Cyclic Stability of Locking Plate Augmented with Intramedullary Polymethyl Methacrylate (PMMA) Strut Fixation for Osteoporotic Humeral Fractures: A Biomechanical Study
Abstract
1. Introduction
2. Materials and Methods
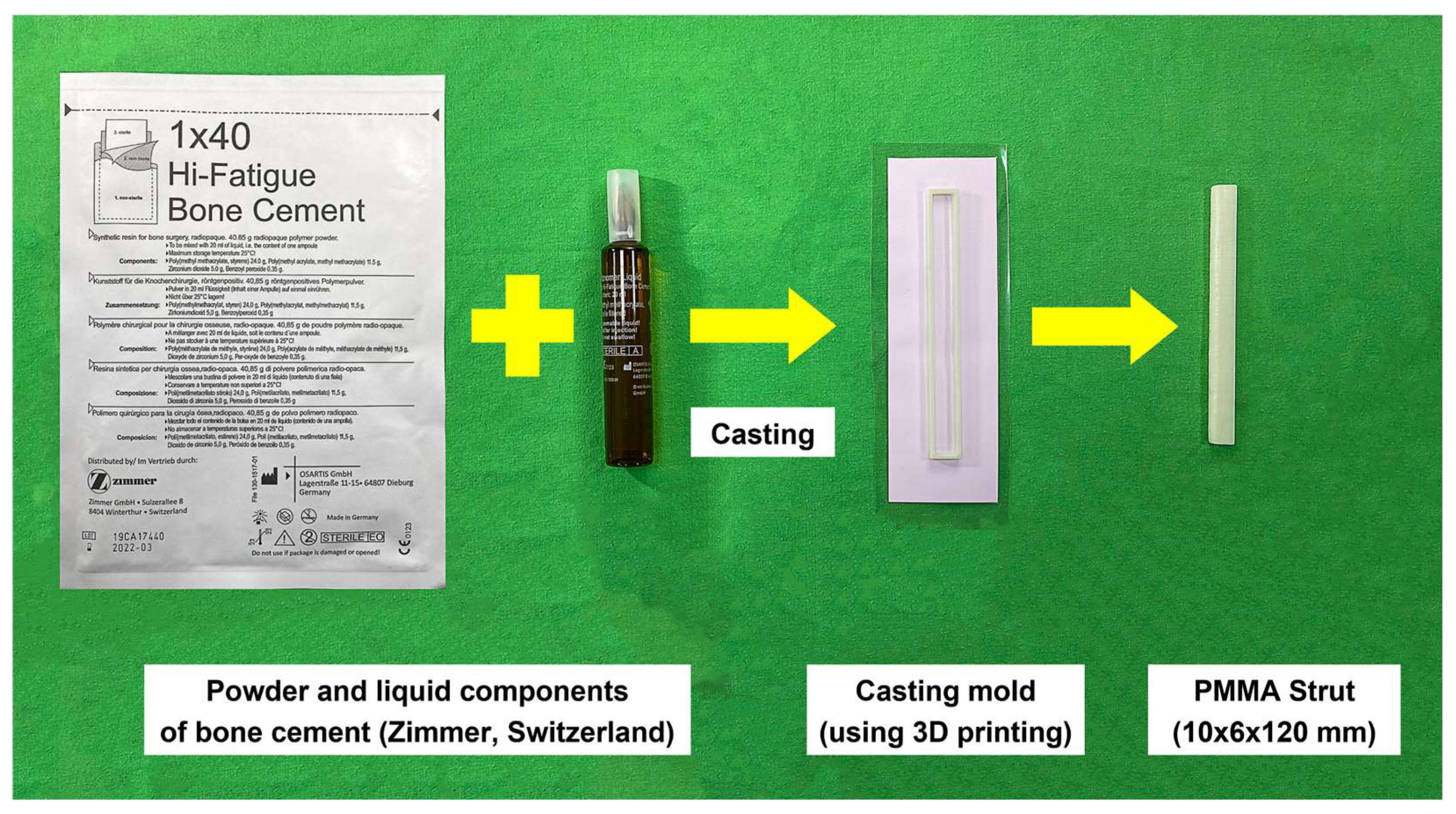
2.1. PMMA Intramedullary Strut
2.2. Artificial Bone Model, Implants and Fixations
2.3. Mechanical Testing
- Intercyclic motion: fragment gap amplitude (peak-to-peak displacement in 1 cycle) at 100, 1000, 2000, 4000, 6000, 8000 and 10,000 cycles;
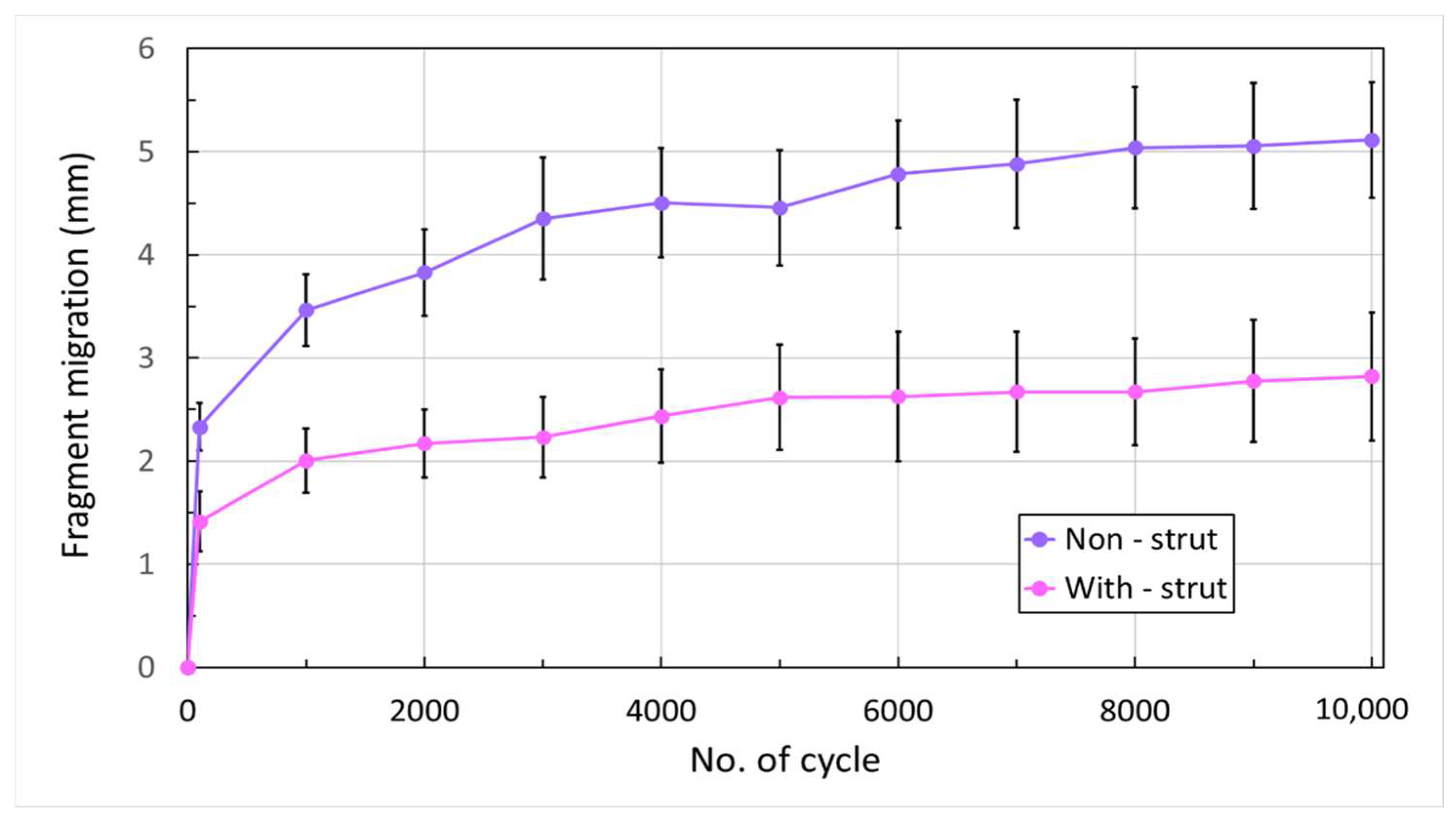
- Cumulated fragment migration: the total change in gap distance at 450 N loading condition from the initial cycle to each 1000-cycle interval up to 10,000 cycles;
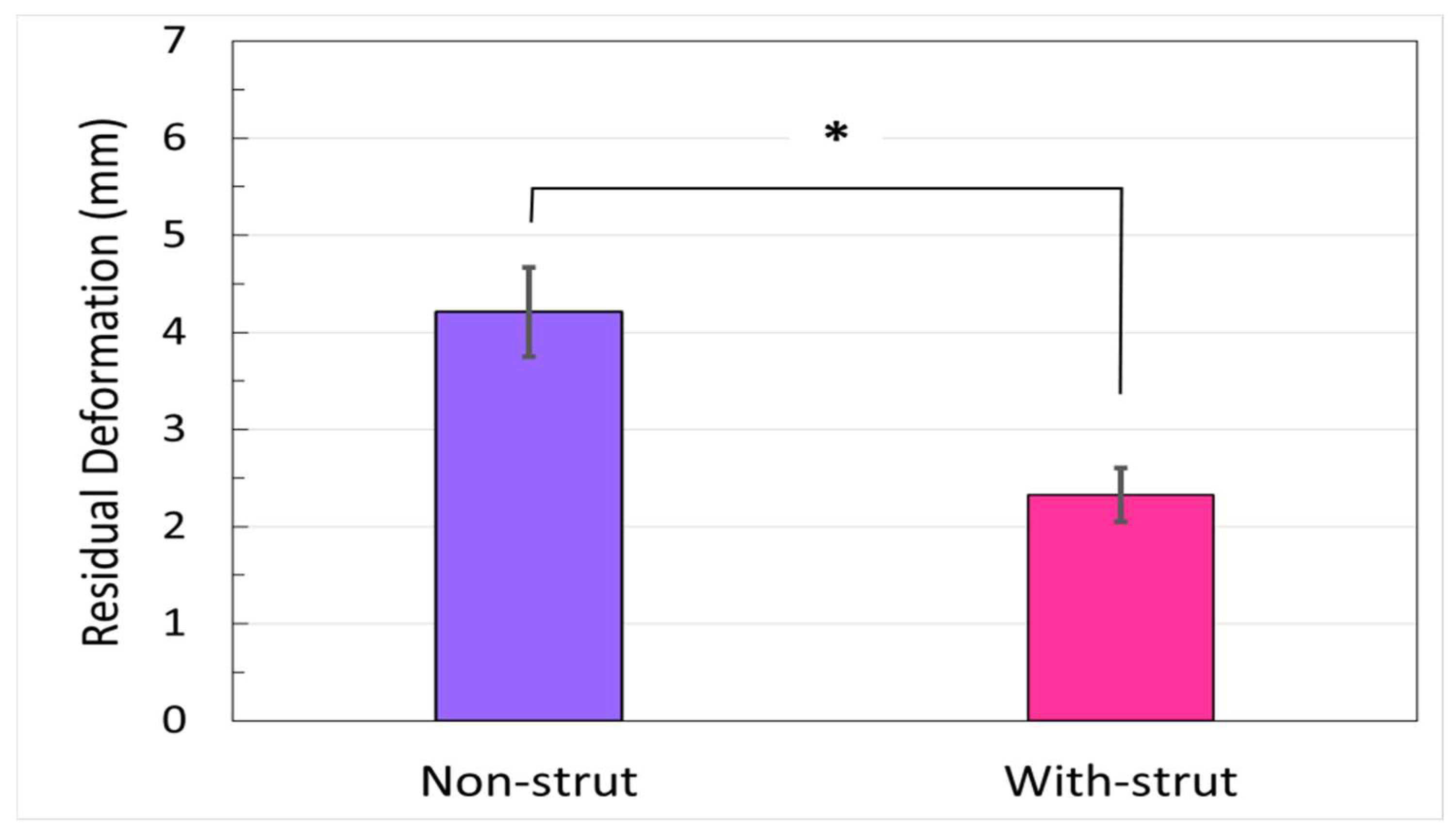
- Residual osteotomy gap deformation: cumulated plastic deformation at osteotomy gap from 100 to 10,000 cycles in the unloaded condition.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, H.P.; Gutbrod, J.; Strelzow, J.A.; Maassen, N.H.; Shi, L. Management of Proximal Humerus Fractures in Adults—A Scoping Review. J. Clin. Med. 2022, 11, 6140. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, T.F.; Hawkins, R.J. Displaced proximal humeral fractures: Evaluation and treatment. J. Am. Acad. Orthop. Surg. 1994, 2, 54–78. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Wilson, N.A.; Zhang, L.Q.; Flores, S.; Merk, B.R. Two-part surgical neck fractures of the proximal part of the humerus. A biomechanical evaluation of two fixation techniques. J. Bone Jt. Surg. Am. 2006, 88, 2258–2264. [Google Scholar]
- Laux, C.J.; Grubhofer, F.; Werner, C.M.L.; Simmen, H.P.; Osterhoff, G. Current concepts in locking plate fixation of proximal humerus fractures. J. Orthop. Surg. Res. 2017, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Seide, K.; Triebe, J.; Faschingbauer, M.; Schulz, A.; Püschel, K.; Mehrtens, G.; Jürgens, C. Locked vs. unlocked plate osteosynthesis of the proximal humerus—A biomechanical study. Clin. Biomech. 2007, 22, 176–182. [Google Scholar] [CrossRef]
