. Infection fatality rate of COVID-19 in community-dwelling elderly populations. Eur. J. Epidemiol. 2022, 37, 235–249. [Google Scholar] [CrossRef]
- Law-Decree March 24 2022, n. 24 [Urgent Dispositions to Overcome the Measures to Contrast the Spread of the COVID-19 Epidemic, as a Consequence of the Termination of the Emergency State]. 2022. Available online: https://www.gazzettaufficiale.it/eli/id/2022/03/24/22G00034/sg (accessed on 21 August 2023).
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Porru, S.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Caliskan, G.; Violán, C.; Torán-Monserrat, P.; Vimercati, L.; Tafuri, S.; Boffetta, P.; et al. Incidence and Determinants of Symptomatic and Asymptomatic SARS-CoV-2 Breakthrough Infections After Booster Dose in a Large European Multicentric Cohort of Health Workers-ORCHESTRA Project. J. Epidemiol. Glob. Health 2023, 13, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Pellegrini, G.; Cereda, D.; Bortolan, F.; Leoni, O.; Pavesi, G.; Galli, M.; Valenti, G.; Corrao, G. Natural and vaccine-induced immunity are equivalent for the protection against SARS-CoV-2 infection. J. Infect. Public Health 2023, 16, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.F.; Amicizia, D.; Marchini, F.; Astengo, M.; Grammatico, F.; Battaglini, A.; Sticchi, C.; Paganino, C.; Lavieri, R.; Andreoli, G.B.; et al. Who Is at Higher Risk of SARS-CoV-2 Reinfection? Results from a Northern Region of Italy. Vaccines 2022, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Almadhi, M.; Alsayyad, A.S.; Conroy, R.; Atkin, S.; Awadhi, A.A.; Al-Tawfiq, J.A.; AlQahtani, M. Epidemiological assessment of SARS-CoV-2 reinfection. Int. J. Infect. Dis. 2022, 123, 9–16. [Google Scholar] [CrossRef]
- Vitale, J.; Mumoli, N.; Clerici, P.; De Paschale, M.; Evangelista, I.; Cei, M.; Mazzone, A. Assessment of SARS-CoV-2 Reinfection 1 Year after Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021, 181, 1407–1408. [Google Scholar] [CrossRef]
- Comelli, A.; Consonni, D.; Lombardi, A.; Viero, G.; Oggioni, M.; Bono, P.; Uceda Renteria, S.C.; Ceriotti, F.; Mangioni, D.; Muscatello, A.; et al. Nasopharyngeal Testing among Healthcare Workers (HCWs) of a Large University Hospital in Milan, Italy during Two Epidemic Waves of COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 8748. [Google Scholar] [CrossRef]
| Total Sample | Reinfections a | ||
|---|---|---|---|
| (n = 121,412) | (n = 6541) | ||
| Mean time after the primary infection, days (SD) | 326 (191) | 518 (204) | -- |
| Months since first infection | n | % (n) | 95% CI (%) |
| - Less than 6 (<183 days) | 121,412 | 0.84 (1019) | (0.79–0.89) |
| - 6–12 (183–365 days) | 101,446 b | 3.12 (3165) | (3.01–3.23) |
| - 13–18 (366–548 days) | 44,235 c | 2.05 (905) | (1.92–2.18) |
| - 19–24 (549–730 days) | 15,322 d | 7.34 (1125) | (6.93–7.77) |
| - 24+ (731–1076 days) | 8555 e | 3.82 (327) | (3.43–4.25) |
| Total Sample | Reinfections a | Reinfection | |
|---|---|---|---|
| Variables | n | % (n) | AHR (95% CI) |