- Walsh, S.; Reindl, R.; Harvey, E.; Berry, G.; Beckman, L.; Steffen, T. Biomechanical comparison of a unique locking plate versus a standard plate for internal fixation of proximal humerus fractures in a cadaveric model. Clin. Biomech. 2006, 21, 1027–1031. [Google Scholar] [CrossRef]
- Egol, K.A.; Ong, C.C.; Walsh, M.; Jazrawi, L.M.; Tejwani, N.C.; Zuckerman, J.D. Early complications in proximal humerus fractures (OTA Types 11) treated with locked plates. J. Orthop. Trauma 2008, 22, 159–164. [Google Scholar] [CrossRef]
- Bjorkenheim, J.M.; Pajarinen, J.; Savolainen, V. Internal fixation of proximal humeral fractures with a locking compression plate: A retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop. Scand. 2004, 75, 741–745. [Google Scholar] [CrossRef]
- Krappinger, D.; Bizzotto, N.; Riedmann, S.; Kammerlander, C.; Hengg, C.; Kralinger, F.S. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011, 42, 1283–1288. [Google Scholar] [CrossRef]
- Brunner, F.; Sommer, C.; Bahrs, C.; Heuwinkel, R.; Hafner, C.; Rillmann, P.; Babst, R. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: A prospective multicenter analysis. J. Orthop. Trauma 2009, 23, 163–172. [Google Scholar] [CrossRef]
- Foruria, A.M. Plate Fixation of Proximal Humerus Fractures: How to Get It Right and Future Directions for Improvement. Curr. Rev. Musculoskelet. Med. 2023, 16, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Owsley, K.C.; Gorczyca, J.T. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J. Bone Jt. Surg. Am. 2008, 90, 233–240, Erratum in J. Bone Jt. Surg. Am. 2008, 90, 862. [Google Scholar] [CrossRef] [PubMed]
- Siebenbürger, G.; Neudeck, R.; Daferner, M.P.; Fleischhacker, E.; Böcker, W.; Ockert, B.; Helfen, T. It Is Always the Same—A Complication Classification following Angular Stable Plating of Proximal Humeral Fractures. J. Clin. Med. 2023, 28, 2556. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kang, S.W.; Jung, K.H.; Oh, Y.K. The potential of locking plate with intramedullary fibular allograft to manage proximal humeral fracture with an unstable medial column. Arch. Orthop. Trauma Surg. 2022, 142, 91–97. [Google Scholar] [CrossRef]