| Overall | 121,412 | 5.39 (6541) | -- |
| Age class, years | |||
| 60+ | 28,761 | 5.49 (1579) | 1 (ref.) |
| 30–59 | 62,009 | 6.17 (3829) | 1.45 (1.34–1.57) |
| 10–29 | 30,642 | 3.70 (1133) | 1.17 (1.07–1.28) |
| Mean age in years (SD) | 46.1 ± 19.8 | 43.7 ± 18.1 | 1.00 (1.00–1.00) |
| Gender | |||
| - Males | 55,888 | 4.69 (2619) | 1 (ref.) |
| - Females | 65,524 | 5.99 (3922) | 1.37 (1.30–1.44) |
| Severe COVID-19 after the first infection | |||
| - No | 118,125 | 5.22 (6171) | 1 (ref.) |
| - Yes | 3287 | 11.3 (370) | 0.88 (0.79–0.98) |
| Diabetes b | |||
| - No | 115,515 | 5.40 (6239) | 1 (ref.) |
| - Yes | 5897 | 5.12 (302) | 1.04 (0.91–1.18) |
| Hypertension b | |||
| - No | 106,711 | 5.50 (5871) | 1 (ref.) |
| - Yes | 14,701 | 4.56 (670) | 1.15 (1.04–1.27) |
| Cardiovascular diseases b | |||
| - No | 110,945 | 5.31 (5895) | 1 (ref.) |
| - Yes | 10,467 | 6.17 (646) | 0.93 (0.85–1.03) |
| COPD b | |||
| - No | 116,246 | 5.34 (6210) | 1 (ref.) |
| - Yes | 5166 | 6.41 (331) | 1.14 (1.02–1.27) |
| Kidney diseases b | |||
| - No | 119,339 | 5.36 (6400) | 1 (ref.) |
| - Yes | 2073 | 6.80 (141) | 1.23 (1.03–1.47) |
| Cancer b | |||
| - No | 115,008 | 5.43 (6247) | 1 (ref.) |
| - Yes | 6404 | 4.59 (294) | 1.05 (0.93–1.19) |
| At least one chronic condition c | |||
| - No | 93,920 | 5.39 (5062) | 1 (ref.) |
| - Yes | 27,492 | 5.38 (1479) | 1.13 (1.05–1.20) |
| SARS-CoV-2 vaccine d | |||
| - No doses | 15,030 | 11.2 (1681) | 1 (ref.) |
| - 1 or 2 doses | 38,741 | 8.14 (3154) | 0.69 (0.65–0.74) |
| - 3 or more doses | 67,641 | 2.52 (1706) | 0.71 (0.66–0.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuti Martellucci, C.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Carota, R.; Rosso, A.; De Benedictis, M.; Di Marco, G.; Di Luzio, R.; Lisbona, F.; et al. Risk of SARS-CoV-2 Reinfection 3 Years after the Start of the Pandemic: A Population-Level Observational Study. Life 2023, 13, 2111. https://doi.org/10.3390/life13112111
Acuti Martellucci C, Flacco ME, Soldato G, Di Martino G, Carota R, Rosso A, De Benedictis M, Di Marco G, Di Luzio R, Lisbona F, et al. Risk of SARS-CoV-2 Reinfection 3 Years after the Start of the Pandemic: A Population-Level Observational Study. Life. 2023; 13(11):2111. https://doi.org/10.3390/life13112111
Chicago/Turabian StyleAcuti Martellucci, Cecilia, Maria Elena Flacco, Graziella Soldato, Giuseppe Di Martino, Roberto Carota, Annalisa Rosso, Marco De Benedictis, Graziano Di Marco, Rossano Di Luzio, Francesco Lisbona, and et al. 2023. "Risk of SARS-CoV-2 Reinfection 3 Years after the Start of the Pandemic: A Population-Level Observational Study" Life 13, no. 11: 2111. https://doi.org/10.3390/life13112111
APA StyleAcuti Martellucci, C., Flacco, M. E., Soldato, G., Di Martino, G., Carota, R., Rosso, A., De Benedictis, M., Di Marco, G., Di Luzio, R., Lisbona, F., Caponetti, A., & Manzoli, L. (2023). Risk of SARS-CoV-2 Reinfection 3 Years after the Start of the Pandemic: A Population-Level Observational Study. Life, 13(11), 2111. https://doi.org/10.3390/life13112111