- Mathison, C.; Chaudhary, R.; Beaupre, L.; Reynolds, M.; Adeeb, S.; Bouliane, M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin. Biomech. 2010, 25, 642–646. [Google Scholar] [CrossRef]
- Osterhoff, G.; Baumgartner, D.; Favre, P.; Wanner, G.A.; Gerber, H.; Simmen, H.P.; Werner, C.M. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: An in vitro study with synthetic bone. J. Shoulder Elb. Surg. 2011, 20, 740–746. [Google Scholar] [CrossRef]
- Rojas, J.T.; Rashid, M.S.; Zumstein, M.A. How to treat stiffness after proximal humeral fractures? EFORT Open Rev. 2023, 1, 651–661. [Google Scholar] [CrossRef]
- Kwisda, S.; Imiolczyk, J.P.; Imiolczyk, T.; Werth, M.; Scheibel, M.; Lee, C.W.; Shin, S.S. A Standardized Operative Protocol for Fixation of Proximal Humeral Fractures Using a Locking Plate to Minimize Surgery-Related Complications. J. Clin. Med. 2023, 3, 1216. [Google Scholar] [CrossRef]
- Pak, P.; Eng, K.; Page, R.S. Fixed-angle locking proximal humerus plate: An evaluation of functional results and implant-related outcomes. ANZ J. Surg. 2013, 83, 878–882. [Google Scholar] [CrossRef]
- Bae, J.H.; Oh, J.K.; Chon, C.S.; Oh, C.W.; Hwang, J.H.; Yoon, Y.C. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J. Bone Jt. Surg. Br. 2011, 98, 937–941. [Google Scholar] [CrossRef]
- Chow, R.M.; Begum, F.; Beaupre, L.A.; Carey, J.P.; Adeeb, S.; Bouliane, M.J. Proximal humeral fracture fixation: Locking plate construct ± intramedullary fibular allograft. J. Shoulder Elb. Surg. 2012, 21, 894–901. [Google Scholar] [CrossRef]
- Lescheid, J.; Zdero, R.; Shah, S.; Kuzyk, P.R.; Schemitsch, E.H. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J. Trauma 2010, 69, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Brianza, S.; Plecko, M.; Gueorguiev, B.; Windolf, M.; Schwieger, K. Biomechanical evaluation of a new fixation technique for internal fixation of three-part proximal humerus fractures in a novel cadaveric model. Clin. Biomech. 2010, 25, 886–892. [Google Scholar] [CrossRef]
- Hsiao, C.K.; Tsai, Y.J.; Yen, C.Y.; Lee, C.H.; Yang, T.Y.; Tu, Y.K. Intramedullary cortical bone strut improves the cyclic stability of osteoporotic proximal humeral fractures. BMC Musculoskelet. Disord. 2017, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Paolin, A.; Romualdi, C.; Romagnoli, L.; Trojan, D. Analysis of potential factors affecting allografts contamination at retrieval. Cell Tissue Bank. 2017, 18, 539–545. [Google Scholar] [CrossRef]
- Roberti Di Sarsisa, T.; Fiore, M.; Coco, V.; Govoni, M.; Vivarelli, L.; Rani, N.; Del Piccolo, N.; Dallari, D. Fresh Osteochondral Allograft Transplantation in Osteochondritis Dissecans in the Knee Joint. Life 2021, 8, 1205. [Google Scholar] [CrossRef] [PubMed]
- Traore, A.; Yombi, J.C.; Tribak, K.; Cornu, O. Risk of virus transmission through femoral head allografts: A Belgian appraisal. J. Clin. Orthop. Trauma 2013, 4, 119–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaishya, R.; Chauhan, M.; Abhishek, V.A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Chan, E.K.S.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef]
- Wähnert, D.; Hoffmeier, K.L.; Klos, K.; Stolarczyk, Y.; Fröber, R.; Hofmann, G.O.; Mückley, T. Biomechanical characterization of an osteoporotic artificial bone model for the distal femur. J. Biomater. Appl. 2012, 26, 565–579. [Google Scholar] [CrossRef]
- Shin, S.S.; Della Valle, C.J.; Ong, B.C.; Meere, P.A. A simple method for construction of an articulating antibiotic-loaded cement spacer. J. Arthroplasty 2002, 17, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Patel, M.; Braden, M. Use of prepolymerized cement spacers to augment the femoral component in revision total knee arthroplasty: A clinical outcome report and laboratory experiment. J. Arthroplasty 2003, 18, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Pitto, R.P.; Spika, I.A. Antibiotic-loaded bone cement spacers in two-stage management of infected total knee arthroplasty. Int. Orthop. 2004, 28, 129–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Durbhakula, S.M.; Czajka, J.; Fuchs, M.D.; Uhl, R.L. Spacer endoprosthesis for the treatment of infected total hip arthroplasty. J. Arthroplasty 2004, 19, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Meyer, C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. Biomed. Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef]
- Wilson, D.; Frei, H.; Masri, B.A.; Oxland, T.R.; Duncan, C.P. A biomechanical study comparing cortical onlay allograft struts and plates in the treatment of periprosthetic femoral fractures. Clin. Biomech. 2005, 20, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hertel, R. Fractures of the proximal humerus in osteoporotic bone. Osteoporos. Int. 2005, 16, 65–72. [Google Scholar] [CrossRef]
- Schumer, R.A.; Muckley, K.L.; Markert, R.J.; Prayson, M.J.; Heflin, J.; Konstantakos, E.K.; Goswami, T. Biomechanical comparison of a proximal humeral locking plate using two methods of head fixation. J. Shoulder Elb. Surg. 2010, 19, 495–501. [Google Scholar] [CrossRef]
- Varga, P.; Inzana, J.A.; Fletcher, J.W.A.; Hofmann-Fliri, L.; Runer, A.; Südkamp, N.P.; Windolf, M. Cement augmentation of calcar screws may provide the greatest reduction in predicted screw cut-out risk for proximal humerus plating based on validated parametric computational modelling: Augmenting proximal humerus fracture plating. Bone Jt. Res. 2020, 3, 534–542. [Google Scholar] [CrossRef]
- Kralinger, F.; Gschwenter, M.; Wambacher, M.; Smekal, V.; Haid, C. Proximal humeral fractures: What is semi-rigid? Biomechanical properties of semi-rigid implants, a biomechanical cadaver based evaluation. Arch. Orthop. Trauma Surg. 2007, 128, 205–210. [Google Scholar] [CrossRef]
- Zettl, R.; Müller, T.; Topp, T.; Lewan, U.; Krüger, A.; Kühne, C.; Ruchholtz, S. Monoaxial versus polyaxial locking systems: A biomechanical analysis of different locking systems for the fixation of proximal humeral fractures. Int. Orthop. (SICOT) 2011, 35, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Praagman, M.; Stokdijk, M.; Veeger, H.E.; Visser, B. Predicting mechanical load of the glenohumeral joint, using net joint moments. Clin. Biomech. 2000, 15, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Laursen, B.; Jensen, B.R.; Nemeth, G.; Sjogaard, G. A model predicting individual shoulder muscle forces based on relation-ship between electromyographic and 3D external forces in static position. J. Biomech. 1998, 31, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Beirer, M.; Crönlein, M.; Venjakob, A.J.; Saier, T.; Schmitt Sody, M.; Huber Wagner, S.; Biberthaler, P.; Kirchhoff, C. Additional calcar support using a blade device reduces secondary varus displacement following reconstruction of the proximal humerus: A prospective study. Eur. J. Med. Res. 2015, 20, 82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lautenschlager, E.P.; Jacobs, J.J.; Marshall, G.W.; Meyer, P.R.J. Mechanical properties of bone cements containing large doses of antibiotic powders. J. Biomed. Mater. Res. 1976, 10, 929–938. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wang, J.C.; Pei, Y.L. Adding vancomycin to bone cement: Research on its influence on mechanical and fixation strength using rabbit femoral prostheses. Orthop. Surg. 2011, 3, 265–267. [Google Scholar] [CrossRef]
- Paz, E.; Sanz-Ruiz, P.; Abenojar, J.; Vaquero-Martín, J.; Forriol, F.; Del Real, J.C. Evaluation of Elution and Mechanical Properties of High-Dose Antibiotic-Loaded Bone Cement: Comparative “In Vitro” Study of the Influence of Vancomycin and Cefazolin. J. Arthroplasty 2015, 30, 1423–1429. [Google Scholar] [CrossRef]
- Muratlı, S.K.; Karatosun, V.; Uzun, B.; Günal, İ. Biomechanical comparison of tigecycline loaded bone cement with vancomycin and daptomycin loaded bone cements. Acta Orthop. Traumatol. Turc. 2020, 54, 535–540. [Google Scholar] [CrossRef]
- Vallier, H.A.; Cureton, B.A.; Patterson, B.M. Randomized, prospective comparison of plate versus intramedullary nail fixation for distal tibia shaft fractures. J. Orthop. Trauma 2011, 25, 736–741. [Google Scholar] [CrossRef]
- De Giacomo, A.F.; Tornetta, P. III Alignment after intramedullary nailing of distal tibia fractures without fibula fixation. J. Orthop. Trauma 2016, 30, 561–567. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-K.; Chiu, Y.-W.; Hsiao, H.-Y.; Tsai, Y.-J.; Lee, C.-H.; Yen, C.-Y.; Tu, Y.-K. Cyclic Stability of Locking Plate Augmented with Intramedullary Polymethyl Methacrylate (PMMA) Strut Fixation for Osteoporotic Humeral Fractures: A Biomechanical Study. Life 2023, 13, 2110. https://doi.org/10.3390/life13112110
Hsiao C-K, Chiu Y-W, Hsiao H-Y, Tsai Y-J, Lee C-H, Yen C-Y, Tu Y-K. Cyclic Stability of Locking Plate Augmented with Intramedullary Polymethyl Methacrylate (PMMA) Strut Fixation for Osteoporotic Humeral Fractures: A Biomechanical Study. Life. 2023; 13(11):2110. https://doi.org/10.3390/life13112110
Chicago/Turabian StyleHsiao, Chih-Kun, Yen-Wei Chiu, Hao-Yuan Hsiao, Yi-Jung Tsai, Cheng-Hung Lee, Cheng-Yo Yen, and Yuan-Kun Tu. 2023. "Cyclic Stability of Locking Plate Augmented with Intramedullary Polymethyl Methacrylate (PMMA) Strut Fixation for Osteoporotic Humeral Fractures: A Biomechanical Study" Life 13, no. 11: 2110. https://doi.org/10.3390/life13112110
APA StyleHsiao, C.-K., Chiu, Y.-W., Hsiao, H.-Y., Tsai, Y.-J., Lee, C.-H., Yen, C.-Y., & Tu, Y.-K. (2023). Cyclic Stability of Locking Plate Augmented with Intramedullary Polymethyl Methacrylate (PMMA) Strut Fixation for Osteoporotic Humeral Fractures: A Biomechanical Study. Life, 13(11), 2110. https://doi.org/10.3390/life13112110





